Abstract
The ideal antifungal agent remains an elusive goal for treatment of life-threatening systemic fungal infections. Such an agent would have broad antifungal activity, low rates of resistance, flexible routes of administration, few associated adverse events, and limited drug-drug interactions. Only three of the seven classes of antifungal agents currently available are suitable for treatment of systemic infection: the polyenes, the azoles, and the echinocandins. None match all the characteristics of an ideal agent, the Holy Grail of antifungal therapy. Academia and industry need to collaborate in the search for new lead antifungal compounds using traditional screening methods as well as the new pharmacogenomics methods. Enhancing efficacy and reducing toxicity of the currently available therapeutic agents is also another important avenue of study. As an example, the Mycosis Research Center at the University of Mississippi Medical Center has identified pyogenic polyenes in commercial preparations of amphotericin B deoxycholate which correlate with infusion related toxicities. A highly purified formulation of amphotericin B appears promising, with a better therapeutic index compared to its parent compound as evidenced by results of in vitro and in vivo studies reviewed in this presentation.
SPECTRUM OF FUNGAL INFECTIONS
Fungi are eukaryotic organisms characterized by non-motile bodies (thalli) composed of elongating walled filaments (hyphae). Fungal cells lack chlorophyll and have a rigid cell wall composed of chitin and various polysaccharides (i.e., β1, 4- and β1, 6-glucans, and a cell membrane containing ergosterol) (1). They have complex life cycles with both sexual and asexual reproduction, usually from a common thallus. Haploid thalli result from zygotic meiosis.
The pathogenic fungi exist in two forms, which are not mutually exclusive: unicellular yeasts and filamentous moulds. Several of the important agents of systemic mycoses, such as Histoplasma capsulatum and Blastomyces dermatitidis, are thermally dimorphic and typically grow as a filamentous mould at 25° C and yeast at 37° C. Fungi are ubiquitous in our environment and may also act as commensals, either as part of our normal flora or transient colonizers. Infection may result from both endogenous and exogenous environmental sources. Candida species, which are part of the normal flora in humans, are a frequent cause of serious fungal infection in immunocompromised patients. In contrast, blastomycosis results from inhalation of fungal conidia of Blastomyces dermatitidis from environmental exposure.
Fungal infections can be clinically classified into five major groups: superficial, subcutaneous, cutaneous, systemic and opportunistic infections. Superficial infections involve the outermost keratinized layers of the skin and hair, do not elicit an inflammatory response and are generally cosmetic problems. The cutaneous infections extend into the epidermis, may involve the nails and are associated with signs and symptoms of inflammation. The subcutaneous infections are typically associated with inoculation of the pathogen into the dermis and subcutaneous tissues. Sporotrichosis is the prototypical example of a subcutaneous infection. Systemic disease involves deep tissue invasion of one or more internal organs, usually after inhalation of the fungus into the lungs. Opportunistic infections in immunocompromised patients may be the result of disseminated disease associated with superficial, cutaneous or subcutaneous infections.
Invasive fungal infections requiring systemic antifungal therapy continue to be a significant cause of morbidity and mortality in critically ill patients including those receiving hematopoietic stem cell transplantation (HSCT) (2), or solid organ transplantation (3) and those with hematological malignancy (4), and those who are receiving immunosuppressive therapy (5) (Table 1). Although C. albicans and Aspergillus remain the most common etiology of nosocomial yeast and mould infections in immunocompromised patients, therapy has been complicated by a changing epidemiology of fungal pathogens and an increasing frequency of organisms resistant to antifungal drugs (6–11). For example, non-albicans Candida species (C. glabrata, C. parapsilosis, C. krusei and C. tropicalis) are now isolated in 35–65% of patients with candidemia, compared to 10–40% prior to 1990 (12–15). C. krusei and C. glabrata are frequently resistant to fluconazole and mortality rates of up to 50% have been reported in patients infected with these species as compared to 28% of patients with invasive C. albicans infections (12). It should be noted, however, that differences in patient characteristics may play a larger role in clinical outcome than do differences in the Candida species (6–8, 16–18).
TABLE 1.
Factors Associated With an Increased Incidence of Serious Fungal Infections
|
Invasive pulmonary aspergillosis, most commonly due to A. fumigatus and A. flavus (19), continues to cause significant morbidity and mortality in patients receiving allogeneic HSCT (16), especially those with prolonged neutropenia and/or patients being treated for graft-versus-host disease (3). Despite a modicum of success of the older triazoles in preventing invasive aspergillosis in high-risk patients, infections caused by new and emerging filamentous fungi, such as Scedosporium species, Fusarium species, and Zygomycetes including Rhizopus, Mucor, Rhizomucor, and Cunninghamella species, have been observed. The emergence of these fungal agents complicates treatment and prevention owing to their resistance to the imidazoles, older triazoles, and amphotericin B (20, 21).
THE SEARCH FOR THE HOLY GRAIL
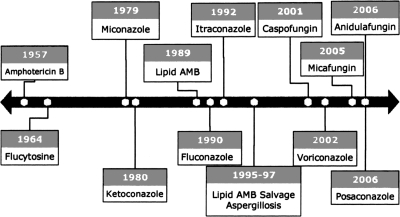
The discovery and development of the ideal antifungal drugs have been a long and difficult journey, beginning with FDA approval of amphotericin B in 1957, the first azole in 1980, lipid formulations of amphotericin B in 1989, and the first echinocandins in 2001 (Figure 1). Currently, seven structurally distinct antifungal drug classes are available, with some members under clinical investigation (Table 2). These compounds exert their antifungal effects in a number of different ways: binding to ergosterol with concomitant disruption of the cell membrane (amphotericin B); blocking ergosterol biosynthesis by inhibiting squalene oxidase (allylamines) or cytochrome P450 enzymes (azoles); inhibiting DNA and RNA synthesis (nucleoside analogs) or mitotic spindle formation (Griseofulvin); and blocking cell wall synthesis by inhibition of glucan synthase (echinocandins).
Fig. 1.
Timeline of development of antifungal agents.
TABLE 2.
Structural Classes of Antifungal Agents for Systemic Therapy
| Structure | Mechanism of Action | Primary Use |
|---|---|---|
| Polyenes | ||
| Amphotericin B(AmB) | Disrupt fungal membranes by binding to ergosterol | Systemic and opportunistic mycoses |
| Lipid formulations AmB | ||
| Nystatin | ||
| Allylamines | ||
| Terbinafine | Depress ergosterol biosynthesis by inhibiting squalene oxidase | Treatment of cutaneous dermatophyte infections unresponsive to topical therapy |
| Morpholine | ||
| Amorolfine | Depress biosynthesis of ergosterol by inhibiting cytochrome P450 enzymes | Topical treatment of onychomycosis |
| Nucleoside Analogs | ||
| Flucytosine (in combinations) | Inhibit DNA and RNA synthesis | Combination therapy for cryptococcal disease |
| AZOLES | ||
| Imidazoles: | Depress biosynthesis of ergosterol by inhibiting cytochrome P450 enzymes | Endemic mycoses and systemic opportunistic mycoses |
| Miconazole | ||
| Ketoconazole | ||
| Triazoles: | ||
| Fluconazole | ||
| Itraconazole | ||
| Voriconazole | ||
| Posaconazole | ||
| Ravuconazole | ||
| ECHINOCANDINS | ||
| Micafungin | Depress cell wall formation by inhibiting glucan synthesis | Candidiasis, aspergillosis |
| Caspofungin | ||
| Anidulafungin | ||
| OTHER | ||
| Griseofulvin | Inhibit fungal mitosis by interacting with microtubules | Cutaneous mycoses unresponsive to topical agents |
| SSKI | Unknown | Lymphocutaneous sporotrichosis |
Despite the expanding list of antifungal agents noted, it is our belief that the Holy Grail of the ideal antifungal remains elusive (Table 3). Mortality rates seen in neutropenic patients with invasive fungal infections are still unacceptably high. Invasive nosocomial fungal disease in immunocompromised hosts has been associated with an attributable mortality rate as high as 42% for yeast (candidemia) and greater than 85% for moulds (22). Additionally, each of the antifungal agents has unique microbiologic activities, pharmacokinetics and pharmacodynamic parameters, adverse effects and drug interactions which limit their ability to qualify as the Holy Grail.
TABLE 3.
The Holy Grail. Characteristics of the Ideal Antifungal Agent
| Broad antifungal activity |
| Fungicidal |
| Low frequency of resistance |
| Intrinsic resistance |
| Acquired resistance |
| Available in IV and PO preparations |
| Ease of administration |
| Low frequency of adverse events |
| Minimal drug interactions |
| Reasonable cost |
ANTIFUNGAL AGENTS: CURRENT STATUS
Amphotericin B.
A polyene macrolide, amphotericin B acts by binding to ergosterol in the fungal cell membrane, effectively creating pores which result in cell leakage and depolarization. While amphotericin B has a stronger affinity for binding to ergosterol, it does bind to sterols (i. e., cholesterol) of mammalian cells, which may account for the toxic effects of the drug. Amphotericin B has activity in vitro against a wide variety of clinical fungal isolates, including the dimorphic fungi Histoplasma capsulatum, Blastomyces dermatitidis, and Sporothrix schenckii, as well as Candida spp, Coccidioides immitis, Aspergillus spp, and Cryptococcus neoformans. Both intrinsic and acquired resistance are limited but have been reported in select Candida spp. (e.g., C. lusitaniae) (23–26), Scedosporium (27, 28) and Coccidioides immitis (29).
In addition, amphotericin B stimulates the immune system and oxygen-dependent killing of fungal pathogens (30, 31). Amphotericin B has also been shown to enhance the phagocytic and antibacterial activity of macrophages and to increase colony-stimulating factor concentrations in mice (32). Chapman and coworkers found that amphotericin B enhanced macrophage tumoricidal activity that was independent of its ionophoretic properties (33). Amphotericin B induces production of IL-1β, TNF-α and IL-1Rα in murine and human monocytes and macrophages (34–37) and increases nitric oxide synthesis in human monocytes (38). It has also been shown to increase both IL-12 and IFN-gamma levels in mice with gastrointestinal or systemic Candida infections (39).
Conventional amphotericin B, despite being a broad-spectrum, fungicidal agent with little intrinsic or acquired resistance, is limited by its serious toxicities and lack of an oral formulation for systemic therapy. Improvements in manufacturing over the last 40 years have enhanced tolerability, although infusion-related reactions and renal dysfunction are still commonplace with the use of the deoxycholate solubilized formulation. Formulations using a lipid carrier have significantly improved tolerability. In recent years, three lipid formulations of amphotericin B (amphotericin B lipid complex, amphotericin B cholesteryl sulfate and liposomal amphotericin B) have been developed and approved by the FDA. Lipid formulations allow increased daily dosage of the parent drug with fewer infusion-associated side effects while still achieving high tissue concentrations in the lungs, liver and spleen. Although less nephrotoxic than deoxycholate amphotericin B, lipid amphotericin B nephrotoxicity still limits treatment in comparison to the newer triazoles and echinocandins (40, 41).
Azoles.
The azoles used in systemic therapy include fluconazole, itraconazole, ketoconazole, posaconazole and voriconazole. The azole family can be classified into two groups: the imidazoles (miconazole and ketoconazole) and the triazoles (fluconazole, itraconazole, voriconazole, and posaconazole). The triazoles are the most widely used antifungal agents and have activity against many fungal pathogens with none of the serious nephrotoxic effects observed with amphotericin B. It should be noted that the azoles have some differences among drugs within the class. For example, itraconazole is fungistatic for Candida species while voriconazole and posaconazole are fungicidal for Aspergillus species (42–44). The imidazoles, such as ketoconazole, have generally been replaced by the triazoles for systemic use owing to their superior pharmacokinetics, improved safety profiles and enhanced clinical efficacy in the treatment of systemic mycoses (45–47).
This class of antifungal agents inhibits the cytochrome P-450 (CYP) dependent enzyme C-14α-demethylase necessary for the conversion of lanosterol to ergosterol, an essential component of the fungal cell membrane. At least four genes, ERG 3, 4, 5 and 11, involved in the ergosterol pathway are affected by these agents, causing significant damage to the cell wall and resulting in increased permeability and cell lysis (48).
Resistance to the azoles has been found to be a factor in treatment failures (49, 50). The molecular basis of fluconazole resistance has been most extensively characterized (51–53) and includes drug efflux due to overexpression of ATP-binding cassette (ABC) transporters encoded by CDR1 and CDR2 (54, 55) and major facilitator transporters encoded by MDR1 and FLU1 (56–58). Increased expression of ERG11 (which encodes the target enzyme 14-α-demethylase), mutations in ERG11 (48) and mutations in ERG3 that inactivate α 5,6 desaturase and cause accumulation of growth-supporting 14 α-methylfecosterol (59, 60) have also been noted as causes of resistance. Aneuploidy, particularly of chromosome 5, has been implicated and is considered likely due to increased copy numbers of ERG11 and other genes (61).
Adverse effects vary for the individual formulations of each azole (47, 62). Chapped lips and dry skin occur in patients treated with high-dose fluconazole (≥400 mg/day). Itraconazole may be associated with peripheral edema and may precipitate congestive heart failure. Voriconazole may be associated with visual symptoms which are reversible and not associated with retinal damage. All of the azoles can be associated with clinically significant transaminase elevations, although this side effect is more frequent with voriconazole (e.g., up to 13% of patients); hepatic failure has been associated most frequently with itraconazole (63).
The azole antifungals have many drug-drug interactions with multiple drug classes owing to their interference with hepatic and intestinal cytochrome P-450 enzymes. Drug-drug interactions include those that decrease absorption or increase the metabolism of the azole, thereby reducing blood and tissue concentrations and increasing treatment failure. The azoles can cause a decrease or increase in the serum concentrations of the co-administered drug. Numerous drugs, including H1 -antihistamines, antineoplastics, steroids, antimicrobials, antiretrovirals, opioids, long acting-barbiturates, cardiovascular agents, psychotropics and oral contraceptives, may be affected by co-administration with the azoles, resulting in unexpected toxicity (64–67). Indeed, any drug that shares the samecytochrome P-450 isoenzymes for metabolism may potentially give rise to drug-drug interactions in vivo. Further, patients with specific genetic polymorphisms are at risk for adverse reactions (68). Many combinations require monitoring of both the azole and/or the co-administered drug while some combinations should be avoided completely. Of the triazoles, itraconazole and voriconazole have the highest frequency of drug interactions, while fluconazole and posaconazole are the best tolerated.
Echinocandins.
The echinocandin class of antifungal agents was first developed in the early 1970s, and led to FDA approval for caspofungin (2001), micafungin (2005) and anidulafungin (2006) for intravenous therapy of invasive and superficial candidiasis and primary and salvage therapy for aspergillosis. These compounds are noncompetitive inhibitors of β-(1,3) glucan synthase (69), an essential component of the rigid fungal cell wall, resulting in cell wall damage and cell death (70). Unlike the azoles, the echinocandins have cidal activity against Candida species and static activity against Aspergillus (71). It should be noted that cidal activity does not necessarily translate to superior efficacy in patients.
The echinocandins have a low frequency of toxicity and have proven extremely safe in comparison to the other classes of antifungal agents (72). The most common adverse effects reported with echinocandin use are infusion reactions that manifest as flushing, urticaria and pruritus, rash, thrombophlebitis at the infusion site and elevations of the levels of liver enzymes (67). Caspofungin has been associated with transient elevations in serum liver enzymes and adverse gastrointestinal effects (73).
Unlike the azole antifungals, the echinocandins are poor substrates for drug efflux transporters, which do not appear to be a factor in resistance to these agents (74, 75). Resistance in Candida spp. has been associated with mutations in the Fks1p subunit of the target enzyme (1,3)-α-D-glucan synthase (76–79). Hakki et al. (80) recently reported a case of invasive and oropharyngeal candidiasis caused by a Candida krusei isolate that developed resistance after caspofungin (CFG) therapy. Cryptococcus neoformans and the emerging fungal pathogens Fusarium spp., Scedosporium spp. and members of the Zygomycetes family are intrinsically resistant to echinocandin drugs (MIC > 16 μg/ml) (81, 82). The mechanism of this inherent resistance is not related to target insensitivity, as glucan synthase activity from Cryptococcus and several moulds were strongly inhibited by caspofungin in in vitro assays (83).
DEVELOPMENT OF NEW ANTIFUNGAL AGENTS
The search for the Holy Grail of antifungal therapy must follow many avenues (Table 4). First, a more complete understanding of the pathogenesis of invasive fungal infections is vital. Virulence factors such as adhesins may prove to be excellent targets for new antifungal agents and the development of vaccines for use in high-risk patient populations. Potential new antifungal compounds, whether natural or synthetic, must be found using either traditional screening methods or new genomic analysis-based methods. Currently available therapeutic drugs must be improved to reduce toxicity and enhance efficacy. Such efforts have begun to yield rewards with the advent of lipid formulations of amphotericin B. Other areas of research must address reliable high throughput susceptibility testing to screen potential agents and to identify and counter antifungal resistance as it develops. This, in turn, will require a better understanding of the mechanisms of intrinsic and acquired resistance to antifungal drugs. Finally, studies employing different combinations of antifungal agents or in combination with immunobiologics (e. g., monoclonal antibodies, cytokines, mannan binding lectin replacements, etc.) should be vigorously pursued.
TABLE 4.
Search for the Ideal Antifungal Agent
| A more complete understanding of the pathogenesis of invasive fungal infections |
| Virulence factors (e.g., adhesins) as targets for new antifungals and vaccines |
| Identification of new compounds (natural and synthetic) |
| Traditional screening against fungal pathogens |
| Identification of new targets by genomic analysis (signature) |
| Enhance efficacy and reduce toxicity of older antifungal drugs |
| Improved delivery systems (Lipid AMB, aerosols) |
| High-throughput and reproducible susceptibility testing |
| A better understanding of the mechanisms of resistance to antifungal drugs |
| Intrinsic vs Acquired |
| Combination therapy - synergy |
| AMB + 5FC for cryptococcal meningitis |
| AMB + voriconazole for aspergillosis |
| Echinocandins + voriconazole for filamentous moulds |
| Immune enhancement therapy |
| Monoclonal antibodies |
| Manan binding lectins replacement |
TOOLS FOR THE SEARCH
Identification of New Lead Compounds.
Two approaches commonly used to identify new agents with direct anti-fungal activity are: (a) Undirected Screening, which assesses libraries of products for either whole cell inhibitory activity or activity against a specific molecular target and (b) Directed Screening, which identifies the biochemical processes of the pathogen and engineers compounds that specifically and selectively interact with a target. Such approaches have led to the identification of compounds with anti-fungal activity targeting five new putative mechanisms-of-action: those that inhibit cell wall synthesis, sphingolipid synthesis, protein synthesis, enzyme activity and fatty acid synthase blockade. Research evaluating competitive inhibitors of fungal chitin synthase enzymes (nikkomycins) and morpholines has been hampered due to toxicity and solubility issues. Natural products are a rich source of novel prototype antifungals, particularly as previously unexplored biosphere niches are investigated. New molecular targets for antifungal therapy are being discovered, and more sensitive and robust assay methods for their evaluation are being developed. Technological advances in isolation of such compounds and elucidation of their structure occur daily.
The-Omics: Genomics, Proteomics, and Glycomics.
The chemical codes used by cells fall into three major languages: genomics, proteomics and glycomics. Clinician scientists and the pharmaceutical industry have been quick to embrace the new opportunities offered by the -omics revolution. Genomics is the study of an organism's entire genome, its functional genes, regulatory sequences and the transcription of these sequences. As an extention, proteomics is the study of an organism's complete complement of proteins as they are expressed in response to signals in the genome and the environment. Such studies will play a major role in the validation of information gleaned from genomic analysis. Understanding biological processes will require an appreciation of protein function in cells. The emerging field of glycomics may potentially play a major role in fungal research, given the unique role of glucans and mannans in these organisms. Currently, genomic analysis studies are more common and have had a greater impact on clinical applications.
Pharmacogenomics has been defined as the general study of all of the many different genes that determine drug behavior, both in the target microorganism and in the infected host. Another commonly used term, pharmacogenetics, refers to the study of inherited differences in drug metabolism and response. A transcriptome can be defined as the complete collection of transcribed elements of the genome, whether employed in either of these areas. In addition to mRNAs, it also represents non-coding RNAs that are used for structural and regulatory purposes. Alterations in the structure or levels of expression of any one of these RNAs or their proteins can contribute to disease. An understanding of the transcriptome will provide a valuable tool in the search for novel drugs. Pharmacogenomics can assist in the pursuit of novel pharmaceuticals as an undirected form of screening and develop small interfering RNA (siRNA) which can target biochemical pathways, interrupting transcription and translation of critical proteins.
On The Trail.
The Mycotic Research Center (MRC) at the University of Mississippi Medical Center has as its primary focus the improvement of safety and efficacy of antifungal therapy by 1) screening new compounds, both natural and synthetic; 2) employing pharmacogenomics to study in vitro antifungal activity and to characterize the mechanisms of drug resistance; and 3) performing phase III clinical trials in the treatment of the endemic and opportunistic mycotic diseases.
With the development of lipid formulations of amphotericin B, the new triazoles and echinocandins, some experts have questioned the role of amphotericin B in the treatment of invasive fungal disease. In response to these criticisms, the Mycotic Research Center has explored methods to improve the safety and efficacy of polyene antifungals. Previous publications have shown that the anemia in patients treated with amphotericin B is a result of inhibition of erythropoietin which is independent of renal function (84). Further, using peripheral blood mononuclear cells and THP-1 cells, we have documented that amphotericin B stimulates the production of proinflammatory cytokines IL-1 and TNF which, along with the release of PGE-2 from endothelial cells, mediates the infusion-related reactions (e.g., fever, chills, hypotension) (34). Amphotericin B also induces the expression of numerous genes encoding chemokines and cell adhesion molecules, including IL-8, MCP-1, MIP-1B, ICAM-1 and CD44. Genomic analysis of THP-1 cells showed increased mRNA levels for IL-1β, TNF-α, and IL-1Ra and that the production of the cytokines is calcium-dependent (37).
Our studies in improving the safety of amphotericin B continue with the identification of pyrogenic polyenes which are present in commercial amphotericin B preparations (85). Impurities in amphotericin B are a predictable product of isolation of this polyene from Streptomyces nodosus supernatants. Two key observations led us to examine the composition of commercial amphotericin B preparations. First, different generic formulations of amphotericin B deoxycholate were associated with varying frequencies and severity of adverse effects. Second, the various generic formulations had different purity profiles for contaminating polyenes which correlated with the frequency and severity of amphotericin B toxicity. Thus, we hypothesized that purifying amphotericin B by removing contaminating polyenes would attenuate its adverse effects.
We have employed HPLC to remove impurities from commercial preparations of amphotericin B and supernatants from Streptomyces nodosus. This new amphotericin B formulation, which we designated amphotericin B high purity (AmBHP), was shown to improve in vitro and in vivo safety and efficacy as compared to pharmaceutical or chemical grade amphotericin B (85). When compared with amphotericin B deoxycholate, AmBHP has a higher single dose LD50 in mice, less nephrotoxicity in rats, and equivalent survival which correlated with a reduction in tissue yeast burden in a murine candidiasis model (85).
NEW AVENUES TO EXPLORE: IMMUNE ENHANCEMENT STRATEGIES
As noted earlier, patients at greatest risk for invasive fungal infections are usually immunocompromised (see Table 1). Thus, immune-enhancing strategies, alone or in combination with antifungal drugs, are being actively investigated (86). One such strategy involves treatment with mannan-binding lectins (MBL). MBL, also called mannose-binding protein, is a member of the collectin subfamily. MBL is a calcium-dependent serum protein that opsonizes bacteria and fungi, activates serum complement and is considered an important part of innate immunity (87). Primary and secondary deficiencies in MBL have been associated with increased frequency of bacterial and fungal infections (88–91). It has been estimated that up to one quarter of the human population has mannose-binding lectin deficiency.
The use of monoclonal antibodies is another example of immune-enhancement strategy. A recent double blind, randomized study in patients with invasive candidiasis employing a monoclonal antibody that binds chaperone heat shock protein HSP90, Mycograb (NeuTec Pharma; Enzon Pharmaceuticals), examined the efficacy of lipid-formulation amphotericin B plus Mycograb compared to amphotericin B plus placebo. Candida-attributable mortality was decreased 4-fold in the amphotericin B-Mycograb combination group, 4% versus 18% in the single treatment group (92). There were no significant adverse events associated with Mycograb therapy.
Another example for improving outcomes associated with invasive fungal disease includes adjunctive passive immunotherapy using a monoclonal antibody directed against the capsular polysaccharide or ceramide monohexosides of Cryptococcus neoformans (86). These studies highlight the promise for adjunctive immunotherapy to improve clinical outcomes in patients with serious invasive fungal disease.
ARE WE THERE YET?
The palette of choices of systemic antifungal agents for the treatment of serious, life-threatening disease is growing. Which current therapy is the nearest approximation of the Holy Grail (Table 5)? Each of the different classes of the antifungals has limitations when compared to our ideal agent. The polyenes are limited by their toxicity, although lipid preparations appear improved in this regard. The development of highly-purified amphotericin B has the potential to improve safety and tolerability of this reliable antifungal. The azoles are limited by their drug-drug interactions and development of resistance. Although the new triazole, posaconazole, has activity against the agents of mucormycosis, it is currently available only in an oral formulation. The echinocandins are available only for intravenous therapy and lack significant activity against Cryptococcus and the endemic mycoses. Thus, each agent has an unique niche; all fall short of the ideal. The search must continue to identify new targets of antifungal drugs as outlined in Table 4.
TABLE 5.
Characteristics of Current Antifungal Agents in Comparison with the Holy Grail of Antifungal Agents
| Characteristic | Am B | Imidazoles | 1st Gen. Triazoles | 2nd Gen. triazoles | Echinocandins |
|---|---|---|---|---|---|
| Antifungal activity | |||||
| Yeast | Yes | Yes | Yes | Yes | Candida |
| Moulds | Yes | No | No | Yes | Aspergillus |
| Indications | |||||
| Subcutaneous Disease | |||||
| Sporotrichosis | Yes | Yes | Itraconazole | ? | No |
| Invasive Disease | |||||
| Opportunistic | Yes | Yes | Variable | Yes | Variable |
| Cryptococcus spp | Yes | No | |||
| Candida spp | Yes | Yes | |||
| Coccidioides spp | Yes | No | |||
| Aspergillus spp | Itraconazole | Yes | |||
| Nonopportunistic | Yes | Yes | Yes | Yes | No |
| Histoplasma | |||||
| Blastomyces | |||||
| Resistance | |||||
| Intrinsic | Minimal | Minimal | Minimal | Minimal | Yes (Cryptococcus) |
| Acquired | Minimal | Yes | Yes | Yes | Yes |
| Administration Route | |||||
| Oral | No | Yes | Yes | Yes | No |
| Parenteral | Yes | Yes | Yes | Yes (except posaconazole) | Yes |
| Adverse events | Many | Many | Some | Some | Some |
| Drug interactions | Few | Many | Some | Many | Some |
DISCUSSION
Dismukes, Birmingham: Very nice talk and review. As you are so aware, the drug companies are really at a point in time when their priorities have sort of moved away from the development of new antibiotics, new antifungals, anti-bacterial agents, etc. Cost is enormous. Are you aware of any efforts at this point in time of these or any drug companies that are targeting new foci in fungi that would lead to reduced toxicity, because you would try to come up with a target that would not be in the mammalian cells. To my knowledge, there really has been a marked shutdown or slowdown in the development of new agents, and as you are trying to make improvements with Amphotericin B, are you aware of efforts ongoing by big pharma to address this overall issue to find the Holy Grail of antifungal therapy?
Chapman, Jackson: I think most of the cell wall-active agents are what they are looking at. There are also other enzymes within the fungi that are being approached, elongation factor II inhibitors, Nicomycin which in effect is a chitin synthase inhibitor. I keep hearing about Nicomycin, but I am not sure it is ever going to come to fruition. Were there any specific ones you were referring to Bill?
Dismukes, Birmingham: No, I think that is the problem. There has not been much interest in this field that we are having to rely on newer drugs, the ASOs and the Candins, unless we can go forward with a better preparation of Amphotericin B.
Boxer, Ann Arbor: I was struck by the release to cytokines that you've demonstrated, and clinically, as you are well aware, when we administer Amphotericin, patients are subject to getting febrile responses. In the highly purified preparation, is that response eliminated?
Chapman, Jackson: Yes it is. In vivo or in vitro?
Boxer, Ann Arbor: In vivo.
Chapman, Jackson: Yes, we've looked at that in vivo and it is reduced.
Boxer, Ann Arbor: Thank you.
Mackowiak, Baltimore: Very nice presentation, Stan. Thank you. As a follow up to Larry's question, is that good or bad in terms of a therapeutic effect of the Amphotericin? I mean have you considered the possibility that fever is not a toxic byproduct of therapy but potentially might potentiate the therapeutic effect of Amphotericin?
Chapman, Jackson: Absolutely. In our studies in mice, however, it hasn't been demonstrated but that is certainly a possibility.
Bransome, Aiken: At the beginning of your presentation, you talked about USP Amphotericin, and I wanted to remind everybody that the USP monographs just put forth criteria for identity and purity. Have you and Cumberland Pharmaceuticals been in contact with the U.S. Pharmacopeia to inform them of the probable necessity of changing the criteria?
Chapman, Jackson: Not specifically that I am aware of, but thanks.
Schiffman, Providence: In the past, I have worked with Amphotericin B in an attempt to sharpen it as a chemotherapeutic agent, and working with it as Fungisone with deoxycholate yielded very different affects than pure Amphotericin which we solubalized in DMSO. There is also a literature on sensitizing tumor cell membranes to this drug using ergosterol, incubating them and then having them become a target for Amphotericin. Are you aware of any recent literature on the usage of the drug in this fashion?
Chapman, Jackson: I am not.
REFERENCES
- 1.Kwon-Chong K. J., Bennett J. E. Medical Mycology. Philadelphia: Lea and Febiger; 1992. [Google Scholar]
- 2.Marr D. A., Crippa F., Leisenring W., Hoyle M., Boeckh M., Balajee S. A., Nichols W. G., Musher B., Corey L. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 2004;103:1527–1533. doi: 10.1182/blood-2003-08-2644. [DOI] [PubMed] [Google Scholar]
- 3.Ullmann A. J., Lipton J. H., Vesole D. H., Chandrasekar P., Langston A., Tarantolo S. R., Greinix H., Morais de Azevedo W., Reddy V, Boparai N., Pedicone L., Patino H., Durrant S. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 4.Viscoli C., Girmenia C., Marinus A., Collette L., Martino P., Vandercam B., Doyen C., Lebeau B., Spence D., Krcmery V. Candidemia in cancer patients: A prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC) Clin Infect Dis. 1999;28:1071–1079. doi: 10.1086/514731. [DOI] [PubMed] [Google Scholar]
- 5.Cornely O, Maertens J, Winston D, Perfect J., Ullmann A., Walsh T., Helfgott D., Holowiecki J., Stockelberg D., Goh Y.T., Petrini M., Hardalo C., Suresh R., Angulo-Gonzalez D. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 6.Chandrasekar P. H., Alangaden G., Manavathu E. Aspergillosis: An increasing problem in tertiary care hospital? Clin Infect Dis. 2000;30:984–985. doi: 10.1086/313832. [DOI] [PubMed] [Google Scholar]
- 7.Baddley J. W., Stroud T. P., Salzman D., Pappas P. G. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin Infect Dis. 2001;32:1319–1324. doi: 10.1086/319985. [DOI] [PubMed] [Google Scholar]
- 8.Enoch D. A., Ludlam H. A., Brown N. M. Invasive fungal infections: A review of epidemiology and management options. J Med Microbiol. 2006;55:809–818. doi: 10.1099/jmm.0.46548-0. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen M. H., Peacock J. E., Jr., Morris A. J., Tanner D., Nguyen M., Syndman D., Wagener M., Rinaldi M., Yu V. The changing face of candidemia: Emergence of non-Candida albicans species and antifungal resistance. Am J Med. 1996;100:617–623. doi: 10.1016/s0002-9343(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 10.Singh N. Trends in the epidemiology of opportunistic fungal infections: Predisposing factors and the impact of antimicrobial use practices. Clin Infect Dis. 2001;33:1692–1696. doi: 10.1086/323895. [DOI] [PubMed] [Google Scholar]
- 11.Syndman D. R. Shifting patterns in the epidemiology of nosocomial Candida infections. Chest. 2003;123:500–503. doi: 10.1378/chest.123.5_suppl.500s. [DOI] [PubMed] [Google Scholar]
- 12.Cheson B. D. Infectious and immunosuppressive complications of purine analog therapy. J Clin Oncol. 1995;13:2431–2448. doi: 10.1200/JCO.1995.13.9.2431. [DOI] [PubMed] [Google Scholar]
- 13.Martino R., Parody R., Fukuda T., Maertens J., Theunissen K., Ho A., Mufti G. J., Kroger N., Zander A. R., Heim D., Paluszewska M., Selleslag D., Steinerova K., Ljungman P., Cesaro S., Nihtinen A., Cordonnier C., Vazquez L., López-Duarte M., Lopez J., Cabrera R., Rovira M., Neuburger S., Cornely O., Hunter A. E., Marr K. A., Jürgen Dornbusch H., Einsele H. Impact of the intensity of the pre-transplantation conditions regimen inpatients with prior invasive aspergillosis undergoing allogeneic hematopoietic stem cell transplantation: A retrospective survey of the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2006;108:2928–2936. doi: 10.1182/blood-2006-03-008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mar K. A. The changing spectrum of candidemia in oncology patients: Therapeutic implications. Curr Opin Infect Dis. 2000;13:615–620. doi: 10.1097/00001432-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Pfaller M. A., Diekema D. J., Jones R. N., Sader H. S., Fluit A. C., Hollis R. J., Messer S. A. The Sentry Participant Group. International Surveillance of Bloodstream Infections Due to Candida Species: Frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY Antimicrobial Surveillance Program. J. Clin Micro. 2001;39:3254–3259. doi: 10.1128/JCM.39.9.3254-3259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diekema D. J., Messer S. A., Brueggemann A. B., Coffman S. L., Doern G. V., Herwaldt L. A., Pfaller M. A. Epidemiology of candidemia: Three-year results from the Emerging Infections and the Epidemiology of Iowa Organisms study. J. Clin. Microbiol. 2002;40:1298–1302. doi: 10.1128/JCM.40.4.1298-1302.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Upton A., Kirby K. A., Carpenter P., Boeckh M., Marr K. A. Invasive aspergillosis following hematopoietic cell transplantation: Outcomes and prognostic factors associated with mortality. Clin Infect Dis. 2007;44:531–540. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 18.Roden M. M., Zaoutis T. E., Buchanan W. L., Knudsen T. A., Sarkisova T. A., Schaufele R. L., Sein M., Sein T., Chiou C. C., Chu J. H., Kontoyiannis D. P., Walsh T. J. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis. 2005;41:634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 19.Nucci M., Marr K. A. Emerging fungal diseases. Clin Infect Dis. 2005;41:521–526. doi: 10.1086/432060. [DOI] [PubMed] [Google Scholar]
- 20.Marr K. A., Carter R. A., Crippa F., Wald A., Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 21.Malani A. N., Kauffman C. A. Changing epidemiology of rare mould infections: Implications for therapy. Drugs. 2007;67:1803–1812. doi: 10.2165/00003495-200767130-00001. [DOI] [PubMed] [Google Scholar]
- 22.Wey S.B., Mori M., Pfaller M.A., Woolson R.F., Wenzel R.P. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Archives of Internal Medicine. 1988;148:2642–2645. doi: 10.1001/archinte.148.12.2642. [DOI] [PubMed] [Google Scholar]
- 23.Sanglard D., Ischer F., Parkinson T., Falconer D., Bille J. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob Agents Chemother. 2003;47:2404–2412. doi: 10.1128/AAC.47.8.2404-2412.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young L. Y., Hull C. M., Heitman J. Disruption of ergosterol biosynthesis confers resistance to amphotericin B in Candida lusitaniae. Antimicrob Agents Chemother. 2003;47:2717–2724. doi: 10.1128/AAC.47.9.2717-2724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vandeputte P., Tronchin G., Berges T., Hennequin C., Chabasse D., Bouchara J.-P. Reduced susceptibility to polyenes associated with a missense mutation in the erg6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrob Agents Chemother. 2007;51(3):982–990. doi: 10.1128/AAC.01510-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng S., Clancy C. J., Nguyen K. T., Clapp W., Nguyen M. H. A Candida albicans petite mutant strain with uncoupled oxidative phosphorylation overexpresses mdr1 and has diminished susceptibility to fluconazole and voriconazole. Antimicrob Agents Chemother. 2007;51(5):1855–1858. doi: 10.1128/AAC.00182-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castiglioni B, Sutton DA, Rinaldi MG, Fung J, Kusne S. Pseudallescheria boydii (Anamorph Scedosporium apiospermum). Infection in solid organ transplant recipients in a tertiary medical center and review of the literature. Medicine (Baltimore) 2002;81:333–348. doi: 10.1097/00005792-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Ellis D. Amphotericin B: spectrum and resistance. J Antimicrob Chemother. 2002;49(Suppl 1):7–10. doi: 10.1093/jac/49.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 29.Johnson E. M., Szekely A., Warnock D. W. In vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J Antimicrob Chemother. 1998;42:741–745. doi: 10.1093/jac/42.6.741. [DOI] [PubMed] [Google Scholar]
- 30.Gruda I., Gruda I., Nadeau P., Brajtburg J., Medoff G. Application of different spectra in the UV-visible region to study the formation of amphotericin B complexes. Biochem Biophys Acta. 1980;602:260. doi: 10.1016/0005-2736(80)90309-0. [DOI] [PubMed] [Google Scholar]
- 31.Brajtburg J., Elberg S., Medoff J., Kobayashi G. S., Schlessinger D., Medoff G. Stimulatory, permeabilizing, and toxic effects of amphotericin B on L cells. Antimicrob Agents Chemother. 1984;26:892–899. doi: 10.1128/aac.26.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin H.-S., Medoff D., Kobayashi G. S. Effects of amphotericin B on macrophages and their precursor cells. Antimicrob Agents Chemother. 1977;11:154–160. doi: 10.1128/aac.11.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapman H A, Hibbs J B. Modulation of macrophage tumoricidal capability by polyene antibiotics: Support for membrane lipid as a regulatory determinant of macrophage function. Proc Natl Acad Sci USA. 1978;75:4349–4353. doi: 10.1073/pnas.75.9.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chia J K, Pollack M. Amphotericin B induces tumor necrosis factor production by murine macrophages. J Infect Dis. 1989;159:113–6. doi: 10.1093/infdis/159.1.113. [DOI] [PubMed] [Google Scholar]
- 35.Gelfand J A, Kimball K, Burke J K, Dinarello C A. Amphotericin B treatment of human mononuclear cells in vitro results in secretion of tumor necrosis factor and interleukin-1. Clin Res. 1988;36:456A. [Google Scholar]
- 36.Cleary J D, Chapman S W, Nolan R L. Pharmacologic modulation of interleukin-1 expression by amphotericin B-stimulated human mononuclear cells. Antimicrob. Agents Chemother. 1992;36:977–81. doi: 10.1128/aac.36.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers P D, Jenkins J K, Chapman S W, Ndebele K, Chapman B A, Cleary J D. Amphotericin B activation of human genes encoding for cytokines. J Infect Dis. 1998;178:1726–33. doi: 10.1086/314495. [DOI] [PubMed] [Google Scholar]
- 38.Mozaffarian N, Berman J W, Casadevall A. Enhancement of nitric oxide synthesis by macrophages represents an additional mechanism of action for amphotericin B. Antimicrob Agents Chemother. 1997;41:1825–9. doi: 10.1128/aac.41.8.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cenci E, Mencacci A, Del Sero G, Bistoni F, Romani L. Induction of protective Th1 responses to Candida albicans by antifungal therapy alone or in combination with an interleukin-4 antagonist. J Infect Dis. 1997;176:217–26. doi: 10.1086/514027. [DOI] [PubMed] [Google Scholar]
- 40.Hiemenz J. R., Walsh T. J. Lipid formulations of amphotericin B: Recent progress and future directions. Clin Infect Dis. 1996;22(Suppl 2):S133. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 41.Wong-Beringer A., Jacobs R. A., Guglielmo B. J. Lipid formulations of amphotericin B: Clinical efficacy and toxicities. Clin Infect Dis. 1998;27:603. doi: 10.1086/514704. [DOI] [PubMed] [Google Scholar]
- 42.Law D., Moore C. B., Denning D. W. 1997. Activity of SCH 56592 compared with those of fluconazole and itraconazole against Candida spp. Antimicrob Agents Chemother. 1997;41:2310–2311. doi: 10.1128/aac.41.10.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manavathum E. E., Cutright J. L., Chandrasekar P. H. Organism-dependent fungicidal activities of azoles. Antimicrob Agents Chemother. 1998;42:3018–3021. doi: 10.1128/aac.42.11.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torres H. A., Hachem R. Y., Chemaly R. F., Kontoyiannis D. P., Raad I. I.. Posaconazole: A broad-spectrum triazole antifungal. Lancet Infect Dis. 2005;5(12):775–785. doi: 10.1016/S1473-3099(05)70297-8. [DOI] [PubMed] [Google Scholar]
- 45.Groll A. H., Piscitelli S. C., Walsh T. J. Clinical pharmacology of systemic antifungal agents: A comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998;44:343. doi: 10.1016/s1054-3589(08)60129-5. [DOI] [PubMed] [Google Scholar]
- 46.Kauffman C. A., Carver P. L. Use of azoles for systemic antifungal therapy. Adv Pharmacol. 1997;39:143. doi: 10.1016/s1054-3589(08)60071-x. [DOI] [PubMed] [Google Scholar]
- 47.Perfect J. R., Lindsay M. H., Drew R. H. Adverse drug reactions to systemic antifungals. Drug Saf. 1992;7:323. doi: 10.2165/00002018-199207050-00003. [DOI] [PubMed] [Google Scholar]
- 48.White T. C., Marr K. A., Bowden R. A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbio Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rex J. H., Pfaller M. A., Galgiani J. N., Bartlett M. S., Espinel-Ingroff A., Ghannoum M. A., Lancaster M., Odds F., Rinaldi M. G., Walsh T. J., Barry A. L. and Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Development of interpretive breakpoints for antifungal susceptibility testing: Conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 50.Pfaller M. A., Diekema D. J., Sheehan D. J. Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin. Microbiol Rev. 2006;19:435–447. doi: 10.1128/CMR.19.2.435-447.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franz R., Kelly S. L., Lamb D. C., Kelly D. E., Ruhnke M., Morshhauser J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob Agents Chemother. 1998;42:3065–3072. doi: 10.1128/aac.42.12.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang Y.-L., J.-L. Lo. Mechanisms of antifungal agent resistance. J Microbiol Immunol Infect. 2001;34:79–86. [PubMed] [Google Scholar]
- 53.Morschhauser J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim. Biophys. Acta. 2002;1587:240–248. doi: 10.1016/s0925-4439(02)00087-x. [DOI] [PubMed] [Google Scholar]
- 54.Sanglard D., Ischer F., Monod M., Bille J. Cloning of Candida albicans genes conferring resistance to azole antifungal agents characterization of CDR2, a new multidrug ABC transporter gene. Microbiology. 1997;143:405–416. doi: 10.1099/00221287-143-2-405. [DOI] [PubMed] [Google Scholar]
- 55.Sanglard D., Kuchler K., Ischer F., Pagani J.-L, Monod M., Bille J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calabrese D., Bille J., Sanglard D. A novel multidrug efflux transporter gene of the major facilitator superfamily from Candida albicans (FLU1) conferring resistance to fluconazole. Microbiology. 2000;146:2743–2754. doi: 10.1099/00221287-146-11-2743. [DOI] [PubMed] [Google Scholar]
- 57.Gupta V., K., Avmeet K., Shankarling P., Neeti A., Panwar S., Prasad R. Identification of polymorphic mutant alleles of CaMDRI, a major facilitator of Candida albicans which confers multidrug resistance, and its in vitro transcriptional activation. Curr Genet. 1998;34:192–199. doi: 10.1007/s002940050385. [DOI] [PubMed] [Google Scholar]
- 58.Marichal P., Koymans L., Willemsens S., Bellens D., Verhasselt P., Luyten W., Borgers M., Ramaekers F. C. S., Odds F. C., Bossche H. V. Contribution of mutations in the cytochrome P450 14 α-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology. 1999;145:2701–2713. doi: 10.1099/00221287-145-10-2701. [DOI] [PubMed] [Google Scholar]
- 59.Kelly S. L., Lamb D. C., Kelly D. E., Manning N. J., Loeffler J., Hebart H. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol α5,6-desaturation. FEBS Lett. 1997;400:80–82. doi: 10.1016/s0014-5793(96)01360-9. [DOI] [PubMed] [Google Scholar]
- 60.Nolte F. S., Parkinson T., Falconer D. J., Dix S., Williams J., Gilmore C., Geller R., Wingard J. R. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob Agents Chemother. 1997;41:196–199. doi: 10.1128/aac.41.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaller M., Schafer W., Korting H. C, Hube B. Differential expression of secreted aspartyl proteinases in a model of human oral candidiasis and in patient samples from the oral cavity. Mol Microbiol. 1998;29:605–615. doi: 10.1046/j.1365-2958.1998.00957.x. [DOI] [PubMed] [Google Scholar]
- 62.McEvoy G. K., Litvak K., Welsh O. H., editors. AHFS Drug Information. Bethesda: American Society of Health Systems Pharmacists, Inc; 1996. [Google Scholar]
- 63.Tan K., Brayshaw N., Tomaszewski K., Troke P., Wood N. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J Clin Pharmacol. 2006;46:235–243. doi: 10.1177/0091270005283837. [DOI] [PubMed] [Google Scholar]
- 64.Lomaestro B. M., Piatek M. A. Update on drug interactions with azole antifungal agents. The Annals of Pharmacotherapy. 1998;32:915–928. doi: 10.1345/aph.17271. [DOI] [PubMed] [Google Scholar]
- 65.Gubbins P. O., McConnell S. A., Penzak S. R. Antifungal Agents. In: Piscitelli S. C., Rodvold K. A., editors. Drug Interactions in Infectious Diseases. Totowa, NJ: Humana Press; 2001. [Google Scholar]
- 66.Gubbins P. O, Amsden J. R. Drug-drug interactions of antifungal agents and implications for patient care. Expert Opinin on Pharmacotherapy. 2005;6:2231–2243. doi: 10.1517/14656566.6.13.2231. [DOI] [PubMed] [Google Scholar]
- 67.Kauffman C. A. Clinical efficacy of new antifungal agents. Curr Opin Microbiol. 2006;9:483–488. doi: 10.1016/j.mib.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Kashuba D. M., Bertino J. S. Mechanisms of drug interactions. In: Piscitelli S. C., Rodvold K. A., editors. Drug Interactions in Infectious Diseases. Totowa, NJ: Humana Press; 2001. [Google Scholar]
- 69.Douglas C. M. Fungal beta (1,3)-D-glucan synthesis. Med Mycol. 2001;39(Suppl. 1):55–66. doi: 10.1080/mmy.39.1.55.66. [DOI] [PubMed] [Google Scholar]
- 70.Debono M., Turner W. W., LaGrandeur L., Burkhardt F. J., Nissen J. S., Nichols K.K., Rodriguez M. J., Zweifel M. J., Zeckner D. J., Gordee R. S., Tang J., Parr T. R., Jr Semisynthetic chemical modification of the antifungal lipopeptide echinocandin B (ECB): Structure-activity studies of the lipophilic and geometric parameters of polyarylated acyl analogs of ECB. J Med Chem. 1995;38:3271–3281. doi: 10.1021/jm00017a012. [DOI] [PubMed] [Google Scholar]
- 71.Denning D. W. Echinocandin antifungal drugs. Lancet. 2003;362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 72.Chandrasekar P. H., Sobel J. D. Micafungin: A new echinocandin. Clin Infect Dis. 2006;42:1171–1178. doi: 10.1086/501020. [DOI] [PubMed] [Google Scholar]
- 73.Cappelletty D., Eiselstein-McKitrick E. The echinocandins. Pharmacotherapy. 2007;27:369–388. doi: 10.1592/phco.27.3.369. [DOI] [PubMed] [Google Scholar]
- 74.Bachmann S. P., Patterson T. F., Lopez-Ribot J. L. In vitro activity of caspofungin (MK-0991) against Candida albicans clinical isolates displaying different mechanisms of azole resistance. J. Clin. Microbiol. 2002;40:2228–2230. doi: 10.1128/JCM.40.6.2228-2230.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stone E. A., Fung H. B., Kirschenbaum H. L. Caspofungin: An echinocandin antifungal agent. Clin Ther. 2002;24:351–377. doi: 10.1016/s0149-2918(02)85039-1. Discussion, 24:329. [DOI] [PubMed] [Google Scholar]
- 76.Katiyar S., Pfaller M., Edlind T. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob Agents Chemother. 2006;50:2892–2894. doi: 10.1128/AAC.00349-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laverdiere M., Lalonde R. G., Baril J. G., Sheppard D. C., Park S., Perlin D. S. Progressive loss of echinocandin activity following prolonged use for treatment of Candida albicans oesophagitis. J Antimicrob Chemother. 2006;57:705–708. doi: 10.1093/jac/dkl022. [DOI] [PubMed] [Google Scholar]
- 78.Miller C. D., Lomaestro B. W., Park S., Perlin D. S. Progressive esophagitis caused by Candida albicans with reduced susceptibility to caspofungin. Pharmacotherapy. 2006;26:877–880. doi: 10.1592/phco.26.6.877. [DOI] [PubMed] [Google Scholar]
- 79.Park S., Kelly R., Kahn J. N., Robles J., Hsu M. J., Register E., Li W., Vyas V., Fan H., Abruzzo G., Flattery A., Gill C., Chrebet G., Parent S. A., Kurtz M., Teppler H., Douglas C. M., Perlin D. S. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob. Agents Chemother. 2005;49:3264–3273. doi: 10.1128/AAC.49.8.3264-3273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hakki M., Staab J. F., Marr K. A. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob. Agents Chemother. 2006;50:2522–2524. doi: 10.1128/AAC.00148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pfaller M.A., Marco F., Messer S.A., Jones R.N. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn Microbiol Infect Dis. 1998;30:251–255. doi: 10.1016/s0732-8893(97)00246-0. [DOI] [PubMed] [Google Scholar]
- 82.Singh J., Rimek D., Kappe R. In vitro susceptibility of 15 strains of zygomycetes to nine antifungal agents as determined by the NCCLS M38-A microdilution method. Mycoses. 2005;48:246–250. doi: 10.1111/j.1439-0507.2005.01132.x. [DOI] [PubMed] [Google Scholar]
- 83.Kahn J.N., Hsu M.J., Racine F., Giacobbe R., Motyl M. Caspofungin susceptibility in Aspergillus and non-Aspergillus molds: Inhibition of glucan synthase and reduction of beta-d-1,3 glucan levels in culture. Antimicrob Agents Chemother. 2006;50:2214–2216. doi: 10.1128/AAC.01610-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin A. C., Goldwasser E., Bernare E., Chapman S. W. Amphotericin B blunts the erythropoietin response to anemia. J Inf Dis. 1990;161:348–351. doi: 10.1093/infdis/161.2.348. [DOI] [PubMed] [Google Scholar]
- 85.Cleary J. D., Chapman S. W., Swiatlo E., Kramer R. High purity amphotericin B. J Antimicrob Chem. 2007;60:1331–1340. doi: 10.1093/jac/dkm322. [DOI] [PubMed] [Google Scholar]
- 86.Larsen R. A., Pappas P. G., Perfect J., Aberg J. A., Casadevall A., Cloud G. A., James R., Filler S., Dismukes W. E. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob Agents Chemother. 2005;49:952–958. doi: 10.1128/AAC.49.3.952-958.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kilpatrick D. C. Consensus statement on the future of mannan-binding lectin (MBL)-replacement therapy. Biochem Soc Trans. 2003;31:745–747. doi: 10.1042/bst0310776. [DOI] [PubMed] [Google Scholar]
- 88.Garred P., Madsen H. O., Hoffmann B., Svejgaard A. Increased frequency of homozygosity of abnormal mannan-binding-protein alleles in patients with suspected immunodeficiency. Lancet. 1995;346:941–943. doi: 10.1016/s0140-6736(95)91559-1. [DOI] [PubMed] [Google Scholar]
- 89.Koch A., Melbye M., Sorensen P., Homoe P., Madsen H. O., Molbak K., Hansen C. H., Andersen L. H., Weinkauff Hahn G., Garred P. Acute respiratory tract infections and mannose-binding lectin insufficiency during acute early childhood. JAMA. 2001;285:1316–1321. doi: 10.1001/jama.285.10.1316. [DOI] [PubMed] [Google Scholar]
- 90.Roy S., Knox K., Segal S., Griffiths D., Moore C. E., Welsh K. I., Smarason A., Day N. P., McPheat W. L., Crook D. W., Hill A. V. S. and the Oxford Pneumococcal Surveillance Group. MBL genotype and risk of invasive pneumococcal disease: A case-control study. Lancet. 2002;359:1569–1573. doi: 10.1016/S0140-6736(02)08516-1. [DOI] [PubMed] [Google Scholar]
- 91.Hibberd M. L., Sumiya M., Summerfield J. A., Booy R., Levin M. and the Meningococcal Research Group. Association of variants of the gene for mannose-binding lectin with susceptibility to meningococcal disease. Lancet 1999; 353:1049–1053. Antimicrob Agents Chemother. 2005;49:952–958. doi: 10.1016/s0140-6736(98)08350-0. [DOI] [PubMed] [Google Scholar]
- 92.Pachl J., Svoboda P., Jacobs F., Vandewoude D., van der Hoven B., Spronk P., Masterson G., Malbrain M., Aoun M., Garbino J., Takala J., Drgona L., Burnie J., Matthews R. for the Mycograb Invasive Candidiasis Study Group. A Randomized, Blinded, Multicenter Trial of Lipid-Associated Amphotericin B Alone versus in Combination with an Antibody-Based Inhibitor of Heat Shock Protein 90 in Patients with Invasive Candidiasis. Clin Infect Dis. 2006;42:1404–1413. doi: 10.1086/503428. [DOI] [PubMed] [Google Scholar]