Abstract
Neutrophils are important effector cells in immunity to microorganisms, particularly bacteria. Here, we show that the process of neutrophil apoptosis is delayed in several inflammatory diseases, suggesting that this phenomenon may represent a general feature contributing to the development of neutrophilia, and, therefore, in many cases to host defense against infection. The delay of neutrophil apoptosis was associated with markedly reduced levels of Bax, a pro-apoptotic member of the Bcl-2 family. Such Bax-deficient cells were also observed upon stimulation of normal neutrophils with cytokines present at sites of neutrophilic inflammation, such as granulocyte and granulocyte–macrophage colony-stimulating factors, in vitro. Moreover, Bax-deficient neutrophils generated by using Bax antisense oligodeoxynucleotides demonstrated delayed apoptosis, providing direct evidence for a role of Bax as a pro-apoptotic molecule in these cells. Interestingly, the Bax gene was reexpressed in Bax-deficient neutrophils under conditions of cytokine withdrawal. Thus, both granulocyte expansion and the resolution of inflammation appear to be regulated by the expression of the Bax gene in neutrophils.
The balance between production and death of cells is important in the control of cell numbers within physiologically appropriate ranges. Inflammatory disorders are characterized by an expansion of hematopoietic effector cells. Such cell accumulation may be the consequence of either increased cell production or decreased cell death. Apoptosis is the most common form of physiologic cell death (1–3) and is characterized by a series of distinct morphological changes of the dying cell (4–6). Whereas the concept of increased cell production in inflammation is well established (7, 8), the contribution of decreased apoptosis remains to be determined.
Apoptosis of neutrophils in vitro can be modulated by survival (9, 10) and death (11, 12) factors, although their contribution to the apoptosis regulation in vivo is not clear. Moreover, the intracellular mechanisms by which these factors regulate neutrophil apoptosis are not completely understood. Although it is clear that neutrophils express some members of the Bcl-2 (13, 14) and caspase (15) families, their functional role as potential apoptosis regulators has not been investigated in these inflammatory effector cells under in vivo or ex vivo conditions.
The Bcl-2 family consists of members with either anti-apoptotic or pro-apoptotic function (16). Bax is a member of the Bcl-2 family and functions as a death agonist within apoptotic pathways (17–19). It has been shown that Bcl-2 antagonizes the pro-apoptotic activity of Bax by forming heterodimers with it (20). Since Bax (14), but not Bcl-2 (13), was reported to be expressed in neutrophils, it is likely that neutrophils contain functional Bax homodimers, which are, at least partially, responsible for the short lifespan of these cells. The pro-apoptotic activity of Bax might be mediated by the release of cytochrome c from mitochondria, perhaps by forming channels (19, 21), with subsequent activation of the caspase cascade (22).
In this study, we demonstrate evidence for delayed neutrophil apoptosis in several neutrophilic inflammatory diseases. Delayed neutrophil apoptosis was associated with increased expression of neutrophil survival factors and markedly reduced intracellular Bax levels. The important functional role of Bax in regulating neutrophil apoptosis was directly demonstrated by using specific Bax antisense oligodeoxynucleotides. Therefore, cytokine-mediated reduction of intracellular Bax levels appears to be a key mechanism in many inflammatory disorders, resulting in the expansion of neutrophils.
Materials and Methods
Antibodies.
Anti-Bcl-2 monoclonal antibody (mAb), control IgG1 mAb, swine anti-rabbit fluorescein isothiocyanate (FITC)-conjugated secondary IgG Ab, and control rabbit IgG were from Dako. FITC-conjugated anti-Bcl-2 mAb was from Ancell (Bayport, MN). Polyclonal rabbit Abs against Bcl-x and Bax were purchased from Santa Cruz Biotechnology. Polyclonal rabbit anti-Bcl-xS Ab was from Calbiochem–Novabiochem. Goat anti-rabbit and anti-mouse horseradish peroxidase (HRP)-labeled secondary Ab were obtained from Amersham. FITC-conjugated control IgG1 mAb was from Coulter. mAb to granulocyte colony-stimulating factor (G-CSF) was obtained from R & D Systems. mAb to granulocyte–macrophage colony-stimulating factor (GM-CSF) was purchased from Genzyme for immunohistochemistry and from R & D Systems for neutralization experiments.
Neutrophil Purification.
Peripheral blood neutrophils were purified from patients with various inflammatory diseases (cystic fibrosis, idiopathic fibrosis, acute pneumonia, and cancer associated with neutrophilia) and healthy control individuals as previously described (23). Tissue neutrophils from bronchoalveolar lavage (BAL) fluids and sputum were directly analyzed because the investigated cell populations used in this study contained >95% neutrophils. For cytokine measurements, BAL cells from three patients with allergic alveolitis and two control individuals were used for comparison.
Neutrophil Cultures.
Neutrophils were cultured at 1 × 106 per ml in the presence or absence of cytokines and/or Bax antisense oligodeoxynucleotides for the indicated times in complete culture medium (RPMI medium 1640 supplemented with 10% fetal calf serum). GM-CSF was a kind gift from T. Hartung (Univ. of Konstanz, Konstanz, Germany). G-CSF, interleukin (IL)-3, IL-8, interferon (IFN)-γ, and tumor necrosis factor α (TNF-α) were obtained from R & D Systems. LPS (Escherichia coli O55:B5) was from Calbiochem–Novabiochem. The final cytokine and LPS concentrations were 25 ng/ml. Bax antisense and scrambled oligodeoxynucleotides with a natural phosphodiester backbone were synthesized by Microsynth (Balgach, Switzerland). Sequences used were according to previously published work (24): antisense Bax, 5′-TCG ATC CTG GAT GAA ACC CT-3′ and 5′-TCC CCC CCC ATT CGC CCT GC-3′; and scrambled Bax, 5′-TCA GTC CTG GTA GAA CAC CT-3′ and 5′-CTC CCC CCA CTT CGC CTC GC-3′. Inhibition of Bax protein expression was achieved by using a mixture of the two antisense molecules, both at a final concentration of 5 μM. The two control oligodeoxynucleotides were used in the same way. The oligodeoxynucleotides were added either into cultures of freshly purified or 32-h GM-CSF-pretreated neutrophils, whereby the initial first hour of incubation was performed in medium without serum to increase the uptake of these molecules. For cytokine neutralization experiments, supernatants from BAL cells were added to neutrophil cultures from control individuals, and the effects on cell death were analyzed in the presence or absence of a mixture of 5 μg/ml anti-G-CSF and 5 μg/ml anti-GM-CSF mAbs.
Protein Expression of Bcl-2 Family Members.
Bcl-2, Bcl-xL, Bcl-xS, and Bax protein expressions were studied by immunofluorescence, immunocytochemistry, and immunoblotting as previously described (25).
mRNA Expression of Bax.
mRNA expression of Bax was studied using Southern blot analysis linked to reverse transcription (RT)-PCR as previously described (25).
Cytokine Protein Expression.
G-CSF and GM-CSF protein expressions in inflammatory tissues (cystic fibrosis lungs and cancer) were studied by immunohistochemistry using the alkaline phosphatase–anti-alkaline phosphatase (APAAP) method as previously described (25). Moreover, BAL cells (1 × 106 per ml) were cultured in RPMI medium 1640 plus 10% fetal calf serum for 24 h, and supernatants were harvested. Cytokine concentrations were measured by ELISA using commercial kits (R & D Systems).
Determination of Neutrophil Death and Apoptosis.
Cell death of neutrophils was assessed by uptake of 1 μmol/liter ethidium bromide and flow cytometric analysis (EPICS XL) as previously described (24–31). Oligonucleosomal DNA fragmentation, a characteristic feature of cells undergoing apoptosis, was assessed by flow cytometry (30, 31). To determine whether neutrophil death was apoptosis, neutrophils were also morphologically examined. Cytospin preparations were made, stained with Diff-Quik (Baxter, Düdingen, Switzerland), and analyzed by light microscopy.
Results and Discussion
Delayed Neutrophil Apoptosis Is a General Feature of Neutrophilic Inflammation.
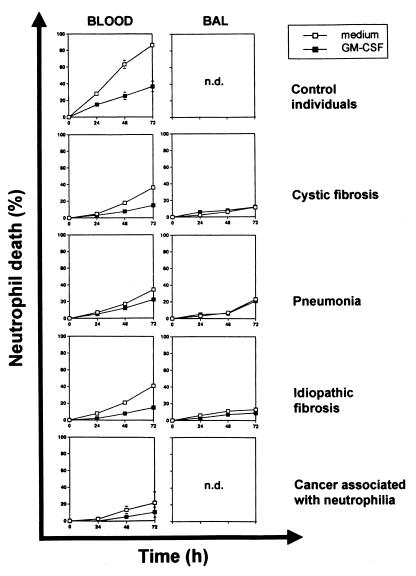
As shown in Fig. 1, purified blood and lung neutrophils from patients with inflammatory and cancer diseases associated with neutrophilia demonstrated, compared with blood neutrophils obtained from control individuals without evidence for an ongoing inflammation, a marked delay of cell death ex vivo. BAL neutrophils died even slower compared with blood neutrophils derived from the same patient. Moreover, because the neutrophil death was already inhibited in the absence of a survival factor, GM-CSF had only little anti-death effect, especially in BAL neutrophils, under chronic inflammatory conditions (Fig. 1). To investigate whether the observed cell death was apoptosis, a DNA fragmentation assay and morphology assessments were performed (data not presented). Although different inflammatory diseases were studied, we consistently observed delayed neutrophil apoptosis, suggesting that the mechanism of delayed apoptosis may represent a general feature of these pathologic conditions.
Figure 1.
Delayed neutrophil death is a general feature of neutrophilic inflammation. Blood and BAL neutrophils from adult patients with three different inflammatory lung diseases (cystic fibrosis, n = 13; pneumonia, n = 1; and idiopathic pulmonary fibrosis, n = 2) associated with neutrophilia were cultured in the presence and absence of the neutrophil survival factor GM-CSF for the indicated times before cell death was measured by flow cytometry. The results were compared with blood neutrophils from normal control individuals (n = 10) and cancer patients with associated blood neutrophilia (n = 2). Values are means ± SEM (SEM is mostly concealed by the symbols). n.d., not done.
Bax Levels Are Reduced in Neutrophils under Inflammatory Conditions.
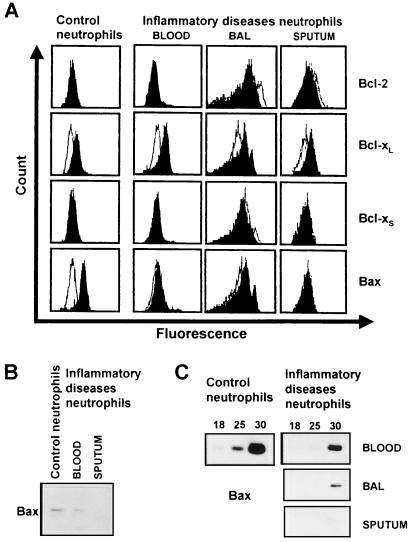
The family of Bcl-2-related proteins is involved in the regulation of apoptosis in many different cells (16). Therefore, we investigated the expression of anti-apoptotic and pro-apoptotic members of this family in blood and tissue neutrophils (BAL fluid and sputum neutrophils) under inflammatory conditions (cystic fibrosis). As assessed by flow cytometry, normal neutrophils significantly expressed Bcl-xL and Bax, but no Bcl-2 or Bcl-xS (Fig. 2A). Interestingly, compared with normal neutrophils, inflammatory diseases neutrophils expressed little or no Bax protein (Fig. 2 A and B). In contrast, Bcl-2, Bcl-xL, and Bcl-xS levels did not appear to be different between these two neutrophil populations. To determine whether the reduced Bax protein levels were the consequence of transcriptional changes, we investigated Bax mRNA levels by semiquantitative RT-PCR. As shown in Fig. 2C, Bax mRNA levels were markedly reduced in neutrophils from neutrophilic patients compared with normal neutrophils. We conclude that Bax deficiency occurs at both mRNA and protein levels and appears to be a general hallmark of neutrophils associated with delayed apoptosis under inflammatory conditions. Moreover, these data confirm previously published work performed in epithelial cancer cells (32) and Bax-deficient mice (17, 18), suggesting that lack of functional Bax is associated with a defect in apoptosis.
Figure 2.
Bax levels are reduced in neutrophils under inflammatory conditions. (A) Flow cytometry. Freshly purified normal blood neutrophils expressed significant amounts of Bcl-xL and Bax, but no Bcl-2 or Bcl-xS protein. In contrast to normal neutrophils, inflammatory diseases neutrophils (cystic fibrosis and cancer with associated neutrophilia, see also Fig. 6B) expressed little or no detectable Bax protein. (B) The markedly reduced Bax expression in inflammatory diseases neutrophils (cystic fibrosis) was confirmed by immunoblotting. (C) Semiquantitative RT-PCR. Bax protein expression correlates with the expression of Bax mRNA. Inflammatory diseases neutrophils (cystic fibrosis) had markedly reduced Bax mRNA levels. Numbers of PCR cycles are given at the top. Glyceraldehyde-3-phosphate dehydrogenase (G3PDH) control amplifications were performed demonstrating equal quality of the cDNA preparations derived from blood, BAL, and sputum neutrophils (not presented). Data are representative of at least five independent experiments.
Bax-Deficient Neutrophils Can Be Generated in Vitro.
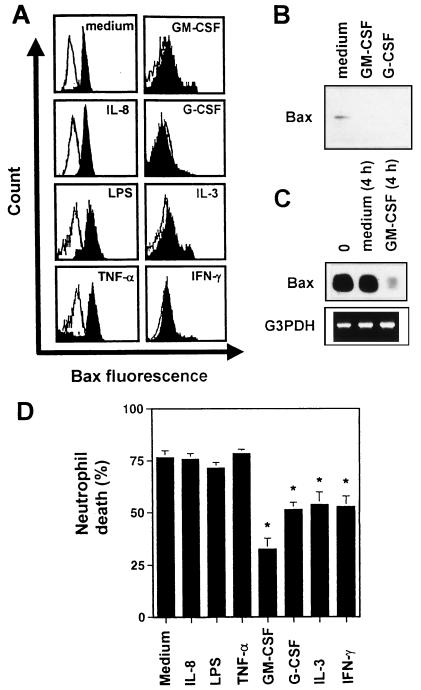
Cytokines are important mediators of inflammatory responses (33–35). To determine whether cytokines might also be involved in the development of Bax-deficient neutrophils, we investigated the effects of several cytokines on the expression of Bax in vitro. As assessed by flow cytometry, GM-CSF, IL-3, G-CSF, and IFN-γ stimulations for 30–50 h markedly down-regulated the expression of Bax in normal neutrophils (Fig. 3A). In contrast, IL-8, LPS, and TNF-α stimulations had no effect on Bax levels in these cells. Cytokine-mediated Bax deficiency in normal neutrophils was also observed when immunoblotting (Fig. 3B) or RT-PCR (Fig. 3C) was used. Only 4 h of survival factor incubation were needed to markedly down-regulate Bax mRNA levels in these cells (Fig. 3C). Furthermore, whereas the neutrophil survival factors (9, 10) GM-CSF, IL-3, G-CSF, and IFN-γ delayed neutrophil death, other mediators previously described as being involved in neutrophilic inflammation, such as IL-8, LPS, and TNF-α, did not increase neutrophil viability in vitro (Fig. 3D). This result suggests that Bax down-regulation is an anti-apoptotic event in neutrophils.
Figure 3.
Bax-deficient neutrophils can be generated by exposure of normal neutrophils to survival factors in vitro. (A) Flow cytometry. Normal neutrophils were cultured in the presence or absence of cytokines and LPS. (B) The effects of GM-CSF and G-CSF on Bax protein levels in normal neutrophils were confirmed by immunoblotting. (C) RT-PCR. GM-CSF exposure of normal neutrophils rapidly reduced Bax mRNA levels. Data from A–C are representative of at least five independent experiments. (D) Cell death assay. Neutrophils were cultured in the presence or absence of cytokines and LPS, respectively, for 72 h before cell death was measured by flow cytometry. Values are means ± SEM of six independent experiments (*, P < 0.05).
Reduced Bax Levels Mediate Delayed Neutrophil Apoptosis.
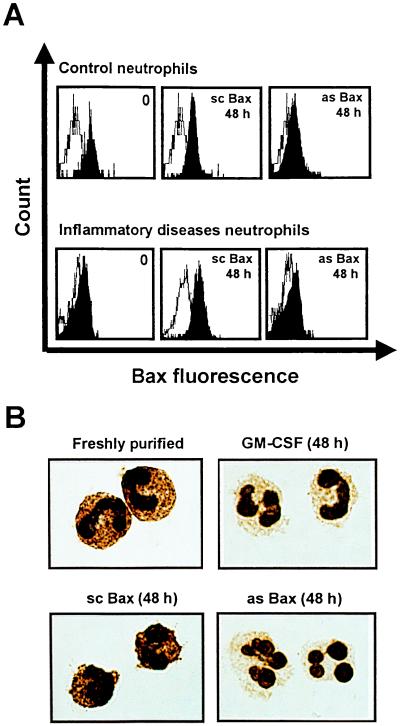
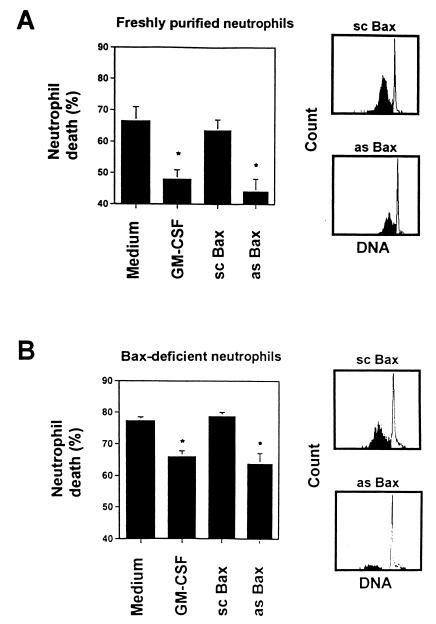
To test the hypothesis that decreased levels of the pro-apoptotic Bax molecule mediate delayed apoptosis in neutrophils, we used Bax antisense oligodeoxynucleotides to specifically decrease intracellular Bax levels. The molecules used have previously been applied to inhibit Bax protein expression in HL-60 cells (24). As demonstrated in Fig. 4 A and B, control neutrophils exposed to an optimal dose of Bax antisense oligodeoxynucleotides for 48 h expressed markedly reduced Bax protein levels that were similar to those observed after GM-CSF treatment. In contrast, Bax scrambled oligodeoxynucleotides did not alter Bax protein levels. Moreover, Bax antisense molecules prevented reexpression of Bax (see below) in inflammatory diseases neutrophils (Fig. 4A Lower), further confirming the efficiency of the oligodeoxynucleotides in reducing Bax levels. The ability of the Bax antisense molecules to decrease intracellular Bax levels allowed exploration of the role of Bax in the regulation of neutrophil apoptosis. As shown in Fig. 5A, Bax antisense, but not scrambled oligodeoxynucleotides, added to freshly purified control neutrophils delayed cell death and apoptotic DNA fragmentation in this system. The anti-apoptotic effects of the Bax antisense molecule and GM-CSF were in the same range. Treatment of in vitro generated Bax-deficient neutrophils with Bax antisense and control oligodeoxynucleotides revealed similar effects (Fig. 5B). These data suggest that reduction of Bax levels in neutrophils is a key mechanism within the anti-apoptotic pathway mediated by cytokines that significantly contributes to neutrophil expansion under inflammatory conditions.
Figure 4.
Bax antisense (as Bax) but not scrambled oligodeoxynucleotides (sc Bax) reduce Bax protein expression in neutrophils. (A) Flow cytometry. Control and inflammatory diseases neutrophils (cystic fibrosis) were cultured in the presence of as Bax or sc Bax for 48 h. (B) Immunocytochemistry. (×1,000.) Control neutrophils were cultured in the presence of GM-CSF, sc Bax, and as Bax for 48 h. Data are representative of at least three independent experiments.
Figure 5.
Bax antisense (as Bax) but not scrambled oligodeoxynucleotides (sc Bax) block neutrophil apoptosis in vitro. (A) Cell death assay. Control neutrophils were cultured at the indicated conditions for 48 h before cell death was measured by flow cytometry. (Right) DNA fragmentation (black) was markedly reduced in as Bax-treated compared to sc Bax-treated neutrophils, suggesting delayed apoptosis in Bax-deficient cells. (B) Cell death assay. Control neutrophils were pretreated with GM-CSF for 32 h to reduce Bax levels (see Fig. 3A). Bax-deficient neutrophils were then cultured under the indicated conditions for an additional 48 h before cell death was measured by flow cytometry. (Right) DNA fragmentation (black) is markedly reduced in as Bax-treated compared to sc Bax-treated neutrophils, suggesting delayed apoptosis in neutrophils where Bax reexpression was prevented. Values in the cell death assays are means ± SEM of three independent experiments (*, P < 0.05).
G-CSF and GM-CSF Are Important Neutrophil Survival Factors in Vivo.
To determine which cytokines are responsible for delayed neutrophil apoptosis in vivo, we investigated cytokine levels produced by BAL cells derived from patients with various inflammatory lung diseases. As shown in Table 1, in diseases associated with neutrophilia, BAL cells released large amounts of G-CSF and GM-CSF, but no significant IFN-γ and IL-3 levels were detected. In contrast to neutrophilic patients, BAL cells from control individuals without neutrophilic lung inflammation did not release significant amounts of G-CSF or GM-CSF. The same results were obtained with RT-PCR for cytokine measurements (not presented). To prove the functional activity of G-CSF and GM-CSF released by BAL cells, the supernatants were added to control neutrophils in the presence or absence of neutralizing mAbs. As shown in Table 2, the supernatants containing significant amounts of G-CSF and GM-CSF delayed neutrophil death as much as an optimal dose of recombinant GM-CSF. The anti-apoptotic effect of the supernatants was completely blocked with a mixture of anti-G-CSF and anti-GM-CSF mAbs, suggesting that no further neutrophil survival factor was present. This finding suggests that G-CSF and GM-CSF are relevant cytokines produced by BAL cells that down-regulate Bax levels in neutrophilic inflammatory responses.
Table 1.
Cytokine release by BAL cells derived from individuals with various neutrophilic lung diseases and control individuals
| Individual
|
Cytokine conc., pg/ml
|
||||
|---|---|---|---|---|---|
| No. | Description | G-CSF | GM-CSF | IL-3 | IFN-γ |
| Individuals with neutrophilic lung inflammation | |||||
| 1 | Cystic fibrosis | 500 | 319 | <5 | <10 |
| 2 | Cystic fibrosis | 406 | 29 | <5 | <10 |
| 3 | Idiopathic fibrosis | 220 | 24 | <5 | <10 |
| 4 | Pneumonia | 417 | 76 | <5 | <10 |
| 5 | Acute allergic alveolitis | 5,362 | 1,076 | ND | ND |
| Individuals without neutrophilic lung inflammation | |||||
| 6 | Control, no lung disease | <5 | <5 | ND | ND |
| 7 | Control, no lung disease | <5 | <5 | ND | ND |
| 8 | Chronic allergic alveolitis | 6 | <5 | ND | ND |
| 9 | Chronic allergic alveolitis | <5 | 7 | ND | ND |
Cytokine concentrations were measured by ELISA in supernatants from untreated BAL cells (106 per ml) after a 24-h culture period. ND, not determined.
Table 2.
Supernatants of BAL cells containing G-CSF and GM-CSF delay death of control neutrophils in vitro that is blocked after cytokine neutralization
| Exp. | Conditions | Neutrophil death, % |
|---|---|---|
| 1 | No treatment | 47 |
| GM-CSF | 61 | |
| Supernatant of patient 1 | 63 | |
| Supernatant of patient 1 + anti-G-CSF/anti-GM-CSF | 41 | |
| Anti-G-CSF/anti-GM-CSF | 46 | |
| 2 | No treatment | 50 |
| GM-CSF | 71 | |
| Supernatant of patient 4 | 60 | |
| Supernatant of patient 4 + anti-G-CSF/anti-GM-CSF | 53 | |
| Anti-G-CSF/anti-GM-CSF | 47 | |
| 3 | No treatment | 53 |
| GM-CSF | 67 | |
| Supernatant of patient 5 | 66 | |
| Supernatant of patient 5 + anti-G-CSF/anti-GM-CSF | 55 | |
| Anti-G-CSF/anti-GM-CSF | 49 |
Control neutrophils were cultured at the indicated conditions for 36 h before neutrophil death measurements. The patient numbers correspond to those in Table 1. The concentration of the neutralizing mAbs was 5 μg/ml each.
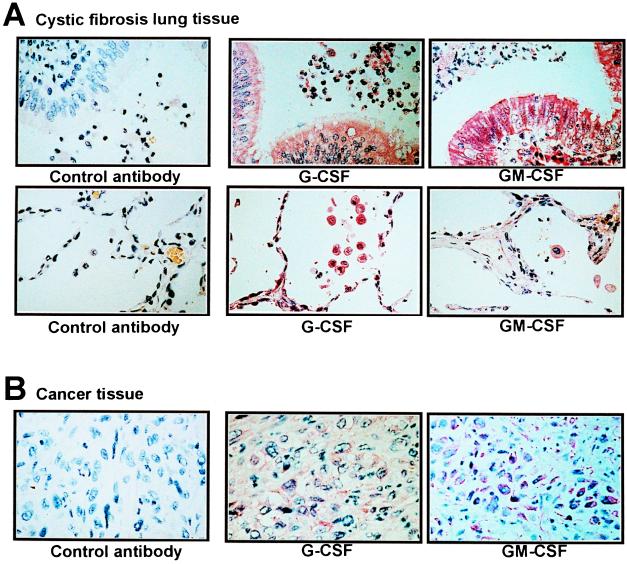
To assess G-CSF and GM-CSF protein localization, we performed immunohistochemistry. As shown in Fig. 6A, cells morphologically resembling epithelial cells, lung macrophages, and neutrophils were seen to stain positive in lungs of cystic fibrosis patients. Similar experiments were performed with tissues from cancer patients with associated neutrophilia. In these tissues, we also observed high G-CSF and GM-CSF levels, although the cellular source of cytokine production was different (cancer cells; Fig. 6B). Taken together, these data suggest that overproduction of G-CSF and GM-CSF is an important feature of neutrophilic inflammation in vivo.
Figure 6.
G-CSF and GM-CSF are significantly expressed in inflammatory responses associated with neutrophilia in vivo. (A) Immunohistochemical staining of cystic fibrosis lung tissues with the indicated antibodies. (×400.) G-CSF and GM-CSF were highly expressed in epithelial cells, macrophages, and neutrophils in patients with cystic fibrosis. (Upper) Central lung sections. (Lower) Lung alveoli. (B) Staining of tissue infiltrated by hypopharynx cancer cells. (×400.) The cancer cells demonstrated high G-CSF and GM-CSF expression. As a consequence, the patient developed a neutrophilia (during the time of these investigations, the peripheral blood neutrophil numbers were between 49,920 and 57,134 per μl of blood).
Bax Is Reexpressed in Neutrophils under Conditions of Survival Factor Withdrawal.
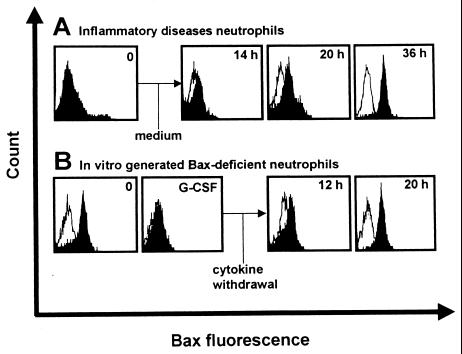
It has been previously suggested that neutrophil apoptosis is an important process for the resolution of an inflammatory response (4–6). It is likely that the resolution of inflammation is associated with decreased cytokine gene expression. Therefore, we investigated whether Bax-deficient inflammatory disease neutrophils may reexpress Bax under in vitro conditions without cytokine support. Moreover, we created Bax-deficient neutrophils from normal donors by stimulation with G-CSF and GM-CSF in vitro, and we measured Bax levels after cytokine withdrawal. As shown in Fig. 7 A and B, both neutrophils from patients with cystic fibrosis and cytokine-stimulated normal neutrophils started to reexpress significant Bax levels 12–20 h after cytokine removal. Normal neutrophil Bax expression was observed after 20–36 h in these experiments. These data suggest that, in the resolution of an inflammatory response when little or no survival factors are present, neutrophils activate the Bax gene and undergo apoptosis, leading to the normalization of neutrophil homeostasis.
Figure 7.
Bax-deficient neutrophils activate the Bax gene under conditions of cytokine withdrawal. (A) Freshly purified Bax-deficient inflammatory diseases neutrophils (0; cystic fibrosis, see Fig. 2A) were cultured without cytokine support for the indicated times, and Bax protein expression was determined by flow cytometry. (B) Freshly purified normal neutrophils (0) were cultured in the presence of G-CSF for 30 h to down-regulate intracellular Bax protein levels (see Fig. 3A). Data are representative of five independent experiments.
In summary, this study demonstrates the important role of delayed apoptosis in inflammatory responses. This process is regulated by cytokines that abolish intracellular Bax, resulting in the expansion of neutrophils. The described cascade of events likely contributes to host defense against infection. In addition, the inflammatory scenario of cytokine overexpression, Bax-deficient neutrophils, and delayed neutrophil apoptosis observed in neutrophilic inflammation can be mimicked in vitro. Moreover, it appears that the resolution of inflammatory responses is also regulated by changes in the levels of Bax. The intracellular mechanism by which Bax transcription is suppressed after the exposure of neutrophils to survival factors remains to be established.
Acknowledgments
We thank S. Gratzl for providing and staining the cancer tissue as well as R. Zinkernagel and M. Baggiolini for discussions and critical reading of the manuscript. This work was supported by the Swiss National Science Foundation (Grant 32-49210.96), the Silva Casa Foundation, Bern, the EMDO Foundation, Zurich, and the OPO Foundation, Zurich.
Abbreviations
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- BAL
bronchoalveolar lavage
- TNF-α
tumor necrosis factor α
- LPS
lipopolysaccharide
- RT-PCR
reverse transcription–PCR
References
- 1.Kerr J F R, Searle J. J Pathol. 1972;107:41–44. doi: 10.1002/path.1711070107. [DOI] [PubMed] [Google Scholar]
- 2.Gerschenson L E, Rotello R J. FASEB J. 1992;6:2450–2455. doi: 10.1096/fasebj.6.7.1563596. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J J. Immunol Today. 1993;14:126–130. doi: 10.1016/0167-5699(93)90214-6. [DOI] [PubMed] [Google Scholar]
- 4.Savill J S, Wyllie A H, Henson J E, Walport M J, Henson P M, Haslett C. J Clin Invest. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigg J M, Savill J S, Sarraf C, Haslett C, Silverman M. Lancet. 1991;338:720–722. doi: 10.1016/0140-6736(91)91443-x. [DOI] [PubMed] [Google Scholar]
- 6.Savill J. J Leukocyte Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 7.Malech H L, Gallin J I. N Engl J Med. 1987;317:687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 8.Haslett C, Savill J S, Meagher L C. Curr Opin Immunol. 1989;2:10–18. doi: 10.1016/0952-7915(89)90091-5. [DOI] [PubMed] [Google Scholar]
- 9.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 10.Lee A, Whyte M K B, Haslett C. J Leukocyte Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 11.Murray J, Barbara J A, Dunkley S A, Lopez A F, Van Ostade X, Condliffe A M, Dransfield I, Haslett C, Chilvers E R. Blood. 1997;90:2772–2783. [PubMed] [Google Scholar]
- 12.Liles W C, Kiener P A, Ledbetter J A, Aruffo A, Klebanoff S J. J Exp Med. 1996;184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwai K, Miyawaki T, Takizawa T, Konno A, Ohta K, Yachie A, Seki H, Taniguchi N. Blood. 1994;84:1201–1208. [PubMed] [Google Scholar]
- 14.Weinmann P, Gaehtgens P, Walzog B. Blood. 1999;93:3106–3115. [PubMed] [Google Scholar]
- 15.Sanghavi D M, Thelen M, Thornberry N A, Casciola-Rosen L, Rosen A. FEBS Lett. 1998;422:179–184. doi: 10.1016/s0014-5793(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 16.Adams J M, Cory S. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 17.Knudson C M, Tung K S K, Tourtellote W G, Brown G A J, Korsmeyer S J. Science. 1995;270:96–99. doi: 10.1126/science.270.5233.96. [DOI] [PubMed] [Google Scholar]
- 18.Yin C, Knudson C M, Korsmeyer S J, van Dyke T. Nature (London) 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- 19.Marzo I, Brenner C, Zamzami N, Jürgensmeier J M, Susin S A, Vieira H L A, Prévost M-C, Xie Z, Matsuyama S, Reed J C, Kroemer G. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 20.Oltvai Z N, Milliman C L, Korsmeyer S J. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu S, Narita M, Tsujimoto Y. Nature (London) 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 22.Green D R, Reed J C. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 23.Yousefi S, Green D R, Blaser K, Simon H-U. Proc Natl Acad Sci USA. 1994;91:10868–10872. doi: 10.1073/pnas.91.23.10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manfredini R, Capobianco M L, Trevisan F, Rauzi F, Barbieri D, Citro G, Tagliafico E, Ferrari S. Antisense Nucleic Acid Drug Dev. 1998;8:341–350. doi: 10.1089/oli.1.1998.8.341. [DOI] [PubMed] [Google Scholar]
- 25.Dibbert B, Daigle I, Braun D, Schranz C, Weber M, Blaser K, Zangemeister-Wittke U, Akbar A N, Simon H-U. Blood. 1998;92:778–783. [PubMed] [Google Scholar]
- 26.Simon H-U, Yousefi S, Dommann-Scherrer C C, Zimmermann D R, Bauer S, Barandun J, Blaser K. J Exp Med. 1996;183:1071–1082. doi: 10.1084/jem.183.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yousefi S, Hoessli D C, Blaser K, Mills G B, Simon H-U. J Exp Med. 1996;183:1407–1414. doi: 10.1084/jem.183.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon H-U, Yousefi S, Dibbert B, Levi-Schaffer F, Blaser K. Eur J Immunol. 1997;27:3536–3539. doi: 10.1002/eji.1830271256. [DOI] [PubMed] [Google Scholar]
- 29.Simon H-U, Yousefi S, Dibbert B, Hebestreit H, Weber M, Branch D R, Blaser K, Levi-Schaffer F, Anderson G P. Blood. 1998;92:547–557. [PubMed] [Google Scholar]
- 30.Hebestreit H, Yousefi S, Balatti I, Weber M, Crameri R, Simon D, Hartung K, Schapowal A, Blaser K, Simon H-U. Eur J Immunol. 1996;26:1775–1780. doi: 10.1002/eji.1830260817. [DOI] [PubMed] [Google Scholar]
- 31.Hebestreit H, Dibbert B, Balatti I, Braun D, Schapowal A, Blaser K, Simon H-U. J Exp Med. 1998;187:415–425. doi: 10.1084/jem.187.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rampino N, Yamamoto H, Ionov Y, Li Y, Sawai H, Reed J C, Perucho M. Science. 1997;275:967–969. doi: 10.1126/science.275.5302.967. [DOI] [PubMed] [Google Scholar]
- 33.Girardin E, Grau G E, Dayer J M, Roux-Lombard P, Lambert P H. N Engl J Med. 1988;319:397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- 34.Martin G D, Danner R L, Ceska M, Suffredini A F. J Exp Med. 1991;173:1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luster A D. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]