Abstract
We trained Japanese macaque monkeys to use tools, an advanced cognitive function monkeys do not exhibit in the wild, and then examined their brains for signs of modification. Following tool-use training, we observed neurophysiological, molecular genetic and morphological changes within the monkey brain. Despite being ‘artificially’ induced, these novel behaviours and neural connectivity patterns reveal overlap with those of humans. Thus, they may provide us with a novel experimental platform for studying the mechanisms of human intelligence, for revealing the evolutionary path that created these mechanisms from the ‘raw material’ of the non-human primate brain, and for deepening our understanding of what cognitive abilities are and of those that are not uniquely human. On these bases, we propose a theory of ‘intentional niche construction’ as an extension of natural selection in order to reveal the evolutionary mechanisms that forged the uniquely intelligent human brain.
Keywords: tool use, body image, mind, self, non-human primates, culture
1. Introduction
Japanese monkeys (Macaca fuscata; an indigenous Old World monkey species) have been deeply incorporated into Japanese culture since the legendary era. Perhaps this long-standing respect and intimacy lay the foundation for Japanese scientists' penchant for viewing monkey behaviour as precursorial to human intellectual ability, rather than as sharply dichotomously sub-human. Japanese ethologists have been leaders in the field of primate research since at least the 1950s when they first described ‘culture’, or ‘proto-culture’, in wild monkeys (Kawamura 1959; Kawai 1965). Such observations led these same researchers to propose the theory of ‘group selection’ or ‘social evolution’ (q.v. §5b below). Thereafter, Japanese neuroscientists took a strong interest in the use of neuronal recording in behaving monkeys as soon as the technique was introduced in the 1960s (Evarts 1966). Currently, more than 50 laboratories in Japan are applying this technique to various higher cognitive experimental paradigms. Thus, modern primate neuroscience in Japan appears to have harmonized its traditional integrative ethological viewpoint with Western reductive analysis.
Macaque monkeys rarely use tools in the wild (Tomasello & Call 1997). Numerous anecdotal observations, such as those that appear in many traditional folk tales, suggest (but do not prove) that Japanese macaques that live in close interaction with human communities do sometimes exhibit primitive tool-use behaviour. Consonant with this general impression of their cleverness, experimental neurophysiologists who work with primates tend to agree that Japanese macaques, compared with other macaque monkey species, are especially rapid and good at acquiring complex cognitive and motor tasks. Whether Japanese macaques are actually cleverer than other species remains unproven, but one certainty is that they are unusually cooperative with humans. Other macaques, such as rhesus monkeys, are thought to tend to be more aggressive. Thus, if Japanese macaques are indeed faster and more efficient at learning tasks under laboratory conditions, it may be due to temperament rather than cognitive superiority. This comparatively gentler, more patient temperament may be an adaptation to life in Japan's severe northern-most territories. Japanese macaques, also known as ‘snow monkeys’, are the farthest north living of all non-human primates (Wolfheim 1983). They famously bathe in the sea and natural hot springs, wash potatoes before eating them (Kawamura 1959; Kawai 1965) and play with snowballs (Eaton 1972). They occasionally use tools (Tokida et al. 1994; Tanaka et al. 2001; Leca et al. 2007) and might learn behavioural acts socially (Kawamura 1959; Kawai 1965; de Waal 2001); the Japanese counterpart for the English word ‘aping’, if translated word by word, would be ‘monkey imitation’.
For these reasons (plus the fact that they are domestically available to us), we chose to use Japanese macaques in our attempts to train monkeys to use tools. Specifically, we trained them to wield handheld rakes to retrieve distant food rewards. It was at first surprisingly difficult to teach them this skill, but in the end they became deft tool users to an extent far surpassing the modest, sporadic instances of tool usage anecdotally noted in the wild. After the monkeys became proficient in this human-like higher cognitive function, we examined their brains and found significant neurobiological changes that bear provocative similarity to connectivity patterns in the human brain, which have been linked to our sophisticated body image and our ability to use tools. Based on these results, we propose a novel research paradigm and an evolutionary hypothesis, which should help explain the evolution of higher cognitive brain functions in primates, and in particular may shed light on the emergence of modern human intellectual functions throughout the course of hominid evolution.
2. Neural mechanisms subserving tool use in trained monkeys
(a) Learning to use tools
The first intuition that monkeys should be able to use tools arose from the observation that they will sometimes pull down on a slender branch to eat a persimmon at its tip. In other words they can, in at least one naturalistic setting, manipulate one object to retrieve a distant target. As noted above, this sort of proto-tool-use behaviour is an exception rather than the rule. Nonetheless, it suggested that the latent precursors of the cognitive ability of apes and humans to wield tools with explicit intention might exist in the monkey brain. Our first step was to test whether training could in fact develop this inchoate capacity further than nature ever ‘called on’ monkeys to develop it. Once we had successfully accomplished that goal (as described next), our second step was to explore whether the monkeys' artificially induced acquisition of tool use could shed light on the evolutionary processes that led to our own species' prodigious ability with tools.
Through trial and error, we developed the following tool-use training protocol (Ishibashi et al. 2000). Initially, food items were placed on a long shafted (approx. 30 cm) spoon, so the monkeys had merely to pull on the shaft to retrieve the reward. This condition, which they accomplish easily, is akin to retrieving fruit from the tip of a branch. Next, the spoon was replaced with the rake-shaped tool and the food was placed on the rake's near-side right next to the shaft. Again, the monkeys had only to pull on the shaft to get the food. Thereafter, we gradually increased the lateral distance between the shaft and the food, so that eventually the monkeys had to learn to move the rake in a slightly curved path to bring the food all the way into arm's reach. The last and by far the most difficult step was to place the food on the far side of the rake. The monkeys had to learn to swing the rake horizontally while pushing it past the food, then stop at the right point and pull it straight in. In every case, it took several days to develop the basic skill. Thereafter, they began to fuse the skill's component actions into a single, smooth, deftly targeted action. They had now learned to control the rake purposefully to accomplish their goal (refer to the line drawings in figure 1a).
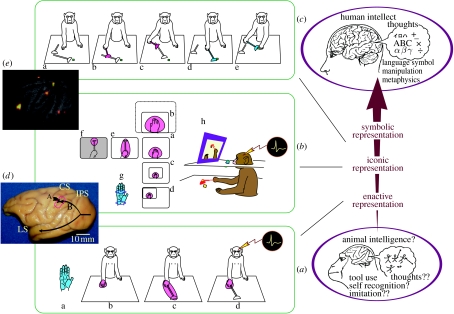
Figure 1.
(a–c) Various modes of cortical body image codings, corresponding to putative hierarchy of internal representations (on the right, see also text). (a) When monkeys use a rake to retrieve distant food, the visual receptive field (b), encompassing the somatosensory receptive field (a), of a representative intraparietal bimodal neuron extended along the rake (c) when using, but did not (d) when not using it. (b) When monkeys use a monitor (experimental set-up shown in h), a visual receptive field of representative intraparietal bimodal neuron was formed around the hand in the monitor (a) encompassing its somatosensory receptive field (g). The visual receptive field altered to match the modified appearance of the hand in the monitor (b–d), extended along the rake when used under the monitor (e), and was confined around the tip of the rake once the image was blotted out except for the tip (f). (c) Combinatory usages (sequentially from a to e) of short and long rakes. (d) The left hemisphere of a monkey brain, with arrows A and B indicating somatosensory and spatial visual processing pathways that merge at the intraparietal area indicated by the red square where neurons were recorded. CS, central sulcus; IPS, intraparietal sulcus; LS, lateral sulcus. (e) Brain activation pattern for sequential combinatory tool usages, showing prefrontal in addition to parietal activation. (a–c and e, Adapted with permission from Maravita & Iriki 2004).
As of this writing, we have rake trained more than 50 monkeys in our laboratory using this procedure and, without a single exception, all have acquired it. At first it took us more than a few months to train each monkey, but as we fine-tuned and streamlined the protocol, we were able to shorten it to approximately 10–14 days per monkey, depending on the criteria used to assess skill acquisition (Ishibashi et al. 2000; Hihara et al. 2003).
(b) Coding of modified body image upon tool use by parietal neurons
People have long commented that as one becomes deft with a tool, introspectively it begins to feel as though the tool has been incorporated into one's body image as an extended hand or forearm. This flexibility has long been regarded as unique to human intelligence. Clinical experience suggested that the sensorimotor semantics of the body image were formed and stored in the parietal cortex (Head & Holmes 1911). In this intraparietal cortical area (figure 1d, red rectangle), somatosensory (tactile, joint, deep muscular, etc.; figure 1d, arrow A) and visual information (figure 1d, arrow B) about the spatial configuration of the body merge (Ungerleider & Mishkin 1982) to form a multimodal model of the body in relation to its surroundings. Yet the concrete neural mechanisms behind the body image, including its ability to flexibly assimilate tools, remained a puzzle until 1996, when we discovered the following phenomenon (Iriki et al. 1996).
We were studying intraparietal bimodal neurons that respond both to tactile stimulation on the hand (a neuron's tactile receptive field; figure 1aa) and to visual stimuli presented in the same spatial vicinity as the tactile receptive field (the same neuron's visual receptive field; figure 1ab). These visual receptive fields were not confined to any region of the retina, but followed the hand around everywhere it was moved in the three-dimensional space. We interpreted these neuronal response properties as coding the image of the hand in space (Iriki et al. 1996; Maravita & Iriki 2004). Our next observation was surprising. When our rake-trained monkeys wielded the rake in order to retrieve food, these same neurons' visual receptive fields extended outwards along the axis of the tool (ac) to include the rake's head. In other words, it appeared that either the rake was being assimilated into the image of the hand or, alternatively, the image of the hand was extending to incorporate the tool. Whenever a monkey was not regarding the rake as a tool and just held it passively as an external object (ad), the visual receptive field withdrew from the rake head and was again limited to the space around the hand. Condition ad is physically identical to ac, but mentally it is equivalent to ab.
Activation in this region of parietal cortex during tool use was confirmed by PET imaging (Obayashi et al. 2001). The neural recording data had revealed the parietal neurons' receptive field properties, but because each such measurement took several minutes to complete, they had not revealed their real-time dynamics during tool use. In combination, these complementary methods demonstrated that intraparietal neurons are flexibly coding modified body image upon tool use.
(c) Neural codings of the advanced modes of body image
This encoding of a modifiable body image seems very close to being the long theorized ‘enactive representation’ (Bruner et al. 1966) or ‘internal model’ (Kawato 2008), which contributes to embedded control of the body parts' movements. As such, it comprises the human animal's most basic, first developed mode of representation (Bruner et al. 1966; figure 1, right; figure 2, virtual axis) and goes on further to serve as the foundation for two higher modes: iconic (which develops approx. 9–10 years old) and symbolic (which develops through adolescence) representations. Having successfully ‘constructed’ the neural circuitry for tool use in the monkey brain (q.v. §2d), which represents the upper limit of enactive representation (figure 2, virtual axis), our next question was whether monkeys could be trained to acquire any of the more advanced modes of representation. In other words, how high up Bruner's scale could the monkey brain be ‘pushed’ through properly structured training?
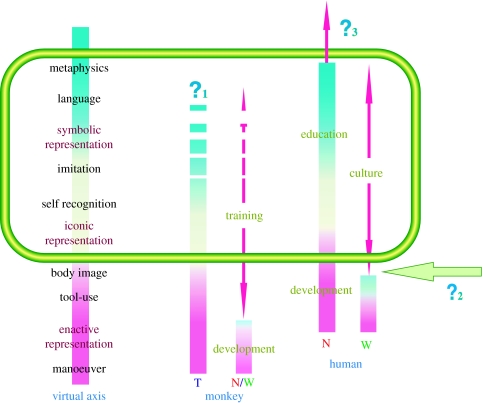
Figure 2.
Framework for the ‘biological science of human intellect’. See text (§2e) for details.
We attempted to train the monkeys on a sort of ‘video game’. Much as with physical tool use, when we play video games, it often feels introspectively as if our own images are being projected onto our on-screen avatar, a ‘virtual tool’ (Iriki et al. 2001). We wondered whether monkeys could make a similar leap of visuomotor abstraction. As before, the monkeys had to collect food with a rake. But this time their view of the table and of their own arms was blocked by an opaque horizontal sight barrier. The only cue available for guiding their reaching was the video feed from a camera mounted under the barrier, which was projected onto a TV monitor in front of them (figure 1bh). The rake was necessary for this training because, if allowed to use just their hands, the monkeys would simply grope for the food on the table until they found it by chance. Once the monkeys had grasped the abstraction involved in using the monitor view to guide their reaching, we again explored the receptive field properties of their parietal bimodal neurons. When we superimposed an artificial dot over the image of the hand on the monitor, and thereby scanned the whole area of the monitor screen, we found that neurons with tactile receptive fields on the hand (figure 1bg) were now endowed with visual receptive fields around the image of the hand (ba). Furthermore, these visual receptive fields could extend to the head of the video image of the rake (be), changed congruently with changes in hand image size (bb,bc) and location (bc,bd) on the monitor, and also matched the electronically modified appearance in the monitor (bf).
These parietal neurons were further demonstrated to code the image of the hand while it remained invisible under the sight barrier groping for food on the table. Wherever the hand was moved, whether actively or passively, the cells' visual receptive fields moved to follow it, even though it was never visible (Obayashi et al. 2000). Thus, monkeys can encode and modify their mental body image in the absence of visual feedback, thanks to their parietal neurons.
Given all these abilities, we expected that the monkeys should be able to use their introspective body image to plan and sequentially combine the usages of their body parts in their minds before actually acting. When rake-trained monkeys were exposed to a food retrieval challenge that required a pre-planned, sequential combination of different tools, to our surprise, they quickly solved it within a few trials. This stands in sharp contrast to the initial tool-use training, which as described earlier took approximately two weeks of enforced, intensive daily practice. In this situation, the food was placed at a distance that could not be reached by the rake the monkey could pick up at the start of the trial. The food could only be reached by a long handled rake that lay beyond arm's reach but within the range of the shorter rake (figure 1ca). Without hesitation, the monkeys used the short rake (cb) to pull in the long rake (cc), then switched rakes (cd) and retrieved the food (ce; Hihara et al. 2003). PET imaging showed intraparietal activation during single tool use, and during combinatorial tool use revealed additional prefrontal activation (Obayashi et al. 2002; figure 1e).
(d) Induction of gene expression and cortical circuit reorganization by tool-use training
The irreducible 10- to 14-day period required for tool-use training suggested that the process was not merely based on functional plasticity within the existing neural circuitry, but involved larger scale neuroplastic reorganization. This is indeed what we found. In the bank of the intraparietal sulcus, where the bimodal neurons described above reside, the expression of immediate early genes (Iriki 2005) and the elevation of neurotrophic factors and their receptor was synchronized with the time course of the cognitive learning process, and then returned to control levels once the learning process was completed (Ishibashi et al. 2002a,b). These training-induced genetic expressions turned out to be a part of morphological modification of the intraparietal neural circuitry.
In order to pinpoint and visualize this reorganization, a retrograde tracer (Fast Blue) was injected into intraparietal area of two groups of monkeys: one naive and the other rake trained (Hihara et al. 2006). Then each cerebral hemisphere was explored in its entirety in search of locations with neuronal cell bodies sending axons to the bank of intraparietal sulcus. Comparing the two groups, two cortical areas were uniquely labelled in the rake-trained monkey brains: ventral prefrontal cortex and the temporoparietal junction area. Next, an anterograde tracer (BDA) was injected into temporoparietal junction, which allowed the axonal arborization and synaptic connection patterns in intraparietal cortex to be explored at both the light and electron microscopic levels. Compared with control monkeys, in which efferent axons arising from temporoparietal junction were confined to the deep layers at the fundus of the intraparietal sulcus, additional axons in trained monkeys were also found to extend farther, perhaps by a millimetre or so, into superficial layers of a shallower portion of the bank of the intraparietal sulcus to form active excitatory synapses with postsynaptic neurons. This novel connection between distant cortical areas apparently sets up a novel mode of multimodal integration in the intraparietal cortex, which in turn endows the monkeys with the capacity to use tools as extensions of their innate body parts.
How might tool-use learning drive such intensely stepped-up interaction between temporoparietal junction and intraparietal cortex? One possibility is that the use of a tool as an extension of innate body parts initially induces a mismatch within the body image coded in the intraparietal region. This mismatch requires recalibration to correct, but the monkey, being ignorant of its own potential, would never undertake the necessary effort on its own initiative. However, our rake training protocol may work by repeatedly forcing the monkey into explicit awareness of its own body and intentions (or mind). Coupled with the high motivational value of the food rewards, the Hebbian mechanisms might at first reinforce, then amplify and even create, through additional neurobiological mechanisms, any functional connections that tended to improve the monkey's ability to focus its own awareness on the task and, by the same token apparently, to incorporate the external object (tool) into its internal body self-representation (Hihara et al. 2006; Iriki 2006). Evidently, a novel set of cortico–cortical connections fits the bill.
If external objects can be reconceived as belonging to the body, it may be inevitable that the converse reconceptualization, i.e. the subject can now objectify its body parts as equivalent to external tools, becomes likewise apparent. Thus, tool use may lead to the ability to disembody the sense of self from the literal flesh-and-blood boundaries of one's skin. As such, it might be precursorial to the capacity to objectify the self. In other words, tool use might prepare the mind for the emergence of the concept of the meta-self, which is another defining feature of human intelligence (q.v. §4b below). And, indeed, in human subjects, activation of homologous circuitry at the temporoparietal junction is detected in self-objectification paradigms (Corradi-Dell'Acqua et al. 2008).
(e) The brain's latent potential for training-induced higher cognitive function
To date, most schemes for comparing the cognitive abilities of humans and various non-human primates have been rather simplistic. For example, apes' mental ability is said to be comparable with that of 7-year-old human children, while monkeys equate with 2-year-olds and so on. But our research, as described above, makes clear that the reality cannot be so simple. Monkeys possess latent cognitive abilities that can be realized by exposure to the proper environment, thereby widening their ‘spectrum’ of cognitive functions. Figure 2 shows a schematic comparison between the human's and monkey's spectrum under this view.
The left column in figure 2 lists various cognitive abilities roughly ranked from lower (bottom) to higher (top) complexity. The centre and right columns show the spectrum of abilities of monkeys and humans, respectively. ‘N’ refers to ‘normal conditions’, which in the case of humans means having grown up in a sophisticated culture. ‘W’ refers to living in the ‘wild’ and ‘T’ refers to a situation under ‘training’. Human biological development can advance an individual's cognitive abilities up to a certain point, but that point can be greatly surpassed through education and other cultural experience. Our ‘default’ is to be hunter-gatherers, but through cultural evolution we have unlocked a huge amount of latent cognitive potential that evolution never ‘intended’ our brains to be able to process. By contrast, monkeys in the wild never develop far past the point to which their biological developmental processes carries them. They achieve dexterous movement and a certain social savvy, but do not advance much further. Yet, as we have shown, through an ‘artificial’ training process they can acquire the ability to use tools and, at times, attain even higher intellectual abilities, such as imitation and reference vocal control (q.v. §3b). This creates an overlap (shown by green rectangle in figure 2) between the cognitive abilities of monkeys that have received tool-use training and the intellectual functions of humans. We propose that this overlap provides a novel experimental platform for studying the evolution and operation of human intellectual functions from a biological perspective.
This platform should open up at least three aspects of human intelligence to biological analysis.
What are the structural innovations (e.g. connection patterns between brain regions), and molecular genetic mechanisms subserving it, that give rise to key intellectual brain functions? One way to address these questions is to entrain human-like intelligent brain functions in monkeys to the maximum extent achievable (as shown by ?1 in figure 2), and then examine the modifications that enabled those enhancements.
Why were humans able to bootstrap their own way spontaneously to educational and cultural achievement while monkeys require artificial training? It is impossible to know what a true ‘wild child’—an individual that somehow managed to survive from infancy without ever meeting another person—would turn out. In reality, all human development occurs embedded within social systems that impart cultural knowledge, skills and education. More so than any other primate, humans have innate social instincts that draw us into joint attention and mutual emulation and thus ensure the propagation of culture (as shown by ?2 in figure 2). It would be extremely valuable to discover the nature of the neural mechanisms that underlie these instincts.
What are the limits and future directions of education and training for our own species (as shown by ?3 in figure 2)? Up to this point in our history, most of our achievements in training-induced brain enhancement have been the results of trial and error, serendipity and common sense. Only recently have we reached a point where we can contemplate using our emerging knowledge of how our brains actually work to intentionally design our educational systems and make the most of our remaining latent potential.
3. The evolutionary emergence of the ‘mind’ in the brain
(a) Evolution of the primate brain's capacity through mere natural selection
The human brain is unique, but it did not evolve ex nihilo. Like every other organ of the body, it is the product of continuous, gradual evolution. How did the human brain acquire its unique set of cognitive functions?
Brains are by no means necessary for life to flourish. Plants, for example, have prospered for ages without the benefit of nervous systems. By contrast, the animal way of life requires the ability to rapidly respond to environmental stimuli, often of the order of just a few milliseconds. The nervous system evolved as a mechanism for translating the information acquired by the sense organs into physical changes in bodily tissues to produce appropriate movements. The most primitive nervous systems are completely decentralized. As animal bodies became larger and their senses and anatomies became more complex, the benefits of more sophisticated information processing also increased. Natural selection began to favour organized neural clusters and tracts, which eventually led to full-blown nervous system centralization.
For almost all animals, from single-celled organisms to mammals, the basic mode of movement is locomotion or intransitive movement of the self. An animal's motor effectors are optimized for efficiently moving towards food and conspecifics, evading or avoiding danger, and getting food into the digestive tract. In animals capable of only intransitive movement, there is an indivisible unity between the brain/nervous system, the ‘subject’ that controls the body's movement, and the physical organs for movement that are the ‘object’ of this control. No division between subject and object arises in such a nervous system.
In the vast majority of species, with the exception of humans and perhaps apes, we cannot attribute much of a ‘mind’ to the information processing that mediates between sensation and action, at least not in the sense of possessing a significant measure of free will or explicit intention. At times we anthropomorphize the actions of non-human animals, but given the limited control they have over their own nervous systems' responses, this is at best a stretch.
A few animals, notably primates, have had their hands freed from the primary task of moving the body. This allowed them to acquire exquisite manual dexterity and sensitivity, enabling them to manipulate external objects. This was a key step in the origin of the human mind (Iriki in press). Primates' physical movements began to include the ‘transitive action’, i.e. transferring purposeful motion to objects. The distinction between the body as the subject and the physical, outer object that is being moved became sharper and more meaningful. However, there is still no necessity to assume a ‘will’ or ‘mind’ at this point. Non-human primates merely move objects in response to the direct requirements of their environments. Conventional Darwinian natural selection is sufficient to explain their evolution to this plateau.
(b) Precursors of mind acquired in our tool-using primate ancestors
The situation changed significantly when one group of our primate ancestors began picking up (and later, fashioning) external objects in their hands and moving these objects as extensions of their own body (Sakura & Matsuzawa 1991). This is the beginning of transitive movement, i.e. tool use. At this point, tools began sharing in the self-representation of the body, and the deep equivalence between the body and the tools it could wield became established. As mentioned earlier, it seems probable that the ability to literally incorporate external objects and the ability to ‘objectify’ the body as another object are just the two sides of the same coin. As soon as one's own body becomes objectified and separate, one must assume a subject with an independent status that is orchestrating the movements of both the body and its tools (Iriki 2006). Thus, the ‘mind’ emerges naturally as a sort of ‘virtual concept’, a placeholder for the link between the subject and the objects of manipulation, which includes the body itself.
Indeed, several recent studies report how laboratory-raised, non-human primates trained in tool use can exhibit a number of other intelligent behaviours, such as imitation and reference vocal control, that are never seen in their wild counterparts (Iriki 2006). The novel cortico–cortical connections induced by tool-use training seem to underlie this boost in capacity and tapping of latent potential in non-human primates that nature does not normally coax into full expression. Although tool-use training is patently non-naturalistic, its marked effects on brain organization and behaviour could shed light on the evolution of higher intelligence in humans.
Once a nervous system has acquired a mind in this sense, how might higher cognitive functioning be effected? The subject might become aware of the continuity of the body across time, further strengthening the concept of a ‘self’ that is non-identical with the physical here and now. This, along with the transferability of tools between individuals, might inevitably lead to the conceptualization of other selves in other bodies, in other words, to a ‘theory of mind’ (Premack & Woodruff 1978). When selves are able to interface at this level, rich culture and complex society become possible. At this point, biological evolution can take a back seat while society evolves on its own, forging novel connections between brain regions wherever possible. These effects of the mind eventually gave birth to a spiritual civilization that is based on self-control and tempering selfishness, and imbues a sense of mutualism and social responsibility. The latest phase in cultural evolution has seen the advent of a scientific and technical civilization, in which nature is an object to be manipulated.
The evolutionary path that led from the monkey brain to the human brain must have proceeded through a continuous, incremental process of natural selection. Nothing completely new should have been added to the primate brain. Evolution has limited the means for reorganizing so complex a structure; these means mainly involve tinkering with size and developmental timetables. One of our main claims here is that the precursors of the mental functions that allowed the human intellect achieve a cultural snowball effect are present, even if only in latent or inchoate forms, in our primitive primate ancestors. A corollary claim is that certain forms of training can produce incremental but functionally significant changes in the non-human primate brain that mimic, perhaps even recapitulate, some of the key neurogenetic changes our ancestors underwent during their long march towards becoming us. As described earlier, by exposing monkeys to an intensive, highly structured training environment, we induced genetic expression and long-range axogenesis and synaptogenesis in the brain, which reorganized the neural circuitry of the parietal lobe and led to novel patterns of behaviour never observed in wild monkeys. Could this be recapitulating one of the key steps in primate evolution, which led them to us? As monkey common ancestors evolved into apes, they may have faced new environmental challenges that drove similar (possibly identical) neurogenetic change in individuals that would as a result reap reproductive advantage. In such a scenario, natural selection would favour the ability to forge these sorts of novel connections more easily. Eventually, it would have become more of a developmental default than a distinct skill that had to be acquired de novo in each adult.
(c) Role of behaviour in evolutionary theory: niche construction
It has been repeatedly emphasized that since changes in behaviour precede morphological changes, behaviour must be viewed as one of the prime ‘engines’ of the evolutionary process (see articles in Plotokin (1988) for review), rather than simply the end product of it. Apart from some classical philosophical arguments, this kind of argument originated with Darwin (1881) himself, and has been recently re-evaluated as the ‘niche construction theory’ (Odling-Smee et al. 2003).
Two points, however, are still open questions. First, how far is behavioural change able to contribute to phenotypic evolution? Some researchers state that an organism's reaction to its environment should be regarded as being on par in importance with natural selection itself, while the others insist that its role is negligible. Darwin himself speculated on this issue and came out in favour of the former viewpoint, but this part of his theory was neglected for more than 100 years and has only recently begun to be re-evaluated. Likewise, his argument about the importance of sexual selection was at first neglected. We believe that the ‘trend’ will continue and vindicate him fully.
Assuming behaviour is a significant force in evolution, the second question is what physiological mechanisms realize the process? The neurobiological mechanism described above may constitute a part of the niche construction process during the course of evolution. The structures and functions of the central nervous system vary among species, and presumably these variations account for much of the variety in different species' behaviours in their environments. But human evolution has always been harder to get a handle on, because we do not fully understand how human intentionality affects the evolutionary process. Our theory of human evolution lags behind our theory of non-human evolution (Laland & Brown 2002; Shennan 2002) because we still do not know how to model the interaction between our brains, our intellects and the physical and cultural environments we construct for ourselves. It seems that in order to adequately characterize the neural mechanisms of human intellectual evolution, we will need to discover some additional factors.
4. Neurobiology of intellectual evolution
(a) Limitations of passive niche construction
Tool use sets up mutual interaction between the organisms and their environments. Tools become embedded cultural traces that are used to modify the environment in which subsequent generations develop and learn. This constructed environment puts selection pressure on the species, favouring individuals with phenotypes (whether morphological features or neural circuitry) that match the usages of such traces. A classic example of this is the beavers that are adapted for life inside the elaborate dams they build. In all non-human species, the process of organism–environment interaction proceeds through a finite number of cycles, which eventually reaches an equilibrium point and then stops. Such interaction is purely passive, a ratchet process prefigured by the combined characteristics of the subject and the environment to which it must adapt. Thus, we can call this process passive niche construction.
For a long time, our hominid ancestors were no exception to this passivity in their own evolution. Indeed, the earliest primitive stone tools they used did not change for over 1.5 Myr (Shennan 2002). Like the beavers with their dams, our ancestors must have stabilized in their mode of interaction. They were not yet capable of actively (intentionally) modifying their environment or their tools through insight. This may have been because they did not have a sufficiently developed sense of the ‘subjective self’, and so could not explicitly imitate (Iriki 2006) or intentionally plan for the future. Thus, once such an additional factor was added on top of a pre-existing stable mode of environment, a novel mode of evolutionary circulation would initiate by succession of sequential niche construction processes. These evolutionary traces of successive additional factors throughout the past evolutionary history could possibly be found in the structures of the present ‘civilized environment’, perhaps in various artificially manufactured tools as ‘mental fossils’.
(b) Stepwise mastery of higher classes of tools: intentional niche construction
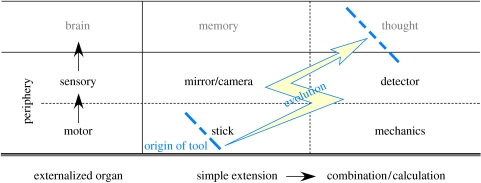
To date, nearly all studies on tool use have focused solely on the class of tools that augment functions of our physical organs, i.e. on ‘motor tools’. Indeed, most definitions of tools include in this class only (Beck 1980). These tools cover a wide range of complexity, from a rake as a mere spatial extension of the hand, to high-tech machinery that requires multistep actions to use. But, in addition to motor tools, humans (and only humans) can use another category of tools, ‘sensory tools’, which extends functions of another class of physical, namely sensory, organs (Asano 1994; Goldenberg & Iriki 2007; figure 3). These tools can be as simple as a prism or mirror, which merely shift one's angle of gaze, but include many advanced technological devices for exploring information undetectable by our own innate sensory organs, such as a radio detector or sonar (cf. a similar but slightly different classification of tools has been proposed by Asano (1994), which is based on the class of functions, rather than apparatus, that are extended).
Figure 3.
Hierarchical structure of various classes of tools.
Human presumably acquired the ability to wield the full range of sensorimotor tools by gradually evolving brain mechanisms for functionally ‘externalizing’ different parts of the innate body. Again, we wondered whether the same ability could be acquired by monkeys through training. Initially, we trained the monkeys with a simple sensory tool (Yamazaki et al. 2006). Monkeys that had been rake trained using the process described earlier were given a new rake with a tiny forward-facing video camera mounted at the tip—an ‘externalized eye’. Again, an opaque horizontal sight barrier prevented them from seeing the tabletop directly, and forced them to rely on the image on the monitor in front of them to search for food. But now rather than a fixed ‘bird's eye’ camera view of the tabletop, the monitor showed a ‘rake's eye’ view that changed every time the rake was moved. Although seemingly somewhat similar to the earlier condition, the result was disappointing. The monkeys never learned to associate the dynamic sensory cues with motor control. We tried multiple ways to assist them, such as introducing supportive sensory clues into the ‘scenery’ on the table. But the monkeys' success rate at retrieving food never rose above chance even after more than three months. Thus, it became clear that they could not acquire sensory tool use through mere association.
We went back to the drawing board and devised a new training process that proceeded in several small, incremental steps. At first we gave the monkeys rakes (motor tools) with a plate at the tip made of mirrors (primitive sensory tools) facing the near side. They learned how to actively use the mirror rake to find and take food hidden behind barriers with surprising ease. Subsequent steps gradually separated the motor and sensory tools, both physically and functionally, further until visual cues became completely divorced from their actual origins in visuomotor space. By the end, exploration, reaching and food retrieval were completely guided by the video image, projected on the monitor, as captured by a camera attached at the tip of the rake. Sensory tool use had at last been successfully acquired. Thus, the hominoids' unique degree of cognitive development might be simulated in monkeys by reconstructing the environmental conditions, in which pre-existing tools are embedded as default, and such an advanced environment, which comprise a novel mode of cognitive load, in turn, stimulated monkey brain to develop successively further advanced tools. In other words, early/prehistoric anatomically modern humans must have spontaneously achieved well-designed operant conditioning situations. Among non-human primates, there is a continuity in the use of motor tools, but not in the use of sensory tools. Thus, this induction of sensory tool use in a non-human primate constitutes a significant modification in cognitive functioning. It was achieved through a circular interaction between individual and environment, and thus offers a novel paradigm for the empirical study of human cultural evolution.
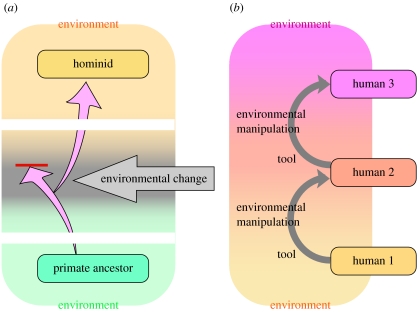
As our ancestors' array of tools and tool-enabled skills increased in size and complexity, selective pressure would have favoured individuals that were more adept at acquiring and mastering them. Although each step in this evolution might have been a simple association, even after just a few such steps, it may have produced something beyond a mere association, when the whole scheme was organized into a certain structure. One such candidate could be the concept of the self, which would have emerged through the self-objectification process described earlier. Thus, niche construction became ‘intentional’, which remarkably accelerated the speed of evolution. With the advent of intentional niche construction, the direction of evolution was no longer passively determined by the natural environment (figure 4a). Now the organisms themselves could decide how the environment should and could be remade. And, of course, each new iteration of the human-altered environment influenced the development of (as well as the selection pressures on) the next generation (figure 4b).
Figure 4.
Comparison of evolutionary mechanisms (a) before early hominids by ‘natural selection’ and (b) modern human intellect by ‘intentional niche construction’.
Beyond the motor and sensory tools lies a third category: metaphysical tools that extend the brain's non-sensory, non-motor physical functions (Goldenberg & Iriki 2007). One example is writing, which augments human memory. Writing has marked effects on the brain, even going so far as to create letter- and reading-specific functional areas in the cortex. The existence of these novel areas may have wider effects on various other cognitive functions, such as on our neural networks for memory and our concept of time and resulting historical self. More recently developed examples of metaphysical tools are tape recorders and silicon memory chips. Computers are continually improving in their capacity as external thinking devices. These ‘externalized’ brains can be expected to continue the same trends of mutual modification and assimilation that drove the explosion of cultural and intellectual development of the last several millennia. As our machines become ‘smarter’ and more deeply integrated into every corner of human life, evolution may well be poised to accelerate yet again. We might be entering into a critical new epoch of human evolution (cf. Moravec 1988; Shennan 2002).
(c) Potential neurobiological bases of intentional niche construction
The biological mechanisms behind intentional niche construction are still mostly unknown, but there are some promising hints. Although the training-induced axogenesis and synaptogenesis in monkey intraparietal cortex (Hihara et al. 2006) are not proved to result in macroscopic changes, human VBM (voxel-based morphometry, using structural MRI data) findings might be consistent with this notion, i.e. portions in the parietal cortex enlarge following extensive sensorimotor association practice by skilled performers such as musicians (Gaser & Schlaug 2003) and jugglers (Draganski et al. 2004). The degree of cortical thickening correlates with the performer's skill level. Furthermore, even purely mental activity, such as mathematical thinking, can increase cortical density in the prefrontal and parietal cortices (Aydin et al. 2007), and vocabulary size correlates with the thickness of intraparietal cortex (Lee et al. 2007). These results evince impressive experience- and demand-dependent structural plasticity in the adult human brain. Another potential factor is neurogenesis. Spontaneous neurogenesis in the naive adult macaque monkey brain has been reported in the parietal and prefrontal cortices (Gould et al. 1999). Although spontaneous neurogenesis induction requires careful examination (Gould et al. 2001; Kornack & Rakic 2001), it might be plausible that the demands of tool-use training could well recruit this process. Neurogenesis is indeed elevated by various environmental factors in the hippocampal dentate gyrus of various mammals (Gould 2007).
Early on in the evolution of primates, parietal cortex underwent extensive expansion. Parietal cortex is the brain's centre for visuomotor integration that primates needed to develop keenly for jumping, moving and hunting for insects among the upper branches of the forest canopy. In the process they became even more deft at manual manipulation and, as noted earlier, may have also become preadapted for tool use and other higher cognitive functions (Iriki in press). The prefrontal cortex, important to the control of behaviour in complex social living contexts, also expanded in early primates. These areas expanded dramatically further in early hominids, as inferred from endocasts of their skulls (Holloway 1996). This pattern is consonant with the findings on primate neurogenesis noted earlier. It also matches the staging of myelination seen during the course of human development: the areas that were most recently expanded in our evolution are the last to be myelinated during development (von Bonin 1950). Many uniquely human higher cognitive functions, including tool use, mathematics and other complicated and abstract mental functions, appear to be localized within these brain areas (Iacoboni 2000). In this view, human intellectual evolution has been a long process of exacting and elaborating latent functional capacities in these brain areas. This process eventually led to full-blown culture and artificial environments, opening up a positive feedback cycle of gene–culture coevolution.
5. Conclusion
(a) Neurobiology of laboratory-trained monkeys
Most research on the neural and evolutionary bases of human intellectual functions involves drawing comparisons between the modern human brain and the brains of non-human primates. Here we have provided the blueprint for a novel variation on this approach, training non-human primates to acquire high-level cognitive functions (in our case, tool use) normally regarded as uniquely human, and then examining their brains to find out what kinds of modification has been induced. In doing so, we hoped to gain insight into the concrete neural modifications that emerged in our pre-hominid ancestors and lay the groundwork for the evolution of modern human intellectual functions. The brains of our tool-trained monkeys had indeed undergone significant changes that were demonstrated first neurophysiologically, then morphologically and then molecular neurogenetically. These changes showed sufficient parallels with human behavioural–neurological data to give us confidence in this new research paradigm. We hope that further studies of ‘enhanced’ monkey brain structures and functions will continue to show overlap with human structures and functions. Finally, as this approach pans out, it should provide us with a scientific platform for challenging purely ‘biological’ mechanisms of intelligence.
Taking into consideration the similarities, equivalences and differences between enhanced monkey and modern human intellectual brain functions, we proposed a novel evolutionary mechanism, intentional niche construction, which we think is necessary, in addition to the mechanisms of Darwinian natural selection and passive niche construction, conceptually proposed by Darwin (1881) and later formalized by Odling-Smee et al. (2003), to account for the full course of human intellectual evolution. Intentional niche construction offers concrete biological mechanisms to explain the hominids' tremendous two million year-long spurt of encephalization (post-Homo habilis) that culminated in an explosion of diversity and complexity in higher cognitive functions over the last few tens of thousands of years. This latter period saw the emergence of the mind through the establishment of the concept of self, which allowed human culture and civilization to reach its present heights of sophistication.
To conclude this article, we will recount how the precursor of the idea of intentional niche selection originated from the tradition of Japanese primatology ca 60 years ago as Kinji Imanishi's group selection theory. This pioneering idea has been largely forgotten in the history of social science—and when it is remembered, it is usually unfortunately misunderstood—and has been awaiting the emergence of concrete biological evidence to revive it and lend it empirical grounding. That time has finally come. In what follows, we review how this original idea has been recently re-evaluated, discuss how our own findings fit into and extend this original concept, and outline the next direction in which we expect this novel biological framework will develop.
(b) Japanese tradition of primatology and its unique group selection theory
Japanese biologists were already emphasizing the active role behaviour in the evolutionary process ca 60 years ago. Kinji Imanishi, an ecologist and primatologist, is a typical example (e.g. Imanishi 1941/2002). Imanishi started his career as a professional entomologist and ecologist ca 1940, and then following World War II he established the foundation of primatology in Japan. He gradually developed his unique evolutionary theory, ‘group selection (also called social evolution)', which was initially based on his own observations of habitat segregation in mayflies. The theory stated that evolution is induced not solely by competition, as the Darwinian theory posited, but also by cooperation among organisms. This theory had great influence on Japanese ecology and evolutionary biology, and remains popular among the public to date (Halstead 1988; Itô 1991; Sakura 1998; de Waal 2001).
Imanishi's formulation of group selection theory was based solely on ecological observations. Due in part to technical limitations of his time, he did not present any genetic, physiological or quantitative evidence to support it. His theory soon came to be misinterpreted as the rather non-biological idea that the identity of an organism itself is the prime driver of the evolutionary process. According to the mainstream evolutionary theory, the emergence of cooperation among organisms is nonetheless driven by fundamentally competitive mechanisms. Individual identity remains difficult to analyse within the modern biological framework. Although empirical evidence for Imanishi's original idea is rather thin by today's standards, it must be appreciated that Imanishi started developing his ideas during the 1930s, well before formal analysis (e.g. game theory) provided a robust framework to explain the existence of cooperative phenomena in the world of living things. Similar theories emerged around the same time even in Western countries. For example, the Chicago school of ecologists in the USA preferred the notion of harmony and accord within ecosystems (see Mitiman 1992), cell biologists recognized how deeply integrated and interdependent the components of biological systems were (Novikoff 1945), and systems theorists and ethologists in Europe championed a holistic view (e.g. von Bertalanffy 1969). Just-mentioned schools of thoughts asserted that cooperation evolved through group selection and accepted it within the Darwinian framework.
But for Imanishi, cooperative group selection and Darwinism appeared inconsistent. He would later criticize Darwinian theory, which is ironic, given that his initial proposed modification of Darwinian theory was more accurate and modern than most Western schools of thought at that time (Sakura 2000). This may have been associated with the establishment of the ‘evolutionary synthesis’, or modern neo-Darwinian theory, ca 1940. Many Western biologists felt strong academic pressure to defend Darwinism as the theoretical core of the newly born evolutionary framework. Imanishi, in the Far East, apparently felt more free to question whether Darwinism was actually sufficient to explain cooperation and reciprocity between organisms. Even though Imanishi went too far in his criticism of Darwinism, this explains why he so passionately searched for alternatives, and why the evolutionary idea he eventually came up with seems to be rather consistent with the newly extended evolutionary theory that emerged in the 1980s, which includes neo-group selection (Sober & Wilson 1998), the extended phenotype (Dawkins 1982) and niche construction (Odling-Smee et al. 2003).
Imanishi could not come up with a reasonable mechanism to explain cooperative phenomena such as habitat segregation. At one point, he hypothesized that multiple mutations might occur simultaneously among many of the individuals within a population, but this is an anachronism in the same vein as the ‘hopeful monster’ hypothesis presented by Richard Goldschmidt in the 1940s. Although Imanishi's anti-Darwinism has been criticized as having hindered the development of evolutionary biology in Japan (see Sakura et al. (1986) and Itô (1991) as reviews), it does not follow that every aspect of his theory deserves dismissal. Some of his ideas were clearly wrong, but others were fruitful and inspired, and deserve reconsideration. We propose that the neurobiological framework described in this paper supports Imanishi's idea that each organism acts ‘somewhat intentionally’ in response to its environment, or niche, under ordinary conditions, and thus upholds a core part of his theory, rendering it compatible with modern evolutionary theory.
(c) Future directions for neurobiology of human intellect
Once goal-directed intentional niche construction was introduced into the evolutionary process, biological and cultural processes became intertwined to an unprecedented degree. The study and elucidation of this process requires a multidisciplinary scientific approach. The sciences of mind, brain, body and society must cooperate in this effort, guided by the insights of philosophy. This should include not just the inductive and reductionist framework of the Western philosophical tradition, but also an increased contribution from the abductive and holistic framework of Eastern philosophy.
The process of intentional niche construction has been steadily accelerating throughout the modern era, and there are few reasons to think it will not continue to do so. This raises the worry that in the not-too-distant future, cultural, social and technological changes might outpace our ability to adapt to it, resulting in chaos or some degree of social breakdown. A countervailing point against this worry is the fact that any new environment we create for ourselves will be formed within the biological and information processing limits of our primate prefrontal and parietal cerebral cortices. Nonetheless, it is an open question as to what kind of novel ‘future’ brain functions we might be capable of developing (figure 2, ‘?3’). As mentioned earlier, we might be able to gain a preliminary sense of our latent potential (as well as our ultimate limits) if we can develop a sufficiently deep understanding of the neurobiological mechanisms subserving our past and present intellectual brain functions plus the evolutionary processes that brought us from the Stone Age to our present level of sophistication. Mind once emerged in our brains. What might emerge next? Humanity faces the unprecedented situation in which numerous ‘minds’ possess external thinking devices (figure 3, top right) linked simultaneously via the Internet. In such a situation, might the will of individual ‘subjects’ become separate from their bodies and act mutually, through the interdependent functioning of the Internet, with the shards of a thousand selves forming the community of an imaginary society? In such an event, perhaps the advanced, virtual concept of ‘multi-selves’ will emerge, evolving through the neurobiological mechanisms depicted here as they carry us into the future.
Footnotes
One contribution of 17 to a Theme Issue ‘Japan: its tradition and hot topics in biological sciences’.
References
- Asano T. Tool using behavior and language in primates. In: Hayes S.C, et al., editors. Behavior analysis of language and cognition. Context; Reno, Nevada: 1994. pp. 145–148. [Google Scholar]
- Aydin K, Ucar A, Oguz K.K, Okur O.O, Ahayev A, Unal Z, Yilmaz S, Ozturk C. Increased gray matter density in the parietal cortex of mathematicians: a voxel-based morphometry study. Am. J. Neuroradiol. 2007;28:1859–1864. doi: 10.3174/ajnr.A0696. doi:10.3174/ajnr.A0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B.B. Garland; New York, NY: 1980. Animal tool behavior. [Google Scholar]
- Bruner J.S, Olver R.R, Greenfield P.M.Studies in Cognitive Growth1966Wiley; New York, NY [Google Scholar]
- Corradi-Dell'Acqua C, Ueno K, Ogawa A, Cheng K, Rumiati R.I, Iriki A. Effects of shifting perspective of the self: an fMRI study. NeuroImage. 2008;40:1902–1911. doi: 10.1016/j.neuroimage.2007.12.062. doi:10.1016/j.neuroimage.2007.12.062 [DOI] [PubMed] [Google Scholar]
- Darwin C. John Murray; London, UK: 1881. The formation of vegetable mould through the action of worms, with observations on their habits. [Google Scholar]
- Dawkins R. Freeman; Oxford, UK: 1982. The extended phenotype. [Google Scholar]
- de Waal F. Basic Books; New York, NY: 2001. The Ape and the Sushi Master: cultural reflections by a primatologist. [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. doi:10.1038/427311a [DOI] [PubMed] [Google Scholar]
- Eaton G. Snowball construction by a feral troop of Japanese macaques (Macaca fuscata) living under seminatural conditions. Primates. 1972;13:411–414. doi: 10.1007/BF01793660. doi:10.1007/BF01793660 [DOI] [PubMed] [Google Scholar]
- Evarts E.V. Methods for recording activity of individual neurons in moving animals. In: Rushmer R.F, editor. Methods in medical research. Year Book; Chicago, IL: 1966. pp. 241–250. [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J. Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg G, Iriki A. From sticks to coffee-maker: mastery of tools and technology by human and non-human primates. Cortex. 2007;43:285–288. doi: 10.1016/s0010-9452(08)70454-4. doi:10.1016/S0010-9452(08)70454-4 [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. doi:10.1038/nrn2147 [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves A.J, Graziano M.S, Gross G.G. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. doi:10.1126/science.286.5439.548 [DOI] [PubMed] [Google Scholar]
- Gould E, Vail N, Wagers M, Gross C.G. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc. Natl Acad. Sci. USA. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. doi:10.1073/pnas.181354698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead L.B. Tsukiji Shokan; Tokyo, Japan: 1988. Kinji Imanishi—the view from the mountain top: a critique of Imanishi's evolutionary theory. in Japanese. [Google Scholar]
- Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain. 1911;34:102–254. doi:10.1093/brain/34.2-3.102 [Google Scholar]
- Hihara S, Obayashi S, Tanaka M, Iriki A. Rapid learning of sequential tool use by macaque monkeys. Physiol. Behav. 2003;78:427–434. doi: 10.1016/s0031-9384(02)01006-5. doi:10.1016/S0031-9384(02)01006-5 [DOI] [PubMed] [Google Scholar]
- Hihara S, Notoya T, Tanaka M, Ichinose S, Ojima H, Obayashi S, Fujii N, Iriki A. Extension of corticocortical afferents into the anterior bank of the intraparietal sulcus by tool-use training in adult monkeys. Neuropsychologia. 2006;44:2636–2646. doi: 10.1016/j.neuropsychologia.2005.11.020. doi:10.1016/j.neuropsychologia.2005.11.020 [DOI] [PubMed] [Google Scholar]
- Holloway R.L. Evolution of the human brain. In: Lock A, Peters C.R, editors. Handbook of human symbolic evolution. Clarendon; Oxford, UK: 1996. pp. 74–125. [Google Scholar]
- Iacoboni M. Mapping cognition—thinking, numerical abilities, theory of mind, consciousness. In: Toga A.W, Mazziotta J.C, editors. Brain mapping—the systems. Academic; San Diego, CA: 2000. pp. 523–534. [Google Scholar]
- Imanishi K. Routledge; London, UK: 1941/2002. A Japanese view of nature: the world of living things by Kinji Imanishi. [Originally in Japanese (1941), English transl. by P. J. Asquith et al. 2002.] [Google Scholar]
- Iriki A. A prototype of Homo faber: a silent precursor of human intelligence in the tool-using monkey brain. In: Dehaene S, et al., editors. From monkey brain to human brain. MIT Press; Cambridge, MA: 2005. pp. 253–271. [Google Scholar]
- Iriki A. The neural origins and implications of imitation, mirror neurons and tool use. Curr. Opin. Neurobiol. 2006;16:660–667. doi: 10.1016/j.conb.2006.10.008. doi:10.1016/j.conb.2006.10.008 [DOI] [PubMed] [Google Scholar]
- Iriki, A. In press. Posterior parietal cortex & tool usage & hand shape. In The new encyclopedia of neuroscience, (eds L. Squire et al.).
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;14:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Obayashi S, Iwamura Y. Self-images in the video monitor coded by monkey intraparietal neurons. Neurosci. Res. 2001;40:163–173. doi: 10.1016/s0168-0102(01)00225-5. doi:10.1016/S0168-0102(01)00225-5 [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Hihara S, Iriki A. Acquisition and development of monkey tool-use: behavioral and kinematic analyses. Can. J. Physiol. Pharmacol. 2000;78:958–966. doi:10.1139/cjpp-78-11-958 [PubMed] [Google Scholar]
- Ishibashi H, Hihara S, Takahashi M, Heike T, Yokota T, Iriki A. Tool-use learning selectively induces expression of brain-derived neurotrophic factor, its receptor trkB, and neurotrophin 3 in the intraparietal cortex of monkeys. Cogn. Brain Res. 2002a;14:3–9. doi: 10.1016/s0926-6410(02)00056-3. doi:10.1016/S0926-6410(02)00056-3 [DOI] [PubMed] [Google Scholar]
- Ishibashi H, Hihara S, Takahashi M, Heike T, Yokota T, Iriki A. Tool-use learning induces BDNF in a selective portion of monkey anterior parietal cortex. Mol. Brain Res. 2002b;102:110–112. doi: 10.1016/s0169-328x(02)00201-2. doi:10.1016/S0169-328X(02)00201-2 [DOI] [PubMed] [Google Scholar]
- Itô Y. Development of ecology in Japan, with special reference to the role of Kinji Imanishi. Ecol. Res. 1991;6:139–155. doi:10.1007/BF02347158 [Google Scholar]
- Kawai M. Newly acquired pre-cultural behavior of the natural troop of Japanese monkeys on Koshima Islet. Primates. 1965;6:1–30. doi:10.1007/BF01794457 [Google Scholar]
- Kawato M. From ‘Understanding the Brain by Creating the Brain’ toward manipulative neuroscience. Phil. Trans. R. Soc. B. 2008;363:2169–2182. doi: 10.1098/rstb.2008.2272. doi:10.1098/rstb.2008.2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S. The process of sub-culture propagation among Japanese macaques. Primates. 1959;2:43–60. doi:10.1007/BF01794457 [Google Scholar]
- Kornack D.R, Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. doi:10.1126/science.1065467 [DOI] [PubMed] [Google Scholar]
- Laland K.N, Brown G.R. Oxford University Press; Oxford, UK: 2002. Sense and nonsense: evolutionary perspectives on human behaviour. [Google Scholar]
- Leca J.-B, Gunst N, Huffman M.A. Japanese macaque cultures: inter- and intra-troop behavioural variability of stone handling patterns across 10 troops. Behaviour. 2007;144:251–281. doi:10.1163/156853907780425712 [Google Scholar]
- Lee H.-L, et al. Anatomical traces of vocabulary acquisition in the adolescent brain. J. Neurosci. 2007;27:1184–1189. doi: 10.1523/JNEUROSCI.4442-06.2007. doi:10.1523/JNEUROSCI.4442-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita A, Iriki A. Tools for the body (schema) Tr. Cogn. Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. doi:10.1016/j.tics.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Mitiman G. The University of Chicago Press; Chicago, IL: 1992. The State of nature: ecology, community, and American social thought, 1900–1950. [Google Scholar]
- Moravec H. Harvard University Press; Cambridge, MA: 1988. Mind children. [Google Scholar]
- Novikoff A.B. The concept of integrative levels and biology. Science. 1945;101:209–215. doi: 10.1126/science.101.2618.209. doi:10.1126/science.101.2618.209 [DOI] [PubMed] [Google Scholar]
- Obayashi S, Tanaka M, Iriki A. Subjective image of invisible hand coded by monkey intraparietal neurons. Neuroreport. 2000;16:3499–3505. doi: 10.1097/00001756-200011090-00020. [DOI] [PubMed] [Google Scholar]
- Obayashi S, Suhara T, Kawabe K, Okauchi T, Maeda J, Akine Y, Onoe H, Iriki A. Functional brain mapping of monkey tool use. Neuroimage. 2001;14:853–861. doi: 10.1006/nimg.2001.0878. doi:10.1006/nimg.2001.0878 [DOI] [PubMed] [Google Scholar]
- Obayashi S, Suhara T, Nagai Y, Maeda J, Hihara S, Iriki A. Macaque prefrontal activity associated with extensive tool use. Neuroreport. 2002;13:2349–2354. doi: 10.1097/00001756-200212030-00036. doi:10.1097/00001756-200212030-00036 [DOI] [PubMed] [Google Scholar]
- Odling-Smee F.J, Laland K.N, Feldman M.W. Princeton University Press; Princeton, NJ: 2003. Niche construction: the neglected process in evolution. [Google Scholar]
- Plotokin, H. C. (ed.) 1988 The role of behavior in evolution, Cambridge, MA: MIT Press.
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978;4:515–526. [Google Scholar]
- Sakura O. Similarities and varieties: a sketch on the acceptance of Darwinism and sociobiology in Japan. Biol. Phil. 1998;13:341–357. doi:10.1023/A:1006504623820 [Google Scholar]
- Sakura O. [Between science and non-science: how Japanese is Japanese primatology]? In: Sugiyama Y, editor. [Primate ecology: dynamism of interaction among environment and behavior] Univesity of Kyoto Press; Kyoto, Japan: 2000. pp. 427–448. [Google Scholar]
- Sakura O, Matsuzawa T. Flexibility of wild chimpanzee nut-cracking behavior using stone hammers and anvils: an experimental analysis. Ethology. 1991;87:237–248. [Google Scholar]
- Sakura O, Sawaguchi T, Kudo H, Yoshikubo S. Declining support for Imanishi. Nature. 1986;323:586. doi:10.1038/323586a0 [Google Scholar]
- Shennan S. Thames & Hudson; London, UK: 2002. Genes, memes and human history: Darwinian archeology and cultural evolution. [Google Scholar]
- Sober E, Wilson D.S. Harvard University Press; Cambridge, MA: 1998. Unto others: the evolution and psychology of unselfish behavior. [Google Scholar]
- Tanaka I, Tokida E, Takefushi H, Hagiwara T. Tube test in free-ranging Japanese macaques: use of sticks and stones to obtain fruit from a transparent pipe. In: Matsuzawa T, editor. Primate origins of human cognition and behavior. Springer; Tokyo, Japan: 2001. pp. 509–518. [Google Scholar]
- Tokida E, Tanaka I, Takefushi H, Hagiwara T. Tool-using in Japanese macaques: use of stones to obtain fruit from a pipe. Anim. Behav. 1994;47:1023–1030. doi:10.1006/anbe.1994.1140 [Google Scholar]
- Tomasello M, Call J. Oxford University Press; Oxford, UK: 1997. Primate cognition. [Google Scholar]
- Ungerleider L.G, Mishkin M. Two cortical visual systems. In: Ingle D.J, editor. Analysis of visual behavior. MIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- von Bertalanffy L. G. Braziller; New York, NY: 1969. General system theory: foundations, development, applications. [Google Scholar]
- von Bonin G. Thomas; Springfield, UK: 1950. Essay on the cerebral cortex. [Google Scholar]
- Wolfheim J.H. University of Washington Press; Seattle, WA: 1983. Primates of the world: distribution, abundance, and conservation. [Google Scholar]
- Yamazaki, Y., Namba, H., Iriki, A. 2006 Learning to use tools to extend motor and sensory functions by Japanese monkeys. Program no. 64.2. Neuroscience meeting planner Atlanta, GA: Society for Neuroscience, 2006. Online.