Abstract
The dynamics of infectious diseases are highly variable. Host ranges, host responses to pathogens and the relationships between hosts are heterogeneous. Here, we argue that the use of animal sentinels has the potential to use this variation and enable the exploitation of a wide range of pathogen hosts for surveillance purposes. Animal sentinels may be used to address many surveillance questions, but they may currently be underused as a surveillance tool and there is a need for improved interdisciplinary collaboration and communication in order to fully explore the potential of animal sentinels. In different contexts, different animal hosts will themselves vary in their capacity to provide useful information. We describe a conceptual framework within which the characteristics of different host populations and their potential value as sentinels can be evaluated in a broad range of settings.
Keywords: sentinel, surveillance, infectious diseases, epidemiology
1. Introduction
The dynamics of infectious disease systems are inherently variable. The outcome of any infection depends on multiple factors relating to pathogen characteristics, host susceptibility, infecting dose and routes of transmission, all of which can vary widely for any particular infectious organism. Many of the major diseases of medical, veterinary and conservation importance (such as highly pathogenic avian influenza (HPAI), foot-and-mouth disease, bluetongue and rabies) are caused by pathogens with wide host ranges (Woolhouse & Gowtage-Sequeria 2005), which introduces further complexity.
While the complex epidemiology of multi-host pathogens presents considerable challenges for understanding infection dynamics and implementing disease control, heterogeneities in host range and infection outcome also provide opportunities for disease surveillance. In this paper, we develop a conceptual framework that can be applied to examine those characteristics of host populations that influence their potential value as sentinels for disease surveillance in different ecological and epidemiological settings.
Surveillance is defined by the World Health Organization as ‘the ongoing systematic collection, collation, analysis and interpretation of data and the dissemination of information to those who need to know in order for action to be taken’ (World Health Organization 2001). The aim of disease surveillance is to identify changes in the infection and/or health status of animal and human populations and is essential to provide rigorous evidence of the absence of disease or to determine the prevalence of a pathogen when present (Salman 2003). A critical element of surveillance is that an identified response is made on the basis of the surveillance data generated to allow appropriate action to be taken. Sentinel surveillance is one form of surveillance in which activities focus on specific subpopulations to enhance detection of disease and/or improve the cost-effectiveness of surveillance (McCluskey 2003). The aim of the sentinel surveillance is to obtain timely information in a relatively inexpensive manner rather than to derive precise estimates of prevalence or incidence in the general population (Centers for Disease Control and Prevention 2002). It has long been recognized that animal populations have the potential to act as sentinels for environmental health hazards (CAMEH 1991), but, given the importance of domestic and wild animal hosts in emerging human diseases, it is clear that surveillance in animals is also critical for understanding and managing emerging disease threats (Kuiken et al. 2005; Woolhouse & Gowtage-Sequeria 2005; Kahn 2006). Animal sentinels almost certainly represent an important but underused surveillance tool (Rabinowitz et al. 2005) that may be capable of accommodating and capitalizing on the variability that exists in infectious disease processes.
Animal sentinels may potentially be used to address a range of surveillance questions including (i) detection of a pathogen in a new area, (ii) detection of changes in the prevalence or incidence of a pathogen or disease over time, (iii) determining the rates and direction of pathogen spread, (iv) testing specific hypotheses about the ecology of a pathogen, and (v) evaluating the efficacy of potential disease control interventions (McCluskey 2003). The appropriate use of animal sentinels can facilitate the early detection and identification of outbreaks that is of critical importance for the success of control and prevention efforts (Chomel 2003; Kahn 2006) and reducing the magnitude of subsequent outbreaks (Ferguson et al. 2005). However, the potential of animal sentinel surveillance can only be realized if the information provided from animal populations is acted upon. For example, in an Ebola outbreak in central Africa, few preventive health measures were taken despite warnings of an imminent human outbreak being provided from monitoring of Ebola deaths in primate sentinels (Rouquet et al. 2005).
The term ‘sentinel’ is widely used in both epidemiological and veterinary clinical literature and is implicitly understood but rarely defined. While all uses invoke the common concept of standing guard or keeping watch, existing definitions tend to be context-specific. The classic example of an animal sentinel is that of the coal miner's canary. In this case, an individual animal of a different species is deliberately selected and placed in a situation where it can provide evidence of increased risk to the human population on the basis of its greater sensitivity and obvious observable response to the presence of carbon monoxide. Since the mid-twentieth century, it has been recognized that animals can act as important sentinels for a wide range of environmental health hazards (CAMEH 1991). For example, domestic dogs and the tumours they develop may facilitate identification of environmental carcinogens that affect humans (Thrusfield 2005). Sentinels can vary from individual animals to herds or larger populations, from animals of the same species to different, more susceptible, more expendable or more accessible species, and from animals deliberately placed or introduced to those already existing in a particular location. The sentinel concept can also refer to a physical location, such as a farm, abattoir, veterinary practice or laboratory (the ‘sentinel unit’) which is selected to monitor a particular disease (table 1). Throughout this paper, we use ‘animal sentinels’ as an umbrella term for the topic in general and ‘sentinel population’ to refer to the unit of observation in a particular case.
Table 1.
Summary of applications of animal sentinels for environmental and infectious hazards.
| type of sentinel | example | reference |
|---|---|---|
| individual animal | coal miner's canary used to detect the presence of carbon monoxide | Burrell & Seibert (1916); Schwabe (1984) |
| herd/population | sentinel cattle herds and chicken flocks used to monitor the distribution of arboviruses and their vectors in Australia and the USA | National Arbovirus Monitoring Program (2003–2004); Loftin et al. (2006) |
| same species | unvaccinated chickens placed within vaccinated flock to detect HPAI | Suarez (2005) |
| different, more susceptible species | feral pigs released into New Zealand to detect the presence of bovine TB—more susceptible than possums; coal miner's canary (as above) | Nugent et al. (2002) |
| sentinel application | example | reference |
| deliberately placed (experimental) | standard laboratory mice sentinel programmes using outbred mice, sacrificed and tested to detect presence of a panel of rodent pathogens in the core experimental or breeding colony | Institute of Laboratory Animal Resources (US). Committee on Infectious Diseases of Mice and Rats (1991) |
| use of sentinel chickens to evaluate the effectiveness of cleaning and disinfection procedures for eradication of Newcastle disease | McCluskey et al. (2006) | |
| in natural habitat (observational) | wildlife as detectors of DDT and PCB toxicityevaluation of white-tailed deer as natural sentinels for Anaplasma phagocytophilum, the cause of human granulocytic anaplasmosis | CAMEH (1991) Dugan et al. (2006) |
| mesothelioma in pet dogs associated with exposure of their owners to asbestos | Glickman et al. (1983) | |
| sentinel unit | equine premises used to investigate presence of vesicular stomatitis in Colorado | McCluskey et al. (2002) |
Despite the apparent potential for animal sentinels to inform decisions about risk to both human and animal populations, animal sentinels appear underutilized, particularly in the context of infectious disease surveillance (Rabinowitz et al. 2005), and their value has been discussed primarily in the context of environmental health (CAMEH 1991). A basic lack of integration between disciplines, most noticeably between human and veterinary medicine and also between different branches within these fields, is likely to have contributed to this underuse of animal sentinels (Rabinowitz et al. 2005). There are currently no standard criteria which are applied for the evaluation of animal sentinels, limiting the ease with which data can be transferred between disciplines (Rabinowitz et al. 2005). The existing infectious disease literature regarding animal sentinels consists largely of descriptive studies that have generated hypotheses regarding animal sentinel use (Rabinowitz et al. 2005), but as yet includes few studies that were purposefully designed to evaluate their potential. The major exception is the extensive research that has been carried out into the use of animal sentinels in the surveillance of West Nile virus (WNV) in North America (see box 1). The utility of different animal sentinel populations varies enormously according to both the ecological context and the aim of the surveillance programme, and in many cases animal sentinels may not prove a useful surveillance tool.
Box 1. West Nile virus surveillance in North America: animal sentinel case study.
West Nile virus (WNV), an arbovirus of the genus Flaviviridae, is maintained in a mosquito–bird–mosquito cycle primarily involving Culex sp. mosquitoes (Campbell et al. 2002). Humans and other mammal species are incidental dead-end hosts. The majority of human infections with WNV are asymptomatic or result in transient febrile illness but in a small proportion of cases, meningoencephalitis can occur (Mostashari et al. 2001). The geographical range of WNV has historically included Africa, Europe, Asia and Australia (Campbell et al. 2002). In 1999, the first North American cases of WNV were reported in New York and since then the virus has spread across the continental United States and into Canada, Latin America and the Caribbean (Hayes & Gubler 2006). The surveillance of WNV in North America has included investigation of the utility of different animal sentinels. Some of the findings of these studies are described below with reference to the sentinel framework.
Sentinel response to pathogen
A number of North American bird species including corvids, house sparrows, house finches and grackles are competent reservoirs for mosquito infection with WNV (Komar et al. 2003). Among these potential sentinel species, corvids and specifically American crows (Corvus brachyrhynchos) are particularly susceptible to infection with WNV and have a high mortality rate (McLean et al. 2001; Komar et al. 2003; Yaremych et al. 2004). In 2000, it was established that dead crow reports preceded both the confirmation of viral activity (through laboratory analysis) and the onset of human cases by several months (Eidson et al. 2001b). Subsequent spatial analyses using data collected in New York have identified a positive association between the risk of human disease caused by WNV and elevated local dead crow reports in the previous one to two weeks (Mostashari et al. 2003; Eidson et al. 2005; Johnson et al. 2006). The thorough characterization of this temporal association ensures that the observation of crow deaths can be acted upon immediately without the need for time-consuming laboratory analyses. The observation of clusters of high crow mortality can therefore be used to predict human risk early enough to implement targeted mosquito control and personal protection warnings (Mostashari et al. 2003; Eidson et al. 2005; Johnson et al. 2006).
Relationship between sentinel and target populations
Domestic dogs have also been evaluated as sentinels of WNV presence (Komar et al. 2001; Kile et al. 2005). This sentinel choice is informed by the particular relationship that domestic dogs have with humans, which means that they are well suited to act as indicators of the infectious disease risks that their owners are likely to encounter. North American domestic dogs consistently show higher seroprevalence of anti-WNV antibodies than humans (Komar et al. 2001; Kile et al. 2005) and one analysis revealed that outdoor dogs were nearly 19 times more likely to have seroconverted to WNV than indoor-only pet dogs (Kile et al. 2005). The pattern of human exposure to the arthropod vectors of WNV is likely to be more similar to that of indoor-only dogs, but within the context of broad spatial association with humans; this divergence from the human niche means that outdoor-only dogs are more sensitive sentinels of WNV presence and human risk than indoor-only dogs (Kile et al. 2005).
Transmission route
The role played by different mosquito species (predominantly of the genus Culex) in the transmission of WNV between birds and to humans is apparently quite variable (Kilpatrick et al. 2005; Molaei et al. 2006). At one study site in Maryland and Washington DC, over 90% of all Culex mosquitoes identified were of the species Culex pipiens (Kilpatrick et al. 2006). At this site, the rise in human WNV cases that occurs in late summer and early autumn is apparently caused by a marked shift in the feeding preferences of this vector species from birds to humans (Kilpatrick et al. 2006) that is associated with the dispersal of a preferred host, the America robin (Turdus migratorius). This temporal variation in vector feeding preferences means that the transmission of WNV to bird hosts (including corvids) occurs earlier in the season than transmission to humans and explains the capacity for bird die-offs to provide an early warning of human risk. A similar shift in feeding patterns associated with a rise in human cases is also seen in Culex tarsalis mosquitoes in Colorado and California (Kilpatrick et al. 2006).
Detectability
Although the pathogenicity of WNV to birds including crows has been demonstrated within the historical geographical range of WNV (Work et al. 1955), bird die-offs are not typically associated with human WNV outbreaks within this historical geographical range and the very high mortality seen in American corvid populations is apparently unusual (Eidson et al. 2001a). Clearly, this difference may limit the application of corvids as useful sentinels of WNV to contexts within the Americas. Even within North America, there is variation in the suitability of corvids to act as a sentinel for WNV activity according to the density of human populations. A study using decoy crows revealed that both detection and reporting rates were lower in rural areas compared with urban areas (Ward et al. 2006). Spatial analyses have also identified reduced capacity of dead crow density measures to forecast human infections in rural areas (Eidson et al. 2005). These effects are seen because the capacity of crows to act as useful sentinels depends upon the likelihood that bird deaths are observed and reported by people. The power of dead crow sentinel surveillance to predict human risk is greatly reduced in rural areas as a consequence of a reduced detectability of the sentinel response.
2. Identifying and assessing animal sentinels
For any population to be useful for surveillance, it must be under observation and must be capable of developing a detectable response to a particular pathogen. Sentinel populations are distinguished from other populations by having attributes that enhance detection of the disease or of the etiological agent and/or improve the cost-effectiveness of surveillance (McCluskey 2003). In most cases, this means that the sentinel population is more likely to be exposed to, or to respond to the pathogen than other populations. This sentinel concept encompasses the variety of uses described above and can refer to any level of grouping from an individual to a larger unit, such as a herd or even a species.
Various authors have compiled lists of attributes of an ‘ideal’ sentinel (CAMEH 1991; Komar 2001), but these have invariably been created with a particular sentinel application in mind and there exists little or no consensus about the common characteristics or defining features of ‘the sentinel’. This ambiguity, of course, reflects the fact that there is no innate quality of sentinel suitability that particular species or populations have. Instead, the criteria against which the usefulness of a given sentinel population is assessed are influenced by the aim of surveillance and the context in which the sentinel would be used. We describe a conceptual framework which we believe can be used to evaluate potential sentinel populations for any combination of surveillance aim and ecological context (figures 1 and 2).
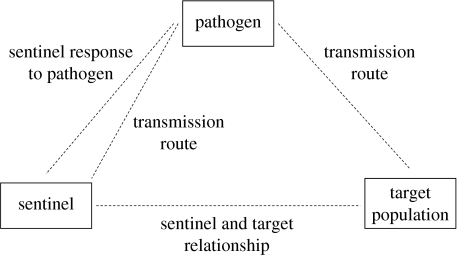
Figure 1.
Key components and attributes of the sentinel framework.
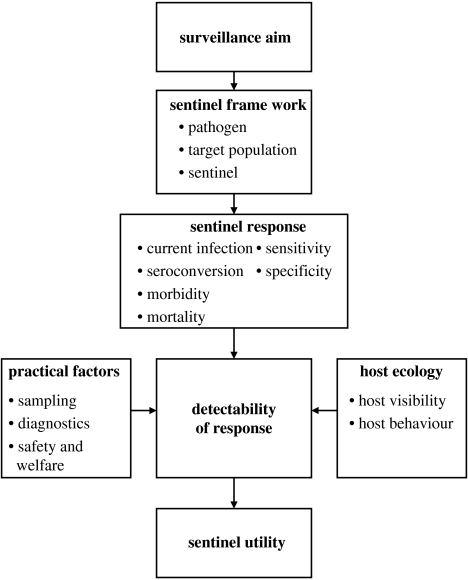
Figure 2.
The sentinel framework in context.
3. The sentinel framework
Within any surveillance context, the sentinel population must always interact with both the pathogen and the target population and it is essential to consider and describe the interactions between these fundamental components (figure 1). The following are the three components of the sentinel framework.
Pathogen. The pathogen that is under surveillance.
Target population. The population of concern to which information gathered from the sentinel is applied.
Sentinel population.
This framework is not intended to represent the transmission dynamics of a pathogen, but rather the ways in which the components are associated. Three critical attributes of this system must be considered in order to assess the utility of a potential sentinel for a particular surveillance aim and in any given ecological context: (i) the sentinel response to the pathogen, (ii) the relationship between sentinel and target populations, and (iii) routes of transmission to both target and sentinel populations. The conceptual issues raised are discussed with reference to the surveillance of WNV in North America (box 1).
3.1 Sentinel response to pathogen
The sentinel response to a pathogen can range from the production of antibody in an otherwise healthy individual, through morbidity and ultimately to mortality. It may also be possible to detect the presence of the pathogen in a sentinel population before other responses develop and sentinel responses can therefore include the following.
Current infection/presence of pathogen.
Seroconversion.
Morbidity.
Mortality.
There is a clear intuitive distinction between sentinel populations that develop high levels of morbidity or mortality in response to pathogen exposure and those that remain healthy. Sick or dying sentinels show an obvious and dramatic response to a pathogen and provide a readily appreciable signal of the presence of a pathogen within an ecosystem (see the discussion of crow mortality as a sentinel of WNV presence in box 1). At the other end of the spectrum, apparently healthy sentinels that develop a subclinical response are often more useful for investigating the maintenance patterns and transmission dynamics of a pathogen within the sentinel and target populations. Following the consumption of prey infected with rabbit haemorrhagic disease virus (RHDV), foxes in northern Germany developed antibody responses that declined after just two weeks. Serosurveillance of this fox population therefore reveals the proportion of the population that has been exposed in the one to two weeks prior to testing. These serological data can provide a good indication of the incidence patterns of RHDV in the sympatric rabbit population (Frölich et al. 1998). In cases in which healthy sentinels are used, it may be desirable to resample the same individuals or populations over time. It is also important that the observation and sampling of the sentinel population, and perhaps also the sentinel response itself, has minimal impact upon the study system.
This example also demonstrates the influence of the temporal characteristics of the sentinel response to a pathogen upon the choice and application of sentinel populations. Sentinel populations which respond to a pathogen prior to the exposure of the target population may be useful for those surveillance programmes that aim to prevent the exposure of the target population. For other sentinel uses, the rapid development of a response may not be required. The duration of the potential sentinel's response can also influence the types of question which can be usefully addressed. An equivalent sentinel population (to that of the foxes) that developed a longer lasting antibody response in the above RHDV example would be of limited use for investigating the incidence of disease in the rabbit population on this immediate time-scale.
The sentinel response can be viewed as a test for the presence of the pathogen within the target population and as such has properties that are analogous to test sensitivity and specificity.
Sentinel sensitivity. The sensitivity of the sentinel refers to its capacity to respond to the presence of the pathogen in the target population and effectively translates as susceptibility to infection. An insensitive population would be unlikely to display evidence of infection with the pathogen even if it were present in the target population and would therefore be poorly suited for use as a sentinel.
Sentinel specificity. The specificity of the sentinel response relates to the ease with which a sentinel response can be interpreted and attributed to a particular pathogen. Specificity is thus closely linked to the response type. Morbidity and mortality are generally less specific indicators of the presence of a particular pathogen than molecular responses that are observed using a test or assay unique to the pathogen in question. Across parts of rural Africa and Asia, for example, bird die-offs due to pathogens other than H5N1 avian influenza virus can be relatively common occurrences, reducing the specificity of bird mortality as an indicator of H5N1 presence (World Health Organization 2005).
Whatever the type of response a particular sentinel population mounts to a pathogen, it is important that the individual members of that population are consistent in the development of the response. Excessive variation within a sentinel population would greatly complicate the interpretation of surveillance findings and it may therefore be important to ensure that members of the sentinel population are of similar age, sex or other relevant characteristics, depending upon the type of response measured.
3.2 Relationship between sentinel and target populations
The relationship that exists between the sentinel and target populations may include behavioural, epidemiological or spatial aspects or any other form of ecological association. Detailed understanding of the associations between the sentinel and target populations is not required to address all questions. However, a comprehensive understanding of the relationship between a sentinel and a target population will allow for the investigation of more complex epidemiological questions and better-informed interpretation of the data collected through surveillance of that sentinel. The minimum association that must exist between a sentinel and a target population is a spatial association. This need not imply spatial overlap however. If the pathogen is spreading on a wavefront or emanating from a focal source, then a sentinel population may be selected on the basis of its closer proximity to the focus at the target population.
At the other extreme, the sentinel population may consist of a specific subset of the target population, ensuring a very close relationship between the two populations. A subpopulation that experiences high-transmission risk, or is particularly sensitive to infection with a particular pathogen, may serve as a sentinel for the wider population and can clearly provide a more accurate assessment of risk to the target than a population occupying a dissimilar ecological niche and consequently experiencing a very different pattern of exposure to the pathogen (e.g. unvaccinated sentinel birds are used to detect the presence of HPAI viruses within the otherwise vaccinated flock; Suarez 2005). The sentinel and target population may also be epidemiologically linked such that the sentinel may act as a source of infection for the target population, as is the case with arthropod vector surveillance.
3.3 Transmission routes
This attribute is essentially a component of the relationship between the sentinel and target populations that explicitly considers the route or routes through which the two populations can become infected with the pathogen. In circumstances where the target and sentinel are exposed to infection via the same route, the relative intensity and patterns of exposure of the two populations to the source of infection are important (Estrada-Franco et al. 2006). It may be desirable to select a sentinel that has higher levels of exposure and which is therefore more likely to show evidence of a pathogen if it is present than to directly survey the target population itself. For pathogens that are transmitted by a vector or vectors, the feeding preferences of the vector(s) can therefore be important in informing sentinel selection. Domestic dogs are the preferred source of blood meals for Triatoma infestans, one of the main vectors of Trypanosoma cruzi in Mexico. A comparative serosurvey revealed overall anti-T. cruzi IgG prevalence of 16% in dogs compared with a 2% prevalence in humans, and a strong positive correlation between human and dog seropositivity within the study area. These data suggest that the feeding preferences of this vector make the domestic dog population a good sentinel for identifying areas of human seropositivity and monitoring prevalence in this context (Estrada-Franco et al. 2006).
There are also circumstances in which the route of exposure of the sentinel and target population may differ. A number of emerging zoonoses, including WNV and HPAI H5N1 viruses can be transmitted through the ingestion of infected material (Komar et al. 2003; Austgen et al. 2004; Rimmelzwaan et al. 2006). Carnivore and scavenger species that are exposed through consumption of infected prey may prove useful sentinels for a wide range of pathogens, specifically because of this additional route of exposure that is not shared with the target population (Cleaveland et al. 2006). A single predator or scavenger typically consumes material from multiple individuals, increasing the probability of exposure to pathogens circulating within the prey population. Predators and scavengers can effectively sample from the prey population, leading to a ‘bioaccumulation’ effect whereby pathogens present at relatively low prevalence in the prey population may be detected at higher prevalence in the predator/scavenger species (Cleaveland et al. 2006). An understanding of the predator–prey relationships between the target population and potential sentinels may prove useful in sentinel selection. The principal transmission route of bluetongue virus, which infects wild and domestic ruminants across East Africa, is via Culicoides midge vectors. Serosurveillance of free-ranging African carnivores revealed that both the seroprevalence and the virus serotype identified varied dramatically across carnivore species (Alexander et al. 1994). This study suggested that the most probable route of infection of carnivores with bluetongue was via consumption of infected prey, and that the variation seen between species was attributable to dietary differences. Different carnivore species may therefore vary in their utility as sentinels for the presence of bluetongue virus in different ruminant species.
4. Placing the sentinel framework in context
The sentinel response can be viewed as the output of the sentinel framework. The nature of this response, in combination with other sentinel host factors and practical influences which depend upon the context in which surveillance is conducted, determines the overall detectability of the sentinel response (figure 2). Unlike the attributes which operate within the sentinel framework, detectability is a quality of the interaction between the sentinel and the observer. The overall utility of any potential sentinel can only be assessed by considering both the sentinel framework and the influences of the context in which it would be applied (figure 2; box 2).
Box 2. Simplified application of the conceptual framework represented in figures 1 and 2 to the evaluation of potential sentinel populations for the surveillance of HPAI H5N1.
Surveillance aim
To establish if H5N1 viruses have been introduced into a country with underdeveloped disease surveillance and reporting structure.
Should sentinels be used?
Cross-sectional survey–may be expensive and time consuming.
Sentinel surveillance–potentially cost-effective alternative
↓
Sentinel framework
pathogen=HPAI H5N1 virus.
target population=the national poultry population.
Potential sentinels
Backyard chicken populations in areas of perceived high risk of virus introduction, e.g. close to areas of wild bird congregation or to livestock markets.
Backyard ducks in similar locations.
Wild bird populations.
Domestic cats.
Domestic dogs.
Other potential sentinels are excluded altogether on the basis of a lack of response to the pathogen or of any type of meaningful relationship with the target population.
Relationship between sentinel and target populations
Chickens
Subset of target population.
Ducks
Occupy a very similar niche to target population.
May act as silent carrier of viruses (Hulse-Post et al. 2005).
Wild birds
May act as source of infection for domestic species.
May not occupy the same geographical areas as the target population (especially true for large congregations of migratory birds).
Cats and dogs
Spatial correspondence with target population.
Cats and dogs may prey upon the target population.
Transmission routes
Chickens, ducks and wild birds
Bird–bird transmission.
Environmental contamination.
Cats and dogs
Consumption of infected birds (Keawcharoen et al. 2004; Kuiken et al. 2004).
Horizontal transmission in cats (Rimmelzwaan et al. 2006).
↓
Sentinel response
Chickens
Consistent, rapid and widespread mortality.
Die-offs provide a prompt indication of virus presence.
Ducks
Variable pathogenicity and thus mortality (Sturm-Ramirez et al. 2005).
Isolation of virus from healthy birds (Hulse-Post et al. 2005).
Wild birds
Variable pathogenicity (Ellis et al. 2004).
Isolation of virus from healthy birds (Chen et al. 2006).
Cats
Experimental evidence of mortality response (Rimmelzwaan et al. 2006).
Mortality reports associated with bird die-offs (Butler 2006a, Songserm et al. 2006a, Yingst et al. 2006).
High seroconversion rates (Butler 2006b).
Subclinical infections (Leschnik et al. 2007).
Dogs
High seroconversion rates (Butler 2006b).
Mortality report associated with bird infection (Songserm et al. 2006b).
Sensitivity and specificity of responses
Chickens
✓✓✓ Highly sensitive but specificity of mortality response is low, as
××× chicken die-offs not necessarily unusual where poultry are not routinely vaccinated against other pathogens, e.g. Newcastle disease virus.
✓✓✓ High specificity of laboratory analyses.
Ducks
✓/× Variable mortality response limits sensitivity.
✓✓✓ High specificity of laboratory analyses.
Wild birds
✓/× Variable mortality response limits sensitivity.
××× Very low prevalence in healthy birds limits sensitivity (Chen et al. 2006).
✓✓✓ High specificity of laboratory analyses.
Cats and Dogs
✓/× Serological analyses non-specific for distinguishing high- and low-pathogenicity viruses.
+
Host ecology
✓✓✓ Domestic species are all highly observable as a consequence of their close association with humans.
××× Wild birds are considerably less visible and may occupy relatively remote and inaccessible areas.
+
Practical factors
Risk to sampling personnel must be considered as a priority when developing all sampling protocols.
✓✓✓ Domestic species approachable and handleable.
✓/× Distribution of cats and dogs relatively to poultry may vary according to factors such as urbanization and religion.
××× Considerable investment of money, time and expertise required to sample sufficient numbers of wild birds.
✓✓✓ For the identification of virus presence, standard test protocols include RT-PCR and virus isolation (World Organization for Animal Health 2005) which are generally adaptable across species.
✓/× Serological analyses may not be well developed for wild birds, cats or dogs.
↓
Detectability
Chickens
✓✓✓ Mortality response easily appreciated.
✓✓✓ High visibility within human communities.
××× Low specificity of mortality limits detectability.
Ducks
××× Mortality response variable.
✓✓✓ Additional responses detectable through laboratory analysis.
✓✓✓ High visibility within human communities.
Wild birds
××× Mortality response variable.
××× Low visibility compared with domestic species.
Logistically complex and time-consuming sampling required.
Cats and Dogs
✓✓✓ High visibility within human communities.
✓✓✓ Sudden and widespread morbidity or mortality uncommon.
✓/× Non-mortality responses less detectable.
In all cases, a comprehensive network of observers is vital and it may be necessary to develop education programmes aimed at improving reporting levels.
↓
Utility
Domestic chicken and ducks sentinels are likely to provide the most rapid and dramatic response to HPAI H5N1 virus within a country. However, in this context in which mortality in domestic birds is not unusual, this mortality may not be reported and the detectability of the response in the context of this surveillance aim may be very low.
To best address this surveillance aim, the specificity of the chicken mortality response to HPAI H5N1 presence could be enhanced by using a combination of sentinels such that priority was given to the investigation of chicken die-offs that were accompanied by morbidity or mortality in cats or dogs (Yingst et al. 2006).
Retrospective analysis of sera collected from ducks, cats and dogs could also be used to identify those areas in which an H5N1 virus had been present.
The visibility of any animal population is determined by the morphology, behaviour, distribution and abundance of the individual animals of which it is comprised. The detectability of the sentinel response includes both the visibility of the animal and its response to a pathogen. The type of response that an animal mounts will directly affect the ease with which it is detected by the observer. For example, lions are being used as sentinels for canine distemper in the Serengeti National Park in Tanzania, as a result of their high visibility to observers and the dramatic manifestations of clinical disease, which include grand mal seizures (Roelke-Parker et al. 1996). Information from lion sentinels would be used to increase disease detection efforts within other wild carnivore populations of the park to establish the extent and impact of any epidemic and initiate a risk–benefit assessment for possible interventions (such as vaccination) for protecting threatened wildlife populations. A wide range of other carnivore species such as hyaenas, bat-eared foxes and leopards are known to be susceptible to canine distemper (Roelke-Parker et al. 1996), but are less suitable as sentinels for disease in the Serengeti owing to ecological and behavioural factors that reduce visibility (e.g. nocturnal behaviour, small body size, den-living characteristics, lower levels of tourist observation). Widespread morbidity or mortality within a sentinel population are more readily appreciable than seroconversion or current infection/presence of pathogen, which can only be detected by the observer after first sampling the sentinel population and then conducting laboratory analysis. In the case of overt sentinel responses such as mortality, the existence of a reliable network of ‘observers’ and a mechanism through which data are reported are crucial. It is equally important to consider the available capacity to detect any less overt responses including the existence of a reliable sampling protocol and a diagnostic test (McCluskey 2003). The majority of diagnostic tests for human and livestock pathogens have not been validated for use in non-target species and the sensitivity and specificity of tests can vary hugely between species (Greiner & Gardner 2000). The existence of a suitable negative control population and recognition of the time required to identify and validate diagnostic tests must be considered in any proposed sentinel surveillance programme.
The practical difficulties involved in sampling any potential sentinel population must also be evaluated and it may often be difficult to reconcile the use of a theoretically ideal sentinel with such practicalities. For a sentinel population to be useful, it must be both logistically feasible and safe to sample sufficient numbers of the population (CAMEH 1991). Since sentinels are often selected on the basis of increased likelihood of exposure to a pathogen, sentinel surveillance can enable targeting of resources and often has improved cost-effectiveness as compared, for example, with more comprehensive cross-sectional surveys (McCluskey et al. 2003). The bioaccumulation effect discussed above suggests that evidence of exposure to a pathogen may effectively accumulate within carnivore populations (Cleaveland et al. 2006). The identification of the presence of a pathogen within a particular area can therefore be achieved by sampling relatively few carnivore sentinels, as compared to an exhaustive and costly survey of the prey population within which the pathogen may circulate at very low prevalence, thereby providing a relatively rapid and inexpensive surveillance option (Frölich et al. 1998; Leighton et al. 2001; Csángó et al. 2004; Cleaveland et al. 2006). In addition to consideration of time and cost, the potential risks to research personnel and the public that are associated with the desired sampling strategy must be evaluated, as well as the effects of sampling upon the sentinel population itself in the context of animal welfare and conservation status (CAMEH 1991).
5. Applications of animal sentinels
Many of the questions addressed through the use of animal sentinels, such as the assessment of pathogen control efforts, the monitoring of prevalence fluctuations over time and the demonstration of the absence of a pathogen, require only the basic qualities of a sentinel as defined above. While the more specific requirements of any particular sentinel are unique to the context and aim to which it is applied, there are some general qualities and subtypes of sentinels that correspond to major applications of animal sentinels. For example, sentinels in which the response to a pathogen and the detection of that response occur prior to exposure, or cases in the target population, can provide early warning of pathogen presence. Early warning sentinels are used to provide a predictive signal of risk to the target population. Sentinels that are exposed and which respond to a pathogen before the exposure of the target population may provide an opportunity to implement pre-emptive control measures and to prevent the infection of the target population (see discussion of WNV surveillance in box 1). Other early warning sentinels may respond to the pathogen more rapidly than the target population but not necessarily before the target's exposure (e.g. the coal miner's canary). In such cases, data collected from the sentinel cannot be used to prevent cases in the target population altogether. However, the information they supply can provide advance warning of cases, enabling the prioritization of resources for treatment and the prevention of additional cases. In most cases, early warning sentinels are highly visible and develop a very obvious response to the pathogen. Data provided by sentinels with these qualities can be more rapidly processed, analysed and acted upon than the data from apparently healthy sentinels for which the potentially lengthy processes of sample collection and laboratory analyses must be carried out before any data are available. Ideally, the response of early warning sentinels should also be very specific to minimize the likelihood of false positive responses and consequently improve confidence in decision making based on the sentinel response alone.
Sentinels can also be used retrospectively to provide evidence of the timing of pathogen introduction and spread through a target population. In situations where a number of populations or locations are sampled, this information can be combined to reveal the spatial and temporal pattern of pathogen spread. Following the widespread rinderpest outbreak that occurred in Kenya in 1993–1997, the retrospective serosurveillance of buffalo herds and analysis of age-seroprevalence patterns allowed the estimation of the time of infection in different herds, the identification of the probable point of entry of the pathogen into the wildlife population and the elucidation of where the pathogen had been, how it had spread and where it was likely to move to (Kock et al. 1999). In this case, buffalo herds were selected as sentinels on the basis of the increased susceptibility of the species to this virus (Rossiter 1994), and served as sentinels for the larger livestock population in the affected areas. In such circumstances, the appropriate sentinel population must develop a response to the pathogen that persists and is detectable a long time after exposure. When used retrospectively, it is also important that individuals of the sentinel population can be reliably aged.
6. Conclusion
The objective of this paper has been to provide a consistent and inclusive framework that clarifies our understanding of the role of animal sentinels and their potential value in the surveillance of human and animal infectious diseases, as well as providing a conceptual tool that can be applied to assess and characterize potential sentinels in the future. At present, surveillance of many pathogens involves the target population alone; however, the broad host range of many important human and animal diseases provides opportunities for exploiting a wide range of species for surveillance purposes. The variability of host responses to a pathogen, the heterogeneities in pathogen exposure in different populations and the differing relationships between sentinel and target populations indicate that different animal hosts will themselves vary in their ability to act as effective sentinels in different circumstances.
Animal sentinels may not serve as a useful surveillance tool in all contexts. The generic framework that we have developed in this paper describes the attributes of host species that need to be considered to identify appropriate sentinel populations for different surveillance purposes. This same framework should also be used to identify characteristics of potential sentinels that perhaps make them unsuitable in a particular circumstance. For example, sentinels must by definition be intentionally observed. This classification distinguishes the use of animal sentinels from scenarios in which responses of animal populations to novel pathogens are ‘noticed’. For this reason, animal sentinels cannot provide the solution to the question of how to carry out surveillance for pathogens that are currently unknown. However, as a consequence of greater awareness of the potential of animal sentinels and improved observation of animal populations, instances of unusual morbidity and mortality in animal populations that result from the emergence of novel pathogens would perhaps be more likely to be noticed and their potential significance to other species recognized.
To date, there has been limited appreciation of the data resource that different animal hosts represent for disease surveillance. This paper aims to highlight the variety of surveillance functions for which animal sentinels may be used, the range of animal host species that may usefully be exploited (particularly for human disease surveillance) and the potential benefits of animal sentinels for enhanced pathogen detection and improved cost-effectiveness of surveillance. The potential value of animal sentinels in disease prevention and control can only be realized with close integration and effective communication between and within human and animal health sectors; information generated from sentinel populations must be disseminated to those who need to take action, and appropriate responses must be generated as a result of this information to mitigate disease risk.
Acknowledgments
We acknowledge the following agencies for supporting components of this work: the Wellcome Trust, Department for the Environment, Food and Rural Affairs and NIH/NSF Ecology of Infectious Diseases Program (grant no. 0225453). Any opinions, findings, conclusions or recommendations expressed are those of the authors and do not necessarily reflect the views of the National Science Foundation. We would also like to thank two anonymous reviewers for very helpful comments on earlier versions of the manuscript and the members of the Wildlife and Emerging Diseases Section, R(D)SVS, University of Edinburgh, for their many contributions that led to the development of the concepts outlined in this article.
Footnotes
One contribution of 20 to a Theme Issue ‘Cross-scale influences on epidemiological dynamics: from genes to ecosystems’.
References
- Alexander K.A, et al. Evidence of natural bluetongue virus-infection among African carnivores. Am. J. Trop. Med. Hyg. 1994;51:568–576. [PubMed] [Google Scholar]
- Austgen L.E, Bowen R.A, Bunning M.L, Davis B.S, Mitchell C.J, Chang G.-J.J. Experimental infection of cats and dogs with West Nile virus. Emerg. Infect. Dis. 2004;10:82–86. doi: 10.3201/eid1001.020616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell G.A, Seibert F.M. Miner's circular. vol. 14. Bureau of Mines, Department of the Interior; Washington, DC: 1916. Gases found in coal mines. [Google Scholar]
- Butler D. Can cats spread avian flu? Nature. 2006a;440:135–135. doi: 10.1038/440135a. [DOI] [PubMed] [Google Scholar]
- Butler D. Thai dogs carry bird-flu virus, but will they spread it? Nature. 2006b;439:773–773. doi: 10.1038/439773a. [DOI] [PubMed] [Google Scholar]
- Campbell G.L, Marfin A.A, Lanciotti R.S, Gubler D.J. West Nile virus. Lancet Infect. Dis. 2002;2:519–529. doi: 10.1016/S1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention 2002 Manual for the surveillance of vaccine-preventable diseases, 3rd edn. See http://www.cdc.gov/nip/publications/surv-manual/default.htm.
- Chen H, et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: implications for pandemic control. Proc. Natl Acad. Sci. USA. 2006;103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel B.B. Control and prevention of emerging zoonoses. J. Vet. Med. Educ. 2003;30:145–147. doi: 10.3138/jvme.30.2.145. [DOI] [PubMed] [Google Scholar]
- Cleaveland S, Meslin F.X, Breiman R. Dogs can play useful role as sentinel hosts for disease. Nature. 2006;440:605. doi: 10.1038/440605b. [DOI] [PubMed] [Google Scholar]
- Committee on Animals as Monitors of Environmental Hazards 1991 Animals as sentinels of environmental health hazards. Washington, DC: National Academy Press.
- Csángó P.A, Blakstad E, Kirtz G.C, Pedersen J.E, Czettel B. Tock-borne encephalitis in southern Norway. Emerg. Infect. Dis. 2004;10:533–534. doi: 10.3201/eid1003.020734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan V.G, Yabsley M.J, Tate C.M, Mead D.G, Munderloh U.G, Herron M.J, Stallknecht D.E, Little S.E, Davidson W.R. Evaluation of white-tailed deer (Odocoileus virginianus) as natural sentinels for Anaplasma phagocytophilum. Vector-Borne Zoonot. Dis. 2006;6:192–207. doi: 10.1089/vbz.2006.6.192. [DOI] [PubMed] [Google Scholar]
- Eidson M, Komar N, Sorhage F, Nelson R, Talbot T, Mostashari F, Mclean R. Crow deaths as a sentinel surveillance system for West Nile virus in the Northeastern United States, 1999. Emerg. Infect. Dis. 2001a;7:615–620. doi: 10.3201/eid0704.010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson M, Kramer L, Stone W, Hagiwara Y, Schmit K. Dead bird surveillance as an early warning system for West Nile virus. Emerg. Infect. Dis. 2001b;7:631–635. doi: 10.3201/eid0704.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson M, Schmit K, Hagiwara Y, Anand M, Backenson P.B, Gotham I, Kramer L. Dead crow density and West Nile virus monitoring, New York. Emerg. Infect. Dis. 2005;11:1370–1375. doi: 10.3201/eid1109.040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T.M, et al. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004;33:492–505. doi: 10.1080/03079450400003601. [DOI] [PubMed] [Google Scholar]
- Estrada-Franco J.G, Bhatia V, Diaz-Albiter H, Ochoa-Garcia L, Barbabosa A, Vazquez-Chagoyan J.C, Martin-Perez M.A, Guzman-Bracho C, Garg N. Human Trypanosoma cruzi infection and seropositivity in dogs, Mexico. Emerg. Infect. Dis. 2006;12:624–630. doi: 10.3201/eid1204.050450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.M, Cummings D.A.T, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke D.S. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- Frölich K, Klima F, Dedek J. Antibodies against rabbit hemorrhagic disease virus in free-ranging red foxes from Germany. J. Wildl. Dis. 1998;34:436–442. doi: 10.7589/0090-3558-34.3.436. [DOI] [PubMed] [Google Scholar]
- Glickman L.T, Domanski L.M, Maguire T.G, Dubielzig R.R, Churg A. Mesothelioma in pet dogs associated with exposure of their owners to asbestos. Environ. Res. 1983;32:305–313. doi: 10.1016/0013-9351(83)90114-7. [DOI] [PubMed] [Google Scholar]
- Greiner M, Gardner I.A. Epidemiologic issues in the validation of veterinary diagnostic tests. Prev. Vet. Med. 2000;45:3–22. doi: 10.1016/S0167-5877(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Hayes E.B, Gubler D.J. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu. Rev. Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- Hulse-Post D.J, et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proc. Natl Acad. Sci. USA. 2005;102:10 682–10 687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (US). Committee on infectious diseases of mice and rats 1991 Infectious diseases of mice and rats. Washington, DC: National Academy Press.
- Johnson G.D, Eidson M, Schmit K, Ellis A, Kulldorff M. Geographic prediction of human onset of West Nile virus using dead crow clusters: an evaluation of year 2002 data in New York State. Am. J. Epidemiol. 2006;163:171–180. doi: 10.1093/aje/kwj023. [DOI] [PubMed] [Google Scholar]
- Kahn L.H. Confronting zoonoses, linking human and veterinary medicine. Emerg. Infect. Dis. 2006;12:556–561. doi: 10.3201/eid1204.050956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keawcharoen J, et al. Avian influenza H5N1 in tigers and leopards. Emerg. Infect. Dis. 2004;10:2189–2191. doi: 10.3201/eid1012.040759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile J.C, Panella N.A, Komar N, Chow C.C, Macneil A, Robbins B, Bunning M.L. Serologic survey of cats and dogs during an epidemic of West Nile virus infection in humans. J. Am. Vet. Med. Assoc. 2005;226:1349–1353. doi: 10.2460/javma.2005.226.1349. [DOI] [PubMed] [Google Scholar]
- Kilpatrick A.M, Kramer L.D, Campbell S.R, Alleyne E.O, Dobson A.P, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg. Infect. Dis. 2005;14:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick A.M, Kramer L.D, Jones M.J, Marra P.P, Daszak P. West Nile virus epidemics in North America are driven by shifts in mosquito feeding behavior. PLoS Biol. 2006;4:606–610. doi: 10.1371/journal.pbio.0040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kock R.A, Wambua J.M, Mwanzia J, Wamwayi H, Ndungu E.K, Barrett T, Kock N.D, Rossiter P.B. Rinderpest epidemic in wild ruminants in Kenya 1993–97. Vet. Rec. 1999;145:275–283. doi: 10.1136/vr.145.10.275. [DOI] [PubMed] [Google Scholar]
- Komar N. West Nile virus surveillance using sentinel birds. In: White D.J, Morse D.L, editors. West Nile virus: detection, surveillance, and control. Academy of Sciences; New York, NY: 2001. pp. 58–73. [DOI] [PubMed] [Google Scholar]
- Komar N, Panella N.A, Boyce E. Exposure of domestic mammals to West Nile virus during an outbreak of human encephalitis, New York City, 1999. Emerg. Infect. Dis. 2001;7:736–738. doi: 10.3201/eid0704.010424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of north American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, Rimmelzwaan G, van Riel D, van Amerongen G, Baars M, Fouchier R, Osterhaus A. Avian H5N1 influenza in cats. Science. 2004;306:241. doi: 10.1126/science.1102287. [DOI] [PubMed] [Google Scholar]
- Kuiken T, Leighton F.A, Fouchier R.A.M, Leduc J.W, Peiris J.S.M, Schudel A, Stohr K, Osterhaus A. Public health—pathogen surveillance in animals. Science. 2005;309:1680–1681. doi: 10.1126/science.1113310. [DOI] [PubMed] [Google Scholar]
- Leighton F.A, Artsob H.A, Chu M.C, Olson J.G. A serological survey of rural dogs and cats on the southwestern Canadian prairie for zoonotic pathogens. Can. J. Public Health. 2001;92:67–71. doi: 10.1007/BF03404848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leschnik M, et al. Subclinical infection with avian influenza A (H5N1) virus in cats. Emerg. Infect. Dis. 2007;13:243–247. doi: 10.3201/eid1302.060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftin K.C, et al. Five-year surveillance of West Nile and Eastern equine encephalitis viruses in southeastern Virginia. J. Environ. Health. 2006;68:33–40. [PubMed] [Google Scholar]
- McCluskey B.J. Use of sentinel herds in monitoring and surveillance systems. In: Salman M.D, editor. Animal disease surveillance and survey systems: methods applications. Iowa State Press; Iowa, IA: 2003. pp. 119–133. [Google Scholar]
- McCluskey B.J, Mumford E.L, Salman M.D, Traub-Dargatz J.J. Use of sentinel herds to study the epidemiology of vesicular stomatitis in the state of Colorado. In: Gibbs E.P.J, Bokma R.H, editors. Domestic animal/wildlife interface: issue for disease control, conservation, sustainable food production, and emerging diseases. Academy of Sciences; New York, NY: 2002. pp. 205–209. [DOI] [PubMed] [Google Scholar]
- McCluskey B.J, Burgess B, Glover J, Kinde H, Hietala S. Use of sentinel chickens to evaluate the effectiveness of cleaning and disinfection procedures in noncommercial poultry operations infected with exotic Newcastle disease virus. J. Vet. Diag. Invest. 2006;18:296–299. doi: 10.1177/104063870601800313. [DOI] [PubMed] [Google Scholar]
- McLean R.G, Ubico S.R, Docherty D.E, Hansen W.R, Sileo L, Mcnamara T.S. West Nile virus transmission and ecology in birds. In: White D.J, Morse D.L, editors. West Nile virus: detection, surveillance, and control. Academy of Sciences; New York, NY: 2001. pp. 54–57. [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis T.A, Armstrong P.M, Anderson J.F, Vossbrinck C.R. Host feeding patterns of Culex mosquitoes and West Nile virus transmission, northeastern United States. Emerg. Infect. Dis. 2006;12:468–474. doi: 10.3201/eid1203.051004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostashari F, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- Mostashari F, Kulldorff M, Hartman J.J, Miller J.R, Kulasekera V. Dead bird clusters as an early warning system for West Nile virus activity. Emerg. Infect. Dis. 2003;9:641–646. doi: 10.3201/eid0906.020794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Arbovirus Monitoring Program 2003–2004 NAMP annual report. See http://www.namp.com.au/reports/report_0304.pdf.
- Nugent G, Whitford J, Young N. Use of released pigs as sentinels for Mycobacterium bovis. J. Wildl. Dis. 2002;38:665–677. doi: 10.7589/0090-3558-38.4.665. [DOI] [PubMed] [Google Scholar]
- Rabinowitz P.M, Gordon Z, Holmes R, Taylor B, Wilcox M, Chudnov D, Nadkarni P, Dein F.J. Animals as sentinels of human environmental health hazards: an evidence-based analysis. EcoHealth. 2005;2:26–37. doi: 10.1007/s10393-004-0151-1. [DOI] [Google Scholar]
- Rimmelzwaan G.F, van Riel D, Baars M, Bestebroer T.M, van Amerongen G, Fouchier R.A.M, Osterhaus A.D.M.E, Kuiken T. Influenza A virus (H5N1) infection in cats causes systemic disease with potential novel routes of virus spread within and between hosts. Am. J. Pathol. 2006;168:176–183. doi: 10.2353/ajpath.2006.050466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelke-Parker M.E, et al. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter P.B. Rinderpest. In: Coetzer J.A.W, Thomson G.R, Tustin R.C, editors. Infectious diseases of lifestock. Oxford University Press; Oxford, UK: 1994. pp. 735–757. [Google Scholar]
- Rouquet P, et al. Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003. Emerg. Infect. Dis. 2005;11:283–290. doi: 10.3201/eid1102.040533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman M.D. Surveillance and monitoring systems for animal health programs and disease surveys. In: Salman M.D, editor. Animal disease surveillance and survey systems: methods and applications. Iowa State Press; Iowa, IA: 2003. pp. 3–13. [Google Scholar]
- Schwabe C.W. Williams and Wilkins; Baltimore, MD: 1984. Veterinary medicine and human health. [Google Scholar]
- Songserm T, Amonsin A, Jam-on R, Sae-Heng N, Meemak N, Pariyothorn N, Payungporn S, Theamboonlers A, Poovorawan Y. Avian influenza H5N1 in naturally infected domestic cat. Emerg. Infect. Dis. 2006a;12:681–683. doi: 10.3201/eid1204.051396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songserm T, et al. Fatal avian influenza A H5N1 in a dog. Emerg. Infect. Dis. 2006b;12:1744–1747. doi: 10.3201/eid1211.060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm-Ramirez K.M, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J. Virol. 2005;79:11 269–11 279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez D.L. Overview of avian influenza DIVA test strategies. Biologicals. 2005;33:221–226. doi: 10.1016/j.biologicals.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Thrusfield M.V. 3rd edn. Blackwell Science; Oxford, UK: 2005. Veterinary epidemiology. [Google Scholar]
- Ward M.R, Stallknecht D.E, Willis J, Conroy M.J, Davidson W.R. Wild bird mortality and West Nile virus surveillance: biases associated with detection, reporting, and carcass persistence. J. Wildl. Dis. 2006;42:92–106. doi: 10.7589/0090-3558-42.1.92. [DOI] [PubMed] [Google Scholar]
- Woolhouse M.E.J, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work T.H, Hurlbut H.S, Taylor R.M. Indigenous wild birds of the Nile-Delta as potential West Nile virus circulating reservoirs. Am. J. Trop. Med. Hyg. 1955;4:872–888. doi: 10.4269/ajtmh.1955.4.872. [DOI] [PubMed] [Google Scholar]
- World Health Organization 2001 Protocol for the evaluation of epidemiological surveillance systems WHO/EMC/DIS/97.2. See http://www.who.int/vaccines-documents/DocsWord/word577.doc.
- World Health Organization 2005 Avian influenza and the pandemic threat in Africa: risk assessment for Africa. See http://www.who.int/csr/disease/avian_influenza/riskassessmentAfrica/en/index.html.
- World Organization for Animal Health 2005 Manual of diagnostic tests and vaccines for terrestrial animals. Ch. 2.7.12. Avian influenza. See http://www.oie.int/Eng/Normes/Mmanual/A_00037.htm.
- Yaremych S.A, Warner R.E, Mankin P.C, Brawn J.D, Raim A, Novak R. West Nile virus and high death rate in American crows. Emerg. Infect. Dis. 2004;10:709–711. doi: 10.3201/eid1004.030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst S.L, Saad M.D, Felt S.A. Qinghai-like H5N1 from domestic cats, northern Iraq. Emerg. Infect. Dis. 2006;12:1295–1297. doi: 10.3201/eid1208.060264. [DOI] [PMC free article] [PubMed] [Google Scholar]