Abstract
A reduced left ventricular ejection fraction measured by echocardiography in a patient with clinical features of heart failure demonstrates that the patient has a cardiac abnormality and that the clinical picture is, in fact, due to heart failure. As such, a reduced ejection fraction (< 0.30 or 0.35) has been used as entry criteria for almost all the large clinical trials that guide our therapy of patients with heart failure. However, it has been recently recognized that a substantial and increasing proportion of patients with heart failure have a normal ejection fraction (> 0.50). Such patients are typically elderly women with systolic hypertension. These patients are subject to the sudden development of pulmonary congestion (flash pulmonary edema). The finding of heart failure in patients with a normal ejection fraction has focused attention on the role of diastolic dysfunction in producing symptomatic heart failure. The optimal treatment of patients with heart failure and normal ejection fraction has not yet been defined, but the control of systolic hypertension and the avoidance of fluid overload are important.
Introduction
The finding of a reduced left ventricular (LV) ejection fraction (EF) in a patient with typical signs and symptoms of heart failure provides objective proof that the patient has a cardiac abnormality and makes it very likely that the patient's clinical syndrome is indeed heart failure. Accordingly, a clearly reduced EF (< 0.30 or 0.35) has been used as an entry criterion for almost all large randomized clinical trials that provide the database that allows evidence-based therapy of patients with heart failure. (1) However, over the last decade, it has become clear that many patients with heart failure do not have a reduced EF. Instead, their EF is in the normal range (> 0.50). Such patients with heart failure and a normal EF have been termed as having diastolic heart failure. In contrast, heart failure and a reduced EF has been termed systolic heart failure. Patients with diastolic heart failure are more commonly women, elderly, and affected with hypertension. The incidence of diastolic heart failure is increasing, and such patients may now make up the majority of patients admitted with heart failure. (2)
Is Diastolic Heart Failure Real Heart Failure?
Since patients with diastolic heart failure have a normal EF, it is possible that they may not have “real” heart failure; their symptoms may be due to lung disease, obesity, anemia and/or deconditioning and not due to a cardiac abnormality. To address this issue, we evaluated patients with mild to moderate heart failure in association with normal and reduced EFs and compared these patients to age-matched controls. (3) We found that the pathophysiologic characteristics of patients with diastolic and systolic heart failure were similar. Furthermore, we have found that the clinical and radiographic findings are similar in diastolic and systolic heart failure. (4,5) We conclude that the syndrome of heart failure is the same whether associated with a normal or reduced EF.
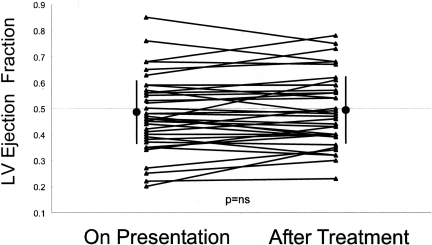
Patients with diastolic heart failure frequently present with sudden exacerbations of their symptoms and with acute pulmonary edema in association with hypertension. It is possible that their decompensation is due to transient systolic dysfunction from ischemia and/or hypertension or mitral regurgitation that had resolved by the time the LV EF was measured. Accordingly, we hypothesized that many patients hospitalized with acute pulmonary edema in association with hypertension have transient LV systolic dysfunction, which is not present when the LV EF is evaluated after the patient has been treated. To test this hypothesis, we used Doppler echocardiography to evaluate LV EF, regional wall motion, and mitral regurgitation in 38 patients both during acute episode of hypertensive pulmonary edema and 24–72 hours after treatment when the hypertension and pulmonary congestion had resolved. (6) In contrast to our hypothesis, we found that LV EF and regional wall motion were similar, both during the acute episode of hypertensive pulmonary edema and after resolution of the congestion and control of the blood pressure (Figure 1). Half of the patients had EFs in the normal range (> 0.50). The LV EF was similar during acute heart failure and after therapy, both in patients with normal and reduced EF. Thus, acute pulmonary edema was not due to transient systolic dysfunction in diastolic heart failure or worsening of pre-existing systolic dysfunction in the patients with reduced EFs. This suggests that diastolic dysfunction is an important contributor to hypertensive pulmonary edema in patients with both normal and abnormal systolic function. (1;6)
Fig. 1.
Left ventricular ejection fraction on presentation with acute pulmonary edema and after treatment. The ejection fraction is similar in both circumstances. (Data from: Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, Little WC. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med 2001; 344:17–22.)
Causes of Diastolic Heart Failure
There are many clinical conditions that cause heart failure with a normal EF. These include diastolic dysfunction, valvular diseases, pericardial diseases, and an intracardiac mass; among them, diastolic dysfunction is the most common cause of heart failure with a normal EF. (7) The term diastolic heart failure most accurately denotes the patient population whose heart failure is associated with LV diastolic abnormalities. It is important to recognize that although patients with diastolic heart failure have diastolic dysfunction, they frequently also have systolic contractile abnormalities (despite the normal EF). (8) In addition, comorbidities such as hypertension, anemia, and renal dysfunction are commonly seen in patients with diastolic heart failure and may contribute to the development of heart failure. (9)
Comparison of Pathophysiology in Systolic and Diastolic Heart Failure
Table 1 compares LV structural and functional characteristics in systolic and diastolic heart failure. Systolic heart failure and diastolic heart failure have several similarities in LV structural and functional characteristics, including increased LV mass and increased LV end-diastolic pressure. (5) The most significant difference between the two forms of heart failure is the difference in LV geometry and LV function; systolic heart failure is characterized by LV dilatation, eccentric LV hypertrophy, and abnormal systolic and diastolic function, whereas diastolic heart failure is characterized by concentric LV hypertrophy, a normal EF and abnormal diastolic function. Thus, the pathophysiology of systolic heart failure is predominantly dependent on progressive LV dilatation and abnormal systolic function. On the other hand, the pathophysiology of diastolic heart failure is predominantly dependent on concentric LV hypertrophy and abnormal diastolic function.
TABLE 1.
Comparison of left ventricular structural and functional characteristics in systolic and diastolic heart failure
| “Diastolic” Heart Failure | “Systolic” Heart Failure | |
|---|---|---|
| Diastolic Dysfunction | +++ | +++ |
| Systolic Dysfunction | + | +++ |
| Left Ventricular Dilation | −/+ | +++ |
| Left Ventricular Hypertrophy | Concentric | Eccentric |
| Aortic Stiffness | +++ | ++ |
| Decompensation | Acute | Chronic/Subacute |
Adapted from: Fukuta H, Little WC. Contribution of systolic and diastolic abnormalities to heart failure with a normal and a reduced ejection fraction. Prog Cardiovasc Dis 2007; 49:229–240.
Utility of Doppler Echocardiography
Non-invasive assessment of diastolic dysfunction using Doppler echocardiography provides useful information on the severity of heart failure and its prognosis. (7) For example, we examined the association of systolic and diastolic function with severity of heart failure assessed by plasma B-type natriuretic peptide levels and prognosis in 104 heart failure patients with an EF <0.50 and 102 heart failure patients with an EF ≥0.50.(8) The degree of diastolic dysfunction, but not EF, was associated with the severity of heart failure quantified by plasma B-type natriuretic peptide levels. In addition, diastolic dysfunction, but not a reduced EF, was associated with a worse 2-year survival. Other investigators have also found that diastolic dysfunction provides stronger prognostic information than the degree of EF reduction. (10,11)
These observations suggest that diastolic dysfunction is an important part of the pathophysiology of heart failure independent of the EF. (12) Evaluation of diastolic function helps establish the diagnosis of diastolic heart failure; in addition, its measurement with Doppler echocardiography is critical for risk stratification in all heart failure patients. (7,9)
Therapy
There is much less objective information available concerning the treatment of patients with diastolic heart failure than about the therapy of systolic heart failure. (1,13,14) This relative paucity of objective information is reflected in the American College of Cardiology/American Heart Association (15) and the European Society of Cardiology guidelines for the treatment of heart failure. (16) To paraphrase the recommendations of these guidelines: a) the goal of therapy of diastolic heart failure is to control symptoms by reducing ventricular filling pressure without reducing cardiac output; b) diuretics and nitrates are indicated for symptomatic patients; c) it is important to control arterial hypertension; d) calcium channel blockers, beta-blockers, angiotensin converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARB) may be of benefit beyond the treatment of hypertension; e) other possible but unproven therapeutic strategies include myocardial revascularization, avoiding tachycardia, and restoring sinus rhythm. (14)
Treatment of diastolic dysfunction is most effective when it is associated with hypertension. (14) Reduction of systolic arterial pressures acutely reduces pulmonary congestion and cardiac ischemia, and chronically may lead to regression of left ventricular hypertrophy. Patients with left ventricular diastolic heart failure in the absence of hypertension are very difficult to treat. They are prone to develop severe hypotension in response to diuretics or nitrates.
We have conducted a series of studies examining the potential benefit of controlling exercise-induced increases in systolic blood pressure in patients with diastolic dysfunction. (17–19) We found that blunting exercise-induced hypertension using angiotensin receptor blockers improved exercise tolerance more than equally hypotensive doses of verapamil or thiazide. In addition, our observational study suggests that statins may substantially improve survival in patients with diastolic heart failure. (20) This may be due to statins' beneficial effects on the comorbidities frequently associated with diastolic heart failure.
While awaiting the results of ongoing and planned trials of the treatment of diastolic heart failure, the current management should include control of systolic blood pressure, avoidance of fluid overload, and treatment of the frequent comorbidities.
ACKNOWLEDGEMENTS/FINANCIAL SUPPORT
These studies were supported, in part, by grants from the National Institutes of Health (RO1 AG18915 and R01 HL076438).
DISCUSSION
Mackowiak, Baltimore: Terrific presentation Bill. Two quick questions: I hope I am not exposing my ignorance of cardiology. When you calculate ejection fraction, do you allow for that part of the blood which is ejected into the atrium rather into the aorta? Is that subtracted from the ejection fraction?
Little, Winston Salem: The ejection fraction is conventionally measured as the stroke volume divided by the end-diastolic volume. You make a very good point: if there is a regurgitate lesion, mitral or aortic regurgitation, that contributes to the ejected stroke volume but it does not contribute to forward cardiac output.
Mackowiak, Baltimore: Shouldn't you do that?
Little, Winston Salem: An alternate way is to calculate the effective ejection fraction, which is the forward stroke volume divided by the end-diastolic volume. As far as assessing the degree of dilatation and the function of the left ventricle, the uncorrected ejection fraction does that. It does not, as you point out, assess the contribution of the left ventricle to the generation of useful cardiac output.
Mackowiak, Baltimore: Thank you. I noticed you had some mitral regurgitation on one of your videos. The second question has to do with limiting salt intake. Apparently there is no evidence to suggest that it is effective in the management of congestive heart failure?
Little, Winston Salem: The consensus of experts is that limiting salt intake and limiting fluid overload is very important. There are no clear objective data proving that, but I think that is very important. The degree of diastolic dysfunction helps determine how you distribute your blood volume If you have trouble filling the left, ventricle, you are prone to inappropriately distributing you blood volume into your lungs, so avoidance of increases in intravascular volume is very important.
Luke, Cincinnati: I have been very unimpressed with the value of ejection fraction in giving prognosis in heart failure, which is all over the map. If you add your parameters, does it get better— the five year survival? The other question, do ACE and ARBs help diastolic dysfunction?
Little, Winston Salem: Very good questions. First of all, our data and other data consistently show that measures of left ventricular diastolic function are superior to ejection fraction in determining prognosis. ACE inhibitors, ARBs have clearly been demonstrated to be effective in patients with heart failure who have low ejection fractions. In patients with heart failure and more normal ejection fractions. There are two large randomized studies that have been done, one of the ARB, candesartan, second with the ACE inhibitor, perindopril. Both enrolled patients with ejection fractions above 40%. Both demonstrated no effect on mortality. However, Both demonstrated a borderline statistically significant effect on reducing hospitalizations. There are methodologic problems with both of the studies. So, I don't think we have a clear answer to the question.
Weir, Baltimore: Bill, beautiful stuff. Two big questions: first of all, you didn't differentiate between ischemic and non-ischemic heart disease, simply because, obviously, the issue of lusitropy versus inotropy in the oxygen demands. Second quick question, and that has to do with the point I brought up in my presentation, is what is the blood pressure that the heart is really seeing, and have you done any work to look at ambulatory blood pressure monitoring, pulse wave velocity? I am particularly interested in pulse wave reflection and stiffness and its influence, obviously, on diastolic filling.
Little, Winston Salem: Very good points. We have not looked at ambulatory blood pressure. We have looked at exercise blood pressure and some of these patients are prone to marked systolic hypertension during exercise. In a series of studies, we have shown that blunting exertional hypertension improves exercise tolerance in these patients. The issue of reflective wave is very important and may contribute to one of the reasons why elderly women are more prone to develop this disease than elderly men. Women in general have shorter stature, thus they are more prone to have early return of the reflective waves to the central aorta; The final part of your question - clearly the pressure that the ventricle sees is the pressure in the left ventricular outflow tract, the central aortic pressure, not the peripheral pressure. Part of the aging effects on the arterial system, as you said, is the faster pulse wave velocity. The early return of a larger reflective wave contributes to an increased systolic load on the left ventricle.
Hillis, San Antonio: Bill, in the next to last figure that you showed, if they were on statins, they had a two year survival of almost 100%.
Little, Winston Salem: Yes.
Hillis, San Antonio: So my question is very simple, and that is: what is the prognosis, long-term, of people with diastolic heart failure versus those with systolic heart failure? Is it different? Is it better?
Little, Winston Salem: Very good question. There is not a simple answer to the question, and part of the reason is because patients with diastolic heart failure are much older than patients with systolic heart failure. They have many comorbidities, some recognized, some unrecognized, that drive their outcome. Their outcome is poor and may approach the outcome of patients with systolic heart failure. Some of it is driven by the heart failure. Some of it is driven by the comorbidities. Being hospitalized with acute pulmonary edema and having a normal ejection fraction is a very bad prognostic sign. How bad the prognosis is is importantly determined by how many comorbidities you have. The mortality is clearly increased in diastolic heart failure. It is an increasingly prevalent condition, and we need to make real headway in treating it.
Bransome, Aiken: Bill, that was an excellent paper defining the neglected area of diastolic heart failure. May I inquire as to your thoughts on the future direction of assessing the effectiveness of therapies?
Little, Winston Salem: There are several large trials currently underway: one using aldosterone blocker, others using beta-blockers and ARBs. So we are going to have additional information. I believe that the real future of this is on two levels. On a basic level, understanding why in response to the same neurohormonal milieu, some patients dilate their hearts and end up with adverse remodeling, where other patients do not, producing the spectrum of the ejection fraction. On a clinical level, is to finally move away from equating heart failure and low ejection fraction and moving toward understanding what is actually driving the symptoms of heart failure, and understanding the mechanisms. This will allow us to develop more rational therapy.
Billings, Baton Rouge: Bill, I, like Phil Mackowiak, am afraid of exposing my ignorance, except I am almost certain I will do so. I always thought that “diastole” meant no systole. So if the heart was in diastole, how could it be in failure since it was doing nothing but sitting there?
Little, Winston Salem: Right. So people think about diastole in terms of passive filling of the ventricle. The MRI-based contrast demonstrate, in fact, diastole is not a passive process. Normally you have almost explosive filling of the ventricle that is driven by suction all the way to the apex. This is due to recoil of the ventricle from the compression that occurred during the ejection. So actually, in diastole, the left ventricle is working as a suction pump and is most prone to have problems, if there are any problems, with the contractile apparatus in the ventricle. So your concept is the conventional understanding of diastolic function, but that is not actually how it works.
Billings, Baton Rouge: So systole is blow and diastole is suck.
Little, Winston Salem: Your words, not mine, but you accurately describe the concept.
Billings, Baton Rouge: The real reason for my asking the question is I give Adriamycin and Herceptin and am constantly measuring ejection fractions, either by radionucleotide or by echocardiography. I rely on those numbers as if they mean something. Is that reliance as ignorant as my understanding of diastole?
Little, Winston Salem: Several things about the ejection fraction: First, it is prone to substantial error, interobserver variability and repeated measures variability. So the numbers are not nearly as precise as many believe. When we give an ejection fraction of 52%, we are giving precision but not accuracy. That was the first point. The second: in the cardiotoxicity of chemotherapy, by the time ejection fraction falls, there has been substantial damage. So my colleague, Greg Hundley, is working with MRI and other things looking for more sensitive indicators and more direct indicators of the toxicity of Adriamycin. Hopefully I will have a better answer for you in the future.
Henrich, San Antonio: I loved your talk Bill. The living laboratory for diastolic disease is the laboratory of patients with end-stage kidney disease, where 90% of new patients in a dialysis unit are shown to have that lesion. Now, the only therapeutic benefit that I know of that has worked in prospective trials is to use beta-blockers in those patients and not ACEs or ARBs. So I would like you to comment on that. The second comment about it is that when biopsies of the heart have been done in that population of patients, there is a great deal of fibrosis, inter-myocytic deposition of fibrosis and bizarrely-shaped myocytes. So you wonder if there have been correlative studies done in the patients who don't have end-stage kidney disease, who have this lesion and what their anatomy shows. So comment about beta-blockers and maybe a comment about what the anatomy shows.
Little, Winston Salem: Thank you. Those are very good questions. I think in reverse order, first there are limited biopsy data in patients. This comes from studies from the Netherlands, and it shows very similar findings as you talked about in the dialysis patients. There are substantial fibrosis changes in the extra-myocyte components of the ventricle. There are also changes in the myocytes in patients with diastolic heart failure. I think beta-blockers are effective. They are effective therapy for treating systolic hypertension. These were effective in the SHEPS study that I showed you in preventing the development of heart failure. The intervention there to treat hypertension was thiazide and atenolol. On theoretical grounds, the ACE and ARBs may be better. They can also treat systolic hypertension, but more directly, address the things that are driving, or least driving some of the fibrosis, but the data to prove what I just said are not there. It is a hypothesis and not proven. Certainly beta-blockers are effective therapy for systolic hypertension and prevent the development of heart failure.
REFERENCES
- 1.Little WC. Hypertensive pulmonary oedema is due to diastolic dysfunction. Eur Heart J. 2001;22:1961–1964. doi: 10.1053/euhj.2001.2665. [DOI] [PubMed] [Google Scholar]
- 2.Gaasch WH, Little WC. Assessment of left ventricular diastolic function and recognition of diastolic heart failure. Circulation. 2007;2007:591–593. doi: 10.1161/CIRCULATIONAHA.107.716647. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Stewart KP, Marburger CT, Brosnihan B, Morgan TM, Wesley DJ. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 4.Fukuta H, Ohte N, Brucks S, Carr JJ, Little WC. Contribution of right-sided heart enlargement to cardiomegaly on chest roentgenogram in diastolic and systolic heart failure. Am J Cardiol. 2007;99:62–67. doi: 10.1016/j.amjcard.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 5.Fukuta H, Little WC. Diastolic versus systolic heart failure. In: Smiseth OA, Tendera M, editors. Diastolic Heart Failure. London: Springer; 2007. in press. [Google Scholar]
- 6.Gandhi SK, Powers JC, Nomeir AM, Fowle K, Kitzman DW, Rankin KM, Little WC. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17–22. doi: 10.1056/NEJM200101043440103. [DOI] [PubMed] [Google Scholar]
- 7.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. 2006;47:500–506. doi: 10.1016/j.jacc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 8.Brucks S, Little WC, Chao T, Kitzman DW, Wesley-Farrington D, Gandhi S, Shihabi ZK. Contribution of left ventricular diastolic dysfunction to heart failure regardless of ejection fraction. Am J Cardiol. 2005;95:603–606. doi: 10.1016/j.amjcard.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Fukuta H, Little WC. Diastolic heart failure: general principles, clinical definition, and epidemiology. In: Klein AL, Garcia MJ, editors. Diastolic Heart Failure. Burlington: Elsevier; 2007. in press. [Google Scholar]
- 10.Rihal CS, Nishimura RA, Hatle LK, Bailey KR, Tajik AJ. Systolic and diastolic dysfunction in patients with clinical diagnosis of dilated cardiomyopathy. Relation to symptoms and prognosis. Circulation. 1994;90:2772–2779. doi: 10.1161/01.cir.90.6.2772. [DOI] [PubMed] [Google Scholar]
- 11.Wang M, Yip G, Yu CM, Zhang Q, Zhang Y, Tse D, Kong SL, Sanderson JE. Independent and incremental prognostic value of early mitral annulus velocity in patients with impaired left ventricular systolic function. J Am Coll Cardiol. 2005;45:272–277. doi: 10.1016/j.jacc.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 12.Little WC. Diastolic dysfunction beyond distensibility: adverse effects of ventricular dilatation. Circulation. 2005;112:2888–2890. doi: 10.1161/CIRCULATIONAHA.105.578161. [DOI] [PubMed] [Google Scholar]
- 13.Little WC, Warner JG, Jr., Kitzman DW. Treatment of heart failure due to diastolic dysfunction. Contemporary Treatment of Cardiovascular Disease. 1997;2:71–83. [Google Scholar]
- 14.Little WC, Brucks S. Therapy for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:380–388. doi: 10.1016/j.pcad.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC., Jr American College of Cardiology/American Heart Association. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 guidelines for the evaluation and management of heart failure) J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 16.European Society of Cardiology: The Task Force of the Working Group on Heart Failure. The treatment of heart failure. Task Force of the Working Group on Heart Failure of the European Society of Cardiology. Eur Heart J. 1997;18(5):736–753. doi: 10.1093/oxfordjournals.eurheartj.a015339. [DOI] [PubMed] [Google Scholar]
- 17.Warner JG, Jr., Metzger DC, Kitzman DW, Wesley DJ, Little WC. Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. J Am Coll Cardiol. 1999;33:1567–1572. doi: 10.1016/s0735-1097(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 18.Little WC, Zile MR, Klein A, Appleton CP, Kitzman DW, Wesley-Farrington D. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98:383–385. doi: 10.1016/j.amjcard.2006.01.106. [DOI] [PubMed] [Google Scholar]
- 19.Little WC, Wesley-Farrington D, Hoyle J, Brucks S, Robertson S, Kitzman DW, Cheng CP. Effect of candesartan and verapamil on exercise tolerance in diastolic dysfunction. J Cardiovasc Pharmacol. 2004;43:288–293. doi: 10.1097/00005344-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Fukuta H, Sane DC, Brucks S, Little WC. Statin therapy may be associated with lower mortality in patients with diastolic heart failure: a preliminary report. Circulation. 2005;112(3):357–363. doi: 10.1161/CIRCULATIONAHA.104.519876. [DOI] [PubMed] [Google Scholar]