Abstract
Enteric viruses were eluted from estuarine sediments by using four organic mixtures; these solutions, with or without various supplements, were compared by determining their abilities to desorb virus from sediments taken from shellfish-harvesting sites. The least effective eluents consisted of glycine buffer, milk preparations, and beef extract paste. When virus type and sediment composition were taken into consideration, higher percentages of virus recovery were achieved with isoelectric casein, powdered beef extract, and nutrient broth mixtures. In addition to the type of eluent used, variations in virus recovery were due to the pH of the eluent, the composition of the sediment, and the type of virus being extracted. No clear distinction between the values of protein and inorganic ion supplements could be made.
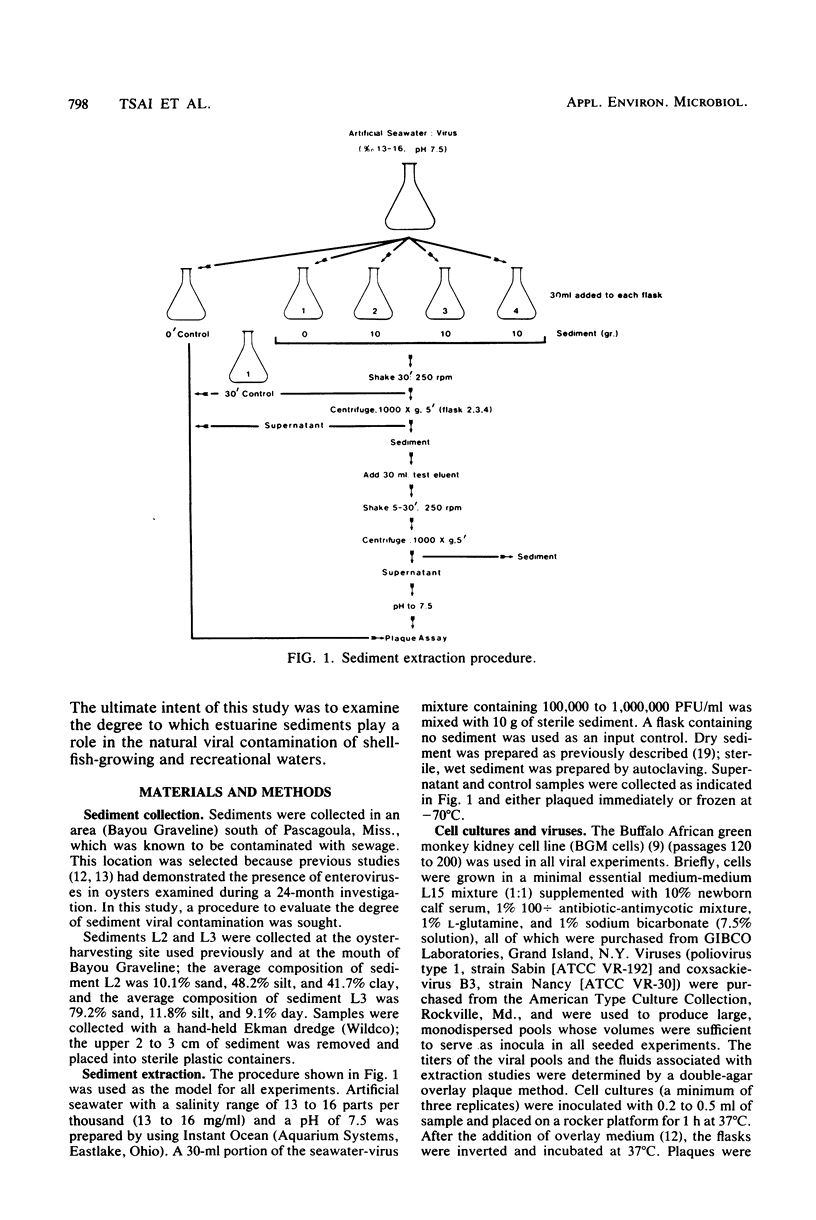
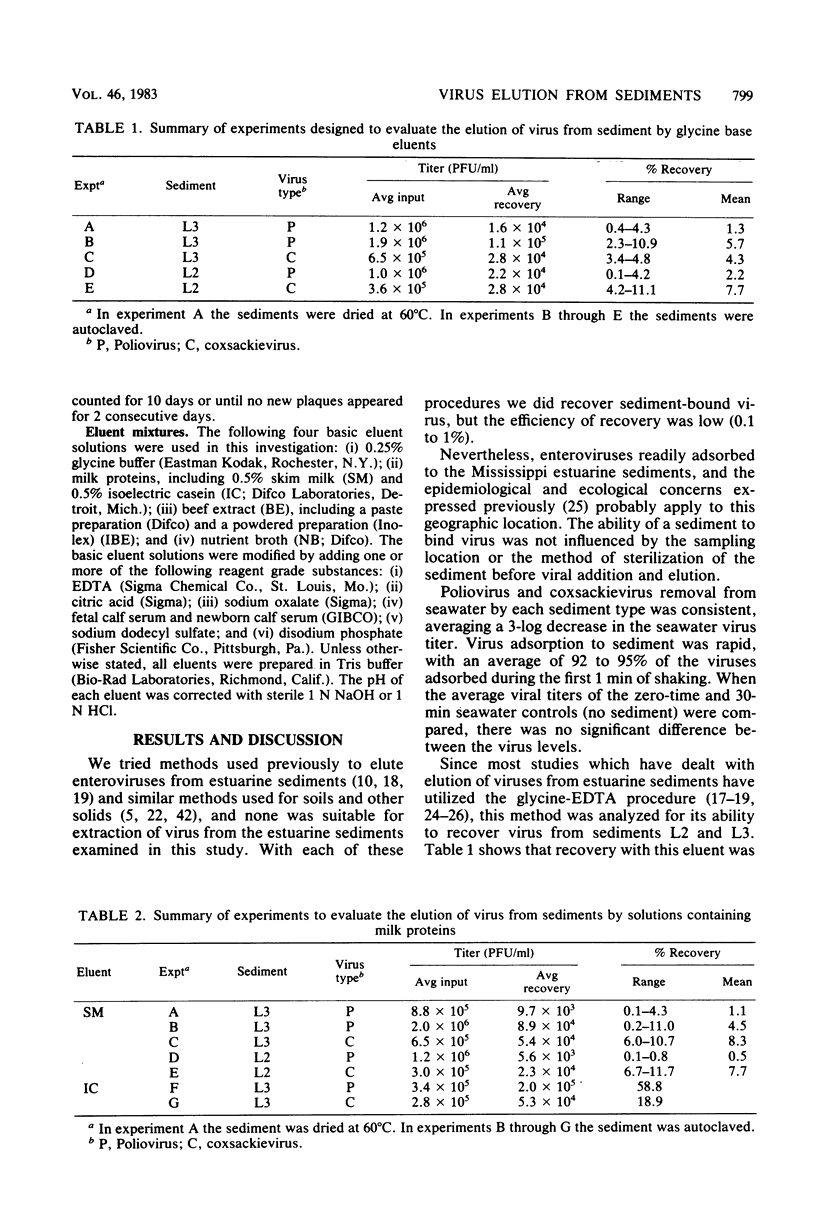
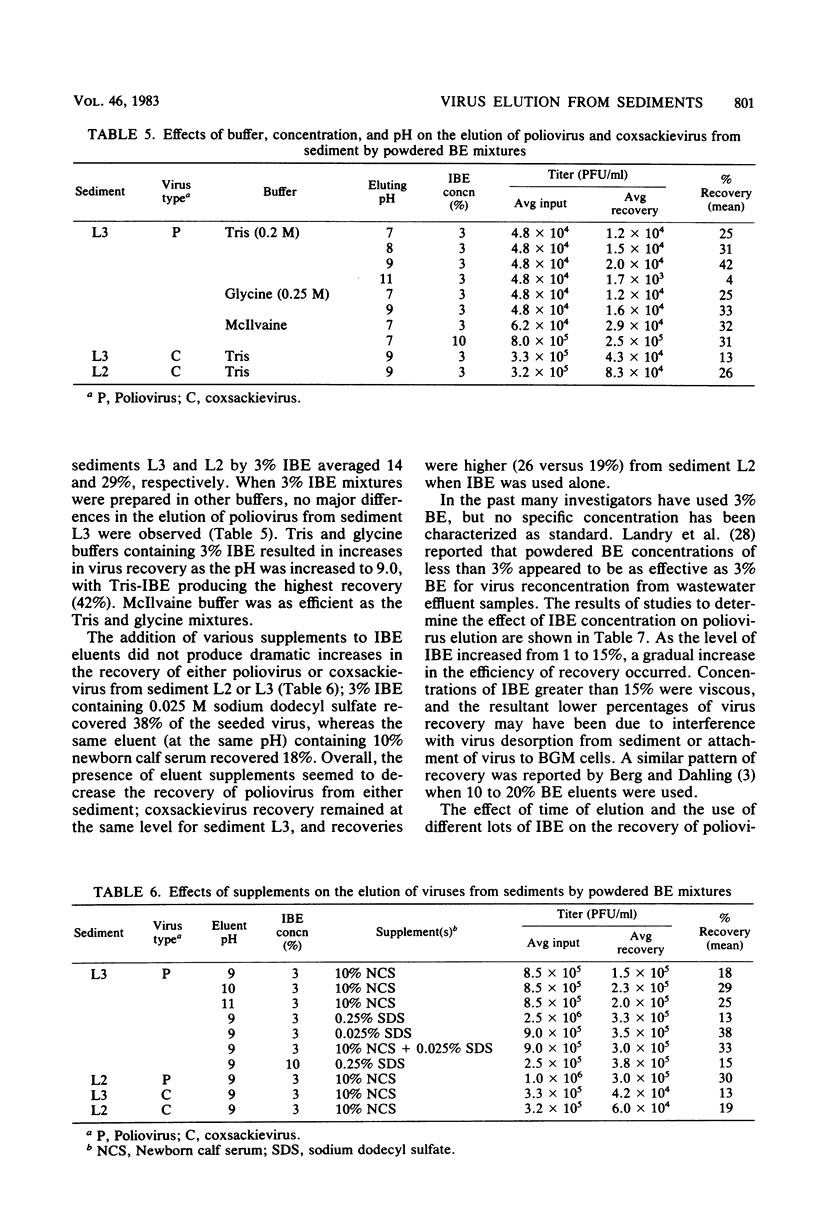
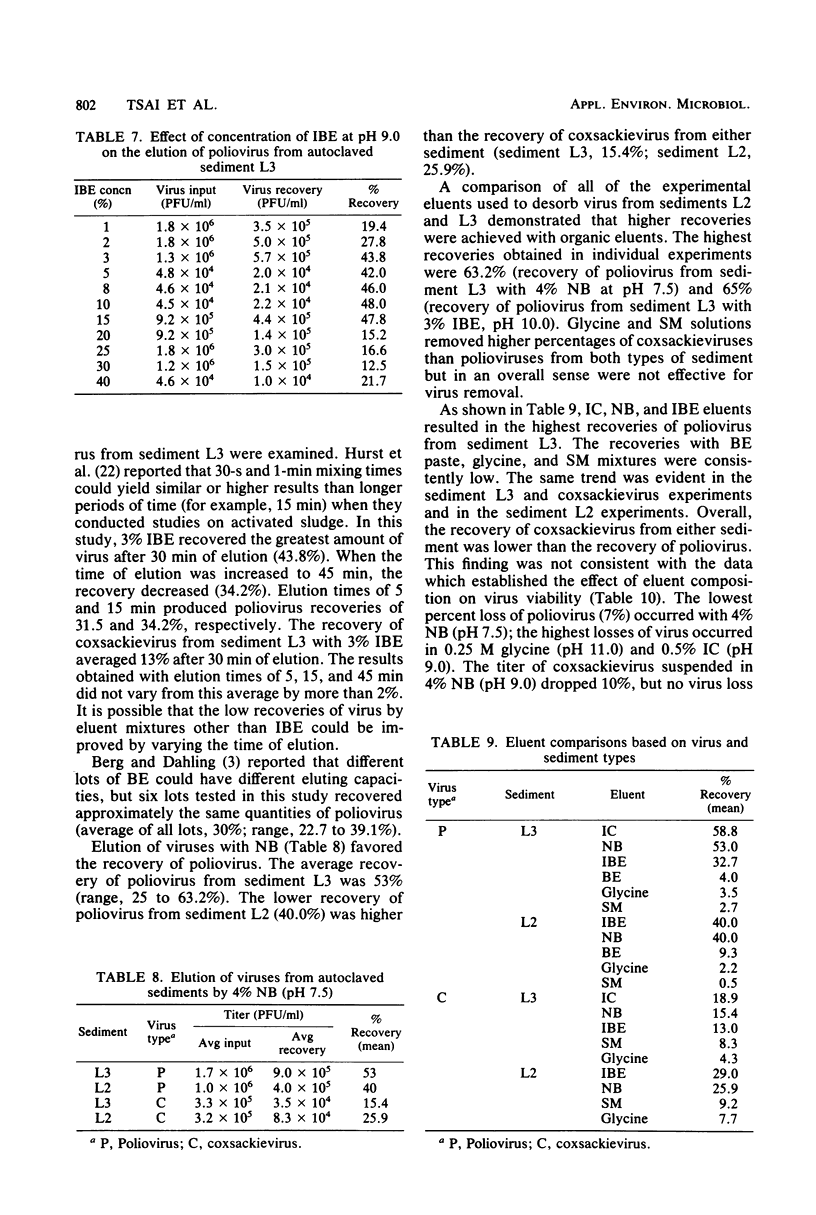
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTELL P., PIERZCHALA W., TINT H. The adsorption of enteroviruses by activated attapulgite. J Am Pharm Assoc Am Pharm Assoc. 1960 Jan;49:1–4. [PubMed] [Google Scholar]
- Berg G., Dahling D. R. Method for recovering viruses from river water solids. Appl Environ Microbiol. 1980 Apr;39(4):850–853. doi: 10.1128/aem.39.4.850-853.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitton G., Charles M. J., Farrah S. R. Virus detection in soils: a comparison of four recovery methods. Can J Microbiol. 1979 Aug;25(8):874–880. doi: 10.1139/m79-130. [DOI] [PubMed] [Google Scholar]
- Bitton G., Chou Y. J., Farrah S. R. Techniques for virus detection in aquatic sediments. J Virol Methods. 1982 Feb;4(1):1–8. doi: 10.1016/0166-0934(82)90048-9. [DOI] [PubMed] [Google Scholar]
- Carlson G. F., Jr, Woodard F. E., Wentworth D. F., Sproul O. J. Virus inactivation on clay particles in natural waters. J Water Pollut Control Fed. 1968 Feb;40(2):R89–106. [PubMed] [Google Scholar]
- De Flora S., De Renzi G. P., Badolati G. Detection of animal viruses in coastal seawater and sediments. Appl Microbiol. 1975 Sep;30(3):472–475. doi: 10.1128/am.30.3.472-475.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILDES P., KAY D. The conditions which govern the adsorption of a tryptophan-dependent bacteriophage to kaolin and bacteria. J Gen Microbiol. 1963 Feb;30:183–191. doi: 10.1099/00221287-30-2-183. [DOI] [PubMed] [Google Scholar]
- Gerba C. P., Goyal S. M., LaBelle R. L., Cech I., Bodgan G. F. Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am J Public Health. 1979 Nov;69(11):1116–1119. doi: 10.2105/ajph.69.11.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C. P., Smith E. M., Melnick J. L. Development of a quantitative method for detecting enteroviruses in estuarine sediments. Appl Environ Microbiol. 1977 Aug;34(2):158–163. doi: 10.1128/aem.34.2.158-163.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal S. M., Gerba C. P. Comparative adsorption of human enteroviruses, simian rotavirus, and selected bacteriophages to soils. Appl Environ Microbiol. 1979 Aug;38(2):241–247. doi: 10.1128/aem.38.2.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst C. J., Farrah S. R., Gerba C. P., Melnick J. L. Development of quantitative methods for the detection of enteroviruses in sewage sludges during activation and following land disposal. Appl Environ Microbiol. 1978 Jul;36(1):81–89. doi: 10.1128/aem.36.1.81-89.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P., Goyal S. M., Melnick J. L., Cech I., Bogdan G. F. Relationships between environmental factors, bacterial indicators, and the occurrence of enteric viruses in estuarine sediments. Appl Environ Microbiol. 1980 Mar;39(3):588–596. doi: 10.1128/aem.39.3.588-596.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P. Influence of estuarine sediment on virus survival under field conditions. Appl Environ Microbiol. 1980 Apr;39(4):749–755. doi: 10.1128/aem.39.4.749-755.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBelle R. L., Gerba C. P. Influence of pH, salinity, and organic matter on the adsorption of enteric viruses to estuarine sediment. Appl Environ Microbiol. 1979 Jul;38(1):93–101. doi: 10.1128/aem.38.1.93-101.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry E. F., Vaughn J. M., Thomas M. Z., Beckwith C. A. Adsorption of enteroviruses to soil cores and their subsequent elution by artificial rainwater. Appl Environ Microbiol. 1979 Oct;38(4):680–687. doi: 10.1128/aem.38.4.680-687.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry E. F., Vaughn J. M., Thomas M. Z., Vicale T. J. Efficiency of beef extract for the recovery of poliovirus from wastewater effluents. Appl Environ Microbiol. 1978 Oct;36(4):544–548. doi: 10.1128/aem.36.4.544-548.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew P. F., Gerba C. P. Thermostabilization of enteroviruses by estuarine sediment. Appl Environ Microbiol. 1980 Aug;40(2):305–308. doi: 10.1128/aem.40.2.305-308.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak P. A., Caraway C. T., Portnoy B. L. Oyster-associated hepatitis: lessons from the Louisiana experience. Am J Epidemiol. 1976 Feb;103(2):181–191. doi: 10.1093/oxfordjournals.aje.a112216. [DOI] [PubMed] [Google Scholar]
- Murray J. P., Laband S. J. Degradation of poliovirus by adsorption on inorganic surfaces. Appl Environ Microbiol. 1979 Mar;37(3):480–486. doi: 10.1128/aem.37.3.480-486.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]
- Schaub S. A., Sagik B. P. Association of enteroviruses with natural and artificially introduced colloidal solids in water and infectivity of solids-associated virions. Appl Microbiol. 1975 Aug;30(2):212–222. doi: 10.1128/am.30.2.212-222.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P., Melnick J. L. Role of sediment in the persistence of enteroviruses in the estuarine environment. Appl Environ Microbiol. 1978 Apr;35(4):685–689. doi: 10.1128/aem.35.4.685-689.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg C. H., Wallis C., Ward C. H. Inactivation of clay-associated bacteriophage MS-2 by chlorine. Appl Environ Microbiol. 1977 Feb;33(2):385–391. doi: 10.1128/aem.33.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Ashley C. S. Inactivation of poliovirus in digested sludge. Appl Environ Microbiol. 1976 Jun;31(6):921–930. doi: 10.1128/aem.31.6.921-930.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings F. M., Lewis A. L., Mountain C. W. Demonstration of solids-associated virus in wastewater and sludge. Appl Environ Microbiol. 1976 Mar;31(3):354–358. doi: 10.1128/aem.31.3.354-358.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



