Abstract
The translocated actin recruiting phosphoprotein (Tarp) is injected into the cytosol shortly after Chlamydia trachomatis attachment to a target cell and subsequently phosphorylated by an unidentified tyrosine kinase. A role for Tarp phosphorylation in bacterial entry is unknown. In this study, recombinant C. trachomatis Tarp was employed to identify the host cell kinase(s) required for phosphorylation. Each tyrosine rich repeat of L2 Tarp harbors a sequence similar to a Src and Abl kinase consensus target. Furthermore, purified p60-src, Yes, Fyn, and Abl kinases were able to phosphorylate Tarp. Mutagenesis of potential tyrosines within a single tyrosine rich repeat peptide indicated that both Src and Abl kinases phosphorylate the same residues suggesting that C. trachomatis Tarp may serve as a substrate for multiple host cell kinases. Surprisingly, chemical inhibition of Src and Abl kinases prevented Tarp phosphorylation in culture and had no measurable effect on bacterial entry into host cells.
Keywords: Chlamydia trachomatis, Src, Abl, tyrosine kinases, Tarp
INTRODUCTION
A Gram-negative obligate intracellular bacterium, Chlamydia trachomatis, is the leading cause of preventable blindness worldwide and the most prevalent bacterial pathogen causing sexually transmitted disease in the western world [1]. Chlamydiae initiate their intracellular developmental cycle by actively gaining entry into host cells. The extracellular infectious form of the developmental cycle is referred to as an elementary body (EB). Once engulfed by the host cell, the EB differentiates into the replicative reticulate body (RB) within the protective confines of a membrane bound parasitophorous vacuole termed an inclusion [2].
EB invasion of non-phagocytic cells is the product of coordinated cytoskeletal remodeling characterized by the formation of pedestal like structures and hypertrophic microvilli that are directly triggered by the invading chlamydiae [3, 4]. A recently identified C. trachomatis type III secreted protein called Tarp, for translocated actin recruiting phosphoprotein, is tyrosine phosphorylated by an unidentified host cell kinase and is spatially and temporally associated with the recruitment of actin at the site of EB invasion [5]. Subsequent in vitro analysis revealed that the C-terminal domain of Tarp nucleated actin filaments independently of tyrosine phosphorylation or additional host factors such as Arp2/3 [6]. Despite all pathogenic Chlamydia species harboring a Tarp ortholog, only C. trachomatis Tarp contains the tyrosine rich repeat domain and is phosphorylated upon cellular contact [7]. The function of Tarp phosphorylation and the signaling molecules that associate with phosphorylated Tarp are unknown.
We demonstrate here that the tyrosine rich repeat region of C. trachomatis L2 Tarp harbors multiple Src kinase target sequences, and is phosporylated by recombinant p60-src. Tyrosine residues 136 and 140 (and the corresponding amino acids in repeats 2–6) are likely the sites of phosphorylation of this kinase. Interestingly, EBs were able to invade cells lacking Src kinases and these cells were capable of phosphorylating Tarp. Our study suggests multiple kinases phosphorylate specific Tarp residues ensuring phosphorylation of this secreted effector irrespective of host kinase repertoires. Furthermore, we demonstrate that EB entry is not dependent on Tarp phosphoryation, as host cells treated with PP2, a Src and Abl family tyrosine kinase inhibitor that effectively blocked Tarp phosphorylation had no effect on bacterial invasion.
MATERIALS AND METHODS
Cloning and Protein Expression
Previously described clones of L2 Tarp served as the template for PCR mutagenesis designed to identify the tyrosine residues phosphorylated by host cell kinases [6]. Individual point mutations of a single L2 Tarp repeat were introduced by site directed mutagenesis.
pGEX-6P-1 plasmids encoding the Tarp fusion proteins were transformed into the BL21 strain of E. coli (Novagen, Madison, WI). Protein expression and purification were performed according to the procedures outlined in the Bulk GST Purification Module(GE Healthcare: Amersham Biosciences AB, Piscataway, NJ).
GST fusion Protein Pull-Down and Kinase Experiments
Recombinant L2 Tarp proteins were phosphorylated by host cellular extracts in pull down assays. HeLa 229 cells were suspended in 100 mM KCl, 10mM HEPES (pH 7.7), 2mM MgCl2, and 2 mM ATP (buffer A) and disrupted by sonication. Insoluble material was removed by centrifugation (12,000 rcf, 25 min, 4°C). Glutathione-sepharose beads were incubated with 10μg of GST fusion proteins or GST for 1 hour at 4°C in PBS (Amersham Biosciences). GST- fusion protein coated sepharose beads were washed twice with PBS and once with buffer A prior to the addition of approximately 100 μg of HeLa extracts. Extracts and beads were incubated together for 2 hours at 4°C , room-temp or 37°C, washed three times with fresh buffer A, and bound proteins were eluted using sample buffer [8]. Kinase assays were performed similarly with HeLa extracts replaced with purified recombinant kinases Src, Yes, Fyn c-Abl, and Zap 70 (Millipore, Temecula, CA).
SDS-PAGE, Immunoblotting, and Antibodies
Proteins were separated on SDS-10% polyacrylamide gels [8] and immunoblotted as previously described [5–7]. The anti-phosphotyrosine 4G10 monoclonal antibody and anti-Src, clone GD11 was purchased from Upstate (Lake Placid, NY). The anti-actin C4 monoclonal antibody was purchased from Chemicon International. The Anti-c-Abl (Ab1) rabbit polyclonal sera was purchased from Calbiochem, EMD Biosciences, (La Jolla CA) Polyclonal rabbit antibodies directed towards the Chlamydia antigens, Tarp was developed at Rocky Mountain Laboratories (Hamilton, MT) and has been described previously [5 ].
Kinase inhibitors
L929 fibroblasts plated into 24 well plates were treated with the kinase inhibitor, PP2 [4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo-[3,4-d]pyrimidine] at media concentrations of 25–200μM for 2 hours (A.G. Scientific, Inc. San Diego, CA and EMD Biosciences La Jolla CA). C. trachomatis L2 was used to infect drug treated fibroblasts at an MOI of 50 for indirect immunofluorescence microscopy and an MOI of 1000 for western blot analysis. A complete list of tyrosine kinase inhibitors tested in culture with indicated concentrations is summarized in Supplemental Table I. Percent of Tarp phosphorylation was determined by densitometry (Image J) of immunoblot signal compared to DMSO control.
RESULTS
Recombinant Tarp proteins are tyrosine phosphorylated by HeLa extracts and purified p60-Src
C. trachomatis Tarp is rapidly tyrosine phosphorylated upon exposure to the host cell cytosol at the site of EB attachment and thought to play a role in the cytoskeletal rearrangements accompanying internalization. Presumably, phosphorylation of Tarp, as with the phosphorylation of other known bacterial secreted effectors from other organisms, initiates signal transduction cascades important for the establishment of intracellular residence [9]. To better ascertain the significance of Tarp phosphorylation, we sought to identify the host cell kinase(s) responsible for this in vivo modification. Glutathione S transferase (GST) fusions to the tyrosine rich N-terminal repeat region as well as other domains of the Tarp protein wre previously constructed (Fig. 1A) [6]. The addition of soluble HeLa extracts to Tarp proteins harboring the tyrosine rich domain resulted in phosphorylation as shown by immunoblotting with phosphotyrosine specific monoclonal antibody [6]. C. trachomatis L2 Tarp harbors six tandem tyrosine rich repeats of similar sequence (Fig. 1B). Sequence analysis of the six individual repeats has revealed that each contains at least one predicted Src-like substrate site (Fig. 1C) [9]. Interestingly, three of the six repeats appear to contain two overlapping Src-like substrate sites (Fig. 1C). To determine if a Src family member was capable of phosphorylating Tarp, recombinant Tarp proteins were incubated with purified p60-Src and immunoblotted with antisera specific for phosphorylated tyrosine. Similar to results with Tarp proteins ectopically expressed in HeLa cells, only Tarp proteins containing the tyrosine rich repeat regions were phosphorylated (Supplemental Fig 1) [7].
Figure 1. Schematic of GST-TARP fusions employed in this study and sequence of the tyrosine rich repeats harboring Src-like kinase substrate sites.
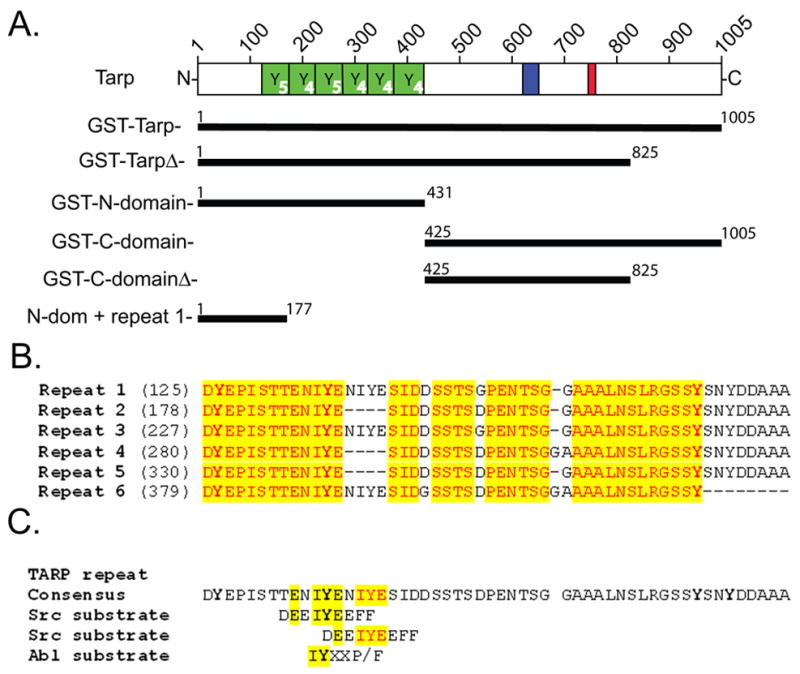
L2 Tarp harbors an N-terminal tyrosine rich repeat region (YYYYY, green boxes), which are contained within the GST fusions; GST-Tarp, GST-TarpΔ, and GST-N-domain. Each repeat contains either 4 or 5 tyrosines indicated in the lower right corner of each green box. N-dom + repeat 1 harbor the first tyrosine rich repeat. A proline dense domain (blue box) and actin binding domain (red box) are contained within the C-terminal half of Tarp located in the GST fusions; GST-C-domain and GST-C-domainΔ. Numbers on each bar indicate L2 Tarp amino acids contained within the GST fusion protein. B. An alignment of the 6 tyrosine rich repeats of C. trachomatis L2 Tarp. Each repeat contains at least 4 tyrosine residues that are indicated in bold type. Identical residues are highlighted in yellow. B. The consensus Tarp repeat harbors two overlapping peptide sequences similar to a Src and Abl substrate.
Mutation of tyrosine residues within the Src substrate sites prevents phosphorylation
To corroborate the observation that Tarp was phosphorylated by a Src family kinase member, point mutations were introduced into a newly constructed GST-Tarp fusion containing only the first tyrosine rich repeat (residues 1–177) named N-dom + repeat 1 (Fig. 1). N-dom + repeat 1 was phosphorylated by both HeLa lysates and purified Src kinase (Fig. 2). A series of GST-Tarp point mutants and double mutants were generated (Fig. 2A) and phosphorylation levels were determined following incubations with HeLa lysates or purified Src kinase (Fig. 2 B). Surprisingly, the double mutant Y136,140F was not phosphorylated indicating that at least one of the two tyrosine residues within the overlapping Src kinase sites were necessary for Tarp phosphorylation or that potentially both residues were required. These data support a role for Src family kinases in the phosphorylation of Tarp.
Figure 2. The double mutant (Y136,140F) prevents Tarp phosphorylation.
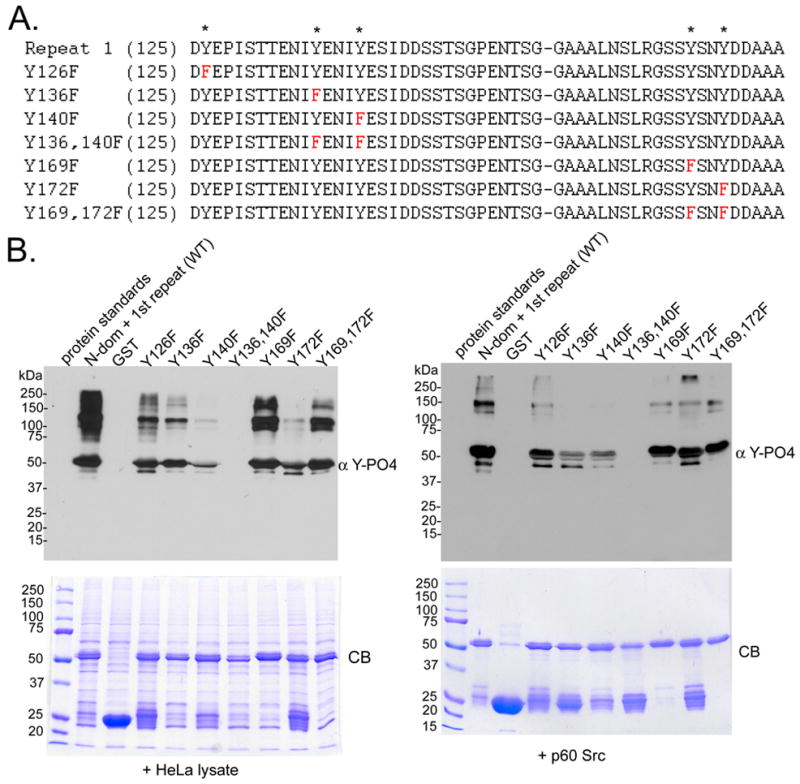
A. A series of tyrosine point mutations were introduced into the GST-N-dom + repeat 1. The mutations are indicated in red. Asterisks on the top line indicate the location of the tyrosine residues found within the first tyrosine repeat. B. Extracts from HeLa cells (HeLa) or purified Src kinase (p60 c-src) were incubated with GST or GST fusions to the N-domain of Tarp harboring the first repeat only (N-dom + repeat 1) or a series of point mutants (Y126F, Y136F, Y140F, Y136,140F, Y169F, Y172F, Y169,172F). Tarp and specifically bound proteins were resolved by SDS-PAGE and visualized by Coomassie blue staining (CB). Phosphorylation was detected by immunobloting with 4G10 (α Y-PO4).
Elementary bodies and Tarp are phosphorylated by Src deficient cells
To further investigate a role for Src family kinases in Tarp phosphorylation, embryonic derived fibroblast-like SYF cells deficient in the Src family kinase members Src, Yes and Fyn were tested for their ability to phosphorylate native Tarp following a chlamydia infection (Supplemental fig 2A) [10]. Interestingly, EBs were observed to co-localize with phosphotyrosine labeled proteins as determined by indirect immunofluorescence staining of fixed SYF cells immediately following invasion. Tyrosine phosphorylation in association with EBs was also detected in all of the control cell lines (Supplemental fig 2A). To confirm that the phosphorylated proteins were Tarp and not another host cell protein phosphorylated and recruited to the site of entry, immunoblots were performed on replicate samples (Supplemental fig 2B). Native Tarp was found to be phosphotyrosine positive. These results indicate that another kinase potentially other than a Src, Yes, or Fyn is able to phosphorylate Tarp following secretion into the host cell. This finding further suggests that Tarp phosphorylation may result from redundant kinases ensuring phosphorylation of this secreted effector regardless of host cell kinase repertoires.
Abl kinase phosphorylates Tarp
The C-terminal portion of Tarp harbors an actin binding domain and nucleates actin monomers at the time of EB attachment following secretion into the host cell [6]. Abl is a nonreceptor associated tyrosine kinase implicated in the regulation of actin dynamics and shares a consensus target sequence which is similar to Src family tyrosine kinases [9, 11]. To determine if Tarp could be phosphorylated by Abl, in addition to Src family kinases, GST-Tarp fusion proteins harboring point mutations of all tyrosine residues (Supplemental fig. 3) were incubated with purified Abl kinase and phosphorylation was determined by immunoblot analysis. All Tarp proteins were phosphorylated except the double mutant Y136,140F indicating that the Src family kinases and Abl phosphorylated the same residues. Abl induced Tarp phosphorylation was reduced with the Y136F mutation more so than Src induced phosphorylation of the same mutant, suggesting that Abl kinase may prefer tyrosine 136 over tyrosine 140. Interestingly, Abl protein expression appears to be elevated in the SYF cells, further suggesting that Abl may play a compensatory role in the Src deficient cells (Supplemental fig. 2B).
Additional attempts at kinase identification focused on fractionating HeLa cells and determining which fractions harbored kinase activity (fraction 15 & 18, Supplemental fig 3). Anion exchange of a soluble HeLa lysate routinely produced two peaks of greater kinase activity. Surprisingly, Western blots of fractions harboring increased kinase activity were negative for Src protein (data not shown), but correlated with the presence of Abl protein (Supplemental fig 3).
To investigate whether Tarp was a more suitable substrate for Src family kinases compared to Abl, a kinase assay was performed with equal quantities (equal mass) of purified Src, Yes, Fyn, Abl and Zap70 kinases. The Src family of kinases, Src, Yes and Fyn were capable of phosphorylating Tarp to a greater extent compared to Abl in the in vitro assay (Fig. 3) These data suggest that C. trachomatis Tarp may serve as a substrate for multiple host cell kinases ensuring immediate phosphorylation of this secreted effector.
Figure 3. Src, Yes and Fyn phosphorylate recombinant Tarp.
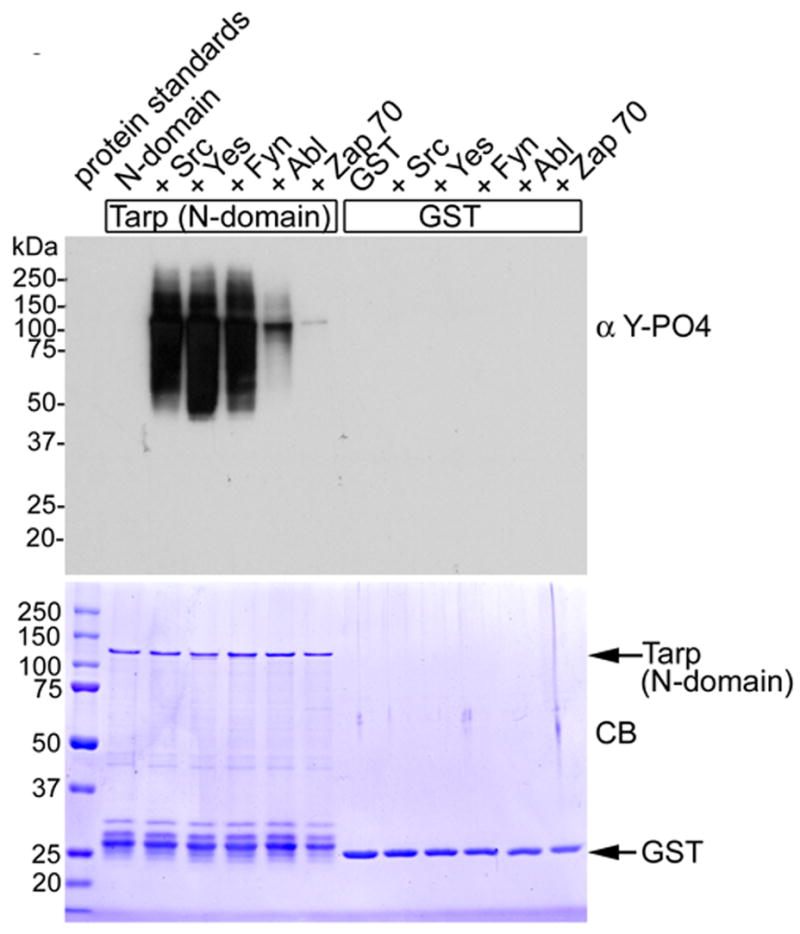
Src, Yes, Fyn , Abl and Zap 70 were added to purified recombinant GST or GST-N-domain Tarp. The SFKs phosphorylated Tarp to a greater extent compared to Abl. Zap 70 was used as a negative control and only demonstrated trace phosphorylation. Proteins were resolved by SDS-PAGE and visualized by Coomassie blue staining (CB). Phosphorylation was detected by immunobloting with 4G10 (α Y-PO4).
Tarp phosphorylation is not required for Chlamydia trachomatis entry
To investigate any requirement for Tarp phosphorylation during infection of host cells, several kinase inhibitors were tested in vitro and in vivo. Tyrosine kinase inhibitors, SU6656 and Lavendustin A, which target SFKs only partially inhibited Tarp phosphorylation, while broad spectrum PTKs inhibitors such as AG213 completely prevented phosphorylation in vitro suggesting that multiple kinases may phosphorylate Tarp (Supplemental Table 1). The tyrosine kinase inhibitor PP2, a potent Src and Abl family kinase inhibitor, was employed in an attempt to disrupt bacterial entry. Tarp phosphorylation was reduced to below the level of detectable limits for western blot analysis at concentrations of PP2 above 200μM (Fig. 4). Despite inhibition of Tarp phosphorylation at these concentrations of PP2, no effect on bacterial entry was observed (Fig. 4). Inclusion development was perturbed at PP2 concentrations above 12μM and completely arrested above 50μM (data not shown). These data indicate that Tarp phosphorylation is not essential for C. trachomatis entry, but Src and Abl family kinase activity may serve a role in subsequent events in chlamydial intracellular development.
Figure 4. Chlamydia trachomaitis L2 entry is independent of Tarp phosphorylation.
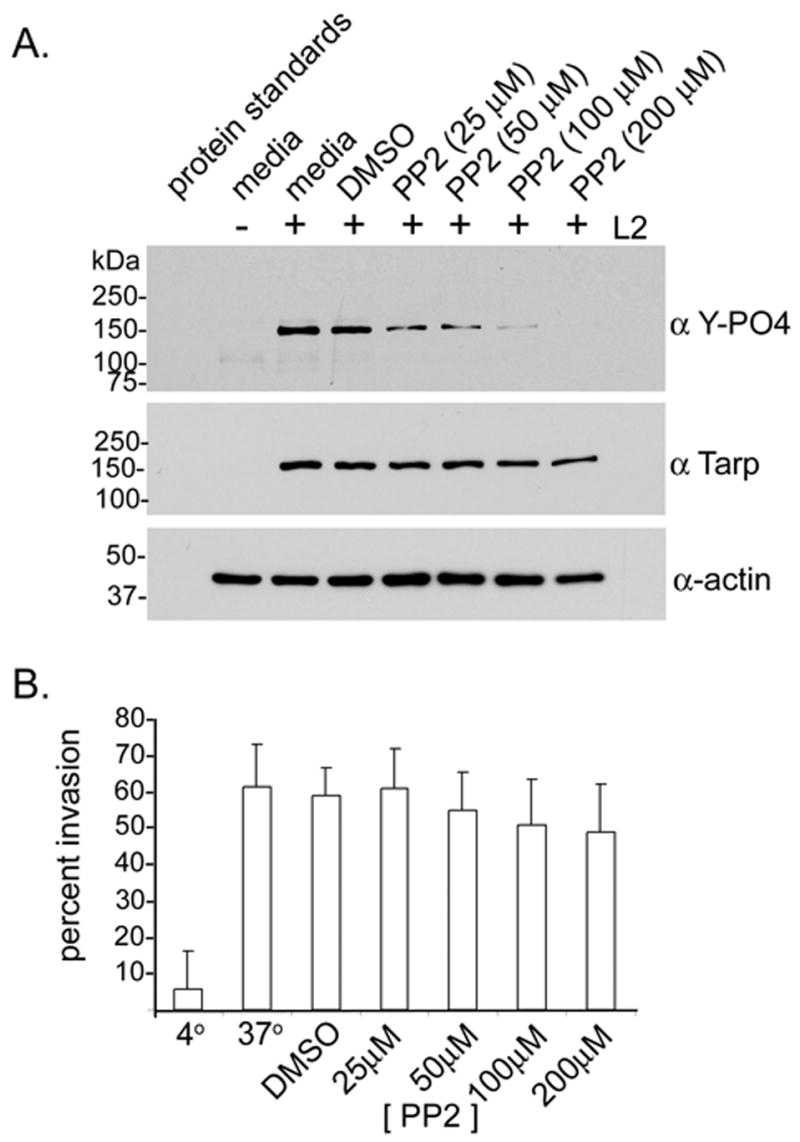
A. Tarp phosphorylation levels were inhibited during an L2 infection of L929 fibroblasts in the presence of the tyrosine kinase inhibitor PP2 [25–200μM]. Cells and bacteria (+L2) from each well of a 24 well plate were suspendered in protein sample buffer following a 30 minute infection. Media alone (media) and media with DMSO (DMSO) were employed as kinase inhibitor solvent controls. Proteins were resolved by SDS-PAGE and Immunoblots were performed with actin (α actin), Tarp (α Tarp) and phosphotyrosine specific antisera (α Y-PO4). B. Graphical representation of EB entry in PP2 treated host cells. The percent of EB invasion (percent invasion) was determined for PP2 [25–200μM] treated cells, media alone (37°), media and DMSO (DMSO), and cells kept on ice to inhibit EB entry (4°). L929 fibroblasts were infected with cmtpx stained EBs (red). Host cells and bacteria were fixed and extracellular EBs was counter stained with anti-EB sera and alexa-fluor conjugated secondary antibody (green). The percent of EB invasion per cell was determined as the number of extracellular EBs (green) subtracted from the total number of EBs (red) divided by the total number of EBs (red) multiplied by 100.
DISCUSSION
Although all Chlamydia species harbor Tarp orthologs, the similarity occurs primarily over the actin binding domain and only C. trachomatis Tarp is tyrosine phosphorylated [5–7]. Strains of C. trachomatis encode Tarp proteins with varying numbers of tyrosine rich repeats [5, 7, 12]. Specifically, C. trachomatis L2 is composed of 6 tandem tyrosine rich repeats of similar sequence while serovars D and A have 3 partial repeat units [5, 7, 12]. Sequence analysis indicates that each repeat contains at least one Src-like consensus target and that three repeats, harbor two overlapping Src-like consensus targets [9]. Mutational analysis of one tyrosine rich repeat harboring the overlapping Src-like target sequences indicated that both tyrosine residues are phosphorylated by HeLa extracts and purified Src kinase. The significance of overlapping kinase sites remains unknown. Additionally, it is unclear if all potential tyrosines are phosphorylated in vivo, or if the different repeats are preferentially phosphorylated by a specific kinase family member. Purified Src, Yes, and Fyn, members of the Src family kinases (SFKs) as well as Abl kinase were all capable of phosphorylating Tarp to detectable levels in in vitro kinase assays. Other Chlamydia species harbor Tarp proteins that are not phosphorylated upon entry, and therefore are presumed to initiate alternate signaling pathways.
Multiple tyrosine kinases appear capable of Tarp phosphorylation. Other secreted effectors such as Tir from Enteropathogenic E. coli (EPEC), are also promiscuous with tyrosine kinases. EPEC Tir was found to be phosphorylated in Fyn deficient cells and Abl was subsequently identified as the alternate tyrosine kinase for Tir [13]. Similarly, Tarp is phosphorylated by Abl in vitro in addition to phosphorylation by SFKs. The same amino acids required for Src phosphorylation of Tarp are also required for Abl-mediated phosphorylation. Fractionation of HeLa extracts demonstrated that fractions containing Abl harbored increased Tarp phosphorylation. Interestingly, Tarp phosphorylation likewise occurred in a number of fractions that did not contain Abl suggesting that additional unidentified kinases may also be involved in Tarp phosphorylation. Kinase redundancy also appears to play a significant role in Helicobacter pylori infection. H. pylori CagA is phosphorylated by Src and Abl [14–16].
One kinase inhibitor, PP2 [4-Amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo-[3,4-d]pyrimidine], was found to inhibit Tarp phosphorylation in L929 fibroblasts. At high concentrations, PP2 has been shown to inhibit tyrosine kinases other than Src and Abl as much as 60% [17]. Therefore the lack of Tarp phosphorylation observed in the presence of PP2 can not be directly attributed to one particular tyrosine kinase or family of proteins. Nevertheless, Tarp phosphorylation was inhibited but EB entry was not significantly affected indicating that Tarp phosphorylation is not required for bacterial entry. However, inclusion development was disrupted with PP2 at concentrations exceeding 12μM, consistent with a model necessitating Src and or Abl family of tyrosine kinases for chlamydial growth and potentially establishing a link between Tarp phosphorylation and later signaling events required for chlamydial survival.
C. trachomatis Tarp consists of at least two functionally distinct domains; a tyrosine-rich domain of unknown function that is absent from other chlamydial species and a conserved C-terminal domain that directly nucleates actin filament formation [5–7]. Actin nucleation by Tarp is insufficient in itself to promote internalization of EBs as it is known that the host Arp2/3 complex is also necessary [18]. A model was proposed in which the linear actin filaments induced by Tarp served as a scaffold for Arp 2/3, which nucleates branching actin filaments from existing actin filaments, to promote changes in the cytoskeletal dynamics favoring internalization of EBs [6]. The signals that activate the Rac-dependent recruitment of the Arp2/3 complex are controversial. Very recent studies implicate a requirement for Abl kinase-mediated phosphorylation of Tarp [19] and subsequent recruitment of multiple guanine nucleotide exchange factors [20] also thought to be required for the Rac activation which appears to be required for Chlamydia uptake. In the absence of genetic methods to mutate Tarp in Chlamydia to eliminate the tyrosine residues phosphorylated, we sought to identify the host kinase(s) responsible such that we could determine the role of Tarp tyrosine phosphorylation in entry. Inhibition of Src and Abl kinases efficiently blocked Tarp phosphorylation but surprisingly had no measurable effect on internalization. Hence, we favor a model which requires C. trachomatis Tarp phosphorylation for post entry events.
An increasing number of secreted effectors from pathogenic bacteria such as Enteropathogenic E. coli, Citrobacter, Helicobacter pylori and Bartonella henselae have been shown to be phosphorylated by host cell tyrosine kinases shortly after translocation into susceptible target cells [9]. Dissecting the signal transduction pathways that initiates these events are crucial to our understanding of infectious diseases. Identification of the kinases mediating Tarp phosphorylation is an initial step in mapping the signal transduction networks initiated by C. trachomatis to establish residence within an intracellular niche.
Supplementary Material
Acknowledgments
The authors would like to thank members of the Hackstadt lab, Mollie W. Jewett and Jean Celli for helpful discussions as well as acknowledge the technical assistance of Tina Clark and Janet Sager. This work is supported by the intramural research program of the NIAID, NIH.
References
- 1.Schachter J. Infection and disease epidemiology. In: Stephens RS, editor. Chlamydia; Intracellular biology, pathogenesis, and immunity. ASM Press; Washington, D.C: 1999. pp. 139–169. [Google Scholar]
- 2.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dautry-Varsat A, Subtil A, Hackstadt T. Recent insights into the mechanisms of Chlamydia entry. Cell Microbiol. 2005;7:1714–1722. doi: 10.1111/j.1462-5822.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 4.Carabeo RA, Grieshaber SS, Fischer E, Hackstadt T. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect Immun. 2002;70:3793–3803. doi: 10.1128/IAI.70.7.3793-3803.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifton DR, Fields KA, Grieshaber S, Dooley CA, Fischer E, Mead D, Carabeo RA, Hackstadt T. A chlamydial type III translocated protein is trosine phosphorylated at the site of entry and associated with recruitment of actin. Proc Natl Acad Sci U S A. 2004;101:10166–10171. doi: 10.1073/pnas.0402829101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jewett TJ, Fischer ER, Mead DJ, Hackstadt T. Chlamydial TARP is a bacterial nucleator of actin. Proc Natl Acad Sci U S A. 2006;103:15599–15604. doi: 10.1073/pnas.0603044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clifton DR, Dooley CA, Grieshaber SS, Carabeo RA, Fields KA, Hackstadt T. Tyrosine phosphorylation of the chlamydial effector protein Tarp is species specific and not required for recruitment of actin. Infect Immun. 2005;73:3860–3868. doi: 10.1128/IAI.73.7.3860-3868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 9.Backert S, Selbach M. Tyrosine-phosphorylated bacterial effector proteins: the enemies within. Trends Microbiol. 2005;13:476–484. doi: 10.1016/j.tim.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Klinghoffer RA, Sachsenmaier C, Cooper JA, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. Embo J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernandez SE, Krishnaswami M, Miller AL, Koleske AJ. How do Abl family kinases regulate cell shape and movement? Trends Cell Biol. 2004;14:36–44. doi: 10.1016/j.tcb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Carlson JH, Porcella SF, McClarty G, Caldwell HD. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect Immun. 2005;73:6407–6418. doi: 10.1128/IAI.73.10.6407-6418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swimm A, Bommarius B, Li Y, Cheng D, Reeves P, Sherman M, Veach D, Bornmann W, Kalman D. Enteropathogenic Escherichia coli use redundant tyrosine kinases to form actin pedestals. Mol Biol Cell. 2004;15:3520–3529. doi: 10.1091/mbc.E04-02-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selbach M, Moese S, Hauck CR, Meyer TF, Backert S. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J Biol Chem. 2002;277:6775–6778. doi: 10.1074/jbc.C100754200. [DOI] [PubMed] [Google Scholar]
- 15.Stein M, Bagnoli F, Halenbeck R, Rappuoli R, Fantl WJ, Covacci A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol Microbiol. 2002;43:971–980. doi: 10.1046/j.1365-2958.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 16.Poppe M, Feller SM, Romer G, Wessler S. Phosphorylation of Helicobacter pylori CagA by c-Abl leads to cell motility. Oncogene. 2007;26:3462–3472. doi: 10.1038/sj.onc.1210139. [DOI] [PubMed] [Google Scholar]
- 17.Bain J, McLauchlan H, Elliott M, Cohen P. The specificities of protein kinase inhibitors: an update. Biochem J. 2003;371:199–204. doi: 10.1042/BJ20021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carabeo RA, Grieshaber S, Hasenkrug A, Dooley CA, Hackstadt T. Requirement for the Rac GTPase in Chlamydia trachomatis invasion of non-phagocytic cells. Traffic. 2004;5:418–425. doi: 10.1111/j.1398-9219.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 19.Elwell CA, Ceesay A, Kim JH, Kalman D, Engel JN. RNA Interference Screen Identifies Abl Kinase and PDGFR Signaling in Chlamydia trachomatis Entry. PLOS Pathogens. 2008;4:e1000021. doi: 10.1371/journal.ppat.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane BJ, Mutchler C, Khodor SA, Grieshaber SS, Carabeo RA. Chlamydial Entry Involves TARP binding of Guanine Nucleotide Exchange Factors. PLOS Pathogens. 2008;4:e1000014. doi: 10.1371/journal.ppat.1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


