Abstract
Zoonoses are infections that are spread from animals to humans. Most often, humans are “dead-end” hosts, meaning that there is no subsequent human-to-human transmission. If one considers most of the emerging infections that were recognized at the end of the last century and the beginning of this century, they would fall into the category of zoonoses. One of the most important common traits exhibited by infections that have been or can be eliminated from the face of the earth (e.g. smallpox, measles, polio) is the absence of any host other than humans. Therefore, zoonses represent infections that can never be eliminated and must be considered as permanent and recurrent factors to be dealt with in protecting human health.
Zoonoses are infections transmitted from animal to humans either directly or via an insect vector. There are hundreds of zoonoses some of which can be propagated human-to-human, but I want to concentrate today on those for which humans represent a dead-end host (i.e. there is little or no human-to-human transmission).
There are two primary reasons for being particularly interested in zoonoses. The first is that most of the diseases that today would be classified as “emerging infectious diseases”-ones of which we are newly becoming aware-are, in fact, zoonoses. The second reason relates to the fact that zoonotic infections (those that have an animal reservoir) can probably never be eradicated, while those for which humans are the only hosts, such as smallpox, measles and polio, can theoretically be eliminated from the face of the earth.
Zoonoses can be categorized by organism causing the disease, by animal reservoir and by the manner in which the disease is contracted; insect bite, animal bite, direct contact or ingestion. I will give examples of each of these categories.
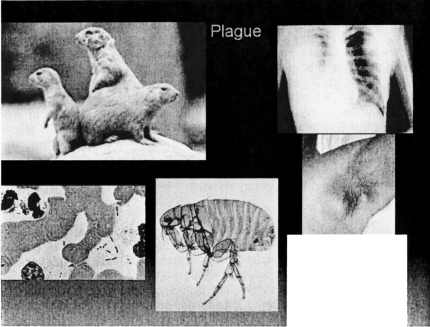
The first zoonosis I would like to discuss is one that is endemic to the geographic area near this meeting site: namely plague (Figure 1). In the middle ages, the animal host for plague was the rat; now in the Southwestern United States, it's the prairie dog. The offending organism is a Gram negative rod, Yersinia pestis, which is transmitted to humans through the bite of a flea that lives on the skin of the prairie dog. The flea bite can result in either a local infection of lymph nodes (bubonic plague—shown in the axilla in Figure 1) or may result in bacteremia causing pneumonia (pneumonic plague), and associated disseminated intravascular coagulopathy (DIC) with attendant gangrene of the extremities.
Fig. 1.
Infectious cycle and clinical presentations of plague. Counterclockwise from top left: Prairie dogs (columbia.edu); Yersinia pestis (mja.com.au); flea vector (mod.uk); axillary bubo (zabout.com); chest radiograph of plague pneumonia (chip.med.nyu).
Two such cases were seen at my former hospital in New York City. A married couple had traveled to New York for a vacation and within hours of arriving, the woman developed lymphatic swelling in the groin and the man developed pneumonia. The diagnosis was made rapidly and antibiotic treatment instituted within hours. The woman made a rapid and uneventful recovery, but the man went on to develop DIC and lost parts of both upper and lower extremities. The appearance of these cases in NYC immediately raised the specter of bioterrorism, but an accurate travel history quickly dispelled those fears.
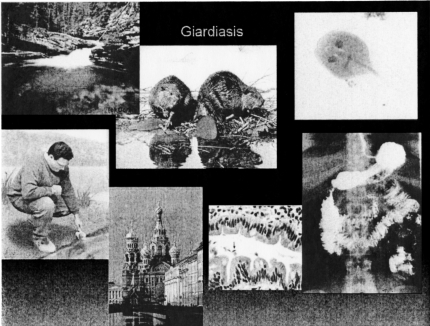
The next zoonosis I would like to discuss, is giardiasis (Figure 2). This infection is also indigenous to the western U.S., where, it is associated with hiking and camping with the common activity being drinking water from beautiful crystal clear flowing streams. The problem results from the fact that upstream from where one is drinking, lives a colony of beavers. These animals harbor the protozoan organism, Giardia lamblia, shown in the upper right hand corner of Fig. 2, asymptomatically and defecate it into the streams where the unknowing camper ingests it with his or her drink of “clean mountain water”. Giardia is resistant to chlorine, so boiling or filtering the water is the only way to get rid of it. Giardia takes up residence in the small bowel, which results in bloating, abdominal pain and chronic diarrhea. As shown in Figure 2, Giardiasis is endemic in the water supply of St. Petersburg, Russia and one should never drink anything but bottled water or vodka in this city. Again a complete travel history coupled with the otherwise non-specific symptoms should prompt stool examination, which will confirm the diagnosis. Treatment with metronidazole is usually curative.
Fig. 2.
Epidemiology and clinical presentation of giardiasis. Counterclockwise from top left: clear mountain stream (away.com); man sampling water (students.stewards.edu); St Petersburg, Russia (geraldbrimacombe.com); small intestine with organism[arrow] and small bowel barium study (medspain.com); Giardia lamblia (nlm.nih.gov); beavers upstream (jasperadventurecentre.com).
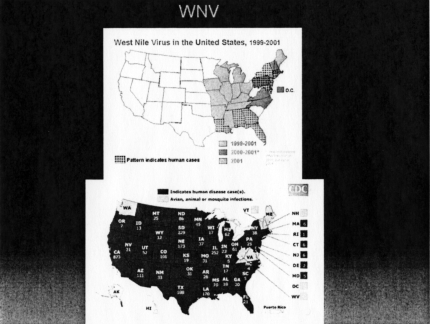
On to West Nile Disease. This entity was unknown in the Americas until 1999 when it first made its appearance in the Northeast United States—New York to be exact. Since that time, the disease has spread rapidly West across the US with Washington State being the only area free of human infection as of 2007 (Figure 3). As it has spread westerly, the incidence in the Northeast declined, either because of the prevalence of antibodies in the population or because of a decrease in the number of susceptible birds.
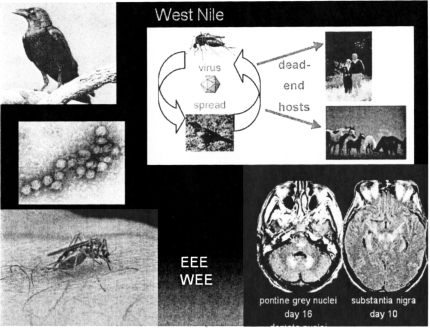
Fig. 3.
West Nile Disease: Epidemiology and Clinical syndrome. Counterclockwise from top left: Crow (healthline.com); West Nile virus (lib.uiowa.edu); mosquito biting (ec.gc.ca); MRI of brain with changes of encephalitis [arrows]; life cycle (wildhorseandburro.blm.gov).
The natural host for West Nile Virus (WNV), an RNA virus and a member of the flavivirus genus, is wild birds. It is spread from bird-to-bird by the bite of a mosquito(culex or aedes). Some birds carry the infection more-or-less asymptomatically, while others, like the crow, succumb to the infection, Dead crows in fact, are sentinel animals for the presence of the virus in a given environment. Humans and animals such as horses are dead-end hosts for this infection.(Figure 4) This pathophysiology is similar to that of the equine encephalitides (Eastern and Western Equine Encephalitis) and of St. Louis Encephalitis. The clinical presentation runs the gamut from sub-clinical disease through paralytic disease, to encephalitis—primarily in elderly patients.
Fig. 4.
West Nile human infection 1999-2001 (top) and 2005 (bottom) (cdc.gov).
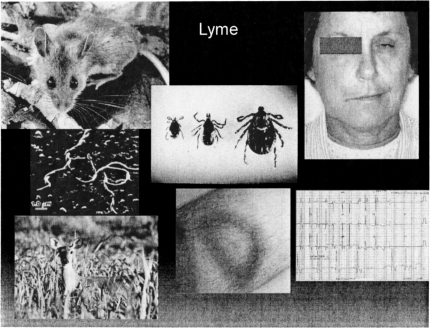
Another scourge primarily affecting the Northeastern U.S., is Lyme disease. Recognized first in Lyme, Connecticut, this infection has been diagnosed in tens of thousands of patients and has entered the differential diagnosis list for any syndrome with multifaceted presentation. The infectious agent is a spirochete, Borrelia burgdorferi, and the most common animal reservoir hosts are the deer and the deer mouse. The insect vector for Lyme disease is the tick—usually of the ixodes species, but the common wood tick can also carry the organism. The tick bite induces an annular spreading target-like rash (erythema chronicum migrans) in over 50% of cases, with the bite site at the center of the rash. This rash is painless and non-pruritic and if in a non-obvious location, may be missed altogether. After a variable latent period, arthralgias and arthritis, central nervous system or cardiac abnormalities may ensue (Figure 5). The most common cardiac syndrome is heart block; central nervous system involvement includes facial and other cranial nerve palsies, meningitis and lower limb paralysis. This disease recently has become quite politicized, with many interest groups attempting to implicate it as the cause of a chronic fatigue-like syndrome.
Fig. 5.
Lyme disease. Epidemiology and clinical manifstations. Counterclockwise from top left: deermouse (chat.carelton.ca); Borrelia burgdorferi, scanning electron micrograph (utopiasilver.com); deer (gardenpa.com); erythema chronicum migrans (antibiotikamonitor.at); ECG showing heart block (childrenshospital.org); facial palsy caused by Lyme disease (lib.uiowa.edu); deerticks, male and female (health.state.mn.us).
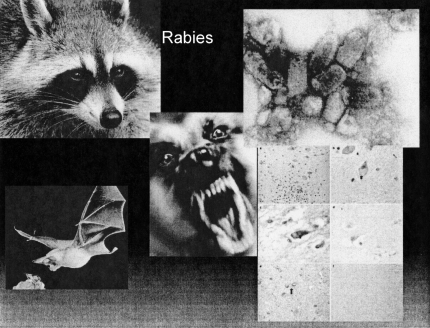
Rabies is perhaps the most well known and most feared zoonosis. It is spread by the bite of a rabid animal. In previous times feral animals such as raccoons and skunks were the prime source of infection, but today the most common rabid bite is from bats (Figure 6).
Fig. 6.
Rabies. Counterclockwise from top left: Raccoon (usanimal.control); bat (ma-watertown.civicplus.com); Negri bodies in the brain (nature.com); rabies virus (wadsworth.org); mad dog (pharmj.com).
These mammals can carry the virus, an RNA bullet-shaped virus member of the lyssavirus genus, asymptomatically, and the bite of the bat may be painless. These two facts have given rise to the recommendation that a sleeping person who awakens in the presence of a bat, should be immunized against rabies even if there is no evidence of a bite. Because the virus is spread along nerves, the incubation period is roughly proportionate to the distance of the bite from the brain. An exception is a bite on the hand, which probably because of the plentiful enervation of the hand, may have a short incubation period. The current post-exposure prophylaxis involves 5 vaccinations with a recombinant vaccine over an 18 week period, and unlike vaccination with the previous monkey spinal cord-derived vaccine is painless and without serious side effects. Rabies infection leads to encephalitis; pathological examination reveals pathognemonic Negri bodies.
In India, where rabies is still relatively common, roaming wild dogs are the prime source for human rabies. For this reason, pre-exposure vaccination is recommended if a trip to rural India is planned.
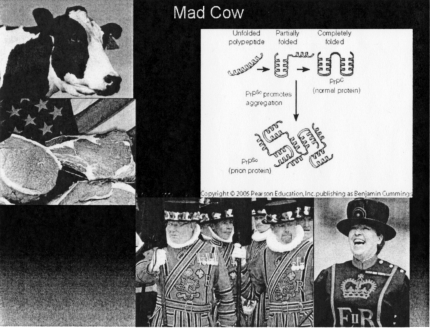
Inclusion of Mad Cow Disease or human variant bovine spongiform encephalitis among the zoonoses is controversial. However, I believe it is justifiable. The cause of the disease is an entity called a prion—itself a controversial “infectious agent”. Unlike all other known infectious agents, the prion does not contain any nucleic acid (RNA or DNA). It is a protein that does not so much replicate, as it induces other normal proteins to rearrange their tertiary structure, thereby creating non-functional or disease-related proteins. The disease is contracted by ingestion of prion-containing food, and the most common source for these prions in the human diet is raw or undercooked beef—hence the Beefeaters in Figure 7, although prions also have been found in elk, and sheep. After a latent period that may last decades, the infected individual develops progressive spongiform encephalopathy eventually leading to death. There is no cure for this disease.
Fig. 7.
Mad Cow Disease. Counterclockwise from top left: cow (us.emb.japan.go); raw steak (cbsnews.com); beefeaters (businessinovationinsider.com; smh.com) prions (Wellesley.edu).
Raw beef is also a prime source of another zoonosis, toxoplasmosis that I won't have time to discuss here.
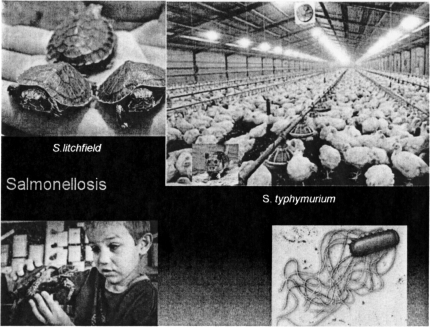
Finishing of the group of zoonoses wherein the mode of acquisition is ingestion, are a group of salmonella infections. Salmonellae are flagellated Gram negative rods. Reptiles, including small pet turtles carry salmonella in their intestines. One of these salmonella species is S. litchfield. There have been many case reports of salmonella infections in young children who have handled small pet turtles, and sale of these turtles is prohibited for this reason in most states. These animals continue to surface from time to time and cause diarrheal disease.
Another salmonella, S. typhimurium is found as a contaminant of almost 100% of commercially raised chickens. The organism derives its name from the fact that it is indigenous to many mice. These mice, in turn, inhabit hen houses (Figure 8) and the chickens ingest the organism along with their feed, which is contaminated with murine feces. The two things that protect us from diarrheal illness every time we eat chicken are cooking the meat thoroughly and inactivation of some of the residual organisms by our gastric acid.
Fig. 8.
Salmonella Infection. Counterclockwise from top left: turtles (blog.nola.com.cdc.gov); salmonella bacterium (geocities.com); chicken farm (source unknown).
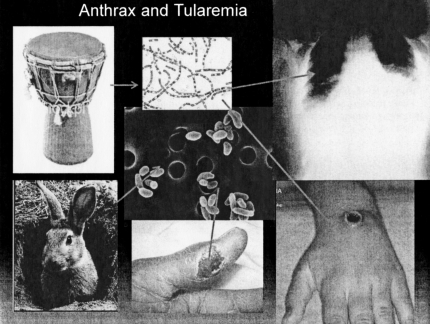
Finally, we have a group of zoonotic organisms that produce severe disease either by direct skin contact or through inspiration of the offending microbe. One of these diseases that has gained recent notoriety is anthrax. Bacillis anthracis is a Gram positive spore-forming rod found in nature on the skins of many animals. Actually, the most common infection caused by this organism is a necrotizing skin ulcer caused by introducing the bug directly through abrasion of the skin. There have been several recent reports of drum makers acquiring infection from contaminated hides. Much less common is the inhalation of the anthrax spores resulting in pneumonia. Tularemia, caused by a Gram negative rod, Francisella tularensis also causes both a skin ulcer, primarily in hunters and trappers who skin animals, and again less often, systemic disease and pneumonia (Figure 9.). What ties these two organisms even more closely is their potential ability to be weaponized so that they could be spread through the air by a bioterrorist causing mass casualties (Figure 10).
Fig. 9.
Anthrax and tularemia. Counterclockwise from top left: drum (l.istockphoto.com); rabbit (gatos.mascotia.com); tularemia skin ulcer (anthropozoonoses.it); anthrax ulcer (io.com) anthrax pneumonia (chip.med.nyu.edu); anthrax bacillus (health.utah.gov): tularemia organism (health.utah.gov).
Fig. 10.
Anthrax as a bioterrorist agent. Letter containing anthrax organisms (dshs.state.tx.us); testing samples on Capital Hill (news.service.stanford.edu); crop duster (skysailing.com).
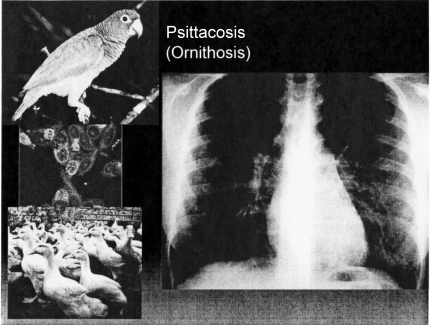
Psittacosis and ornithosis are spread only by inhalation. The hosts are birds either psittacine birds such as parrots, macaws, parakeets, or non-psittacine birds (ducks and geese), The organism is an obligate intracellular parasite, Chlamydophila pneumonia— similar to the organisms that cause trachoma, inclusion conjunctivitis and lymphogranuloma venereum. The cytologic hallmark of all chlamydial infections is the finding of typical intra-cytoplasmic inclusion bodies in infected cells (Figure 11). Commonly, infection leads to an interstitial pneumonia indistinguishable from mycoplasma or other atypical pneumonias. There is little if any person-to-person spread. It is this infection that provides the infectious disease consultant with his or her most potent weapon in acquiring a significant piece of medical history that others have missed: the question, “do you have a pet at home?” The bird may seem entirely healthy. Because many of the psittacine birds are members of endangered species, there is a black market in these birds, and the regulation that legally imported birds be quarantined and treated with tetracycline to eliminate infection, is often circumvented by smugglers.
Fig. 11.
Psittacosis/Ornithosis. Counterclockwise from top left: parrot (rainforrestinn.com) chlamydophila pneumoniae infecting cells (Newcastle.edu); ducks (madgettsfarm.co.uk); Chlamydia pneumonia (cielo.cl).
I would like to conclude with a brief discussion of the 800 pound gorilla of respiratory zoonoses: avian influenza. Ordinary pandemic influenza does not fit into my criteria for inclusion in this presentation in that, even though it has animal reservoirs, it has been fully adapted to person-to-person spread. It is well to remember that in a 2–3 year period from 1918 to 1921, pandemic influenza A, so called Spanish flu, killed more people than have died of AIDS in the 25 years since it was first described. Many of the victims of this pandemic were young adults as opposed to the elderly and infirm who have the highest mortality in most other infectious outbreaks. Because influenza pandemics often start in the hog raising centers of China, it has been postulated for many years that pigs and the foul that live in close proximity to them serve as animal reservoirs for the influenza virus. In 1997 we experienced the first documented cases of direct spread from foul to humans. A newly recognized avian influenza virus with type 1 hemagglutinin and type 5 neuraminidase (H1N5) caused 18 cases of influenza in Hong Kong. The majority of cases were in children, the common epidemiologic factor was recent exposure to live chickens; the mortality was a staggering 33%. The outbreak was truncated by rapid epidemiological analysis and the slaughtering of millions of chickens. Things were pretty quiet after that until 2003-4 when 37 cases were reported throughout Southeast Asia with 26 deaths (a 66% mortality rate).
By the middle of 2007 there had been 329 cases reported throughout Asia with 201 deaths—a 61% mortality. There have been only two documented person-to-person infections, although a family outbreak in Indonesia implicated some person-to-person spread. Oseltamiver, an anti-neuraminidase antiviral agent seems to reduce mortality, but the level is still at about 50% in treated patients. The high mortality in young healthy people in both the 1918 and current Avian outbreaks seems to be attributable to a so called cytokine storm in which an overwhelming immune-modulatory response is evoked by the virus. Spread to birds in this country in the near future is almost a certainty; spread from birds to humans in this country is highly likely at some point; but adaptation to person-to-person spread here or elsewhere remains a daunting threat.
The reservoir for avian influenza virus is migratory shore birds. As with all zoonoses, the existence of a large animal reservoir means that we will never be able to eliminate these agents from our environment as we can with organisms for which humans are the only host.
FINANCIAL SUPPORT AND CONFLICT OF INTEREST
I have received no financial support referable to any aspect of this presentation, but I do love my dog.
DISCUSSION
Mackowiak, Baltimore: Terrific presentation, Steve. Just a quick thought and a question that relates to West Nile virus …rather than developing immunity, I wonder if the decrease in incidence of the disorder in the East is related more to a die-off of susceptible black birds, and that's the reason why Washington State has no cases. I have been following West Nile virus activity regularly in the Weekly Morbidity and Mortality Report, and I think there still are no cases in Washington. I would be interested in your thoughts as to why Washington State might be protected from West Nile Virus.
Baum, New York: I have no idea. Sorry but I really have no idea.
Chapman, Jackson: With respect to Lyme disease, STARI is another disease that can mimic Lyme disease and which we are probably seeing in the Southeastern United States. Would you comment about that, about the long-term sequelae? Do we know anything more about the long-term sequelae of STARI, Southern Tick-Associated Rash Illness?
Baum, New York: I think Lyme has become such a touchy and politicized issue this year in the Infectious Diseases Society. They are worried about people being over-treated, because the chronic disease syndrome is now attributed to Lyme in many cases as it was attributed to herpes a decade and a half ago. I really don't know anything about that. I think there may be variants of Lyme. I think there may be others that we don't know about, and this is one of my reasons for picking Zoonoses. I think most of the emerging infections are going to turn out to be Zoonoses and this may be another one that is yet undetected. For those of you who are non-infectious disease folks, the hypothesis is that we only know about 10% of all of the potentially infecting organisms that exist in the world, and obviously, these can tell us at least about some of the other 90%.
Henrich, San Antonio: Just a quick question about other wasting diseases: I think everybody is well familiar with the story about eating meat from cattle and so forth. But you hear about other wasting diseases in elk and other animals. Are those presumed to be prion diseases as well?
Baum, New York: Yes, I think they are. I was going to mention elk, because we are in elk country, and one of the biggest fights I had with one of my dear friends is telling him not to eat elk out here. He lives in Colorado. So I think they are. We knew about Scrapie, and we knew about Kuru, which Dr. Gajdusek described and won the Nobel Prize for. I think many of those wasting diseases probably are prion diseases, and just again for information, you can't kill this stuff. You can boil it; you can radiate it; you can do anything you want, and that is why the controversy is to whether or not it is a living form. Certainly, they can be transmitted from one animal, man, person, whatever to another, and that is my definition of an infectious disease. The organism is really weird.
Jordan, Los Angeles: I am a transplant doctor, and in the last year or so, there have been several reports of rabies being transmitted by organ transplant, and West Nile virus, with basically death in all of the recipients very rapidly in an immunosuppressed patient. I have also been personally involved with patients in Singapore who died after receiving organs from a patient infected with dengue that was not recognized and caused very fulminate hemorrhagic death. For us, this is a real difficult problem in terms of trying to recognize and diagnose people that may be carriers that were unrecognized. Do you have any suggestions in this regard?
Baum, New York: None that apply today. Again, I think PCR-type techniques. I think just as we now screen for HIV, we probably screen for Hep C. We are going to screen for all of these things as time goes by. Jakob-Creutzfeldt has certainly been transmitted with corneal transplants, joint transplants and others. So it is a huge worry with nothing that I think one could do anything about today, except take an incredibly careful history. That would be my major recommendation for this afternoon.
Duma, Daytona Beach: Steve, I enjoyed it very much. I want some personal medical advice from you if I can get it. I am going to play some golf this afternoon. If I stray off the fairway, do you recommend I get a little doxycycline, sort of one capsule prophylactically?
Baum, New York: I don't know, I but again, if a prairie dog has your golf ball in its mouth, try not to take it away.
Lindberg, Bethesda: I wanted to follow up on the question about how to get these fancy lectures available. If the Society makes all of the material freely available through PubMed Central, we will guarantee to get the illustrations, color or back and white, available online pronto. Beyond that, we are interested in experimenting with interactive publications. Many of the surgical journals allow you to see what you would see through instrumentation, and in this case, I think you might be interested in interactive publication such that you could see—“point and click”, so to speak—the data behind the graphs. If the Society wants to engage in that experiment, I would be happy to work it out with you.
Du Pont, Houston: I am going to make one comment about Zoonoses after Steve's nice talk. Jim Steele, as many of you know, who was the Chief Vet at the CDC and who retired from the CDC, came to the Houston School of Public Health with his concept of “one health”. The health of animals and the health of people are linked and you cannot have good human health unless you have good animal health. That is a Steele sort of quote, and I think Steve Baum's presentation certainly emphasizes that.