Abstract
Bovine adrenal zona fasciculata (AZF) cells express bTREK-1 K+ channels that set the resting membrane potential. Inhibition of these channels by adrenocorticotropic hormone (ACTH) is coupled to membrane depolarization and cortisol secretion. Curcumin, a phytochemical with medicinal properties extracted from the spice turmeric, was found to modulate both bTREK-1 K+ currents and cortisol secretion from AZF cells. In whole-cell patch clamp experiments, curcumin inhibited bTREK-1 with an IC50 of 0.93μM by a mechanism that was voltage-independent. bTREK-1 inhibition by curcumin occurred through interaction with an external binding site and was independent of ATP hydrolysis. Curcumin produced a concentration-dependent increase in cortisol secretion that persisted for up to 24 h. At a maximally effective concentration of 50 μM, curcumin increased secretion as much as10-fold. These results demonstrate that curcumin potently inhibits bTREK-1 K+ channels and stimulates cortisol secretion from bovine AZF cells. The inhibition of bTREK-1 by curcumin may be linked to cortisol secretion through membrane depolarization. Since TREK-1 is widely expressed in a variety of cells, it is likely that some of the biological actions of curcumin, including its therapeutic effects, may be mediated through inhibition of these K+ channels.
Keywords: Curcumin, TREK-1, Ion channel, Cortisol, Adrenal
Curcumin (diferulomethane) is a polyphenolic compound found in the curry spice turmeric which is obtained from the roots of the Curcuma Longa plant [1]. In addition to its use as a spice, where daily intake may reach several grams, this phytochemical has been used for centuries in traditional Indian medicine to treat a wide range of diseases and conditions [1,2]. Modern research has shown curcumin to exhibit antioxidant, anti-inflammatory, antiviral, antibacterial, antifungal, and anti-tumor activities [2,3]. As a result, curcumin may be useful as a therapeutic agent in the treatment of diseases, ranging from allergies and arthritis to Alzheimer’s disease, diabetes, malignancies, including prostate cancer, and congestive heart failure [2,4,5]. Curcumin is currently being tested in separate clinical trials to determine its effectiveness in the treatment of colon and pancreatic cancer, multiple myeloma, cystic fibrosis, psoriasis, and Alzheimer’s disease [3].
At the molecular level, the therapeutic properties of curcumin may be mediated through its well-documented effects on a host of intracellular signaling pathways that ultimately modulate the expression or activity of transcription factors, enzymes, growthfactors, and cytokines [1–3]. Curcumin has also been shown to modulate the activity of several types of ion channels. These include the cystic fibrosis transmembrane conductance Cl− channel, and the inositol 1,4,5-triphosphate receptor Ca2+ channel [6,7]. Recently, we showed that curcumin inhibited the voltage-gated Kv1.4 K+ channel of bovine adrenocortical zona fasciculata (AZF) cells with an IC50 of 4.4 μM [8].
The ability of curcumin to modulate the activity of these three unrelated ion channels suggests that this agent could affect the activity of other channels, including those expressed in secretory cells. By modulating the activity of ion channels in secretory cells, curcumin could enhance or inhibit the secretion of hormones and neurotransmitters. In this regard, bovine AZF cells synthesize cortisol from cholesterol in response to stimulation by ACTH or AngII. Although the signaling pathways by which these peptide hormones regulate cortico-steroidogenesis are only partially understood, a role for ion channels and depolarization-dependent Ca2+ entry has been established for both ACTH and AngII [9–12]. Bovine AZF cells express bTREK-1 K+ channels that set the resting membrane potential [9,13]. Inhibition of these leak-type channels by activation of ACTH or AngII receptors is directly coupled to membrane depolarization, activation of T-type Ca2+ channels, and cortisol secretion [9,10].
Experiments were done to characterize the effect of curcumin on bTREK-1 K+ channels of bovine AZF cells. Curcumin potently inhibited these channels and produced a concentration-dependent increase in cortisol synthesis that was present after 1 h and persisted for at least 24 h.
Materials and methods
Materials
Tissue culture media, antibiotics, fibronectin, and fetal bovine sera (FBS) were obtained from Invitrogen (Carlsbad, CA). Coverslips were from Bellco (Vineland, NJ). Curcumin was from Biomol (Plymouth Meeting, PA). Resveratrol was obtained from EMD Biosciences (San Diego, CA). Phosphate-buffered saline (PBS), BSA, enzymes, 1,2 bis-(2-aminophenoxy) ethane-N,N,N′,N″-tetraacetic acid (BAPTA), MgATP, NaUTP, transferrin, and insulin were obtained from Sigma (St. Louis, MO). Cortisol EIA kit was purchased from Diagnostic Systems Laboratories (Webster, TX).
Isolation and culture of AZF cells
Bovine adrenal glands were obtained from steers (age 2–3 yr) at a local slaughterhouse. Isolated AZF cells were obtained and prepared as previously described [14]. After isolation, cells were either resuspended in DMEM/F12 (1:1) with 10% FBS, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and the antioxidants α-tocopherol (1 μM), 20 nM selenite, and 100 μM ascorbic acid (DMEM/F12+) and plated for immediate use, or divided into 1 ml aliquots and stored in liquid nitrogen. To ensure cell attachment, dishes or coverslips were treated with fibronectin (10 μg/ml) at 37 °C for 30 min then rinsed with sterile PBS before adding cells. For patch clamp experiments, cells were plated in DMEM/F12+ in dishes containing 9 mm2 glass coverslips. Cells were maintained at 37 °C in a humidified atmosphere of 95% air–5% CO2.
Secretion experiments
AZF cells were cultured at a density of 3–5 × 105 cells/dish in DMEM/F12+ as described above. After 24 h, the media was aspirated and changed to defined media consisting of DMEM/Ham’s F12 (1:1), 50 μg/ml BSA, 100 μM ascorbic acid, 1 μM tocopherol, 10 nM insulin, and 10 μg/ml transferrin. Curcumin or resveratrol were added directly to the media from concentrated stock in DMSO. Defined media containing DMSO without curcumin served as the control. DMSO alone (up to 0.2%) did not have any effect on secretion.
Media samples from duplicate dishes were taken at specified times and stored at −20 °C until assayed. Cortisol was measured using a Cortisol EIA from Diagnostic Systems Laboratories (Webster, TX) according to the manufacturer’s directions. To rule out cytotoxicity, we determined the number of viable cells at the end of each experiment using trypan blue exclusion.
Patch clamp experiments
Patch clamp recordings of K+ channel currents were made in the whole-cell configuration. The standard pipette solution consisted of 120 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 11 mM BAPTA, 10 mM HEPES, 5 mM MgATP, and 200 μM GTP, with pH titrated to 6.8 using KOH. Pipette solution of this composition yielded a free Ca2+ concentration of 2.2 × 10−8 M, as determined by the Bound and Determined software program [15]. The external solution consisted of 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, 2 mM MgCl2, 10 mM Hepes, and 5 mM glucose, with pH adjusted to 7.4 using NaOH. The standard external and pipette solutions used for single-channel recording from outside-out patches were identical to those used for whole-cell recordings.
Recording conditions and electronics
AZF cells were used for patch clamp experiments 2–12 h after plating. Typically, cells with diameters <15 μm and capacitances of 10–15 pF were selected. Coverslips were transferred from culture dishes to the recording chamber that was continuously perfused by gravity at a rate of 3–5 ml/min. For whole-cell recordings, patch electrodes with resistances of 1.0–2.0 MΩ were fabricated from Corning 0010 glass (World Precision Instruments, Sarasota, FL). These electrodes yielded access resistances of 1.5–4.0 MΩ and voltage-clamp time constants of <100 μs. K+ currents were recorded at room temperature (22–25 °C) according to the procedure of Hamill et al. [16] using a List EPC-7 patch clamp amplifier.
Pulse generation and data acquisition were done using a personal computer and PCLAMP software with Digidata 1200 interface (Axon Instruments, Inc., Burlingame, CA). Currents were digitized at 2–10 KHz after filtering with an 8-pole Bessel filter (Frequency Devices, Haverhill, MA). Linear leak and capacity currents were subtracted from current records using summed scaled hyperpolarizing steps of ½ to ¼ pulse amplitude. Data were analyzed using CLAMPFIT 9.2 (Molecular Devices, Sunnyvale, CA) and SigmaPlot (version 10.01) software. Drugs were applied by bath perfusion, controlled by a six-way rotary valve.
Results
Curcumin inhibits bTREK-1 K+ currents
Bovine AZF cells express two types of K+ channels. These include voltage-gated, rapidly inactivating Kv1.4 K+ channels, and bTREK-1 K+ channels of the two pore, four transmembrane segment (2P/4TMS) family of leak-type K+ channels that we have characterized with patch clamp and molecular cloning techniques [9,13,17]. In whole-cell patch clamp recordings, bTREK-1 K+ current amplitude typically increases spontaneously to a steady-state value and is enhanced by acidifying the pipette solution [13].
The absence of time- and voltage-dependent inactivation allows bTREK-1 K+ current to be isolated in whole-cell recordings using either of two voltage clamp protocols. When voltage steps of several hundred millisecond duration are applied from a potential of −80 mV, bTREK-1 K+ current can be measured near the end of a voltage step when the transient Kv1.4 current has fully inactivated (Fig. 1A and B; left traces). Alternatively, bTREK-1 K+ current can be selectively activated by an identical voltage step applied immediately after a 10 s prepulse to −20 mV has fully inactivated Kv1.4 channels (Fig. 1A and B; right traces).
Fig 1.
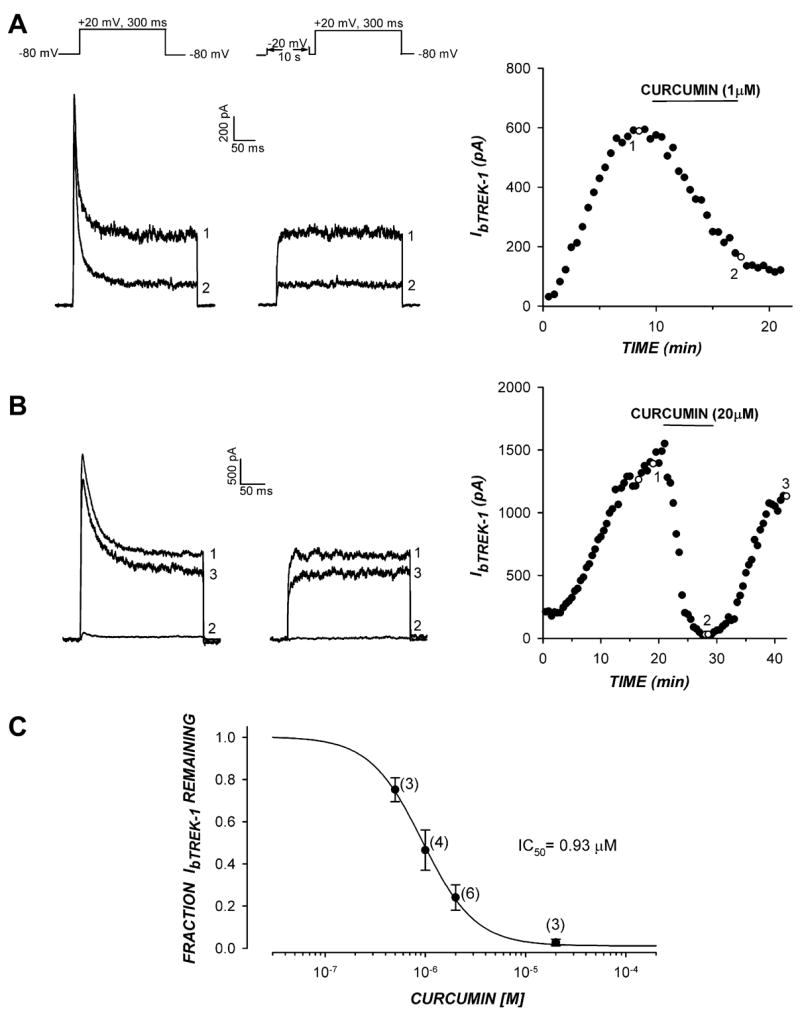
Curcumin inhibits bTREK-1 K+ channels. The inhibition of bTREK-1 K+ current by curcumin was measured in whole-cell patch clamp recordings from bovine adrenocortical cells. K+ currents were recorded at 30 s intervals in response to voltage steps to +20 mV from a holding potential of −80 mV with or without 10 s prepulses to −20 mV. After bTREK-1 reached a stable maximum amplitude, cells were superfused with saline containing curcumin at concentrations ranging from 0.5 to 20 μM. (A,B) K+ currents recorded with (right traces) or without (left traces) 10 s depolarizing prepulses to −20 mV. bTREK-1 amplitudes recorded with (open circles) or without (closed circles) prepulses are plotted against time. Numbers on traces correspond to those on plot. (C) Inhibition curve. Fraction of unblocked bTREK-1 current is plotted against curcumin concentration. Data were fit with an equation of the form: I/Imax = 1/[1 + (X/IC 50)B] were X is the curcumin concentration, B is the Hill coefficient, and IC50 is the concentration that reduces bTREK-1 by 50%. Values are means ± SEM of indicated number of determination.
In a previous study, we found that curcumin inhibited the voltage-gated Kv1.4 channel with an IC50 of 4.4 μM [8]. We now report that curcumin more potently inhibits the structurally unrelated bTREK-1 K+ channels with an IC50 of 0.93 μM (Fig. 1A–C). Fig. 1A shows that, at a concentration of 1 μM, curcumin inhibits the non-inactivating bTREK-1 current by approximately 75%, while the rapidly inactivating Kv1.4 current is largely unblocked. By comparison, at a 20-fold higher concentration, curcumin blocked both Kv1.4 and bTREK-1 current almost completely (Fig. 1B). Inhibition of both K+ currents by curcumin was reversible (Fig. 1B).
The inhibition of bTREK-1 by curcumin appeared to occur through a direct interaction with the channel at the external side of the membrane. When curcumin was applied externally to AZF cells at 20 μM, bTREK-1 was completely inhibited. In contrast, when curcumin was applied intracellularly at the same concentration through the pipette solution, it failed to suppress the time-dependent increase in bTREK-1 expression typically observed in whole-cell recordings (Fig. 2A).
Fig 2.
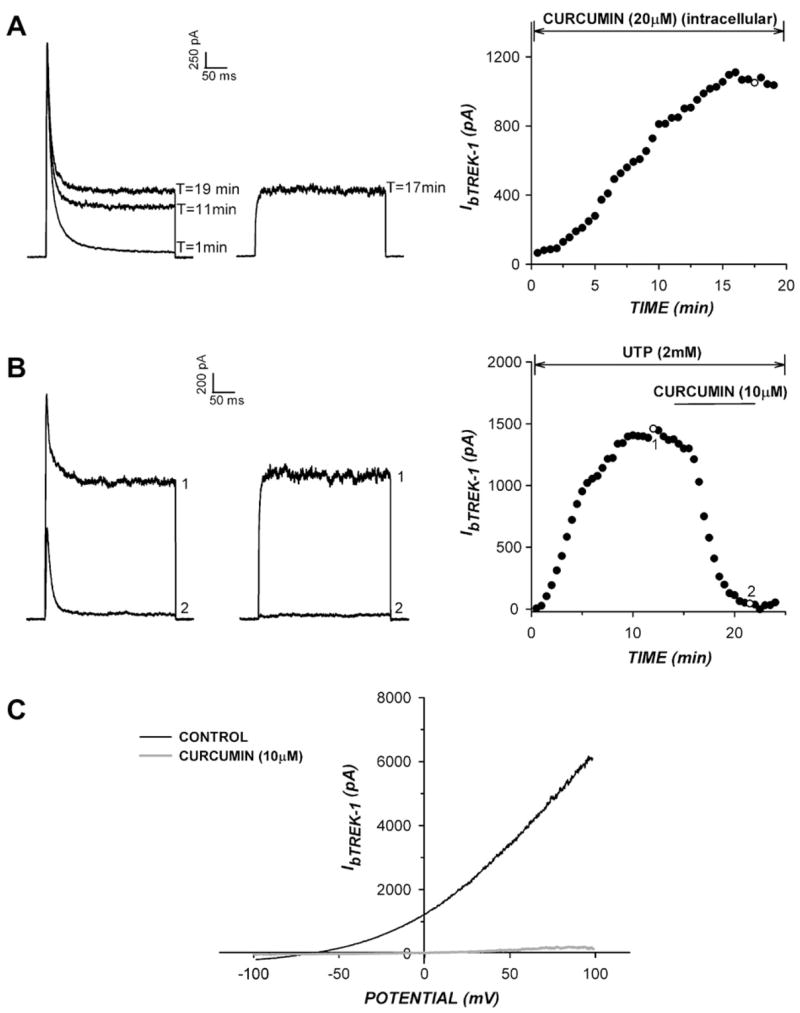
Characteristics of bTREK-1 inhibition by curcumin. (A,B) Curcumin blocks bTREK-1 directly at an external site. Whole-cell K+ currents were recorded in response to voltage steps to +20 mV, applied at 30 s intervals from a holding potential of −80 mV with or without 10 s depolarizing test pulses to −20 mV. (A) Effect of intracellular curcumin on bTREK-1 expression. Whole-cell K+ currents were recorded with (right traces) or without (left traces) depolarizing prepulses with a pipette containing curcumin (20 μM). Current amplitudes are plotted at right. B) Effect of UTP on TREK-1 inhibition by curcumin. K+ currents were recorded with (right traces) or without (left traces) depolarizing prepulses with a pipette containing 2 mM NaUTP in place of MgATP. Current amplitudes are plotted at right. Numbers on plot correspond to those on traces. C) bTREK-1 inhibition by curcumin is voltage-independent. bTREK-1 currents were activated in response to voltage ramps before and after superfusion of curcumin (10 μM). Voltage ramps were applied at 100 mV/s to potentials between +100 and −100 mV from a holding potential of 0 mV. Traces show currents before and after steady state block by curcumin.
The inhibition of bTREK-1 by curcumin occurred independently of kinases or other ATP-dependent signaling pathways. UTP does not serve as a substrate for kinases or ATPases, but enhances the activity of bTREK-1 channels when applied intracellularly through the pipette solution [14]. Substituting NaUTP (2 mM) for MgATP (5 mM) in the pipette had no effect on bTREK-1 inhibition by cur-cumin (Fig. 2B). With UTP in the pipette, curcumin (10 μM) inhibited bTREK-1 by 97.4 ± 1.1% (n = 3).
Although it is a background or leak-type K+ channel, bTREK-1 activation is weakly voltage-dependent, with open probability enhanced at more positive potentials [14]. The inhibition of bTREK-1 by curcumin was voltage-independent over a wide range of test potentials. When bTREK-1 was recorded in response to voltage ramps applied between −100 mV and +100 mV, curcumin (10 μM) blocked the current completely over the entire voltage range (Fig. 2C).
Curcumin stimulates cortisol secretion from AZF cells
The inhibition of bTREK-1 K+ channels by ACTH and AngII is linked to membrane depolarization and cortisol secretion [10]. In addition to inhibiting bTREK-1 K+ channels, curcumin stimulated cortisol secretion by AZF cells. The curcumin-stimulated increase in cortisol secretion was concentration-dependent and persisted for many hours. In the experiment illustrated in Fig. 3A, curcumin (20 μM) induced an increase in cortisol synthesis that was present by 1 h, and reached a value that was 6-fold greater than the time-matched control by 6 h. Curcumin-stimulated increases in cortisol secretion were greatest during the first 6 h, but persisted for up to 24 h (Fig. 3B).
Fig 3.
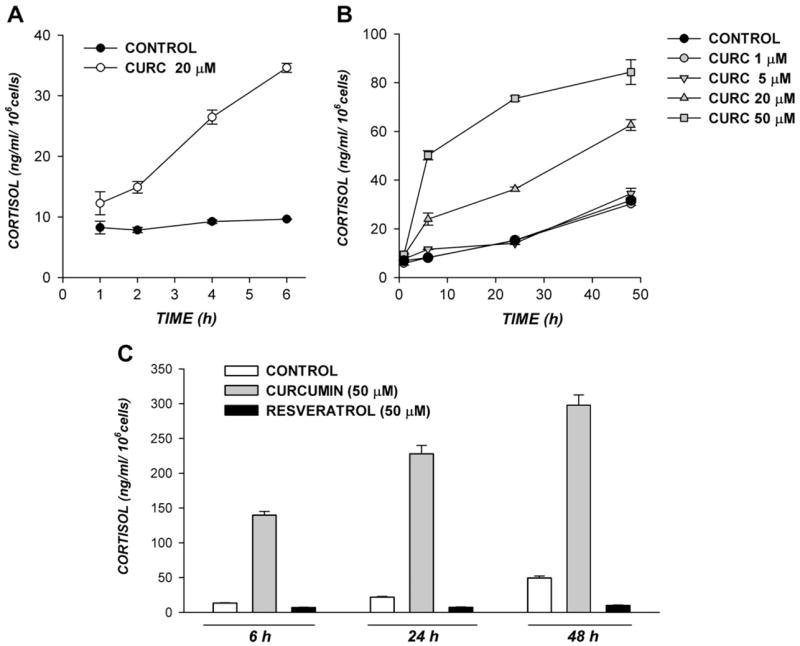
Curcumin stimulates cortisol secretion from AZF cells. Bovine AZF cells in culture were maintained in serum-free, defined media (see Materials and methods) or the same media containing curcumin or resveratrol at the indicated concentrations. Media samples were collected at the indicated times and stored at −20 °C. Cortisol was assayed as described in Methods. Values are expressed as means ± SEM of duplicate cultures, assayed in duplicate.
With respect to potency, curcumin stimulated cortisol secretion at concentrations greater than 5 μM. In the experiment illustrated in Fig. 3B, 50 μM curcumin increased cortisol secretion 6- and 5-fold, respectively, over the control values at 6 and 24 h. Between 24 and 48 h, curcumin stimulated no further increase in cortisol secretion. The effects of curcumin on secretion at concentrations greater than 50 μM were not studied since, at these higher concentrations, curcumin diminished cell viability.
Similar to curcumin, resveratrol is a dietary polyphenol with medicinal properties [18,19]. Resveratrol was compared to curcumin with respect to its ability to stimulate cortisol secretion. Rather than stimulating cortisol secretion, resveratrol inhibited the quantity of cortisol secreted at times ranging from 6 to 48 h by 46% to 77% while diminishing cell viability. In the experiment illustrated in Fig. 3C, at the 24 h time point, curcumin (50 μM) increased the quantity of cortisol synthesized 10-fold over the time-matched control, while resveratrol inhibited cortisol production by 67%.
Discussion
The major findings of this study are that curcumin potently inhibits native bTREK-1 K+ channels and stimulates large increases in cortisol secretion from bovine AZF cells. In this regard, curcumin blocks bTREK-1 channels with an IC50 of 0.93 μM which places it among the most potent inhibitors of this channel identified thus far [20]. With regard to mechanism, curcumin was effective only when applied externally to AZF cells, while inhibition was rapidly reversible and independent of ATP hydrolysis. Thus, although curcumin modulates multiple intracellular signaling pathways, bTREK-1 inhibition appears to occur through a direct interaction with an external binding site on the channel.
The inhibition of hippocampal TREK-1 channels by cAMP reportedly occurs through an A-kinase-dependent mechanism that converts TREK-1 from leak-type to voltage-gated channels. Consequently, cAMP was much less effective at inhibiting TREK-1 K+ channels at increasingly positive test potentials [21]. In contrast, curcumin acts by a different mechanism wherein block is equally effective over a wide range of voltages.
The role of bTREK-1 channels in curcumin-stimulated cortisol secretion is yet to be determined. Curcumin stimulated the synthesis of cortisol at times from 1 to 24 h after which no further increase was observed. In comparison, ACTH and cAMP induce rapid and delayed increases in cortisol synthesis that are mediated by two separate mechanisms. The rapid increases in cortisol production induced by ACTH and cAMP occur in the absence of effects on gene transcription, while the delayed responses involve the enhanced transcription of genes coding for steroidogenic proteins, including steroid hydroxylases that catalyze the conversion of cholesterol to cortisol [22–24]. Curcumin did not increase the expression of steroid hydroxylase mRNAs in bovine AZF cells (unpublished observations). Accordingly, this agent increased cortisol secretion most effectively during the first several hours of exposure.
The rapid stimulation of cortisol secretion by ACTH, cAMP, and curcumin may all occur through mechanisms that involve depolarization-dependent Ca2+ entry in response to bTREK-1 inhibition. However, curcumin inhibited bTREK-1 K+ channels at concentrations lower than those required to stimulate cortisol secretion. In this regard, in the absence of depolarizing inward currents, nearly all bTREK-1 channels would have to be inhibited to effectively depolarize the AZF cell. This would require curcumin concentrations significantly greater than the IC50.
Although our results do not establish a clear, causal relationship between curcumin-mediated bTREK-1 inhibition and curcumin-stimulated cortisol secretion, each of these effects is significant in its own right for reasons that extend beyond actions in the adrenal. First, although a role for bTREK-1 channels in the therapeutic actions of curcumin hasn’t been demonstrated, several interesting possibilities exist. Most notably, curcumin shows promise in the treatment of human prostate cancer, where it induces apoptosis of prostate cancer cells [25–27]. Remarkably, it has been shown that TREK-1 K+ channels are expressed in human prostate cancers, but not in normal prostate cells. Further, suppression of bTREK-1 activity by a non-specific TREK-1 antagonist, sipatrigine, or by over-expression of a dominant negative mutant of this channel restores proliferation to near normal levels [28]. Because curcumin is five times more potent than sipatrigine as a bTREK-1 antagonist [29], it is likely that curcumin may suppress prostate cancer cell growth by an action on bTREK-1 channels.
A role for TREK-1 K+ channels in depression has also been proposed. TREK-1 K+ channels are widely distributed throughout the brain where deletion of TREK-1 in knockout mice produces a depression-resistant phenotype, suggesting that TREK-1 antagonists might display antidepressant actions [30,31]. Accordingly, antidepressant effects of curcumin have been reported in rats [32,33].
In addition to regulating cortisol secretion, curcumin may regulate the release of hormones and neurotransmitters from other secretory cells. These include those that secrete other steroid hormones, including aldosterone, testosterone, and estrogen, as well as those secreting peptide hormones and neurotransmitters. In this regard, the low water solubility and consequent limited bioavailability of curcumin has likely limited its effectiveness and masked its potency as a modulator of cellular signaling pathways, including those involving ion channels. The development of a water-soluble “nano” formulation of curcumin significantly improves its bioavailability, allowing this compound to enter the bloodstream at higher concentrations and interact with intended as well as unintended target sites [1]. Based on the widespread distribution of TREK-1 channels in the CNS, heart, adrenal, and other tissues and the robust stimulation of cortisol secretion induced by curcumin, additional effects of this compound on the physiology of excitable cells will likely be forthcoming.
Acknowledgments
This work was supported by National Institutes of Health Grant R01-DK47875 (to J.J.E.).
References
- 1.Singh S. From exotic spice to modern drug? Cell. 2007;130:765–768. doi: 10.1016/j.cell.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Adv Exp Med Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 3.Corson TW, Crews CM. Molecular understanding and modern application of traditional medicines: triumphs and trials. Cell. 2007;130:769–774. doi: 10.1016/j.cell.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morimoto T, Sunagawa Y, Kawamura T, Takaya T, Wada H, Nagasawa A, Komeda M, Fujita M, Shimatsu A, Kita T, Hasegawa K. The dietary compound curcumin inhibits p300 histone acetyltransferase activity and prevents heart failure in rats. J Clin Invest. 2008 doi: 10.1172/JCI33160. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li HL, Liu C, de Couto G, Ouzounian M, Sun M, Wang AB, Huang Y, He CW, Shi Y, Chen X, Nghiem MP, Liu Y, Chen M, Dawood F, Fukuoka M, Maekawa Y, Zhang L, Leask A, Ghosh AK, Kirshenbaum LA, Liu PP. Curcumin prevents and reverses murine cardiac hypertrophy. J Clin Invest. 2008 doi: 10.1172/JCI32865. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Berger AL, Randak CO, Ostedgaard LS, Karp PH, Vermeer DW, Welsh MJ. Curcumin stimulates cystic fibrosis transmembrane conductance regulator Cl− channel activity. J Biol Chem. 2005;280:5221–5226. doi: 10.1074/jbc.M412972200. [DOI] [PubMed] [Google Scholar]
- 7.Dyer JL, Khan SZ, Bilmen JG, Hawtin SR, Wheatley M, Javed Mu, Michelangeli F. Curcumin: a new cell-permeant inhibitor of the inositol 1,4,5-trisphosphate receptor. Cell Calcium. 2002;31:45–52. doi: 10.1054/ceca.2001.0259. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Danthi SJ, Enyeart JJ. Curcumin potently blocks Kv1.4 potassium channels. Biochem Biophys Res Commun. 2006;344:1161–1165. doi: 10.1016/j.bbrc.2006.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mlinar B, Biagi BA, Enyeart JJ. A novel K+ current inhibited by ACTH and Angiotensin II in adrenal cortical cells. J Biol Chem. 1993;268(12):8640–8644. [PubMed] [Google Scholar]
- 10.Enyeart JJ, Mlinar B, Enyeart JA. T-type Ca2+ channels are required for ACTH-stimulated cortisol synthesis by bovine adrenal zona fasciculata cells. Mol Endocrinol. 1993;7:1031–1040. doi: 10.1210/mend.7.8.8232302. [DOI] [PubMed] [Google Scholar]
- 11.Doronin SV, Potapova IA, Lu Z, Cohen IS. Angiotensin receptor type 1 forms a complex with the transient outward potassium channel Kv4.3 and regulates its gating properties and intracellular localization. J Biol Chem. 2004;279:48231–48237. doi: 10.1074/jbc.M405789200. [DOI] [PubMed] [Google Scholar]
- 12.Enyeart JJ, Danthi SJ, Liu H, Enyeart JA. Angiotensin II inhibits bTREK-1 K+ channels in adrenocortical cells by separate Ca2+- and ATP hydrolysis-dependent mechanisms. J Biol Chem. 2005;280:30814–30828. doi: 10.1074/jbc.M504283200. [DOI] [PubMed] [Google Scholar]
- 13.Enyeart JJ, Xu L, Danthi S, Enyeart JA. An ACTH- and ATP-regulated background K+ channel in adrenocortical cells is TREK-1. J Biol Chem. 2002;277:49186–49199. doi: 10.1074/jbc.M207233200. [DOI] [PubMed] [Google Scholar]
- 14.Enyeart JJ, Gomora JC, Xu L, Enyeart JA. Adenosine triphosphate activates a noninactivating K+ current in adrenal cortical cells through nonhydrolytic binding. J Gen Physiol. 1997;110:679–692. doi: 10.1085/jgp.110.6.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks SP, Storey KB. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal Biochem. 1992;201:119–126. doi: 10.1016/0003-2697(92)90183-8. [DOI] [PubMed] [Google Scholar]
- 16.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 17.Mlinar B, Enyeart JJ. Voltage-gated transient currents in bovine adrenal fasciculata cells II: A-type K+ current. J Gen Physiol. 1993;102:239–255. doi: 10.1085/jgp.102.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence Nature reviews. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 19.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;72:1439–1452. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Enyeart JA, Enyeart JJ. Potent inhibition of native TREK-1 K+ channels by selected dihydropyridine Ca2+ channel antagonists. J Pharmacol Exp Ther. 2007;323:39–48. doi: 10.1124/jpet.107.125245. [DOI] [PubMed] [Google Scholar]
- 21.Bockenhauer D, Zilberberg N, Goldstein SA. KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat Neurosci. 2001;4:486–491. doi: 10.1038/87434. [DOI] [PubMed] [Google Scholar]
- 22.Simpson ER, Waterman MR. Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Ann Rev Physiol. 1988;50:427–440. doi: 10.1146/annurev.ph.50.030188.002235. [DOI] [PubMed] [Google Scholar]
- 23.John ME, John MC, Boggaram V, Simpson ER, Waterman MR. Transcriptional regulation of steroid hydroxylase genes by corticotropin. Proc Nat Acad Sci USA. 1986;83:4715–4719. doi: 10.1073/pnas.83.13.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 25.Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer-I. Curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000;3:84–93. doi: 10.1038/sj.pcan.4500399. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21(/WAF1/CIP1) Cell cycle (Georgetown, Tex) 2007;6:2953–2961. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 28.Voloshyna I, Besana A, Castillo M, Matos T, Weinstein IB, Mansukhani M, Robinson RB, Cordon-Cardo C, Feinmark SJ. TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 2008;68:1197–1203. doi: 10.1158/0008-5472.CAN-07-5163. [DOI] [PubMed] [Google Scholar]
- 29.Meadows HJ, Chapman CG, Duckworth DM, Kelsell RE, Murdock PR, Nasir S, Rennie G, Randall AD. The neuroprotective agent sipatrigine (BW619C89) potently inhibits the human tandem pore-domain K(+) channels TREK-1 and TRAAK. Brain Res. 2001;892:94–101. doi: 10.1016/s0006-8993(00)03239-x. [DOI] [PubMed] [Google Scholar]
- 30.Hervieu GJ, Cluderay JE, Gray CW, Green PJ, Ranson JL, Randall AD, Meadows HJ. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience. 2001;103:899–919. doi: 10.1016/s0306-4522(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 31.Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thümmler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- 32.Wang R, Xu Y, Wu HL, Li YH, Guo JB, Li XJ. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur J Pharmacol. 2008;578:43–50. doi: 10.1016/j.ejphar.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Ku B, Tie L, Yao H, Jiang W, Ma X, Li X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006;1122:56–64. doi: 10.1016/j.brainres.2006.09.009. [DOI] [PubMed] [Google Scholar]


