Abstract
Tumor-specific targeting using achievements of nanotechnology is a mainstay of increasing efficacy of anti-tumor drugs. To improve drug targeting we covalently conjugated for the first time two different monoclonal antibodies, an anti-mouse transferrin receptor antibody and a mouse autoimmune anti-nucleosome antibody 2C5, onto the drug delivery nanoplatform, poly(β-L-malic acid). The active anti-tumor drug components attached to the same carrier molecule were antisense oligonucleotides to vascular protein laminin-8. The resulting drug, a new Polycefin variant, was administered intravenously into glioma-bearing xenogeneic animals. The drug delivery system was targeted across mouse endothelial system by the anti-mouse transferring receptor antibody and to the tumor cell surface by the anti-nucleosome antibody 2C5. The targeting efficacies of the Polycefin variants bearing either two antibodies or each single antibody were compared in vitro and in vivo. ELISA confirmed the co-existence of two antibodies on the same nanoplatform molecule and their functional activities. Fluorescence imaging analysis after 24 h of intravenous injection demonstrated significantly higher tumor accumulation of Polycefin variants with the tandem configuration of antibodies than with single antibodies. The results suggest improved efficacy for tandem configuration of antibodies than for single configurations carried by a drug delivery vehicle.
Keywords: Enhanced tumor targeting, Antibody tandem configuration, Nanobiopolymer, Brain tumor, Poly(β-L-malic acid)
1. Introduction
Specific drug delivery is crucial for treating tumors with minimal side effects for normal cells. Drugs, such as monoclonal antibodies (mAbs), antisense oligonucleotides (AONs) or small molecules, should be able to penetrate the cell membrane for direct targeting of cancer cells. There are three basic methods for intracellular drug delivery, passive diffusion through aqueous channels or pores, passive diffusion of lipid-soluble drugs dissolved in a membrane, and carrier-mediated active transport (viral vectors, liposome-mediated gene transfer system, special chemicals) [1-3].
Polymers able to deliver inhibitory agents to tumor cells are becoming more popular because they are less immunogenic than viral vectors, and therefore, more useful for repetitive treatments [4-7]. The advantages of macromolecular therapeutics compared to small drug molecules are increased efficacy, increased maximum tolerated dose, decreased non-specific toxicity, circumvention of multidrug resistance, increased solubility and tumor targeting, enhanced accumulation in solid tumors, enhanced apoptosis induction, and activation/blocking of different signaling pathways. Targeted and standard combination chemotherapy and photodynamic therapy using macromolecular therapeutics showed enhanced effects on tumors that could not be obtained with either chemotherapy of photodynamic therapy (PDT) alone [8]. The last decade has seen successful clinical application of polymer-protein conjugates (e.g. Oncaspar, Neulasta) and promising results in clinical trials with polymer-anticancer drug conjugates. The realization that nanomedicine may play an important future role in cancer diagnosis and treatment has increased the interest in this emerging field. More than 10 anticancer conjugates have now entered clinical development. Notably, phase I/II clinical trials involving N-(2-hydroxypropyl)methacrylamide (HPMA)-doxorubicin conjugate (PK1; FCE28068) showed a four-to fivefold reduction in anthracycline-related toxicity, and no cardiotoxicity was observed [9].
High-molecular-mass molecules recently caught special attention because tumors can selectively accumulate them. This enhanced permeability and retention (EPR) effect was observed in cancer tissue for macromolecules and liposomes (MW>45 kDa) [9-11] and is used in cancer treatment for specific drug delivery. Unlike currently used small molecule anticancer drugs, macromolecular (or polymeric) drugs can target tumors with high selectivity through the EPR effect [8,9,12]. Amphiphilic block copolymers can significantly enhance passage of proteins through blood brain barrier BBB [55]. Delivery to tumor vasculature was recently demonstrated using a synthetic non-biodegradable polymer, HPMA, conjugated with O-(chloroacetyl-carbamoyl) fumagillol (TNP-470) [8].
Interesting approaches were recently introduced using biodegradable polymersomes for systemic delivery of PEGylated liposomes into tumor cells after systemic administration in vivo. These polymer-based shell-like particles provide a hydrophobic membrane-like compartment and an aqueous lumen to efficiently carry both hydrophobic and hydrophilic drugs, respectively, paclitaxel and doxorubicin [13]. Simultaneous suppression of ‘pump and nonpump’ drug resistance dramatically enhanced the ability of doxorubicin for inducing apoptosis leading to higher in vitro cytotoxicity and in vivo anti-tumor activity [14].
Another promising drug carrier is poly(β-l-malic acid) (PMLA) [15-22]. This polymer is a natural product of Physarum polycephalum [23]. The attractive properties of PMLA as a carrier matrix of biopharmaceuticals are its lack of toxicity in vitro and in vivo, non-immunogenicity, biodegradability, stability in the bloodstream, and easy cellular uptake [18,20-22,24]. The drug Polycefin has been synthesized with PMLA as platform [25]. It is a conjugate of nanoscale size (20-30 nm) carrying a variety of specifically functioning modules: targeting antisense oligonucleotides (AONs) to silence human tumor-specific mRNAs, homing devices such as tumor-specific antibodies for receptor-mediated endocytosis, an endosomal escape system, and a release system for the AONs after entrance into the cytoplasm [22,26]. Besides, tracers can also be conjugated to PMLA such as radioactive groups or fluorescent dyes that render the drug carrier system easy to trace in biological fluids (blood, urine, spinal fluid) and tissues.
Importantly, multiple inhibitors such as AONs of different molecular targets (mRNAs) can be easily attached in tandem configuration (side by side on the same PMLA platform molecule) [27,28]. This especially enables every tumor cell-targeting drug molecule to block efficiently the expression of tumor-specific proteins that consist of more than one chain. Because of their complexity, such proteins as laminins, collagens, etc. could not be previously considered as targets for drug therapy. Moreover, blocking of only one chain of these proteins (e.g., laminin α4 chain) has been found to elicit a compensatory increase in the expression of another, auxiliary functioning laminin chain, α5 [29,30].
Because of the mentioned properties, the Polycefin drug delivery vehicle has many clear advantages over currently tested carriers. Importantly, we have the technology in place to synthesize various modifications regarding the kind and number of the drug species using the same vehicle for targeting a variety of different tumor markers. Eventually, the drug may be customized to a particular tumor using either nucleic acid-based or other drugs to tumor-specific targets.
In order to improve direct tumor targeting and to avoid the damage of non-tumor cells we synthesized a new Polycefin variant with two monoclonal antibodies of different specificities on the same platform molecule and studied the drug accumulation in glioma-bearing animals. We use the combination of anti-nucleosome antibody 2C5 together with mouse anti-transferrin receptor (TfR) antibody [28] to target human glioma in xenogeneic mouse model. In the model used, the Polycefin variant with the combination of mouse anti-TfR [31-37] and human tumor-specific antibody 2C5 [10, 38] provides the most efficient drug delivery through mouse endothelial system and into implanted human brain tumor cells not achieved by variants with single mAbs or devoid of antibodies. Presence of two or more different antibodies at the same time on drug delivery systems, especially on Polycefin variants, may be important for future specific drug delivery and therapeutic efficacy in tumor treatment.
2. Materials and methods
2.1. Reagents
Morpholino™-3′-NH2 antisense oligonucleotides AGC-TCA-AAG-CCA-TTT-CTC-CGC-TGA-C to laminin α4 (MORPH-AON-1) and CTA-GCA-ACT-GGA-GAA-GCC-CCA-TGC-C to laminin β1 (MORPH-AON-2) chains [39] were custom made by Gene Tools (USA) in PBS (phosphate buffered saline). Rat anti-mouse monoclonal TfR antibody R17217 (mTfR) that does not recognize human TfR was purchased from Southern Biotech, USA. Mouse autoimmune monoclonal antibody 2C5 recognizing tumor cell surface-bound nucleosomes (released from neighboring apoptotic tumor cells) was a gift of Prof. V.P. Torchilin (Northeastern University, Boston, MA). Poly(β-L-malic acid) (PMLA), a natural polymer of molecular mass Mw=50,000 (polydispersity Mw/Mn=1.3) from the culture broth of Physarum polycephalum was highly purified and size-fractionated on Sephadex G25. The lyophilized polyacid was devoid of material absorbing at 260 nm and 280 nm wavelengths. If not mentioned otherwise, molar quantities of conjugated groups refer to total malyl residues. Chromatographically pure mPEG5000-amine and maleimide-PEG3400-maleimide were obtained from Nektar Therapeutics (USA). 3-(2-Pyridyldithio)-propionate (PDP) was synthesized as described. Oregon Green and AlexaFluor® 680 C2-maleimide were obtained from Molecular Probes-Invitrogen (USA). All other chemicals of highest purity were bought from Merck (Germany), Sigma (Germany), and Pierce (USA). Glioma cell line U87MG was obtained from the American Type Culture Collection (USA).
2.2. Nomenclature
The term Polycefin denotes the drug delivery device with poly(malic acid) as platform and the various functional groups described previously, specifically the monoclonal mouse anti-rat TfR antibody (OX-26) and leucyl ethylester as the active endosomal escape residue. The newly synthesized versions of Polycefin contain other monoclonal antibodies instead of anti-rat TfR antibody. Polycefin(mTfR) denotes the version with mouse anti-human TfR, Polycefin(2C5) is the version with tumor-specific antibody 2C5 [38,40], and Polycefin(mTfR, 2C5) is the version with both antibodies. For the synthesis, the reader is referred to the Results section.
2.3. Analytical methods for chemical synthesis
Thiol residues attached to poly(malic acid) conjugates were assayed by the method of Ellman after removal of free 2-MEA by diafiltration (5 kDa cutoff). Amounts of maleimido groups were quantified by their reaction with a given amount of 2-MEA. During the preparation of PMLA conjugates, the substitution of NHS was followed by RP-HPLC analysis of the reaction mixtures. Amino acids were quantified by RP-HPLC after hydrolysis of conjugates in 6 M HCl at 100 °C and colorimetry with trinitrofluorobenzene (TNBS) following standard protocols. Reducing SDS-PAGE (with 20 mM DTT) on 10% polyacrylamide gels (samples boiled for 5 min in 5% SDS) and Western blotting were carried out as published [25]. Chromatography was performed on a Merck-Hitachi analytical LaChrom D-7000 HPLC-UVand fluorescence detector system with either Macherey & Nagel C18-Nucleosil reversed phase RP (250/4 mm) or size exclusion Bio-Sil SEC 250-5 (5 μm, 300/7.8 mm) columns. 220 and 260 nm wavelengths were used for the detection of PMLA and its conjugates. Molecular mass markers were polystyrene sulfonates were from Machery-Nagel (Germany) and proteins from Sigma. Precoated silica gel 60 F254 aluminum sheets (Merck) were used in thin layer chromatography with n-butanol, water, and acetic acid (4:2:1 by volume) as solvent.
2.4. In vitro indirect ELISA for Polycefin variants
Tandem configuration of two different antibodies occurs if they are crosslinked to the same polymer platform molecule of the nanoconjugate. For a demonstration of successful conjugation and verification of the functionality of the antibodies in question, ELISA was used. The antibodies (rat anti-mouse TfR and mouse anti-nucleosome 2C5 antibody) that were conjugated to Polycefin(mTfR, 2C5) were analyzed using a Protein Detector™ ELISA Kit (KPL, USA). Mouse transferrin receptor ectodomain [gift from Dr. Chi-Hong (Betty) Chen, (UCLA Department of Chemistry and Biochemistry) to Dr. Manuel Penichet] and Nucleohistone (Worthington Biochemical CO., USA) were used as antigens. Below, a typical procedure is described, with additional details provided in the Results section.
A 100 μl of antigen solution was mixed with × coating buffer at the concentration of 10 μg/ml for mouse TfR or 100 μg/ml for nucleohistone and incubated in 96-well cell culture plates (Corning, USA) for 1 h at room temperature. The antigen-coated plates were washed three times with 1× wash solution, and 3.3× bovine serum albumin (BSA) diluent/blocking buffer was added for 20 min at room temperature. After application of 1× wash solution for 3 times, Polycefin was added at antibody concentrations of 0, 0.01, 0.1, and 1.0 μg/ml in phosphate buffered saline (PBS) and incubated for 1 h at room temperature. Unbound Polycefin variant was removed with five washes in the 1× wash solution wash, and anti-rat or anti-mouse horseradish peroxidase (HPR) conjugate (GeneTex, Inc., San Antonio, TX) was added at portions of 100 μl/well with a dilution of 1/5000 (v/v) in PBS and incubated for 1 h at room temperature. Excess conjugate was washed away five times and the substrate ABTS [2,2′-azino-di-(3-ethyl-benzthia-zoline-6-sulphonic acid)] (KPL, USA) added at 100 μl/well with 0.1 h incubation at room temperature. A405 readings were obtained after the addition of 100 μl of stop solution per well. Controls were assayed in the absence of coated antigens and the presence or absence of Polycefin(mTfR, 2C5).
2.5. Cell lines and culture conditions
Human glioblastoma cell line U87MG was obtained from American Type Culture Collection (USA). Cells were cultured in Eagle’s MEM with 10% fetal bovine serum, l-glutamine, antibiotics, and sodium pyruvate.
2.6. In vitro confocal microscopy
Human glioma cell culture U87MG was treated with Polycefin(mTfR, 2C5). A TCS SP spectral scanner (Leica Microsystems, Mannheim, Germany) was used for confocal microscopy. Image stacks of 160 by 160 μm in size and 7.5 μm in depth of live U87MG glioma cells were acquired with a Leica PlanApo 63/1.2 NA lens. Live cells were placed in an Attofluor incubation chamber (Molecular Probes, USA). A temperature of 37 °C was maintained by a separate lens and chamber heating system. The Oregon Green derivatives of Polycefin conjugated with two antibodies, mTfR and 2C5, were added to cells in serum-free medium at a concentration of 5 μg/ml.
2.7. Animal studies
5×104 of U87MG human glioblastoma cells were stereotactically implanted into the right basal ganglia field of athymic mice (CrTac:NCr-Foxn1nu Homozygous, Taconic, USA).
At day 21 after tumor implantation, 100 μl of AlexaFluor 680 labeled Polycefin variant or unconjugated AlexaFluor 680 was injected intravenously at the concentration of 3 μM. For assessment of drug distribution and localization in nude mice, Xenogen IVIS 200 was used under the Isoflurane anesthesia at different time points (before drug admiistration; 1 h, 3 h, 6 h, 24 h, 48 h after drug administration). Twenty-four hours after drug administration, the mice were euthanized and the circulating drugs in blood vessels were eliminated by intraarterial PBS perfusion for 20 min. The brain was harvested to detect the fluorescent signal. The fluorescent signal intensities in the tumor, tumor adjacent area, normal cerebrum and normal cerebellum were analyzed by Xenogen Living Image®, Version 2.50 (WaveMetrix, USA).
All animal procedures were carried out in accordance with IACUC protocols approved by animal welfare committee at Cedars-Sinai Medical Center.
2.8. Statistical analysis
All experiments were performed in triplicates and the data were subjected to analysis of variance (ANOVA) and the Bonferroni’s test.
3. Results
3.1. Drug synthesis
The two new Polycefin versions were synthesized essentially according to the method described recently for Polycefin [25]. To synthesize the versions with a single molecule antibody attached to the PMLA platform, 25 nmol of the particular (2-pyridyldithio)propionyl-morpholino AONs were coupled as disulfides to the pendant platform preconjugate amidoethylthionyl groups. In the case of Polycefin(mTfR, 2C5), the reaction mixture contained 50 nmol of equimolar mouse monoclonal anti-human TfR antibody and mouse monoclonal tumor-specific anti-nucleosome antibody 2C5. The reaction of the preconjugate with antibodies was followed by HPLC-sec, indicated by a small yet significant shift of the elution peak towards shorter retention times. By this method we were able to follow the mAb conjugation reaction to completion (results not shown). Polycefin variants were conjugated with fluorescent AlexaFluor 680 C2-maleimide (Molecular Probes-Invitrogen, Karlsruhe, Germany) at 1% of the carboxyl groups for in vitro detection and imaging, following the same synthetic route as described [25,27]. Polycefin variants were homogeneous (single symmetrical peaks) by HPLC-sec. If not protected against oxygen, preparations tended to self-crosslink during prolonged storage (several months), especially when kept in solution (Scheme 1).
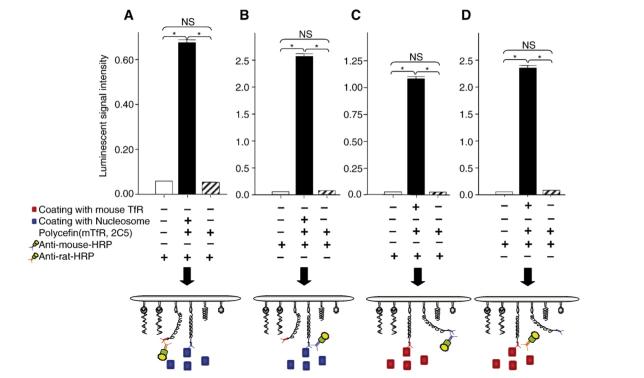
Fig. 1.
ELISA showing tandem configuration of rat anti-mouse TfR antibody and mouse anti-nucleosome antibody 2C5 conjugated with Polycefin(mTfR, 2C5). The level of luminescence indicates the amount of Polycefin variant bound to plate-coated TfR and nucleosome antigens (black boxes), of controls with secondary antibodies to rat anti-TfR antibody or to mouse anti-nucleosome antibody either in the absence of antigens/Polycefin(mTfR, 2C5) (open boxes) or in the absence of antigens only (shaded boxes). The total concentration of antibodies conjugated with Polycefin(mTfR, 2C5) is 1.0 μg/ml. Anti-mouse-HRP, a secondary antibody to mouse anti-nucleosome antibody 2C5, conjugated to horseradish peroxidase. Anti-rat-HRP, a secondary antibody to rat anti-mouse TfR antibody. A and B. Binding of Polycefin(mTfR, 2C5) to coated nucleosome antigen. A. Detection of the tandem anti-mouse TfR antibody. B. Detection of the tandem anti-nucleosome antibody. C and D. Binding of Polycefin(mTfR, 2C5) to coated mouse TfR. C. Detection of the tandem anti-mouse TfR antibody. D. Detection of the tandem anti-nucleosome antibody. SD, error bar. Asterisks, P<0.05. NS, not significant.
3.2. Estimates of stoichiometries and molecular masses
100% Malic acid residues (FW 116)=430 per molecule of PMLA (Mw=50 kD). Fraction of malic acid with free-COOH (FW116) is 45%=193.5×116 Da: 22.5 kDa. Fraction conjugated malic acid (FW 99) is 55%=236.5×99 Da: 23.4 kDa. Fraction 2-MEA (FW 76) is 10%=43×76 Da: 3.27 kDa. Fraction leucine ethyl ester (FW 158.22) is 40%=172×158.22 Da: 27.2 kDa. Fraction mPEG5000 (FW 5000) is 5%=21.5×5000 Da: 107.5 kDa. Fraction Morpholino oligos (FW 8700) is 5%=21.5×8700 Da: 187 kDa. Fraction mAb1 (FW 150 kDa) is 0.23%=1×150 kDa: 150 kDa.Fraction mAb2 (FW 150 kDa) is 0.23%=1×150 kDa: 150 kDa. Fraction Fluorescent dyes (FW 1000) is 1%=4.3×1000 Da: 4.3 kDa. Fraction 3-mercaptopropanonic acid (FW 105) is 3.54%= 15.2×105 Da: 1.6 kDa. Total estimated average Mw of Polycefin ∼677 kDa. Polycefin variants carrying a single mAb molecule had average Mw 527.
3.3. Verification of the function of antibodies and their tandem configuration in Polycefin(mTfR, 2C5)
To confirm the function of antibodies in Polycefin(mTfR, 2C5) and their tandem configuration, we performed ELISA; the results are shown in Fig. 1. Four different types of experiments were performed to verify the antigen binding activity and co-existence of the two different antibodies (rat anti-mouse TfR antibody and mouse anti-nucleosome antibody 2C5) on the drug carrier molecule. Luminescence indicated that Polycefin(mTfR, 2C5) recognized and bound specifically to the coated antigens via both of its mAbs. Signal intensities were dose dependent. Binding was not observed if plates were BSA-blocked and not antigen coated. In particular, results shown in Fig. 1A and B indicate that the 2C5 antibody recognizes and binds specifically to coated nucleosome antigen while the presence of anti-mouse TfR antibody is also shown. Correspondingly, the results in Fig.1C and D indicate that anti-mouse TfR antibody recognizes and binds to coated mouse TfR specifically while the presence of the 2C5 antibody is documented. The results show that the antibodies in Polycefin(mTfR, 2C5), anti-mouse TfR antibody and mouse anti-nucleosome antibody 2C5 were functionally active and were present in tandem configuration on the Polycefin molecule.
3.4. Accumulation of Polycefin variants in cells of cultured human glioma
In agreement with the finding of Torchilin et. al [10] that anti-nucleosome antibody 2C5 recognizes an antigen exposed on tumor cell surfaces we observed that the Oregon Green labeled antibody 2C5 bound to U87MG cell surface in vitro followed by cellular uptake using confocal microscopy (not shown here).
When U87MG cells were treated with Oregon Green labeled Polycefin(2C5) or Polycefin(mTfR, 2C5), fluorescence appeared in endosomes and subsequently in the cytoplasm with the highest drug accumulation after one hour (Fig. 2). The results were similar to those reported previously for Polycefin variant [25] and were again interpreted by a mechanism that involved recognition and binding of the Polycefin(mTfR, 2C5) variant to the 2C5 antigen at the tumor cell surface and subsequent internalization into endosomes. U87MG cells pre-treated with 2C5 antibody were found unable to internalize Polycefin(2C5) or Polycefin(mTfR, 2C5) (results not shown) probably due to masking of 2C5 antigen. Polycefin(mTfR) devoid of the 2C5 antibody was not reactive in agreement with the known absence of the ability of the mouse antibody to recognize and bind to human TfR.
Fig. 2.
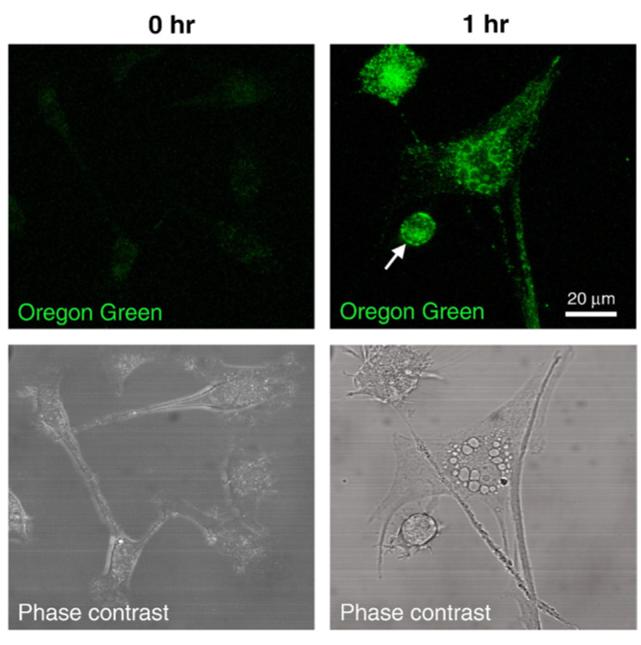
Polycefin accumulation in vitro. At time 0 Polycefin does not accumulate in U87MG glioma cells. Accumulation is detected in U87MG cells in 1 h incubation with Polycefin(mTfR, 2C5) labeled with Oregon Green. Arrow shows Polycefin on the cell membrane; cytoplasmic accumulation is also prominent.
3.5. Polycefin(mTfR,2C5) specifically accumulates in human glioma in vivo
Several AlexaFluor 680-conjugated Polycefin variants and controls were tested: A. Free AlexaFluor 680; B. Variant with conjugated anti-mouse TfR antibody alone, C. variant with conjugated antibody 2C5 alone, D. variant with antibody 2C5 together with anti-mouse TfR antibody (Fig. 3). These samples were injected into nude mice for fluorescence imaging.
Fig. 3.
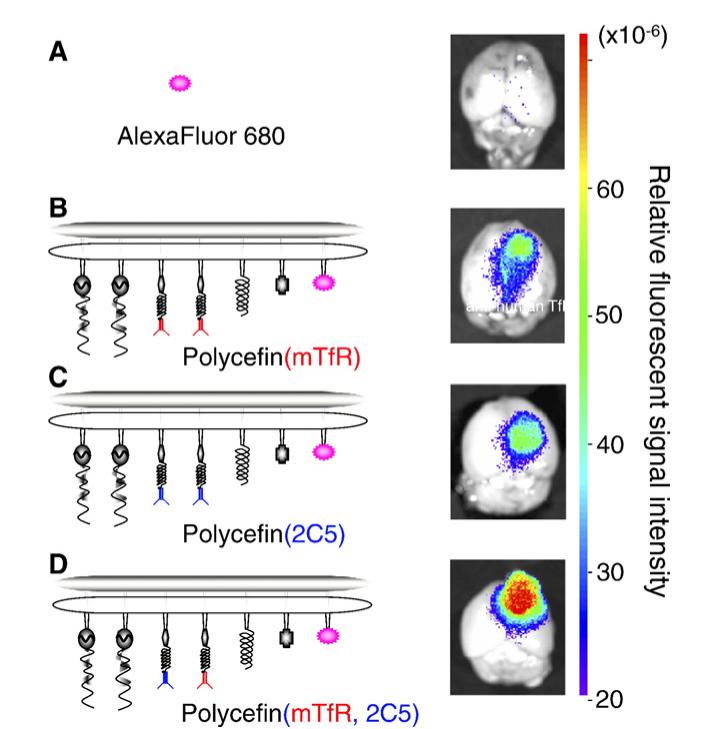
Fluorescence imaging of brain from nude mice bearing human U87MG glioma 24 h after intravenous injection of free AlexaFluor 680 and the indicated AlexaFluor 680-labeled Polycefin variants. Only the tumor contains fluorescent drug. The highest drug accumulation in the tumor is observed for the tandem configuration in Polycefin(mTfR, 2C5). 100 μl of AlexaFluor 680 (0.6 μM) or labeled Polycefin variants at concentrations of 3 μM morpholino AONs were injected intravenously.
The results are shown in Figs. 3, 4. Fluorescence (Polycefin variant) accumulation was seen in tumor and was higher than in adjacent areas as well as in normal non-tumor brain (cortex and cerebellum, Figs. 3, 4). Drug accumulation with only anti-mouse antibody was lower in tumor and adjacent area (Fig. 3B) than with only 2C5 antibody (Fig. 3C); see also Fig. 4. The highest tumor accumulation was achieved for Polycefin(mTfR, 2C5) when the drug contained the tandem combination of anti-mouse TfR and antibody 2C5 (Figs. 3D, 4). The differences were significant (p<0.01). The fluorescence for injected AlexaFluor 680 was insignificant in Fig. 3A and low in Fig. 4. The results are in agreement with the assumption that the highest drug accumulation was achieved by Polycefin(mTfR, 2C5) through the combined action of the two mAbs in tandem, with anti-mouse TfR antibody transporting the drug through the blood-tumor-barrier (BTB) by binding specifically to the endothelium-expressed TfR, and anti-nucleosome 2C5 antibody targeting human tumor cells by binding to a tumor cell surface antigen.
Fig. 4.
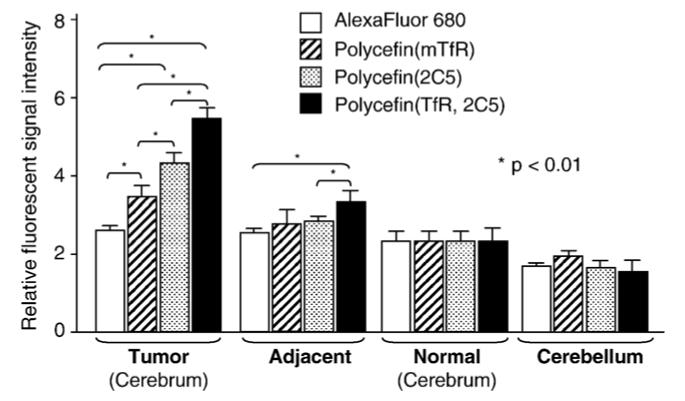
Fluorescence imaging of glial tumor, adjacent areas, normal brain, and cerebellum after 24 h following intravenous injection into glioma-bearing nude mice of the indicated Polycefin variants or free AlexaFluor 680 at the dosages given in Fig. 3. The intensities of fluorescent signal in the tumor, tumor adjacent area, normal cerebrum, and normal cerebellum were measured and analyzed by Xenogen Living Image®, Version 2.50.
4. Discussion
Polycefin consists of modules active in endosomal uptake, endosomal membrane disruption, oligonucleotide release in the cytoplasm, protection from enzymatic degradation, and a fluorescent dye for imaging. Two AONs blocking mRNAs coding for α4 and β1 chain of an invasive tumor marker, laminin-8, were conjugated to be delivered simultaneously to brain tumor cells [25,27]. All these components were covalently conjugated to highly purified poly(malic acid) (Mw 50000, Mw/ Mn 1.3) from Physarum polycephalum. The original Polycefin version contained a single conjugated mAb, anti-rat/human TfR OX-26 antibody, that facilitated transfer of Polycefin though BTB by recognizing and binding to exposed endothelial rat TfR as well as it targeted glioma cells and facilitated endosomal uptake. A cross-reactivity with endothelial BTB TfR and human glioma TfR was not available in a single anti-TfR antibody. To successfully deliver the AONs into nude mouse-inoculated human glioma cells, a mAb tandem had to be established that recognized both the mouse TfR and the glioma cell surface. Together with an anti-mouse TfR mAb, we chose the anti-nucleosome mAb 2C5, previously introduced by Torchilin’s group [10,40] for glioma targeting. A combination of other appropriate mAbs can be also used for tumor targeting.
The successful synthesis of the tandem mAb variant of Polycefin is presented following the established method for Polycefin synthesis [25,27] and requiring no additional preparative measures. ELISA testing indicated the mAb tandem was present and functionally active in the new Polycefin(mTfR, 2C5) variant. The 2C5 antibody recognized the nucleosomes bound to a surface receptor on glioma cells and the drug vehicle was internalized in vitro. These results laid the basis for successful in vivo imaging experiments.
Mouse fluorescence imaging was performed and the contribution of single mAbs and of the mAb-tandem compared. To this end, human glioma cell line U87MG was inoculated intracranially into nude mice. Polycefin variants were injected intravenously. Polycefin variants bearing single and tandem antibodies to TfR and human surface antigen recognized by 2C5 antibody showed significantly different accumulation in the tumor. In the xenogeneic model used, the variant with the combination of mouse anti-TfR and anti-nucleosome 2C5 antibodies provided the most effective drug delivery through mouse endothelial system and into growing human brain tumors [41].
Here, we used anti-TfR antibodies for the purpose of drug transfer from blood vessel to brain parenchyma across the BTB and 2C5 antibodies for xenogeneic human tumor cell targeting. If the drug were not taken up by cancer cell due to the lack of cancer cell-specific antibodies, the drug would have washed out quickly through the cerebrospinal drainage system even after the accumulation in the tumor tissue by EPR effect. Therefore, a tumor cell-targeting agent like 2C5 antibody is important to adhere to the cell surface avoiding removal by the drainage system in the brain. If the drug did not have active BTB transfer mediated by the anti-mouse TfR antibody, it had to rely on mechanism(s) generating the EPR effect. The contribution of such mechanisms, however, cannot be excluded on the bias of our experimental data.
Whatever the detailed mechanisms, our data support the idea that the tandem configuration of specific antibodies enhances targeting of tumor tissue and can be used to increase drug treatment efficacy.
5. Conclusions
The work presented here supports the idea that tandem conjugation of mAbs with different antigen specificities to the platform of a nanoconjugate drug delivery system can enhance tumor cell targeting potential and efficacy. Poly(malic acid) as a polymer with multiple chemically functional pendant groups offers a great potential for future syntheses of such tandem configured carrier system. Though the kind and combination of antibodies can be varied to achieve specific effects, e.g., anti-TfR to direct the conjugate across the BTB and 2C5 antibody to target tumor cells, this system represents an ideal combination for brain tumor treatment. Further investigation of Polycefin variants should provide a more profound understanding of the optimal targeting mechanisms. A tandem type Polycefin might serve in the future as a potential therapeutic intervention for treatment of patients with brain tumor and in experimental cancer studies.
Scheme 1.
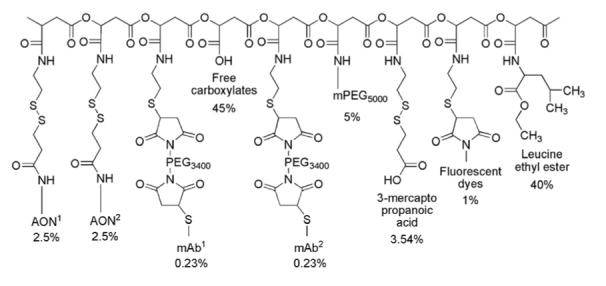
Schematic structure of Polycefin with two antibodies mTfR and 2C5.
Acknowledgements
We thank Prof. V.P. Torchilin (Northeastern University, Boston, MA, USA) for providing 2C5 anti-nucleosome antibody.
This work was supported by grant from NIH (R01 CA123495 to JYL). We thank Dr. Chi-Hong (Betty) Chen, (UCLA Department of Chemistry and Biochemistry) for providing the mouse transferrin receptor.
References
- [1].Mycek MJ, Harvey RA, Champe PC. Pharmacology. 2nd ed. Lippincott-Raven; Philadelphia: 1997. [Google Scholar]
- [2].Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4:95–99. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kanzawa T, Ito H, Kondo Y, Kondo S. Current and future gene therapy for malignant gliomas. J. Biomed. Biotechnol. 2003;1:25–34. doi: 10.1155/S1110724303209013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Satchi-Fainaro R, Puder M, Davies JW, Tran HT, Sampson DA, Greene AK, Corfas G, Folkman J. Targeting angiogenesis with a conjugate of HPMA copolymer and TNP-470. Nat. Med. 2004;10:255–261. doi: 10.1038/nm1002. [DOI] [PubMed] [Google Scholar]
- [5].Duncan R. The dawning era of polymer therapeutics. Nat. Rev. Drug Discov. 2003;2:347–360. doi: 10.1038/nrd1088. [DOI] [PubMed] [Google Scholar]
- [6].Vinogradov SV, Batrakova EV, Li S, Kabanov AV. Mixed polymer micelles of amphiphilic and cationic copolymers for delivery of antisense oligonucleotides. J. Drug Target. 2004;12:517–526. doi: 10.1080/10611860400011927. [DOI] [PubMed] [Google Scholar]
- [7].Kabanov AV, Batrakova EV, Sriadibhatla S, Yang Z, Kelly DL, Alakov VY. Polymer genomics: shifting the gene and drug delivery paradigms. J. Control. Release. 2005;101:259–271. doi: 10.1016/j.jconrel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- [8].Nori A, Kopecek J. Intracellular targeting of polymer-bound drugs for cancer chemotherapy. Adv. Drug Deliv. Rev. 2005;57(4):609–636. doi: 10.1016/j.addr.2004.10.006. [DOI] [PubMed] [Google Scholar]
- [9].Duncan R, Vicent MJ, Greco F, Nicholson RI. Polymer-drug conjugates: towards a novel approach for the treatment of endrocine- related cancer. Endocr. Relat. Cancer Suppl. 2005;1:S189–S199. doi: 10.1677/erc.1.01045. [DOI] [PubMed] [Google Scholar]
- [10].Torchilin VP, Lukyanov AN. Peptide and protein drug delivery to and into tumors: challenges and solutions. Drug Discov. Today. 2003;8:259–266. doi: 10.1016/s1359-6446(03)02623-0. [DOI] [PubMed] [Google Scholar]
- [11].Peterson CM, Shiah J, Sun Y, Kopeckova P, Minko T, Straight RC, Kopecek J. HPMA copolymer delivery of chemotherapy and photodynamic therapy in ovarian cancer. Adv. Exp. Med. Biol. 2003;519:101–123. doi: 10.1007/0-306-47932-X_7. [DOI] [PubMed] [Google Scholar]
- [12].Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int. Immunopharmacol. 2003;3:319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- [13].Ahmed F, Pakunlu RI, Brannan A, Bates F, Minko T, Discher DE. Biodegradable polymersomes loaded with both paclitaxel and doxorubicin permeate and shrink tumors, inducing apoptosis in proportion to accumulated drug. J. Control. Release. 2006;116:150–158. doi: 10.1016/j.jconrel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- [14].Pakunlu RI, Wang Y, Saad M, Khandare JJ, Starovoytov V, Minko T. In vitro and in vivo intracellular liposomal delivery of antisense oligonucleotides and anticancer drug. J. Control. Release. 2006;114:153–162. doi: 10.1016/j.jconrel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- [15].Batrakova EV, Vinogradov SV, Robinson SM, Niehoff ML, Banks WA, Kabanov AV. Polypeptide point modifications with fatty acid and amphiphilic block copolymers for enhanced brain delivery. Bioconjug. Chem. 2005;16:793–802. doi: 10.1021/bc049730c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee BS, Holler E. Effects of culture conditions on β-poly(l-malate) production by Physarum polycephalum. Appl. Microbiol. Biotechnol. 1999;51:647–652. [Google Scholar]
- [17].Korherr C, Roth M, Holler E. Poly(β-l-malate) hydrolase from plasmodia of Physarum polycephalum. Can. J. Microbiol. 1995;1(41 Suppl):192–199. [Google Scholar]
- [18].Lee BS, Vert M, Holler E. In: Water-soluble Aliphatic Polyesters: poly(malic acid)s, Biopolymers. Doi Y, Steinbüchel A, editors. 3a. Wiley-VCH; New York: 2002. pp. 75–103. [Google Scholar]
- [19].Gasslmaier B, Holler E. Specificity and direction of depolymerization of β-poly(l-malate) catalysed by polymalatase from Physarum polycephalum. Fluorescence labeling at the carboxy-terminus of β-poly(l-malate) Eur. J. Biochem. 1997;250:308–314. doi: 10.1111/j.1432-1033.1997.0308a.x. [DOI] [PubMed] [Google Scholar]
- [20].Gasslmaier B, Krell CM, Seebach D, Holler E. Synthetic substrates and inhibitors of β-poly(l-malate)-hydrolase (polymalatase) Eur. J. Biochem. 2000;267:5101–5105. doi: 10.1046/j.1432-1327.2000.01573.x. [DOI] [PubMed] [Google Scholar]
- [21].Braud C, Bunel C, Vert M. Poly(β-malic acid): a new polymeric drug-carrier. Evidence for degradation in vitro. Polym. Bull. 1985;13:293–299. [Google Scholar]
- [22].Cammas S, Béar M-M, Moine L, Escalup R, Ponchel G, Kataoka K, Guérin Ph. Polymers of malic acid and 3-alkylmalic acid as synthetic PHAs in the design of biocompatible hydrolyzable devices. Int. J. Biol. Macromol. 1999;25:273–282. doi: 10.1016/s0141-8130(99)00042-2. [DOI] [PubMed] [Google Scholar]
- [23].Ljubimova JY, Fujita M, Khazenzon NM, Lee BS, Wachsmann-Hogiu S, Farkas DL, Black KL, Holler E. Nanoconjugate based on polymalic acid for tumor targeting. Chem. Biol. Interact. doi: 10.1016/j.cbi.2007.01.015. Electronic publication 2007 Feb 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Domurado D, Fournié P, Braud C, Vert M, Guérin P, Simonnet F. In vivo fates of degradable poly(β-malic acid), and of its precursor, malic acid. J. Bioact. Compat. Polym. 2003;18:23–32. [Google Scholar]
- [25].Lee BS, Fujita M, Khazenon NM, Wawrowsky KA, Wachsmann-Hogiu S, Farkas D.l., Black KL, Ljubimova JY, Holler E. Polycefin, a new prototype of multifunctional nanoconjugate based on poly(β-L-malic acid) for drug delivery. Bioconjug. Chem. 2006;17:317–326. doi: 10.1021/bc0502457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pichon C, Goncalves C, Midoux P. Histidine-rich peptides and polymers for nucleic acid delivery. Adv. Drug Deliv. Rev. 2001;53:75–94. doi: 10.1016/s0169-409x(01)00221-6. [DOI] [PubMed] [Google Scholar]
- [27].Fujita M, Khazenzon NM, Ljubimov AV, Lee BS, Virtanen I, Holler E, Black KL, Ljubimova JY. Inhibition of laminin-8 in vivo using a novel poly(malic acid)-based carrier reduces glioma angiogenesis. Angiogenesis. 2006;9:183–191. doi: 10.1007/s10456-006-9046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ljubimova JY, Fugita M, Khazenzon NM, Das A, Pikul BB, Newman D, Sekiguchi K, Sorokin LM, Sasaki T, Black KL. Laminin-8 association with glial tumor grade, recurrence and patient survival. Cancer. 2004;101:604–612. doi: 10.1002/cncr.20397. [DOI] [PubMed] [Google Scholar]
- [29].Zhou Z, Doi M, Wang J, Cao R, Liu B, Chan KM, Kortesmaa J, Sorokin L, Cao Y, Tryggvason K. Deletion of laminin-8 results in increased tumor neovascularization and metastasis in mice. Cancer Res. 2004;64:4059–4063. doi: 10.1158/0008-5472.CAN-04-0291. [DOI] [PubMed] [Google Scholar]
- [30].Fujita M, Khazenzon NM, Bose S, Sekiguchi K, Sasaki T, Carter WG, Ljubimov AV, Black KL, Ljubimova JY. Overexpression of β1 chain-containing laminins in capillary basement membranes of human breast cancer and its metastases. Breast Cancer Res. 2005;7:411–421. doi: 10.1186/bcr1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shi N, Boado RJ, Pardridge WM. Antisense imaging of gene expression in the brain in vivo. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14709–14714. doi: 10.1073/pnas.250332397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Boado RJ, Kazantsev A, Apostol BL, Thompson LM, Pardridge WM. Antisense-mediated down-regulation of the human huntingtin gene. J. Pharmacol. Exp. Ther. 2000;295:239–243. [PubMed] [Google Scholar]
- [33].Boado RJ, Tsukamoto H, Pardridge WM. Drug delivery of antisense molecules to the brain for treatment of Alzheimer’s disease and cerebral AIDS. J. Pharm. Sci. 1998;87:1308–1315. doi: 10.1021/js9800836. [DOI] [PubMed] [Google Scholar]
- [34].Shi N, Pardridge WM. Noninvasive gene targeting to the brain. Proc. Natl. Acad. Sci. 2000;97:7567–7572. doi: 10.1073/pnas.130187497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kurihara A, Deguchi Y, Pardridge WM. Epidermal growth factor radio-pharmaceuticals: 111In chelation, conjugation to a blood-brain barrier delivery vector via a biotin-polyethylene linker, pharmacokinetics, and in vivo imaging of experimental brain tumors. Bioconjug. Chem. 1999;10:505–511. doi: 10.1021/bc980123x. [DOI] [PubMed] [Google Scholar]
- [36].Bickel U, Yoshikawa T, Pardridge WM. Delivery of peptides and proteins through the blood-brain barrier. Adv. Drug Deliv. Rev. 2001;46:247–279. doi: 10.1016/s0169-409x(00)00139-3. [DOI] [PubMed] [Google Scholar]
- [37].Zhang Y, Pardridge WM. Delivery of β-galactosidase to mouse brain via the blood-brain barrier transferrin receptor. J. Pharmacol. Exp. Ther. 2005;313:1075–1081. doi: 10.1124/jpet.104.082974. [DOI] [PubMed] [Google Scholar]
- [38].Iakoubov LZ, Torchilin VP. A novel class of antitumor antibodies: nucleosome-restricted antinuclear autoantibodies (ANA) from healthy aged nonautoimmune mice. Oncol. Res. 1997;9:439–446. [PubMed] [Google Scholar]
- [39].Khazenzon NM, Ljubimov AV, Lakhter AJ, Fujiwara H, Sekiguchi K, Sorokin LM, Virtanen I, Black KL, Ljubimova JY. Antisense inhibition of laminin-8 expression reduces invasion of human gliomas in vitro. Mol. Cancer Ther. 2003;2:985–994. [PubMed] [Google Scholar]
- [40].Gupta B, Torchilin VP. Monoclonal antibody 2C5-modified doxorubicin-loaded liposomes with significantly enhanced therapeutic activity against intracranial human brain U-87 MG tumor xenografts in nude mice. Cancer Immunol. Immunother. 2007;56:1215–1223. doi: 10.1007/s00262-006-0273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cao Y, Nagesh V, Hamstra D, Tsien CI, Ross BD, Chenevert TL, Junck L, Lawrence TS. The extent and severity of vascular leakage as evidence of tumor aggressiveness in high-grade gliomas. Cancer Res. 2006;66:8912–8917. doi: 10.1158/0008-5472.CAN-05-4328. [DOI] [PubMed] [Google Scholar]