Animal models are invaluable resources for biomedical research, including research on the effects of diet on metabolism and disease. Usually, great care is taken to ensure comparable genetic backgrounds and environmental conditions when performing studies using animal models, since this minimizes introduction of variability that can confound detection of treatment-related phenotypic differences. However, many papers using animal models draw conclusions about dietary effects from comparison of natural ingredient chow with defined diets, despite marked differences in micro- and macronutrient content. When comparing the effects of a chow diet with a high-fat defined diet, the effects of the dietary fat will be confounded with the effects of other components in the diets. This issue is highlighted by a limited literature survey that was conducted to identify common problems in the use and reporting of rodent diets.
All original research papers identified by the keywords "mouse high fat" published in 2007 in five high-impact journals were included in this evaluation. Of the 35 papers examined, only 14% (5 papers) compared diets using identical nutrients differing only in relative amounts of fat and carbohydrate (Figure 1). Specific details regarding the dietary comparisons made are often lacking, and frequently conclusions are drawn from comparisons of defined high-fat diets to chow.
Figure 1.
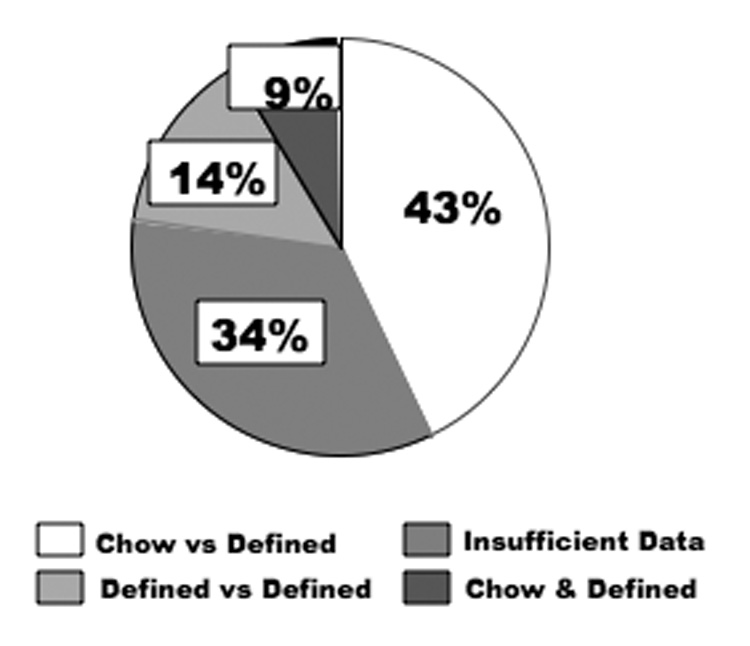
Pie chart showing the percentage of 35 original research papers evaluated that used appropriate diet comparisons (14%), that compared chow and defined high-fat diets (43%), or that presented insufficient information to evaluate diet comparisons (34%). In the Chow & Defined diet category, both diet types were used, but no direct comparison was made between them. The journals examined were Cell Metabolism (7 papers), Diabetes (the first 11 of 36 papers), The Journal of Clinical Investigation (12 papers), Nature (2 papers), and Nature Medicine (3 papers).
Regular chow is composed of agricultural byproducts, such as ground wheat, corn, or oats, alfalfa and soybean meals, a protein source such as fish, and vegetable oil and is supplemented with minerals and vitamins. Thus, chow is a high fiber diet containing complex carbohydrates, with fats from a variety of vegetable sources. Chow is inexpensive to manufacture and is palatable to rodents. In contrast, defined high-fat diets consist of amino acid supplemented casein, cornstarch, maltodextrose or sucrose, and soybean oil or lard, also supplemented with minerals and vitamins. Fiber is often provided by cellulose. Chow and defined diets may exert significant separate and independent unintended effects on the measured phenotypes in any research protocol.
Two important difference between chow and defined diets are the phytoestrogen content from soy that is high but variable in chow diets but is absent from defined diets (reviewed in [1]) and sucrose that is used as a carbohydrate source in defined diets but is absent from chow. Dietary phytoestrogens influence food and water intake, anxiety related behaviors, locomotor activity, learning and memory, fat deposition, blood insulin, leptin and thyroid levels, and lipogenesis and lipolysis in isolated rat adipocytes ([2, 3] and reviewed in [4]). Sucrose is 50% fructose and there is abundant evidence that fructose can exacerbate weight gain and contribute to insulin resistance and dyslipidemia (reviewed in [5]). Other effects that differ between chow and defined diets and that may be related to either phytoestrogen or fructose content include bone-related changes, plasma estradiol, urinary calcium, urinary corticosterone [6], and solution taste preferences [7].
When comparing the effects of chow with a high-fat defined diet, the effects of the dietary fat will be confounded with the effects of other components that differ between the diets. Just as it is essential that mouse strains be specified, constituents of experimental diets must be specified [1, 8, 9]. Specifics to be considered are the objectives of the study, whether the endpoints are affected by several diet components, the maintenance diet, and palatability of the diet [1]. Although this correspondence focuses on high-fat diet comparisons, these issues are applicable to any specific nutrient comparisons.
ACKNOWLEDGEMENTS
The authors sincerely thank Drs. Sean Adams, Jim Cheverud, and Peter Havel for their comments and suggestions. Neither C. H. Warden nor J. S. Fisler has a conflict of interest regarding the contents of this letter. CHW was supported by DK52581 and DK69978.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Thigpen JE, Setchell KD, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. Ilar J. 2004;45:401–416. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- 2.Torre-Villalvazo I, Tovar AR, Ramos-Barragan VE, Cerbon-Cervantes MA, Torres N. Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J Nutr. 2008:462–468. doi: 10.1093/jn/138.3.462. [DOI] [PubMed] [Google Scholar]
- 3.Lephart ED, Porter JP, Lund TD, Bu L, Setchell KD, Ramoz G, Crowley WR. Dietary isoflavones alter regulatory behaviors, metabolic hormones and neuroendocrine function in Long-Evans male rats. Nutr Metab (Lond) 2004;1:16. doi: 10.1186/1743-7075-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lephart ED, Setchell KD, Handa RJ, Lund TD. Behavioral effects of endocrine-disrupting substances: phytoestrogens. Ilar J. 2004;45:443–454. doi: 10.1093/ilar.45.4.443. [DOI] [PubMed] [Google Scholar]
- 5.Stanhope KL, Havel PJ. Fructose consumption: potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr Opin Lipidol. 2008;19:16–24. doi: 10.1097/MOL.0b013e3282f2b24a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tou JC, Arnaud SB, Grindeland R, Wade C. The effect of purified compared with nonpurified diet on bone changes induced by hindlimb suspension of female rats. Exp Biol Med (Maywood) 2005;230:31–39. doi: 10.1177/153537020523000104. [DOI] [PubMed] [Google Scholar]
- 7.Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: implications for large-scale phenotyping projects. J Nutr. 2002;132:2288–2297. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tordoff MG, Bachmanov AA, Friedman MI, Beauchamp GK. Testing the genetics of behavior in mice. Science. 1999;285:2069. author reply 2069–2070. [PubMed] [Google Scholar]
- 9.Everitt JI, Foster PM. Laboratory animal science issues in the design and conduct of studies with endocrine-active compounds. Ilar J. 2004;45:417–424. doi: 10.1093/ilar.45.4.417. [DOI] [PubMed] [Google Scholar]


