Abstract
The interaction between sheep and the nematode Teladorsagia circumcincta is one of the best understood of all host–parasite interactions. Following infection, there is considerable variation among lambs in the number of nematode eggs produced, the number of early fourth-stage larvae and the number of adult worms in the mucosa. These traits have a high variance to mean ratio (i.e. they are overdispersed or aggregated among hosts), they are skewed and approximately negative binomially distributed. The sources of overdispersion are differences among lambs in the ingestion of infective larvae and the immune response. Both forces can produce aggregation but their relative importance is unknown. The key components of variation can be identified by variance analysis. The sum of the average effects of polymorphic genes is known as additive genetic variation and this increases essentially from zero at one month of age to quite high values at six months of age. The major mechanism underlying genetic variation appears to be the differences among individuals in immune responses. Two of the major sources of variation in immune responses are differences in antigen recognition and differences in the type of cytokines produced. Genes that influence both these sources of variation are associated with differences in resistance to nematode infection. Therefore, much of the heterogeneity among animals in parasite transmission appears to be due to genetic variation in immune responsiveness.
Keywords: parasitology, genetics, immunology, Ovis aries, Teladorsagia circumcincta, variance analysis
1. Introduction
Nematodes represent a major disease challenge to most mammals, although their importance is often underestimated because they are seldom a problem for humans in the Western world. They are among the most important diseases affecting livestock (Perry & Randolph 1999; Nieuwhof & Bishop 2005) and consequently they have been intensively studied. Their genetics (Stear et al. 1997a), immune defence mechanisms (Stear et al. 1999b; Balic et al. 2000), pathology (Stear et al. 2003) and methods of disease control (Sayers & Sweeney 2005; Stear et al. 2007) have recently been reviewed. Gastrointestinal nematodes in livestock represent one of the most fully understood of all host–parasite systems (Grenfell et al. 1995a; Stear et al. 1996).
In cool, temperate areas of the world such as the United Kingdom and southern Australia, the dominant nematode is Teladorsagia circumcincta (Stear et al. 1998). Other common taxa in the UK include Trichostrongylus vitrinus, Trichostrongylus axei, Nematodirus battus, Nematodirus filicolis, Nematodirus spathiger and Cooperia spp. (Stear et al. 1998). Among nematode eggs, only Nematodirus spp. are readily distinguishable from the other species and are routinely counted separately. There are several methods that have been used to differentiate the eggs from the remaining species of parasitic nematodes, including culture of eggs to third-stage larvae (Stear et al. 1990), analysis of the size and shape of the eggs (Christie & Jackson 1982) and rate of embryonic development at different temperatures (Gruner et al. 2002, 2004). However, these methods are not in routine use and eggs from the non-Nematodirus species are usually counted together.
This review focuses on T. circumcincta and other non-Nematodirus species in cool, temperate areas. It summarizes the transmission dynamics of nematodes in livestock, the distribution of nematodes among hosts, the sources of aggregation, the key components influencing the heterogeneity among hosts, the influence of genetic variation on parasite transmission and the potential for combining genetic and modelling approaches.
2. Transmission dynamics of nematodes in livestock
The life cycle of the most important nematodes of livestock, such as T. circumcincta or Haemonchus contortus involves only one host (Urquhart et al. 1987). Eggs are produced in the gastrointestinal tract and are then deposited on pasture in the faeces. The eggs hatch into first-stage larvae and subsequently moult into second-stage larvae. Both first- and second-stage larvae feed on bacteria in the faeces. The third-stage larvae leave the faeces and some of these larvae are ingested by sheep during grazing. Within the host the third-stage larvae undergo two further moults to fourth- and fifth-stage larvae. The latter are young adults and once they mature, they breed at species-specific sites within the gastrointestinal tract.
Mature sheep are relatively immune to nematode infection and in the UK, most breeding ewes produce significant numbers of eggs only during the periparturient period (Donaldson et al. 1998; Coop et al. 2001; Huntley et al. 2004; Houdijk et al. 2006). The infection of naive lambs is initiated by the ingestion of infective larvae. These infective larvae arise from the small number of parasites overwintering on pasture and the development of eggs deposited by ewes during the periparturient period. Infection levels increase as warmer weather hastens the development of eggs into infective larvae and as ingested larvae develop into adults and themselves contribute to egg output.
There is considerable variation among regions and farms in husbandry arrangements. In a typical Scottish upland farm, lambs are born in late March to early April, kept with their mothers until three to four months of age and then grazed with other lambs until the end of the grazing season when they are six to seven months of age. At this point transmission of nematodes ceases or falls to low levels. Often, egg counts peak in mid-season, then fall but rise again in October (Thomas & Boag 1972; Stear et al. 2006). However, this pattern is dependent on the weather and the frequency of anthelmintic treatment and is not observed on all farms in all years.
The decline from the mid-season peak has been explained by the onset of immunity in lambs (Stear et al. 1999a). However, different rates of development among nematode species in response to the prevailing weather appear to be responsible for the second late peak in October (Stear et al. 2006).
3. The distribution of parasites among hosts
A good description of the data is not only important for summarizing and comparing results but also for analysis and modelling purposes. A minimally adequate description of a parasite population would include the mean, variance and a description of the class of distribution of parasites among hosts (e.g. Poisson, Weibull, negative binomial or zero-inflated negative binomial and so on). The distribution of parasites also influences the transmission dynamics and the intensity and prevalence of infection and disease. Therefore, the distribution has received considerable attention.
If the infective stages of nematodes are uniformly distributed across the pasture, if there are no differences among grazing lambs and if infection is due to a random encounter between host and parasite, then the distribution of parasites among hosts should follow a Poisson distribution. The variance of Poisson distributions is equal to the mean. However, for most parasite distributions the variance is greater than the mean (Shaw & Dobson 1995); parasites are aggregated among their hosts. An alternative way of describing the phenomenon is to say that the distribution of parasites is overdispersed.
Taylor's power law states that variance=a×meanb (Taylor 1961) where a and b are population-specific parameters. It has been widely used to describe the relationship between the mean abundance of a population and the variability in population size over space and time (Anderson et al. 1982; Keeling 2000). For most natural populations the exponent b lies between 1 and 2 (Taylor 1961). For sheep in Scotland, the exponent was 1.23±0.08 for the relationship between variance and the mean faecal nematode egg count (Stear et al. 2006), suggesting that sheep show substantial variation in egg output and their high level of variation is typical of many populations.
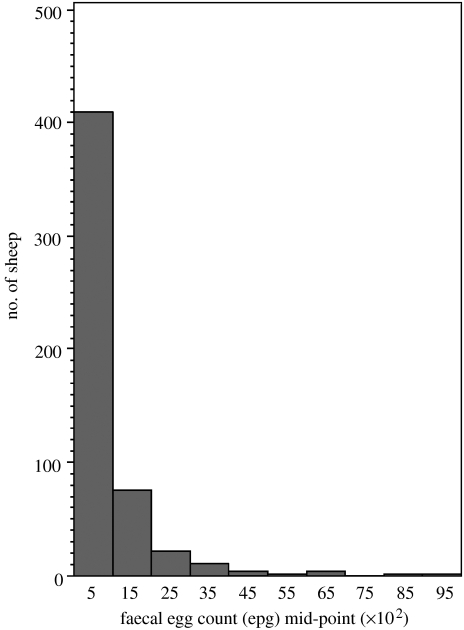
Figure 1 shows the variation among 6.5-month-old naturally infected lambs in egg output expressed as eggs per gram (epg) of faeces. The distribution of egg counts in lambs is typically skewed and overdispersed; most hosts have relatively low egg counts while a small proportion have relatively high egg counts. These individuals with high egg output make a disproportionate contribution to environmental contamination and therefore parasite transmission between hosts.
Figure 1.
Frequency distribution of faecal egg count in 6.5-month-old lambs from a single commercial farm in central Scotland. Naturally infected sheep were necropsied in each of 4 successive years in late October or early November. Nematode eggs were counted in faeces from 530 sheep. The distribution is overdispersed.
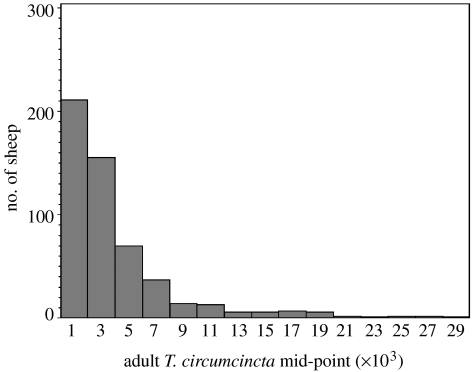
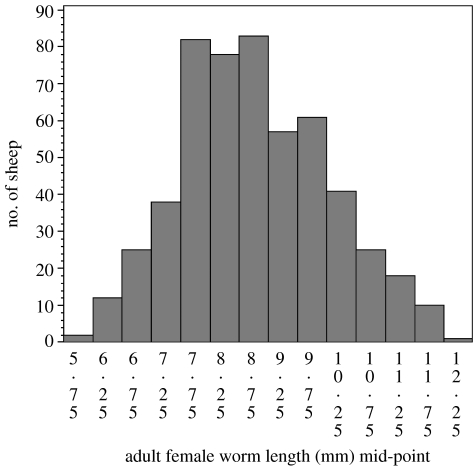
Many but not all responses to parasitic infection are overdispersed. Figure 2 shows the distribution at six to seven months of age among the same animals in the number of adult nematodes of the species T. circumcincta; the distribution of nematode numbers among lambs is also overdispersed. In contrast, figure 3 shows that the distribution of the lengths of adult female T. circumcincta is approximately symmetrical around the mean. This trait has a strong curvilinear relationship with nematode fecundity; longer females lay more eggs per day (Stear & Bishop 1999).
Figure 2.
Frequency distribution of adult T. circumcincta in 6.5-month-old lambs at necropsy. All lambs came from a single commercial farm in central Scotland. Naturally infected sheep were necropsied in each of 4 successive years in late October or early November. Nematodes were counted in the gastrointestinal tract from 533 sheep. The distribution is overdispersed.
Figure 3.
Frequency distribution of adult female T. circumcincta length in 6.5-month-old lambs at necropsy. Naturally infected sheep were necropsied in each of 4 successive years in late October or early November. Nematodes were measured in samples from 533 sheep. All lambs came from a single commercial farm in central Scotland. The distribution is approximately unimodel and symmetrical.
Ever since the pioneering work with nematode egg counts in Scottish sheep (Hunter & Quenouille 1952) and head lice in Indian prisoners (Bliss & Fisher 1953), the negative binomial has been widely used to provide a mathematical description of parasite distributions. The negative binomial is defined by two parameters: mean and k, an inverse index of overdispersion. This distribution has also been used to describe several indirect indicators of infection. For example, mean egg counts in a flock of naturally infected Scottish sheep ranged from 50 to 570 eggs per gram over the grazing season and k ranged from 0.09 to 2.59 (Stear et al. 1995a). There is a strong relationship between the mean egg count and k (Scott 1987; Grenfell et al. 1995).
In contrast, there have been few attempts to directly examine the distribution of parasites (Shaw & Dobson 1995), particularly in livestock (Barger 1985; Stear et al. 1998, 2004). In Australian lambs (Barger 1985), mean nematode burdens were 2102, 5451, 1820 and 506, while k values and their standard error of the mean were 1.22±0.16, 1.46±0.19, 1.49±0.19 and 1.41±0.18 for adult H. contortus, Ostertagia spp. (predominantly T. circumcincta), intestinal Trichostrongylus spp. (predominantly Trichostrongylus colubriformis) and Nematodirus spp. (predominantly N. spathiger), respectively. In Scottish lambs (Stear et al. 1998), the mean number of adult T. circumcincta over 4 successive years were 6570, 2778, 1548 and 2996 while k values and their standard errors were 1.90±0.24, 1.79±0.24, 1.63±0.17 and 1.90±0.20, respectively. The similar k values indicate similar levels of aggregation in the different studies from the two countries. In contrast, the distribution of fourth-stage larvae among lambs was much more aggregated (Stear et al. 2004). The mean number of fourth-stage larvae was 5738, 528, 705 and 3124 while k values and 95% confidence limits in the same Scottish sheep were 0.57 (0.45–0.75), 0.29 (0.22–0.40), 0.22 (0.18–0.28) and 0.52 (0.42–0.67), respectively.
The use of the negative binomial distribution is largely empirical but the use can be justified. If the contributions of different genetic and non-genetic mechanisms are multiplicative then the underlying variation among animals in egg output would be lognormally distributed. The counting process is also likely to produce additional variation; replicate egg counts from the same sample will not necessarily give the same result. This variation in the counting process is likely to follow a Poisson distribution. The combination of the lognormal and Poisson distributions could produce a negative binomial distribution (Hunter & Quenouille 1952). Alternatively, if the underlying variation among animals followed a gamma distribution, the combination of gamma and Poisson distributions would also follow a negative binomial distribution. The distribution of underlying genetic effects is unknown and traditionally assumed to follow a normal distribution (Falconer & Mackay 1996), but recent analysis suggests that the size of gene effects may follow a gamma distribution (Hayes & Goddard 2001).
However, the negative binomial distribution does not always provide an adequate description of nematode egg counts among lambs (Stear et al. 1995a, 2006). In part, this may reflect the fact that the observed distributions, particularly of egg counts, are often mixtures of distributions, reflecting different contributions from different species of nematode. Heterogeneous negative binomial distributions, when combined together, do not necessarily produce a negative binomial distribution (Grafen & Woolhouse 1993). A comparison of different distributions found that the Weibull distribution often provided a better empirical description of the data than the more widely used negative binomial distribution (Gaba et al. 2005).
4. Sources of aggregation
Aggregation arises because there are differences among hosts in the infection process or in the response to infection (Shaw & Dobson 1995). These two processes will be reviewed and discussed separately. For grazing sheep, the infection process is the ingestion of grass or other pasture plants contaminated with infective larvae.
Each mouthful of grass ingested by grazing sheep can be considered a Poisson sample of the infective larvae in the immediate vicinity. However, faeces and infective larvae are not uniformly distributed on pasture and appear to follow a negative binomial distribution (Boag et al. 1989). Therefore, over a short period of time, the intake of larvae is likely to be aggregated among sheep; most sheep will ingest relatively low numbers of infective larvae but some sheep will ingest large numbers of larvae. Therefore, it is plausible that variation in larval ingestion is responsible, at least in part, for the observed aggregation in nematode numbers (figure 2).
There are differences among sheep in grazing behaviour (Boag et al. 1989). For example, faster-growing sheep may eat more grass. These sheep might ingest more infective larvae. This would produce positive correlations between growth rate and intensity of parasitic infection. However, for natural, predominantly T. circumcincta infections, there are only weak phenotypic and strong negative genetic correlations between faecal nematode egg count and growth rate (Bishop et al. 1996; Bouix et al. 1998). Sheep may modify their grazing behaviour in response to natural infection (Stear et al. 2001; Hutchings et al. 2003). Parasitized sheep might eat fewer (Kyriazakis et al. 1998) or richer foods (Hutchings et al. 2003) during infection and these might be adaptive responses to reduce or control infection (Hutchings et al. 2003). Aggregation would decline if heavily infected animals modified their grazing habits to reduce infection but the modifications may not be consistent over time.
In the absence of consistent differences among sheep in grazing behaviour, the ingestion of infective larvae will tend to homogenize over longer periods of time. Sheep that ingest higher than average numbers of larvae on one day may ingest lower than average numbers on another day. However, in sheep undergoing continuous natural infection, the survival time of adult nematodes is quite short (Kao et al. 2000). Aggregated distributions of adult nematodes could still arise if the majority of parasites were recently ingested. In other words, the long-term tendency to homogenization is overwhelmed by the short-term survival of ingested larvae. Temporal variation in the number of infective larvae on pasture (Stear et al. 2006) would magnify the aggregation effects of grazing. Experimentation alone is unable to quantify larval intakes and the precise balance between death rates and homogenization of larval intakes over time. This is an area where more modelling could be fruitful. One promising approach models exposure as a non-homogeneous Poisson process (Isham 1995).
The second force promoting aggregation of the numbers of adult nematodes in sheep is the immune response. In natural, predominantly T. circumcincta infection of lambs, the steadily increasing heritability of egg counts suggests that resistance is largely acquired rather than innate (Stear et al. 1997a). Resistant sheep have fewer adult nematodes, more inhibited larvae and shorter and less fecund adults (Stear et al. 1997a). Sheep with more globule leucocytes (discharged mast cells) have fewer adult T. circumcincta (Seaton et al. 1989; Stear et al. 1995b) and the working hypothesis is that immediate hypersensitivity reactions prevent the establishment of incoming larvae and possibly expel some adults. Variation in globule leucocyte numbers accounts for about one-third of the variation in worm numbers (Stear et al. 1995b).
Immunity to nematodes depends upon exposure and is likely to be more intense in more heavily infected individuals (Dineen 1963). This would tend to reduce aggregation (Galvani 2003). However, in natural, predominantly T. circumcincta nematode infection, differences in immunity due to exposure are likely to be relatively small and swamped by genetic variation in immune responsiveness (Strain et al. 2002).
Sheep with increased mucosal IgA activity against fourth-stage larvae have shorter and less fecund adult worms as well as more inhibited fourth-stage larvae (Stear et al. 1995b, 2004; Strain et al. 2002). This extends previous reports of increased numbers of inhibited larvae and IgA positive cells in efferent gastric lymph in older when compared with younger animals (Smith et al. 1984). IgA activity in the abomasal mucosa also varies extensively among animals. IgA activity in the mucosa is correlated with globule leucocyte numbers (r=0.4) consistent with both responses being influenced by Th2-type cytokines (Grencis 1997, 2001).
The distribution of immune responses mirrors the distribution of the associated parasitological variables; globule leucocytes and adult worm numbers are skewed and overdispersed while both mucosal IgA activity and adult female worm length were consistent with a normal distribution. The observed associations and the similar distributions suggest that the immune system may be, at least in part, responsible for the observed distributions.
Variation in the number of both the ingested larvae and the immune response appears to be capable of generating aggregation in worm numbers among lambs. However, the relative contribution of each to the observed aggregation is unknown. Variation among sheep in the number of ingested larvae would be very difficult to monitor in natural infections. This is another area where more modelling could be useful.
Egg output is also overdispersed. Several different species of nematode contribute to the total egg count and the total egg count can be considered as the sum of the counts for each separate species. The species for which egg counts have been most comprehensively analysed is T. circumcincta. The egg count is determined by the number of adult female worms and their average fecundity. There is a density-dependent effect on fecundity; as the number of adult worms increases fecundity declines (Bishop & Stear 2000). The number of worms is skewed and overdispersed for all major species of sheep nematode (Stear et al. 1998). The distribution of worm length is approximately normal (figure 3) and there is a curvilinear relationship between worm length and fecundity (Stear & Bishop 1999). We assume that the combination of an overdispersed distribution for worm number and an approximately normal distribution for fecundity would produce an overdispersed distribution of egg output for each species but modelling is needed to confirm this. The total egg output is then a combination of overdispersed distributions.
5. Quantitative genetic analysis of variation
The aim of quantitative genetic analysis is to determine how much of the variation among animals is due to the effects of genes and how much is due to other non-genetic sources (Nicholas 1987; Falconer & Mackay 1996; Lynch & Walsh 1998).
A standard genetic model assumes that continuously distributed traits are multifactorial, i.e. influenced by many different factors (Nicholas 1987). Some of these factors will be different genes; others will be non-genetic factors including chance, climate and nutrition. All these non-genetic factors are called environment. The performance of an individual for a particular trait is called the phenotypic value (P) and this is made up of a genetic component G (genotypic value) and an environmental component E (environmental deviation): P=G+E.
The environmental deviation is defined to have a mean of zero and, consequently, the average performance of a particular genotype is the genotypic value. The genotypic value can be broken down into the additive effect (A, also known as the breeding value) plus a deviation due to dominance (D) and a deviation due to interaction between genes at different loci (I, epistatic deviation): G=A+D+I.
The additive genetic component is the sum of the average effects of polymorphic genes. In this context genes include all relevant polymorphisms that contribute to the trait, including variation in coding regions, regulatory regions and elsewhere in the genome. Variation in the number of copies of specific genes and epigenetic changes in DNA such as methylation are included in the additive effect if they differ between individuals and are inherited in a stable manner from the oldest to the youngest generation in the study.
The additive genetic component is the most important for selective breeding while the dominance deviation and epistatic components are used in crossbreeding schemes. The proportion of the variance that is due to genotypic values (VG/VP) is heritability in the broad sense. In contrast, heritability in the narrow sense is the proportion of the variance that is attributable to variance in breeding values (VA/VP). It is the narrow-sense heritability (h2) that predicts the response to selection and this is the term of most value to animal breeders and livestock farmers. The term ‘heritability’ usually refers to the narrow-sense heritability. The heritability of a trait and the breeding value equations can vary between populations, depending on gene frequencies, type of gene action and environmental effects.
The statistical procedure most commonly employed to estimate heritability is variance analysis. Mixed model methodology has been widely used to partition the variance into genetic and non-genetic components. Animal models, which take all known pedigree relationships into account, are preferred (Lynch & Walsh 1998). A simple but relevant general linear mixed model can be described with matrix notation: Y=Xß+Zu+e. Y is a column vector describing the phenotypic values for a trait measured in n individuals, X is the n×p design matrix, ß is a p×1 vector of fixed effects, Z is an n×q incidence matrix, u is a q×1 vector of random effects and e is an n×1 column vector of residual deviations.
There is substantial evidence for genetic variation in humans (Quinnell 2003) and livestock (Stear et al. 1997a; Dominik 2005) in resistance to nematodes. The narrow-sense heritability for a single faecal egg count increases as lambs mature (Bishop et al. 1996; Stear et al. 1997a). In older calves and lambs, the heritability usually falls between 0.1 and 0.4 (Albers et al. 1987; Bishop et al. 1996; Windon et al. 2000; Bisset et al. 2001; Gruner et al. 2001; Woolaston & Windon 2001; Gauly et al. 2002). However, there have been surprisingly few attempts to estimate the heritability of nematode numbers in sheep or nematode fecundity. An examination of 530 lambs from a single commercial farm in Scotland found that the heritability and standard error of adult T. circumcincta was only 0.14±0.10 while the heritability and standard error of adult female worm length (which is strongly associated with fecundity) was much higher at 0.62±0.20 (Stear et al. 1997b). In contrast, heritability estimates following deliberate infection with H. contortus were 0.54±0.20 in 29 Rhön lambs and 0.06±0.14 and 0.11±0.11 in 79 Merinoland lambs, while the heritabilities of worm length were not significantly different from zero (Gauly et al. 2002).
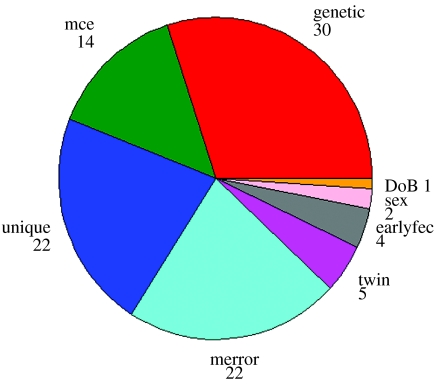
One of the most comprehensive quantitative genetic analyses available for any parasitic infection is a study in approximately 1000 Scottish Blackface lambs (Stear et al. 1996). Figure 4 illustrates the key sources of variation in faecal egg counts at five months of age. The most important component in this study was additive genetic variation. The importance of these components varies over time; for example, the maternal component decreases as the lambs mature. In contrast, the additive genetic component is indistinguishable from zero at one and two months of age but increases to one-third of the total variation at six and seven months of age. The delayed development of genetic resistance is consistent with the hypothesis that resistance is due to the development of protective immune responses (Bishop et al. 1996). A heritability of one-third is similar to the heritability of milk production in dairy cattle or growth rate in beef cattle. Heritabilities of this magnitude mean that selective breeding for decreased egg output is feasible.
Figure 4.
Key components of variation in faecal egg counts from five-month-old lambs from a single commercial farm in central Scotland. Each year from 1992 to 1996, a cohort of 200 lambs was followed but deaths, lost tags, missing or incorrect records meant that only 902 lambs were included in the analysis. Genetic refers to additive genetic variation which accounts for 30% of the variance. DoB is date of birth which accounts for 1% of the variance. Earlyfec is the faecal egg count at one month of age. Young lambs with high egg counts have lower than average egg counts at five months of age. Twin lambs have higher egg counts than singletons. Merror is measurement error, i.e. the discrepancy between replicate counts on the same aliquot. Unique means factors specific to each individual while mce refers to maternal genetics and the maternal common environment.
These heritabilities refer to a single egg count at each time point. In practice, a sensible strategy would be to make replicate counts on each sample and to combine the results from samples taken at different times. A combination of multiple counts and replicate samples would improve the identification of genetically resistant animals and produce faster responses to selection. The more rapid response to selection is a consequence of the fact that the mean of several counts is a better estimate of genetic effects and is therefore under stronger genetic control. This observation suggests that resistance to this parasitic disease is strongly influenced by genetic variation in the host; the relatively low estimates of genetic variation in disease resistance may sometimes be a consequence of the difficulty in defining and accurately measuring resistance to disease.
Traditional quantitative genetic analyses successfully predict the response to selection in production traits such as milk yield or growth. In the case of parasitic and infectious diseases, traditional approaches underestimate the response to selection because they take no account of the reduced contamination of the environment. For example, selected animals will produce fewer nematode eggs and mean egg counts of infected lambs will decrease over time. The combination of reduced environmental contamination and increased resistance in hosts has been addressed using epidemiological models of the transmission dynamics (Bishop & Stear 1997, 2003). The results from these models predict that the response to selection will be much greater than that predicted by traditional genetic theory. There is variation among animals in the transformation of excreted eggs into infective larvae. Eggs from resistant sheep develop into infective larvae less successfully than eggs from susceptible sheep (Jorgensen et al. 1998). This result has still to be incorporated into models that predict the responses to selection. It should lead to even greater benefits from selective breeding.
6. Molecular genetic analysis of variation
Identifying the genes underlying immune responses would improve our understanding of the host–parasite interaction and allow selective breeding to be enhanced by the use of genetic markers.
There are two methods of data analysis used in gene hunting: linkage and association. Their advantages and disadvantages have been extensively reviewed (Thomas 2004). In brief, linkage analysis involves studying joint inheritance in families. It is less powerful but more robust than association analysis which involves the study of unrelated individuals. Ideally, in families, correlations between genotypes and phenotypes will be influenced by causative mutations and linkage, while correlations in unrelated individuals will be a consequence of causative mutations and linkage disequilibrium. However, these distinctions are not so clear-cut in farm animal populations. On sheep and cattle farms, many animals share common ancestors and correlations of genotypes with phenotypes will reflect both linkage disequilibrium and linkage. Most studies have used either linkage (Davies et al. 2006) or a combination of linkage and linkage disequilibrium (Stear et al. 2005). More promising methods combine multipoint linkage and linkage disequilibrium (Farnir et al. 2002; Meuwissen et al. 2002), but these have yet to be widely applied.
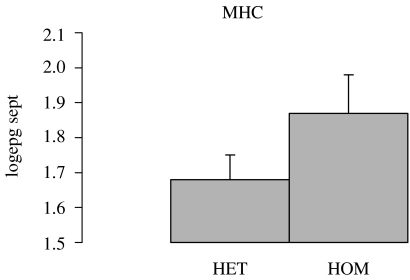
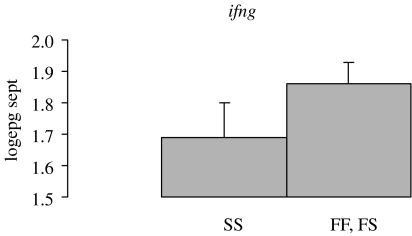
Two of the major sources of variation in immune responses are variation in antigen recognition, which influences the quality of the immune response and variation in cytokine production, which influences the quantity of the immune response. There are two confirmed genetic markers for T. circumcincta. Figure 5 illustrates the influence of heterozygosity at the major histocompatibility complex class II locus DRB1. The association between the mhc and nematode infection has been independently confirmed by several groups (Schwaiger et al. 1995; Paterson et al. 1998; Buitkamp & Epplen 2001; Charon et al. 2002; Sayers et al. 2005; Stear et al. 2005; Davies et al. 2006). The second marker is the interferon gamma locus ifng (figure 6). At this locus, one of the two or three alleles is associated with high nematode egg counts. This association has been independently confirmed by several groups (Coltman et al. 2001; Abuargob 2006; Davies et al. 2006). These markers are biologically plausible candidate genes: the mhc class II region influences antigen recognition, while interferon gamma can influence antigen recognition but its main function is to determine the type of cytokine response (Frank 2002). However for both genes, the causative mutations have still to be identified.
Figure 5.
Heterozygotes (HET) had lower egg counts than homozygotes (HOM) for the DRB1 locus within the sheep MHC. There were 152 homozygotes and 637 heterozygotes. The animals were naturally infected and parents were bred without knowledge of mhc genotypes. All animals came from a commercial farm in southwest Strathclyde. Heterozygous individuals with two different alleles at the DRB1 locus are more resistant to nematode infection than individuals with two copies of the same allele. The y-axis represents faecal egg counts in September that were transformed by taking logarithms to the base 10.
Figure 6.
Influence on faecal egg count of different genotypes at the microsatellite locus within the interferon gamma gene. All 709 animals tested came from the same commercial farm in southwest Strathclyde. There are only two alleles in Scottish blackface sheep. The frequency of the F allele was 0.56. The F allele is dominant and individuals with one or two copies of the F allele have higher egg counts than individuals with two copies of the S allele. The y-axis represents faecal egg counts in September that were transformed by taking logarithms to the base 10.
The F allele at the ifng locus shows dominant susceptibility in male lambs while the polymorphic DRB1 locus shows heterozygote advantage for some of the traits describing relative resistance to nematode infection. These loci therefore show non-additive gene action. The two loci may be markers for other linked loci in linkage disequilibrium and the non-additive gene action may reflect gene action at the true disease resistance loci. Both loci were more strongly associated with differences in the number of adult T. circumcincta than with differences in the number of immature larvae or in worm length or fecundity (unpublished observations). Together these two loci accounted for approximately 50% of the additive genetic variation in worm number.
7. The potential for combining genetic and modelling approaches
Genetic approaches complement the modelling approaches that have been used to study host–parasite systems. Transmission dynamics have been extensively studied using epidemiological models that either treat overdispersed traits phenomenologically (Anderson & May 1978; May & Anderson 1978; Roberts 1995; Roberts et al. 1995) or expose potential sources of heterogeneity that could influence the observed distributions of traits. A combination of observational and theoretical approaches have shown that these sources include variability in exposure, acquired immunity, age, host predisposition and parasite-induced host mortality (Tallis & Leyton 1966; Anderson & Gordon 1982; Pacala & Dobson 1988; Woolhouse 1992; Hudson & Dobson 1995; Roberts 1995; Roberts et al. 1995; Smith et al. 1995). Modelling advances which go beyond the phenomenological approach to create frameworks from which the aggregation emerges naturally have incorporated spatial heterogeneity and stochasticity in the infection process, the explicit representation of acquired immunity, heterogeneity in the host response to infection, dynamically evolving levels of aggregation and multiple infections (Grenfell et al. 1995a,b; Isham 1995; Cornell et al. 2000, 2004; Herbert & Isham 2000; Rosà & Pugliese 2002; Bottomley et al. 2005).
Typically, within these host–parasite transmission models, host predisposition has been treated simply. Somewhat surprisingly, only a few models of nematode infection in livestock have explicitly modelled individual (Louie et al. 2005) or genetic variation among individuals (Bishop & Stear 1997). A genetic approach provides the opportunity to underpin these models with an explicit bottom-up representation of a key source of heterogeneity in infection traits. This will assist in the quantification of the contribution of non-genetic mechanisms to the generation of aggregation. In particular, the integration of genetic and modelling approaches would allow the development of predictive models of host–parasite transmission and control based upon a detailed biological understanding of the interactions between host and parasite.
8. Conclusions
A genetic approach can assist our understanding of disease dynamics by identifying genes and mechanisms that influence variation among animals. However, a genetic analysis in itself may not be sufficient. The next stage is to develop mathematical models that explicitly formulate and test the assumptions inherent in biological approaches. In particular, there is a need for a mechanistic model to determine whether our understanding of genetic and non-genetic variation is sufficient to account for the variation in immunological responses and whether the identified protective immunological responses can account for the observed variation in parasitological traits following natural and deliberate infection.
Acknowledgments
All research was approved by the University of Glasgow Ethical Review Process and licensed by the UK Home Office.
Footnotes
One contribution of 20 to a Theme Issue ‘Cross-scale influences on epidemiological dynamics: from genes to ecosystems’.
References
- Abuargob, O. 2006 The influence of sex on nematode infection in Scottish Blackface lambs. PhD thesis, University of Glasgow 2006.
- Albers G.A.A, Gray G.D, Piper L.R, Barker J.S.F, Lejambre L, Barger I.A. The genetics of resistance and resilience to Haemonchus contortus infection in young Merino sheep. Int. J. Parasitol. 1987;17:1355–1363. doi: 10.1016/0020-7519(87)90103-2. [DOI] [PubMed] [Google Scholar]
- Anderson R.M, Gordon D.M. Processes influencing the distribution of parasite numbers within host populations with special emphasis on parasite-induced host mortalities. Parasitology. 1982;85:373–398. doi: 10.1017/s0031182000055347. [DOI] [PubMed] [Google Scholar]
- Anderson R.M, May R.M. Regulation and stability of host–parasite population interactions. I. Regulatory processes. J. Anim. Ecol. 1978;47:219–247. doi: 10.2307/3933. [DOI] [Google Scholar]
- Anderson R.M, Gordon D.M, Crawley M.J, Hassell M.P. Variability in the abundance of animal and plant species. Nature. 1982;296:245–248. doi: 10.1038/296245a0. [DOI] [Google Scholar]
- Balic A, Bowles V.M, Meeusen E.N.T. The immunobiology of gastrointestinal nematode infections in ruminants. Adv. Parasitol. 2000;45:181–241. doi: 10.1016/s0065-308x(00)45005-0. [DOI] [PubMed] [Google Scholar]
- Barger I.A. The statistical distribution of trichostrongylid nematodes in grazing lambs. Int. J. Parasitol. 1985;15:645–649. doi: 10.1016/0020-7519(85)90010-4. [DOI] [PubMed] [Google Scholar]
- Bishop S.C, Stear M.J. Modelling responses to selection for resistance to gastro-intestinal parasites in sheep. Anim. Sci. 1997;64:469–478. [Google Scholar]
- Bishop S.C, Stear M.J. The use of a gamma-type function to assess the relationship between the number of adult Teladorsagia circumcincta and total egg output. Parasitology. 2000;121:435–440. doi: 10.1017/S0031182099006526. [DOI] [PubMed] [Google Scholar]
- Bishop S.C, Stear M.J. Modeling of host genetics and resistance to infectious diseases: understanding and controlling nematode infections. Vet. Parasitol. 2003;115:147–166. doi: 10.1016/S0304-4017(03)00204-8. [DOI] [PubMed] [Google Scholar]
- Bishop S.C, Bairden K, McKellar Q.A, Park M, Stear M.J. Genetic parameters for faecal egg count following mixed, natural, predominantly Ostertagia circumcincta infection and relationships with liveweight in young lambs. Anim. Sci. 1996;63:423–428. [Google Scholar]
- Bisset S.A, Morris C.A, McEwan J.C, Vlassoff A. Breeding sheep in New Zealand that are less reliant on anthelmintics to maintain health and productivity. N. Zeal. Vet. J. 2001;49:236–246. doi: 10.1080/00480169.2001.36238. [DOI] [PubMed] [Google Scholar]
- Bliss C.I, Fisher R.A. Fitting the negative binomial distribution to biological data. Biometrics. 1953;9:176–200. doi: 10.2307/3001850. [DOI] [Google Scholar]
- Boag B, Topham P.B, Webster R. Spatial distribution on pasture of infective larvae of the gastro-intestinal nematode parasites of sheep. Int. J. Parasitol. 1989;19:681–685. doi: 10.1016/0020-7519(89)90047-7. [DOI] [PubMed] [Google Scholar]
- Bottomley C, Isham V, Basanez M.-G. Population biology of multi-species helminth infection: interspecific interactions and parasite distribution. Parasitology. 2005;131:1–17. doi: 10.1017/S0031182005007791. [DOI] [PubMed] [Google Scholar]
- Bouix J, et al. Genetic resistance to gastrointestinal nematode parasites in Polish long-wool sheep. Int. J. Parasitol. 1998;28:1797–1804. doi: 10.1016/S0020-7519(98)00147-7. [DOI] [PubMed] [Google Scholar]
- Buitkamp J, Epplen J.T. Major histocompatibility and T-cell receptor genes in Artiodactyls: characteriztion, polymorphism and genetic resistance to a helmintic infection. J. Anim. Breed. Genet. 2001;113:287–291. [Google Scholar]
- Charon K.M, Moskwa B, Rutkowski R, Gruszczynska J, Swiderek W. Microsatellite polymorphism in DRB1 gene (MHC class II) and its relation to nematode faecal egg count in Polish Heath Sheep. J. Anim. Feed. Sci. 2002;11:47–58. [Google Scholar]
- Christie M, Jackson F. Specific identification of strongyle eggs in small samples of sheep faeces. Res. Vet. Sci. 1982;32:113–117. [PubMed] [Google Scholar]
- Coltman D.W, Wilson K, Pilkington J, Stear M.J, Pemberton J.M. A microsatellite polymorphism in the gamma interferon gene is associated with resistance to gastrointestinal nematodes in a naturally-parasitized population of Soay sheep. Parasitology. 2001;122:571–582. doi: 10.1017/S0031182001007570. [DOI] [PubMed] [Google Scholar]
- Coop, R. L., Bartley, D., Jackson, E., Houdijk, J. G. M., Kyriazakis, I. & Jackson, F. 2001 Influence of dietary protein on the periparturient relaxation of immunity in parasitized ewes. In Proc. 5th Int. Sheep Veterinary Conf., University of Stellenbosch., South Africa, 21–25 January.
- Cornell S.J, Isham V, Grenfell B.T. Drug-resistant parasites and aggregated infection—early season dynamics. J. Math. Biol. 2000;41:341–360. doi: 10.1007/s002850000051. [DOI] [PubMed] [Google Scholar]
- Cornell S.J, Isham V.S, Grenfell B.T. Stochastic and spatial dynamics of nematode parasites in farmed ruminants. Proc. R. Soc. B. 2004;71:1243–1250. doi: 10.1098/rspb.2004.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G, Stear M.J, Benothman M, Abuargob O, Kerr A, Mitchell S, Bishop S.C. Quantitative trait loci associated with parasitic infection in Scottish Blackface sheep. Heredity. 2006;96:252–258. doi: 10.1038/sj.hdy.6800788. [DOI] [PubMed] [Google Scholar]
- Dineen J.K. Immunological aspects of parasitism. Nature. 1963;197:268–269. doi: 10.1038/197268a0. [DOI] [PubMed] [Google Scholar]
- Dominik S. Quantitative trait loci for internal nematode resistance in sheep: a review. Genet. Select. Evol. 2005;37(Suppl. 1):S83–S96. doi: 10.1051/gse:2004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J, van Houtert M.F.J, Sykes A.R. The effect of nutrition on the periparturient parasite status of mature ewes. Anim. Sci. 1998;67:523–533. [Google Scholar]
- Falconer D.S, Mackay T.F.C. Longman; Harlow, UK: 1996. Introduction to quantitative genetics. [Google Scholar]
- Farnir F, et al. Simultaneous mining of linkage and linkage disequilibrium to fine map quantitative trait loci in outbred half-sib pedigrees: revisiting the location of a quantitative trait locus with major effect on milk production on bovine chromosome 14. Genetics. 2002;161:275–287. doi: 10.1093/genetics/161.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S.A. Princeton University Press; Princeton, NJ: 2002. Immunology and evolution of infectious disease. [PubMed] [Google Scholar]
- Gaba S, Ginot V, Cabaret J. Modelling macroparasite aggregation using a nematode-sheep system: the Weibull distribution as an alternative to the negative binomial distribution? Parasitology. 2005;131:393–401. doi: 10.1017/S003118200500764X. [DOI] [PubMed] [Google Scholar]
- Galvani A.P. Immunity, antigenic heterogeneity, and aggregation of helminth parasites. J. Parasitol. 2003;89:232–241. doi: 10.1645/0022-3395(2003)089%5B0232:IAHAAO%5D2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Gauly M, Kraus M, Vervelde L, van Leeuwen M.A, Erhardt G. Estimating genetic differences in natural resistance in Rhön and Merinoland sheep following experimental Haemonchus contortus infection. Vet. Parasitol. 2002;106:55–67. doi: 10.1016/S0304-4017(02)00028-6. [DOI] [PubMed] [Google Scholar]
- Grafen A, Woolhouse M.E.J. Does the negative binomial distribution add up? Parasitol. Today. 1993;9:475–477. doi: 10.1016/0169-4758(93)90107-Q. [DOI] [PubMed] [Google Scholar]
- Grencis R.K. Th2-mediated host protective immunity to intestinal nematode infections. Phil. Trans. R. Soc. B. 1997;352:1377–1384. doi: 10.1098/rstb.1997.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grencis R.K. Cytokine regulation of resistance and susceptibility to intestinal nematode infection—from host to parasite. Vet. Parasitol. 2001;100:45–50. doi: 10.1016/S0304-4017(01)00482-4. [DOI] [PubMed] [Google Scholar]
- Grenfell B.T, Dietz K, Roberts M.G. Modelling the immuno-epidemiology of macroparasites in naturally-fluctuating host populations. In: Grenfell B.T, Dobson A, editors. Ecology of infectious diseases in natural populations. Cambridge University Press; Cambridge, UK: 1995a. [Google Scholar]
- Grenfell B.T, Wilson K, Isham V.S, Boyd H.E.G, Dietz K. Modelling patterns of parasite aggregation in natural populations: trichostrongylid nematode–ruminant interactions as a case study. Parasitology. 1995b;111:S135–S151. doi: 10.1017/s0031182000075867. [DOI] [PubMed] [Google Scholar]
- Gruner, L., Aumont, G., Bouix, J. & Mandonnet, N. 2001 The genetic resistance to nematode parasites in small ruminants: a more and more well known trait. 8emes Rencontres autour des Recherches sur les Ruminants, Paris, France, 5–6 December 2001–2198. [In French.]
- Gruner L, Cortet J, Sauve C, Limouzin C, Brunel J.C. Evolution of nematode community in grazing sheep selected for resistance and susceptibility to Teladorsagia circumcincta and Trichostrongylus colubriformis: a 4-year experiment. Vet. Parasitol. 2002;109:277–291. doi: 10.1016/S0304-4017(02)00302-3. [DOI] [PubMed] [Google Scholar]
- Gruner L, Cortet J, Sauve C, Hoste H. Regulation of Teladorsagia circumcincta and Trichostrongylus colubriformis worm populations by grazing sheep with differing resistance status. Vet. Res. 2004;35:91–101. doi: 10.1051/vetres:2003043. [DOI] [PubMed] [Google Scholar]
- Hayes B, Goddard M.E. The distribution of the effects of genes affecting quantitative traits in livestock. Genet. Select. Evol. 2001;33:209–229. doi: 10.1051/gse:2001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J, Isham V. Stochastic host–parasite interaction models. J. Math. Biol. 2000;40:343–371. doi: 10.1007/s002850050184. [DOI] [PubMed] [Google Scholar]
- Houdijk J.G.M, Kyriazakis I, Jackson F, Coop R.L. The relationship between protein nutrition, reproductive effort and breakdown in immunity to Teladorsagia circumcincta in periparturient ewes. Anim. Sci. 2006;72:595–606. [Google Scholar]
- Hudson P.J, Dobson A.P. Macroparasites: observed patterns in naturally fluctuating animal populations. In: Grenfell B.T, Dobson A.T, editors. Ecology of infectious diseases in natural populations. Cambridge University Press; Cambridge, UK: 1995. [Google Scholar]
- Hunter G.C, Quenouille M.H. A statistical examination of the worm egg count sampling technique for sheep. J. Helminthol. 1952;26:157–170. [Google Scholar]
- Huntley J.F, Jackson F, Coop R.L, Macaldowie C, Houdijk J.G.M, Familton A.S, Xieh H.L, Stankiewicz M, Sykes A.R. The sequential analysis of local inflammatory cells during abomasal nematode infection in periparturient sheep. Vet. Immunol. Immunopathol. 2004;97:163–176. doi: 10.1016/j.vetimm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hutchings M.R, Athanasiadou S, Kyriazakis I, Gordon I.J. Can animals use foraging behaviour or combat parasites? Proc. Nutr. Soc. 2003;62:361–370. doi: 10.1079/PNS2003243. [DOI] [PubMed] [Google Scholar]
- Isham V. Stochastic models of host–macroparasite interaction. Ann. Appl. Prob. 1995;5:720–740. [Google Scholar]
- Jorgensen L.T, Leathwick D.M, Charleston W.A.G, Godfrey P.L, Vlassoff A, Sutherland I.A. Variation between hosts in the developmental success of the free-living stages of trichostrongyle infections of sheep. Int. J. Parasitol. 1998;28:1347–1352. doi: 10.1016/S0020-7519(98)00092-7. [DOI] [PubMed] [Google Scholar]
- Kao R.R, Leathwick D.M, Roberts M.G, Sutherland I.A. Nematode parasites of sheep: a survey of epidemiological parameters and their application in a simple model. Parasitology. 2000;121:85–103. doi: 10.1017/S0031182099006095. [DOI] [PubMed] [Google Scholar]
- Keeling M.J. Simple stochastic models and their power-law type behaviour. Theor. Popul. Biol. 2000;58:21–31. doi: 10.1006/tpbi.2000.1475. [DOI] [PubMed] [Google Scholar]
- Kyriazakis I, Tolkamp B.J, Hutchings M.R. Towards a functional explanation for the occurrence of anorexia during parasitic infections. Anim. Behav. 1998;56:265–274. doi: 10.1006/anbe.1998.0761. [DOI] [PubMed] [Google Scholar]
- Louie K, Vlassoff A, Mackay A. Nematode parasites of sheep: extension of a simple model to include host variability. Parasitology. 2005;130:437–446. doi: 10.1017/S003118200400678X. [DOI] [PubMed] [Google Scholar]
- Lynch M, Walsh B. Sinauer; Sunderland, MA: 1998. Genetics and analysis of quantitative traits. [Google Scholar]
- May R.M, Anderson R.M. Regulation and Stability of host–parasite population interactions II. Destabilizing processes. J. Anim. Ecol. 1978;47:249–267. doi: 10.2307/3934. [DOI] [Google Scholar]
- Meuwissen T.H.E, Karlsen A, Lien S, Olsaker I, Goddard M.E. Fine mapping of a quantitative trait locus for twinning rate using combined linkage and linkage disequilibrium mapping. Genetics. 2002;161:373–379. doi: 10.1093/genetics/161.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas F.W. Oxford University Press; Oxford, UK: 1987. Veterinary genetics. [Google Scholar]
- Nieuwhof G.J, Bishop S.C. Costs of the major endemic diseases of sheep in Great Britain and the potential benefits of reduction in disease impact. Anim. Sci. 2005;81:23–29. doi: 10.1079/ASC41010023. [DOI] [Google Scholar]
- Pacala S.W, Dobson A.P. The relation between the number of parasites/host and host age: population dynamic causes and maximum likelihood estimation. Parasitology. 1988;96:197–210. doi: 10.1017/s0031182000081762. [DOI] [PubMed] [Google Scholar]
- Paterson S, Wilson K, Pemberton J.M. Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population (Ovis aries L.) Proc. Natl Acad. Sci. USA. 1998;95:3714–3719. doi: 10.1073/pnas.95.7.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry B.D, Randolph T.F. Improving the assessment of the economic impact of parasitic diseases and of their control in production animals. Vet. Parasitol. 1999;84:145–168. doi: 10.1016/S0304-4017(99)00040-0. [DOI] [PubMed] [Google Scholar]
- Quinnell R.J. Genetics of susceptibility to human helminth infection. Int. J. Parasitol. 2003;33:1219–1231. doi: 10.1016/S0020-7519(03)00175-9. [DOI] [PubMed] [Google Scholar]
- Roberts M.G. A pocket guide to host-parasite models. Parasitol. Today. 1995;11:172–177. doi: 10.1016/0169-4758(95)80150-2. [DOI] [PubMed] [Google Scholar]
- Roberts M.G, Smith G, Grenfell B.T. Mathematical models for macroparasites of wildlife. In: Grenfell B.T, Dobson A, editors. Ecology of infectious diseases in natural populations. Cambridge University Press; Cambridge, UK: 1995. [Google Scholar]
- Rosà R, Pugliese A. Aggregation, stability and oscillations in different models for host–parasite interactions. Theor. Popul. Biol. 2002;61:319–334. doi: 10.1006/tpbi.2002.1575. [DOI] [PubMed] [Google Scholar]
- Sayers G, Sweeney T. Gastrointestinal nematode infection in sheep—a review of the alternatives to anthelmintics in parasite control. Anim. Health Res. Rev. 2005;6:159–171. doi: 10.1079/AHR2005108. [DOI] [PubMed] [Google Scholar]
- Sayers G, Good B, Hanrahan J.P, Ryan M, Angles J.M, Sweeney T. Major histocompatibility complex DRB1 gene: its role in nematode resistance in Suffolk and Texel sheep breeds. Parasitology. 2005;131:403–409. doi: 10.1017/S0031182005007778. [DOI] [PubMed] [Google Scholar]
- Schwaiger F.W, Gostomski D, Stear M.J, Duncan J.L, McKellar Q.A, Epplen J.T, Buitkamp J. An ovine major histocompatibility complex DRB1 allele is associated with low faecal egg counts following natural predominantly Ostertagia circumcincta infection. Int. J. Parasitol. 1995;25:815–822. doi: 10.1016/0020-7519(94)00216-B. [DOI] [PubMed] [Google Scholar]
- Scott M.E. Temporal changes in aggregation: a laboratory study. Parasitology. 1987;94:583–595. doi: 10.1017/s0031182000055918. [DOI] [PubMed] [Google Scholar]
- Seaton D.S, Jackson F, Smith W.D, Angus K.W. Development of immunity to incoming radiolabelled larvae in lambs continuously infected with Ostertagia circumcincta. Res. Vet. Sci. 1989;46:241–246. [PubMed] [Google Scholar]
- Shaw D.J, Dobson A.P. Patterns of macroparasite abundance and aggregation in wildlife populations: a quantitative review. Parasitology. 1995;111:s111–s133. doi: 10.1017/s0031182000075855. [DOI] [PubMed] [Google Scholar]
- Smith W.D, Jackson F, Jackson E, Williams J, Miller H.R.P. Manifestations of resistance to ovine ostertagiasis associated with immunological responses in the gastric lymph. J. Comp. Pathol. 1984;94:591–601. doi: 10.1016/0021-9975(84)90063-X. [DOI] [PubMed] [Google Scholar]
- Smith G, et al. Macroparasite group report: problems in modelling the dynamics of macroparasitic systems. In: Grenfell B.T, Dobson A.P, editors. Ecology of wildlife diseases. Cambridge University Press; Cambridge, UK: 1995. pp. 209–229. [Google Scholar]
- Stear M.J, Bishop S.C. The curvilinear relationship between worm length and fecundity of Teladorsagia circumcincta. Int. J. Parasitol. 1999;29:777–780. doi: 10.1016/S0020-7519(99)00019-3. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Hetzel D.J.S, Brown S.C, Gershwin L.J, Mackinnon M.J, Nicholas F.W. The relationships among ecto- and endoparasite levels, class I antigens of the bovine major histocompatibility system, immunoglobulin E levels and weight gain. Vet. Parasitol. 1990;34:303–321. doi: 10.1016/0304-4017(90)90077-O. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Bairden K, Duncan J.L, Gettinby G, McKellar Q.A, Murray M, Wallace D.S. The distribution of faecal nematode egg counts in Scottish Blackface lambs following natural, predominantly Ostertagia circumcincta infection. Parasitology. 1995a;110:573–581. doi: 10.1017/s003118200006529x. [DOI] [PubMed] [Google Scholar]
- Stear M.J, et al. Regulation of egg production, worm burden, worm length and worm fecundity by host responses in sheep infected with Ostertagia circumcincta. Parasite Immunol. 1995b;17:643–652. doi: 10.1111/j.1365-3024.1995.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Park M, Bishop S.C. The key components of resistance to Ostertagia circumcincta in lambs. Parasitol. Today. 1996;12:438–441. doi: 10.1016/0169-4758(96)10069-7. [DOI] [PubMed] [Google Scholar]
- Stear M.J, et al. The genetic basis of resistance to Ostertagia circumcincta in lambs. Vet. J. 1997a;154:111–119. doi: 10.1016/S1090-0233(97)80049-4. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Bairden K, Duncan J.L, Holmes P.H, McKellar Q.A, Park M, Strain S.A.J, Murray M. How hosts control worms. Nature. 1997b;389:27–27. doi: 10.1038/37895. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Bairden K, Bishop S.C, Gettinby G, McKellar Q.A, Park M, Strain S.A.J, Wallace D.S. The processes influencing the distribution of parasitic nematodes among naturally infected lambs. Parasitology. 1998;117:165–171. doi: 10.1017/S0031182098002868. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Strain S.A.J, Bishop S.C. How lambs control infection with Ostertagia circumcincta. Vet. Immunol. Immunopathol. 1999a;72:213–218. doi: 10.1016/S0165-2427(99)00134-8. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Strain S.A.J, Bishop S.C. Mechanisms underlying resistance to nematode infection. Int. J. Parasitol. 1999b;29:51–56. doi: 10.1016/S0020-7519(98)00179-9. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Eckersall P.D, Graham P.A, McKellar Q.A, Mitchell S, Bishop S.C. Fructosamine concentration and resistance to natural, predominantly Teladorsagia circumcincta infection. Parasitology. 2001;123:211–218. doi: 10.1017/S0031182001008253. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Bishop S.C, Henderson N.G, Scott I. A key mechanism of pathogenesis in sheep infected with the nematode Teladorsagia circumcincta. Anim. Health Res. Rev. 2003;4:45–52. doi: 10.1079/AHRR200351. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Bairden K, Innocent G.T, Mitchell S, Strain S.A.J, Bishop S.C. The relationship between IgA activity against fourth-stage larvae and density-dependent effects on the number of fourth-stage larvae of Teladorsagia circumcincta in naturally infected sheep. Parasitology. 2004;129:363–369. doi: 10.1017/S0031182004005736. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Innocent G.T, Buitkamp J. The evolution and maintenance of polymorphism in the major histocompatibility complex. Vet. Immunol. Immunopathol. 2005;108:53–57. doi: 10.1016/j.vetimm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Abuargob O, Ben Othman M, Bishop S.C, Innocent G.T, Kerr A, Mitchell S. Variation among faecal egg counts following natural nematode infection in Scottish Blackface lambs. Parasitology. 2006;132:275–280. doi: 10.1017/S0031182005009029. [DOI] [PubMed] [Google Scholar]
- Stear M.J, Doligalska M, Donskow-Schmelter K. Alternatives to anthelmintics for the control of nematodes in livestock. Parasitology. 2007;134:139–151. doi: 10.1017/S0031182006001557. [DOI] [PubMed] [Google Scholar]
- Strain S.A.J, Bishop S.C, Henderson N.G, Kerr A, McKellar Q.A, Mitchell S, Stear M.J. The genetic control of IgA activity against Teladorsagia circumcincta and its association with parasite resistance in naturally infected sheep. Parasitology. 2002;124:545–552. doi: 10.1017/S0031182002001531. [DOI] [PubMed] [Google Scholar]
- Tallis G.M, Leyton M. A stochastic approach to study of parasite populations. J. Theor. Biol. 1966;13:251–260. doi: 10.1016/0022-5193(66)90020-8. [DOI] [Google Scholar]
- Taylor L.R. Aggregation, variance and the mean. Nature. 1961;189:732–735. doi: 10.1038/189732a0. [DOI] [Google Scholar]
- Thomas D.C. Oxford University Press; Oxford, UK: 2004. Statistical methods in genetic epidemiology. [Google Scholar]
- Thomas R.J, Boag B. Epidemiological studies on gastro-intestinal nematode parasites of sheep. Infection patterns on clean and summer-contaminated pasture. Res. Vet. Sci. 1972;13:61–69. [PubMed] [Google Scholar]
- Urquhart G.M, Armour J, Duncan J.L, Dunn A.M, Jennings F.W. Longman Scientific and Technical; Avon, UK: 1987. Veterinary parasitology. [Google Scholar]
- Windon, R. G., Dineen, J. K. & Wagland, B. M. 2000 Genetic control of immunological responsiveness against the intestinal nematode Trichostrongylus colubriformis in lambs.
- Woolaston R.R, Windon R.G. Selection of sheep for response to Trichostrongylus colubriformis larvae: genetic parameters. Anim. Sci. 2001;73:41–48. [Google Scholar]
- Woolhouse M.E.J. A theoretical framework for the immunoepidemiology of helminth infection. Parasite Immunol. 1992;14:563–578. doi: 10.1111/j.1365-3024.1992.tb00029.x. [DOI] [PubMed] [Google Scholar]