Abstract
The neuroendocrine control of reproduction in all mammals is governed by a hypothalamic neural network of approximately 1,500 gonadotropin releasing hormone (GnRH) secreting neurons that control activity of the reproductive axis across life. Recently, the syndrome of human GnRH deficiency, either with anosmia, termed Kallmann Syndrome (KS), or with a normal sense of smell, termed normosmic Idiopathic Hypogonadotropic Hypogonadism (nIHH), have proven important disease models that have revealed much about the abnormalities that can befall the GnRH neurons as they differentiate, migrate, form networks, mature and senesce. Mutations in several genes responsible for these highly coordinated developmental processes have thus been unearthed by the study of this prismatic disease model. This paper discusses several of the more important discoveries in this rapidly evolving field and puts them into a developmental and physiologic context.
Within all mammals, a single gene, GnRH, and the neuronal network of approximately 1,500 neurons that secrete this decapeptide in a coordinated, pulsatile fashion serve as the pilot light of reproduction. Activity of this neural network initiates full reproductive activity during the neonatal period, silences it throughout most of childhood, only to reactivate full sexual maturation when unknown metabolic cues to the hypothalamus signal the body's readiness to enter puberty (1–4). From an evolutionary perspective, species have had to constantly modify the activity of this critical hypothalamic pacemaker of reproduction to survive and evolve amidst an ever-changing repertoire of environmental threats to the species. Consequently, hypothalamic GnRH neurons have somehow acquired the ability to sense these threats and adapt their secretory behavoir to dramatically changing environmental signals. These contextural changes include wide swings in food availability during feast/famine cycles; dramatic differences in exercise and caloric needs during long migrations; ever-changing light-dark cycles that cue seasonal breeding; and signaling of reproductive readiness in breeding females among numerous other environmental and predatory threats.
This unique biology of GnRH contrasts sharply with other biologic systems crucial for survival wherein large families of genes with overlapping biologic functions have typically evolved to envelope such critical functions in layered, evolutionary redundancy. Such biologic back-up is a hallmark of most growth factors, G-coupled protein receptors, transcription factors, peptide ligands, pheromones, taste and olfactory receptors. With all mammalian reproductive activity being invested solely in a single gene/neuronal system, it is thus likely that genes and pathways underlying GnRH itself must have evolved to encompass many of these modulatory functions to protect the reproductive system in mammals. To date, however, our understanding of these components of this complex developmental biology of GnRH and its genetic control has been limited. The availability of information derived from the Human Genome Project began to change this beginning in the early 1990's with the discovery of the KAL1 gene.
Lessons for GnRH Neuronal Biology from Discovery of The KAL1 Gene
The origins of GnRH neurons are extra-CNS in the nasal placode (5, 6). Once they commit to a fate as GnRH neurons, these neurons leave the nasal placode following the lead of the olfactory epithelium, migrate into the CNS via the olfactory bulb and tract, and ultimately halt their progress at the arcuate nucleus of the hypothalamus. There, they extend axonal processes to communicate with each other forming a network and extend dendrites into the median eminence. There, this GnRH neuronal network somehow synchronizes its secretory activity via the development of a coherent pulsatile pattern of release of GnRH, a decapeptide, into the hypophyseal-portal circulation as is required to evoke physiologic gonadotropin secretion from the pituitary gonadotropes via internal pacemaker activity (7). The entire migratory journey of these GnRH neurons is guided by unknown signals from the olfactory epithelium, bulb, as well as possibly other cells to arrive at their ultimate destination, the arcuate nucleus and median eminence of the hypothalamus.
KAL1 was the first gene discovered to be a critical determinant of the GnRH developmental pathway by the study of a single patient with KS who had a contiguous gene syndrome associated with a deletion of the Xp21 region (8–10). The protein product of KAL1, anosmin, is apparently secreted from olfactory neurons and is required for the formation of the olfactory guidance system for GnRH neurons and/or development of the olfactory track. This function was deduced since in the absence of anosmin (i.e. patients with KS), GnRH neurons arrest their migratory march from the olfactory placode into the CNS at the cribiform plate (10), never entering the CNS nor arriving at the hypothalamus. The study of a single male child with Kallmann Syndrome who inherited a defective X chromosome lacking the Xp21 tip from his mother, who was unaffected since she had presumably selectively inactivated this region (10). This mother subsequently became pregnant with a second male child with an identical Xp deletion. The post-mortem of the second boy revealed that by 19 weeks, his GnRH neurons had differentiated from the olfactory placode and had migrated into the CNS as far as the cribiform plate but had failed to enter the central nervous system. This failure was associated with a lack of the required olfactory epithelium, bulb and tracks over which GnRH neurons must traverse to arrive at their ultimate anatomic destination, the arcuate nucleus of the hypothalamus, where they initiate reproductive competency. These first two brothers heralded the beginning of the genetic era in reproductive endocrine control of reproduction. Ever since the combined study of phenotypes and genotypes of GnRH deficient patients with KS and nIHH have proven remarkably successful in elucidating the biologic complexity of this developmental system (11).
GPR54 and Kisspeptin
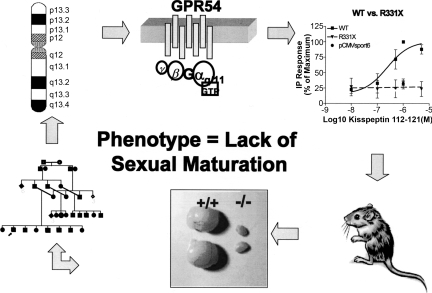
The next critically important new finding about the ontogeny of GnRH neurons came from the discovery of a new and entirely unsuspected system that proved to be an upstream gatekeeper of GnRH neurons (11,12). Upon his return to his native country of Kuwait, a former fellow of ours was charged with finding a Bedouin family with GnRH deficiency and a normal sense of smell (i.e. nIHH). This form of GnRH deficiency, like its anosmic variant, KS, traditionally presents with a lack of sexual maturation by age 18, associated with low gonadotropins and no anatomic reason for this failure of sexual maturation. Earlier presentations of GnRH deficiency may be suggested by the findings of cryptordism, microphallus or other congential anomalies (skeletal, hearing, cardiac, etc), in association with low gonadotropins during the neonatal period of reproductive activity. Such a family was located in Kuwait (Fig. 1). This finding led to a remarkable chain of discoveries, including linkage to and ultimately isolation of a new gene, GPR54, and its cognate ligand, kisspeptin. This ligand/receptor system has now been demonstrated to control sexual maturity in all primate species studied to date (11,12). Animal knock-outs of both receptor and ligand have been recreated, confirming the central role for this system, and their study is yielding a harvest of information about their control in several species, including the human (13–18).
Fig. 1.
Pathway of Discovery of the GPR54 Gene, Mutations in its Sequence Leading to GnRH Deficiency in the Human, and Animal Knock-outs as Occurred in Reference 12. A pedigree (lower left) led to linkage (upper left) which led to demonstration of a biologic defect in the (then) candidate gene, GPR54, (upper right) which led to animal knock-outs (lower right) that demonstrated hypogonadism (lower center).
FGFR1 = KAL2
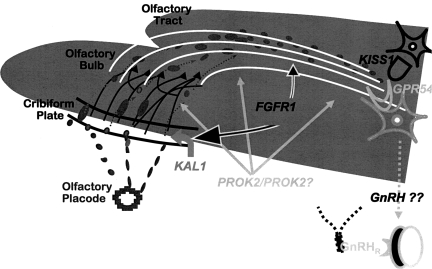
A chromosomal breakpoint associated with a patient with Kallmann Syndrome was located on chromosome 8p, an area of interest since the GnRH gene was somewhat close to this region (19). Careful mapping studies of this breakpoint revealed that a disruption of the FGFR1 gene, one of only 4 tyrosine kinase receptors for a family of 23 ligands required for fibroblast growth, occurred in these families. Mutations in this gene were shown to account for roughly 10% of cases of KS (20). What was a surprise was the finding that nearly an identical number of cases of nIHH were associated with mutations in FGFR1 (21,22). Demonstrating that FGFR1 mutations can cause both KS and nIHH established an important precedent. This finding meant that FGFR1 (and presumably one of its ligands yet to be identified) were critical not only for the migration of GnRH neurons across the olfactory tracts but also demonstrated that an entirely different mechanism must exist for the failure of GnRH in association with those cases with normal olfactory bulbs, tracks and senses of smell. This finding of FGFR1 underlying nIHH added a new dimension to the complexity of the spectrum of GnRH neuronal mechanisms necessary for GnRH neurons to undergo by demonstrating that their olfactory tracking of the GnRH neurons was only a portion of the mechanisms by which GnRH ontogeny could go awry. Similarly, the fact that some GnRH deficient patients with mutations in FGFR1, following a diagnosis of clear-cut nIHH with no evidence of sexual maturation by age 18, subsequently exhibited normal puberty, sexual maturation and fertility after receiving hormonal replacement therapy (23, 24). That is to say, about 10% of such patients underwent complete reversals of their syndrome (24). Still others with FGFR1 mutations demonstrated a secondary failure of their reproductive axis or acquired forms of GnRH deficiency responsive to exogenous GnRH. This additional phenotypic complexity added yet another suggestion that mutations in FGFR1, in addition to presumably playing a critical role in GnRH neuronal maturation, may very well have something to do with neuronal survival and possibly apoptosis in nIHH patients with FGFR1 mutations (Fig. 2).
Fig. 2.
GnRH Neuronal Ontogeny: A Story Told by Patients with GnRH Deficiency.
PROK2 and PROKR2
Most recently, mutations in another pair of ligand/receptor genes have been demonstrated to cause human GnRH deficiency in patients with both a normal sense of smell (nIHH) and anosmia (KS). The prokineticin system is composed of 2 nearly identical (>85% gene holomology) receptors (GPR 73 a & b) in the rhodopsin family of receptors like GPR54 (25). Their two polypeptide ligands, PROK1 and PROK2, have considerably less homology and exhibit quite different anatomic distributions. Whereas PROK1 and its receptor, PROKR1, are primarily restricted to the GI tract and appear responsible for motility, PROK2 and PROKR2 have a decidedly more neuroendocrine profile, being located in the arcuate nucleus, olfactory track and suprachiasmatic nucleus (SCN) (i.e. the home of the CNS' master clock genes) (26,27). Murine knock-outs of both ligand and receptor (27–30) produce a spectrum of defects within the reproductive system, and their human counterparts show GnRH deficiency, again with widespread clinical phenotypes in both the ligand and the receptor (31,32). At the moment, the murine knock-outs of PROK2 require loss of both alleles, whereas the genetics of the receptor, PROKR2, are less clear, particularly in the human (27–32). Information about this ligand/receptor pair will be important to watch as they evolve because of the close links between the body's biologic clock and reproduction.
Digenicity and Oligogenicity
It has become clear that when reviewing single gene defects and their genetic segration both within single GnRH deficient pedigrees or contrasting phenotypes of identical mutations across families from different parts of the world, striking departures from Mendelian expectations occur (33). The most recent evidence from our group suggests that when these phenomenon are encountered, this lack of precise segregation can be attributed to the presence of mutations in a second (or more) gene(s) in the same families whose previous lack of segregation is explainable by the existence of digenic defects in some family members, accounting for the more severe phenotypes. Thus, oligenicity is likely to be an important future trend to seek in GnRH deficiency and other scenarios where rare, single gene variations do not explain all of the findings within a pedigree but rather suggest important interactions with other modifying genes. The emerging notion here is that each of the single gene defects may cause only mild (or even no) defects when occurring on their own but synergize to produce some surprising phenotypes when co-inherited.
Summary
Several new genetic tools, including use of continguous gene syndromes, linkage, animal knock-outs and mapping of chromosomal breakpoints associated with phenotypes, have proven to be unique biology opportunities to understand the spectrum of GnRH deficiency in the human. More broadly, such defects have begun to synergize to produce interesting opportunities to understand the ontogeny of the GnRH neuroendocrine network, a neural network critical to the evolution of species. Subtler defects in these genes may well be responsible for more common reproductive disorders and determine an individual's susceptibility to them, such as stress-related amenorrhea, weight loss related loss or reproductive function, or even polycystic ovarian disease.
DISCUSSION
Nachman, New York: Tell us more about the link between reproduction and the olfactory system that seems so important to many of these defects in GnRH neuronal biology?
Crowley, MA: That's a great question, To re-paraphrase it: How come olfaction and reproduction are so tightly linked? If you really think about this issue, in certain rare species, pheromones are the critical attractants for reproduction. You have to be able to tell when there is another wild bear in estrous five or ten miles away if you are a bear needing to reproduce. If you think about the affinity of the ligand/receptors requiring that pheromones be sensed in low concentrations so many miles away, it is impressive. So olfaction and reproduction are linked for a successful reproductive fitness strategy. Similarly being able to smell predators would have a similar reproductive advantage. A similar sort of evolutionarily thinking about finding mates in an otherwise difficult circumstance might obtain. Hence olfaction and successful speciation are linked tightly. The fact that the GnRH neurons require migration over the olfactory bulb and tracts and that this embryonic pathway is the key linker here, in retrospect, makes an evolutionary story that is rather interesting.
BIBLIOGRAPHY
- 1.Grumbach MM, Kaplan SL. The neuroendocrinology of human puberty: an ontogenetic perspective. In: Grumbach MM, Sizonenko PC, Aubert ML, editors. Control of the onset of puberty. Baltimore: Williams & Wilkins; 1990. pp. 1–68. [Google Scholar]
- 2.Ross JL, Loriaux DL, Cutler GB., Jr Developmental changes in neuroendocrine regulation ofgonadotropin secretion in gonadal dysgenesis. J Clin Endocrinol Metab. 1983;57:288–293. doi: 10.1210/jcem-57-2-288. [DOI] [PubMed] [Google Scholar]
- 3.Conn PM, Hsueh AJW, Crowley WF., Jr Gonadotropin-releasing hormone: Molecular andcell biology, physiology, and clinical applications. Fed Proc. 1984;43:2351–2361. [PubMed] [Google Scholar]
- 4.Conn PM, Crowley WF. Gonadotropin-releasing hormone and its analogues. N Engl J Med. 1991;324:93–103. doi: 10.1056/NEJM199101103240205. [DOI] [PubMed] [Google Scholar]
- 5.Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Molecular Brain Research. 1989;6:311–326. doi: 10.1016/0169-328x(89)90076-4. [DOI] [PubMed] [Google Scholar]
- 6.Wray S, Nieburgs A, Elkabes S. Spatiotemporal cell expression of luteinizing hormone releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain Res Dev Brain Res. 1989;46:309–318. doi: 10.1016/0165-3806(89)90295-2. [DOI] [PubMed] [Google Scholar]
- 7.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 8.Legouis R, Hardelin JP, Levilliers J, et al. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell. 1991;67:423. doi: 10.1016/0092-8674(91)90193-3. [DOI] [PubMed] [Google Scholar]
- 9.Franco B, Guioli S, Pragliola A, et al. A gene deleted in Kallmann's syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353:529. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- 10.Bick D, Franco B, Sherins RJ, Heye B, Pike L, Crawford J, Maddalena A, Incerti B, Pragliola A, Meitinger T, et al. Brief report: intragenic deletion of the KALIG-1 gene in Kallmann's syndrome [see comments] N Engl J Med. 1992;326:1752–1755. doi: 10.1056/NEJM199206253262606. [DOI] [PubMed] [Google Scholar]
- 11.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 13.Navarro VM, Fernandez-Fernandez R, Castellano JM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano JM, Navarro VM, Fernandez-Fernandez R, et al. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 15.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004 Sep;145(9):4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 16.Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant T. M. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148(7):3364–70. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- 17.Seminara SB, Dipietro MJ, Ramaswamy S, Crowley WF, Jr., Plant TM. Continuous human metastin 45–54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male Rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147(5):2122–6. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]
- 18.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148(10):4927–36. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 19.Dode C, Levilliers J, Dupont JM, De Paepe A, Le Du N, Soussi-Yanicostas N, Coimbra RS, Delmaghani S, Compain-Nouaille S, Baverel F, et al. Loss-of-function mutations in FGFR1 cause autosomal dominant Kallmann syndrome. Nat Genet. 2003;33:463–465. doi: 10.1038/ng1122. [DOI] [PubMed] [Google Scholar]
- 20.Sato N, Katsumata N, Kagami M, Hasegawa T, Hori N, Kawakita S, Minowada S, Shimotsuka A, Shishiba Y, Yokozawa M, et al. Clinical assessment and mutation analysis of Kallmann syndrome 1 (KAL1) and fibroblast growth factor receptor 1 (FGFR1, or KAL2) in five families and 18 sporadic patients. J Clin Endocrinol Metab. 2004;89:1079–1088. doi: 10.1210/jc.2003-030476. [DOI] [PubMed] [Google Scholar]
- 21.Pitteloud N, Acierno JS, Jr., Meysing A, Eliseenkova AV, Ma J, Ibrahimi OA, Metzger DL, Hayes FJ, Dwyer AA, Hughes VA, et al. Mutations in fibroblast growth factor receptor 1 cause both Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci US A. 2006;103:6281–6286. doi: 10.1073/pnas.0600962103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trarbach EB, Costa EM, Versiani B, de Castro M, Baptista MT, Garmes HM, de Mendonca BB, Latronico AC. Novel fibroblast growth factor receptor 1 mutations in patients with congenital hypogonadotropic hypogonadism with and without anosmia. J Clin Endocrinol Metab. 2006;91:4006–4012. doi: 10.1210/jc.2005-2793. [DOI] [PubMed] [Google Scholar]
- 23.Pitteloud N, Acierno JS, Jr., Meysing AU, Dwyer AA, Hayes FJ, Crowley WF., Jr Reversible kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J Clin Endocrinol Metab. 2005;90:1317–1322. doi: 10.1210/jc.2004-1361. [DOI] [PubMed] [Google Scholar]
- 24.Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, Hughes VA, Cole LW, Pearce SH, Lee H, Boepple PB, Crowley WF, Pitteloud N. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007 Aug 30;357(9):863–73. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 26.Cheng MY, Leslie FM, Zhou QY. Expression of prokineticins and their receptors in the adult mouse brain. J Comp Neurol. 2006;498:796–809. doi: 10.1002/cne.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 28.Li JD, Hu WP, Boehmer L, Cheng MY, Lee AG, Jilek A, Siegel JM, Zhou QY. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623. doi: 10.1523/JNEUROSCI.3679-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng KL, Li JD., Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, et al. Kallmann Syndrome: Mutations in the Genes Encoding Prokineticin-2 and Prokineticin Receptor-2. PLoS Genet. 2006;2(10):e175. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole L, Plummer L, Jacobson-Dickman EE, Mellon P, Zhou QY, Crowley WF., Jr Loss-of-function mutation in the prokineticin 2 gene cause Kallmann Syndrome and normosmic Idiopathic Hypogonadotropic Hypogonadism. PNAS. 2007;104:1447–1452. doi: 10.1073/pnas.0707173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, et al. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117:457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]