Abstract
Objective
Non-invasive brain stimulation such as transcranial direct current stimulation (tDCS) has been successfully used to induce polarity-specific excitability changes in the brain. However, it is still unknown if anodal tDCS (tDCSanodal) applied to the primary somatosensory cortex (S1) can lead to behavioral changes in performance of tactile discriminative tasks.
Methods
Using an accurate tactile discrimination task (grating orientation task: GOT) we tested the hypothesis that application of 1 mA of tDCSanodal (current density at the electrodes of 0.04 mA/cm2) over the left S1 can lead to an improved tactile spatial acuity in the contralateral index-finger (IF).
Results
Performance in the GOT task with the contralateral IF but not with the ipsilateral IF was enhanced for about 40 min after a 20 min application of tDCSanodal in the absence of changes with sham stimulation.
Conclusion
These results provide the first evidence that tDCSanodal over S1 improves performance in a complex somatosensory task beyond the period of stimulation.
Significance
The ability to induce performance improvement in the somatosensory domain with tDCS applied over S1 could be used to promote functional recovery in patients with diminished tactile perception.
Keywords: Transcranial direct current stimulation (tDCS), tactile acuity, somatosensory cortex, grating orientation task (GOT), perceptual, cortical plasticity
Introduction
Somatosensory input is crucial for skillful motor control and for learning new motor skills (Johansson and Westling, 1984; Pause et al., 1989; Hermsdorfer et al., 2004). In animal experiments, surgically abolished sensation in one of the forelimbs in monkeys leads to a decrease of voluntary motor activity (Taub, 1977). More recently, the same phenomenon has been studied in detail in human subjects. For example, a reduction of somatosensory input by local anesthesia impairs motor control in normal volunteers (Monzee et al., 2003; Duque et al., 2005). Conversely, it has been shown that increased somatosensory input by means of peripheral nerve stimulation modulates cortico-motor excitability in healthy volunteers and enhances transiently motor function in chronic stroke patients (Ridding et al., 2000; Kaelin-Lang et al., 2002; Conforto et al., 2007). Additionally, previous studies demonstrated that it is possible to modulate somatosensory (Tegenthoff et al., 2005; Dieckhofer et al., 2006; Pleger et al., 2006) and motor (Hummel et al., 2005) function by noninvasive brain stimulation. These techniques might therefore play an adjuvant role in rehabilitative treatments of neurological and psychiatric disorders (for review see (Kobayashi and Pascual-Leone, 2003; Ward and Cohen, 2004; Cooke and Bliss, 2006)).
Transcranial direct current stimulation (tDCS), a particular form of noninvasive brain stimulation, is a procedure used to polarize brain regions through the application of weak direct currents (Nitsche and Paulus, 2000; Wassermann and Grafman, 2005). For example, anodal tDCS (tDCSanodal) applied over the primary motor cortex (M1) transiently increases cortical excitability beyond the period of stimulation, while cathodal stimulation often decreases it (Priori et al., 1998; Nitsche and Paulus, 2001). Furthermore, tDCS-induced excitability changes seem to be associated with effects on performance of motor as well as non-motor tasks (Hummel et al., 2005; Hummel and Cohen, 2006).
In the somatosensory cortex, comparable polarity-specific differential effects of tDCS on the high and low frequency components or amplitudes of somatosensory evoked potentials (SEPs) have also been demonstrated (Matsunaga et al., 2004; Dieckhofer et al., 2006). However, it is still unknown if tDCSanodal application over primary somatosensory cortex (S1) is capable to induce changes in tactile spatial acuity in human subjects.
Here, we intended to clarify if application of tDCSanodal centered over the left S1 influences tactile discrimination skills on the index-finger (IF) contralateral to stimulation in healthy volunteers. As a behavioral outcome measure, we choose the grating orientation task (GOT), an accurate and commonly accepted measure of tactile spatial acuity in human subjects (Johnson and Phillips, 1981; Van Boven and Johnson, 1994).
Since tDCS elicits lesser perceptual phenomena than transcranial magnetic stimulation (TMS) or theta burst stimulation (TBS) such as acoustic noise or sudden scalp sensation, tDCS has possibly a greater potential for sham-controlled studies and clinical applications. Furthermore, one technical advantage of tDCS application is that performance changes can be measured during the time of stimulation. Therefore, tDCS could be a useful adjuvant tool when combined with performance of complex cognitive tasks or rehabilitative treatments after brain lesions (Gandiga et al., 2006).
Material and Methods
Experimental procedures
Subjects
We studied ten healthy volunteers between 23 and 34 years of age (26.8 ± 3.9 years (SD); 5/10 females). They gave written informed consent to participate in the experiment according to the declaration of Helsinki and the NINDS Institutional Review Board approved the study. Prior to participation, all volunteers underwent a comprehensive neurological examination. According to the Oldfield questionnaire for the assessment of handedness (Oldfield, 1971), all subjects were right-handed.
Main Experiment
We tested the effect of tDCSanodal centered over left S1 on performance of the GOT (primary outcome measure) in a pseudo-randomized, double-blind sham-controlled cross-over study design. All volunteers participated in two experimental sessions (tDCSanodal and tDCSsham) separated by 8.3 ± 3.5 days (mean ± SD). Half of the volunteers started with tDCSanodal and half of them with tDCSsham. Subjects were told that they would receive two times the same kind of brain stimulation that might, however, differ in stimulus intensity and that there was no difference in the design of the two sessions. Each session consisted of four familiarization GOT measurements in the right index-finger (IF) separated by 2 minutes each (GOT1-4; GOT4=pre), followed by the intervention (tDCSanodal or tDCSsham) and subsequent GOT performance determination (GOT5-7). The time required for each subject to complete each GOT measurement ranged between 3 and 5 minutes dependent of the individual tactile performance. GOT thresholds were defined as the level at which 75% of the responses were correct (see below). GOT5 was measured 10 minutes after the onset of stimulation. GOT performance was tested immediately after (GOT6) the end of the intervention. Additionally, GOT7 was tested 40 minutes after each intervention (see Fig. 1).
Figure 1. Experimental design.
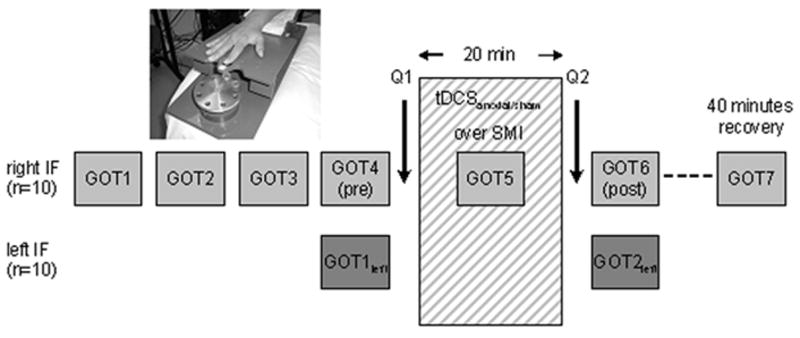
In order to obtain a stable baseline performance for the right IF, GOT thresholds were measured on 4 consecutive sessions (GOT1-4) before tDCSanodal/sham was applied. The left IF served as controls to assess spatial specificity of perceptual effects. GOT thresholds of the left IF were tested only immediately before (GOT1left) and after (GOT2left) the stimulation period because effects of task familiarization are known to generalize across fingers. After initial GOT testing on both fingers, tDCS was applied over the left S1. After tDCS, GOT thresholds on both index fingers (IFs) were measured. GOT7 served to assess the recovery of tDCS-induced effects on tactile GOT thresholds of the right IF.
As a control for the spatial specificity of tDCS-induced changes in tactile acuity, the left IF (ipsilateral to the stimulated left S1) was tested before (GOT1 left) and immediately after (GOT2 left) tDCSanodal or tDCSsham (Fig 1). Using a visual analogue scale (VAS), healthy volunteers rated their attention level toward the task (range 1–10; 1=no attention, 10=high attention), their perception of fatigue (range 1–10; 1=strong fatigue, 10=no fatigue) as well as their discomfort (range 1–10; 1=no discomfort, 10=strong discomfort) two times in each session (before and after each intervention). Additionally, they rated their perception of the intensity (range 1–10; 1=no sensation, 10=strong sensation) and duration of tDCS (range 1–10; 1=very transient, 10=permanent) immediately after the intervention.
Grating Orientation Task (GOT)
The GOT was tested in a two-alternative forced-choice paradigm as described previously (Van Boven and Johnson, 1994). Prior to both sessions, each subject was instructed how to perform the task by visually demonstrating the plastic domes, a set of small hemispherical grating surfaces (see Fig. 1). During the task, subjects were comfortably seated and blindfolded. Gratings were applied with eight hemispherical plastic domes with gratings cut into their surfaces, i.e. parallel ridges and grooves of equal widths for each dome (Tactile Acuity Gratings, MedCore, www.med-core.com). The width of the ridges and grooves varied between domes from 0.35 and 2.5 mm (0.35, 0.5, 0.75, 1, 1.2, 1.5, 2, 2.5 mm). With the help of a custom-made device, gratings were applied with moderate force (resulting in approximately 2 mm of skin displacement) for a duration of 2 sec to the distal pad of the test index-finger (IF). The domes were mounted on a rotating disc that allowed rapid switching between stimuli. To standardize stimulation, the rotating disc was coupled with a platform that was moved up and down by the investigator. The arm and fingers of the subjects were immobilized on the plate so that no active touch could be applied to the dome by the subjects. With an investigator-controlled down-movement of the platform, the domes touched the tip of the IF with the same indentations and force in each trial. Each dome was presented 20 times in one block with the ridges and grooves of the dome randomly oriented in one of two directions (10 times orthogonal and 10 times parallel to the axis of the IF). Immediately after touching the plastic domes subjects had to indicate verbally their perception of the orientation of the respective dome by answering “p” for parallel or “o” for orthogonal. Volunteers did not receive any feedback about their performance during the task. Trials in which the subjects failed to respond immediately after touching the domes were rejected, as well as trials in which they moved.
The test was performed with a series of descending dome widths starting with the largest grating (2.5 mm) and ending when tactile acuity of the IF dropped below 75% correct responses within one block. The gratings discrimination threshold was defined as the level at which 75% of the responses were correct. Performance at this level is midway between chance and perfect performance and is a standard psychophysical criterion for tactile acuity threshold determination (Van Boven and Johnson, 1994). For the GOT threshold the following formula was used:
In this formula, ThresholdG75 = the estimated threshold for the grating spacing on which the subject scored 75% correct responses; G = the grating spacing; P = trials correct/number of trials; below = the grating spacing or probability of correct response on the highest grating spacing on which the subject responded correctly less than 75% of the time; above = grating spacing or the probability of a correct response on the lowest grating spacing on which the subject responded correctly more than 75% of the time.
Transcranial Direct Current Stimulation and blinding procedures
tDCS was delivered using a Phoresor® II Auto (Model No. PM850, IOMED®, Salt Lake City, Utah 84120 USA) through two sponge surface electrodes (TransQE from IOMED®; surface area was 25 cm2 for each electrode) embedded in a saline-soaked solution (normal saline with 9.0 mg sodium chloride/liter). One electrode was positioned at the C3′ position, 2 cm posterior to C3 (10–20 EEG system) and the other electrode at the forehead above the contralateral orbit. Projections of that point (C3′ position) over the brain fall over the primary somatosensory cortex. The stimulation electrodes were fixed by elastic bands.
As described previously, for both interventions (tDCSanodal and tDCSsham) current was increased in ramp-like fashion from 0 to 1 mA at the onset of stimulation eliciting a transient tingling sensation on the scalp (Hummel et al., 2005). The current density at the stimulation electrodes at our maximum setting of 1 mA was 0.04 mA/cm2. tDCS was delivered for 20 minutes in the tDCSanodal session (total charge as expressed by current density × total stimulation duration (s) was 0.048 C/cm2) and for up to 30 seconds in the tDCSsham session. 20 minutes after the onset of tDCSanodal or sham, currents were turned off slowly over a few seconds out of the field of view of the volunteers precluding sensory perceptual differences between both sessions (Nitsche et al., 2003a; Nitsche et al., 2003b). This strategy has recently demonstrated efficiency in blinding the procedure (Hummel et al., 2005; Gandiga et al., 2006).
Statistical analysis
Data were analyzed using the SPSS software package for Windows version 12.0.1. We used mixed effects MANOVA, repeated measures ANOVA (ANOVARM) with post hoc analysis (Bonferroni corrected), Students’ Paired T-Test and Mann-Whitney U Test (nonparametric Test) to compare tactile performance before and after tDCSanodal or tDCSsham. All figures rep resent group data. Error bars refer to the standard error (s.e.m.) of the measurements.
Results
All volunteers achieved a stable baseline performance in the GOT task on the right IF prior to tDCS application (see Fig. 2a and b). There was no significant difference between GOT thresholds on the right IF within the familiarization measurements (GOT1-4) between tDCSanodal and tDCSsham (tDCSanodal: 1.60 ± 0.15 mm; tDCSsham: 1.63 ± 0.14 mm; repeated measures ANOVA with factor INTERVENTION: F(1,18)=0.021; p=0.888, see also Table 1 and 2 for individual subject data). ANOVARM revealed a significant INTERVENTION (tDCSanodal, sham) × TIME (GOT1-7) interaction (F(6,54)=6.124; p<0.0001) on GOT thresholds. tDCSanodal resulted in GOT thresholds improvements of 0.48 ± 0.07 mm after the first 10 minutes and of 0.48 ± 0.09mm immediately after tDCSanodal (Mixed-effects MANOVA, Bonferroni-corrected post hoc comparison (GOT4-6, p=0.018; Figure 2a). All volunteers but one showed a gain in GOT threshold in the tDCSanodal session (see Fig. 3 and Table 2). GOT thresholds 40 minutes after tDCSanodal were still lower relative to baseline (ANOVARM with factor TIME (GOT4, GOT7): F(1,9)=8.548; p=0.017, Fig. 2a).
Figure 2.
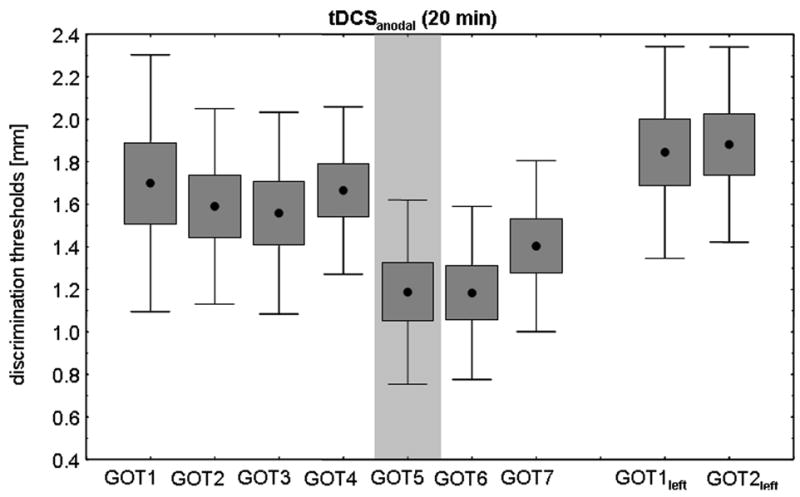
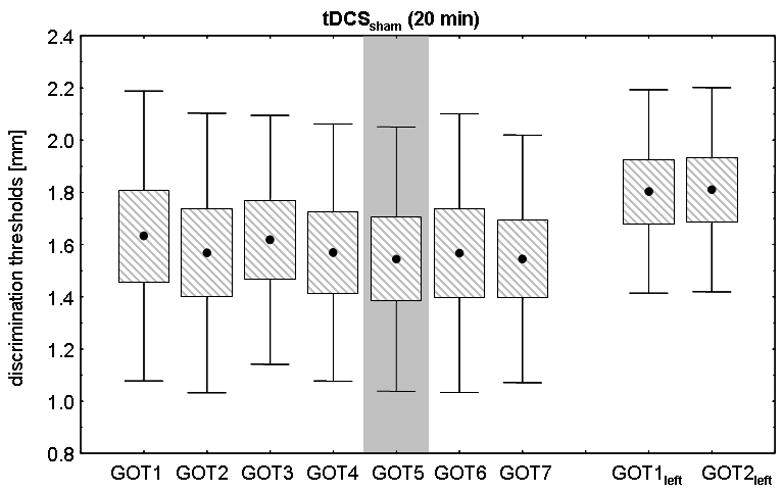
Figure 2a. Effect of tDCSanodal (20 min) on right IF GOT thresholds. All subjects achieved a stable baseline performance prior to tDCS. Ten minutes after the onset of tDCSanodal, subjects showed a significant discrimination improvement that persisted for at least 40 minutes. The GOT thresholds on the left IF ipsilateral to tDCSanodal was not affected.
Figure 2b. Effect of tDCSsham (20 min) on right IF GOT thresholds. In comparison to tDCSanodal, there was no change in GOT thresholds neither on the right nor on the left IF.
Table 1.
Individual subject data (n=10) for GOT thresholds on the right (GOT1-7) and left (GOT1left – GOT2left) IF in the tDCSsham session.
| tDCSsham | Right IF | Left IF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Gender | Age | GOT1 | GOT2 | GOT3 | GOT4 | GOT5 | GOT6 | GOT7 | GOT1left | GOT2left |
| 1 | M | 31 | 1.75 | 1.20 | 1.77 | 1.75 | 1.70 | 1.68 | 1.6 | 2.08 | 2.00 |
| 2 | F | 34 | 1.44 | 1.40 | 1.35 | 1.32 | 1.20 | 1.35 | 1.41 | 1.30 | 1.30 |
| 3 | M | 24 | 1.05 | 1.03 | 1.08 | 1.10 | 1.10 | 1.04 | 1.03 | 1.87 | 1.75 |
| 4 | F | 25 | 0.81 | 1.06 | 1.06 | 1.00 | 1.04 | 1.1 | 1.04 | 1.50 | 1.50 |
| 5 | F | 23 | 1.31 | 1.43 | 1.35 | 1.41 | 1.36 | 1.45 | 1.5 | 1.45 | 1.40 |
| 6 | F | 24 | 2.08 | 1.93 | 2.00 | 2.00 | 1.83 | 2.14 | 1.7 | 1.64 | 1.81 |
| 7 | F | 25 | 2.16 | 2.00 | 1.91 | 1.50 | 1.75 | 1.7 | 1.83 | 2.00 | 2.00 |
| 8 | M | 28 | 2.53 | 2.53 | 2.35 | 2.57 | 2.53 | 2.57 | 2.33 | 2.53 | 2.62 |
| 9 | M | 31 | 2.00 | 2.10 | 2.16 | 1.92 | 2.00 | 1.83 | 2.12 | 2.16 | 2.10 |
| 10 | M | 23 | 1.20 | 1.00 | 1.15 | 1.12 | 0.93 | 0.81 | 0.89 | 1.50 | 1.62 |
|
| |||||||||||
| Mean ± s.e.m. | 26.8 ± 1.24 | 1.63 ± 0.18 | 1.57 ± 0.17 | 1.61 ± 0.15 | 1.57 ± 0.16 | 1.54 ± 0.16 | 1.57 ± 0.17 | 1.55 ± 0.15 | 1.83 ± 0.12 | 1.81 ± 0.12 | |
Table 2.
Individual subject data (n=10) for GOT thresholds on the right (GOT1-7) and left (GOT1left – GOT2left) IF in the tDCSanodal session.
| tDCSanodal | Right IF | Left IF | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | Gender | Age | GOT1 | GOT2 | GOT3 | GOT4 | GOT5 | GOT6 | GOT7 | GOT1left | GOT2left |
| 1 | M | 31 | 1.71 | 1.75 | 1.42 | 1.71 | 1.40 | 1.44 | 1.66 | 2.25 | 2.25 |
| 2 | F | 34 | 2.42 | 2.16 | 2.00 | 2.00 | 1.83 | 1.40 | 1.64 | 1.50 | 1.91 |
| 3 | M | 24 | 1.08 | 1.00 | 1.00 | 1.50 | 0.79 | 0.82 | 0.95 | 1.35 | 1.32 |
| 4 | F | 25 | 1.20 | 1.30 | 1.10 | 1.50 | 0.87 | 0.92 | 1.20 | 2.68 | 2.57 |
| 5 | F | 23 | 0.97 | 1.13 | 1.05 | 1.00 | 0.48 | 0.47 | 0.70 | 1.30 | 1.30 |
| 6 | F | 24 | 1.80 | 1.66 | 2.00 | 2.00 | 1.20 | 1.38 | 1.50 | 1.75 | 1.66 |
| 7 | F | 25 | 1.66 | 1.70 | 1.75 | 1.80 | 1.13 | 1.20 | 2.00 | 2.25 | 2.16 |
| 8 | M | 28 | 2.50 | 2.00 | 2.10 | 2.00 | 1.27 | 1.25 | 1.35 | 2.00 | 2.16 |
| 9 | M | 31 | 2.50 | 2.20 | 2.08 | 2.08 | 1.85 | 1.95 | 1.83 | 2.16 | 2.18 |
| 10 | M | 23 | 1.15 | 1.00 | 1.08 | 1.06 | 1.05 | 1.00 | 1.20 | 1.20 | 1.30 |
|
| |||||||||||
| Mean ± s.e.m. | 26.8 ± 1.24 | 1.70 ± 0.19 | 1.59 ± 0.14 | 1.56 ± 0.14 | 1.67 ± 0.12 | 1.19 ± 0.13 | 1.18 ± 0.12 | 1.40 ± 0.12 | 1.84 ± 0.15 | 1.88 ± 0.14 | |
Figure 3. Effect of tDCSanodal and tDCSsham on GOT thresholds.
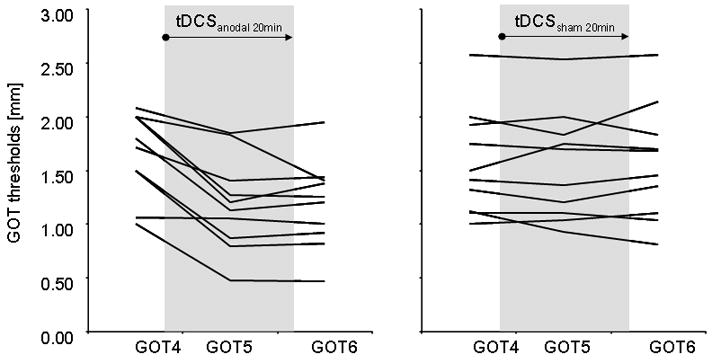
Please, note a decrease in GOT thresholds in the right IF with tDCSanodal in all but one subject.
Subjects who received tDCSanodal first experienced a return of GOT thresholds to baseline values at the time of their second visit (8.3 ± 3.5 days later; paired T-Test GOT7 (tDCSanodal) vs. GOT1 (tDCSsham), p=0.759).
GOT thresholds at baseline were comparable in the IF ipsilateral and contralateral to the stimulated S1 (paired T-Test, n.s., see Fig. 2a and 2b). ANOVARM revealed no significant effects of INTERVENTION (tDCSanodal vs. tDCSsham;F(1,9)=0.136; p=0.720) or TIME (GOT1-2 left IF; F(1,9)=0.546; p=0.479), nor their interaction on GOT thresholds in IF ipsilateral to the stimulated S1.
Neither tDCSanodal nor tDCS sham modified attention (change in VAS rating after tDCSanodal: 0.60 ± 0.42; Sham: 0.50 ± 0.40), fatigue (tDCSanodal: 0.5 ± 0.74; tDCSsham: 0.1 ± 0.48) and discomfort (tDCSanodal: 0.20 ± 0.20; tDCSsham: 0.10 ± 0.27). Subjects were unable to discriminate the difference in stimulation intensity between the two interventions (tDCSanodal: 3.50 ± 0.58; tDCSsham: 2.30 ± 0.55) but felt a mild difference in the perception of the duration of stimulation (tDCSanodal: 6.60 ± 1.11; tDCSsham: 2.70 ± 0.53; p=0.009). This effect was mainly driven by four subjects that rated the period of 20 min of tDCSanodal stimulation as permanent. Stratification of data analysis acquired during tDCSanodal showed that the individual changes in GOT thresholds with tDCSanodal were comparable in the four subjects who felt longer duration of stimulation relative to sham (n=4) and in those who felt comparable durations (n=6) (GOT4 vs. GOT5, Mann-Whitney U Test: p=0.522; GOT4 vs. GOT6, Mann-Whitney U Test: p=0.748).
Discussion
Our results demonstrate that a short period of tDCSanodal applied over the human S1 enhances tactile spatial acuity in the contralateral hand relative to sham stimulation. This performance improvement lasts for at least 40 minutes after the end of the stimulation period and is not present in the hand ipsilateral to the stimulated S1.
Improvements in tactile performance after non-invasive brain stimulation have been reported in previous studies. For example, 5 Hz rTMS, paired-associative stimulation or TBS applied over S1 could improve tactile performance, as quantified by two-point discrimination (2PD) thresholds (Ragert et al., 2003; Tegenthoff et al., 2005; Litvak et al., 2007; Ragert et al., 2007). It has been hypothesized that these performance improvements might be associated with excitability changes within the stimulated body part representation in the somatosensory cortex (Tegenthoff et al., 2005).
Of particular interest is that tDCSanodal centered over M1 results in an amplitude increase of the P25/N33, N33/P40 (parietal) and P22/N30 (frontal) components of the somatosensory evoked potentials (SEPs) elicited by stimulation of the median nerve of the contralateral hand whereas tDCScathodal lacks such an effect (Matsunaga et al., 2004). It has been proposed that the areas most likely polarized by tDCS centered over M1 included the surface of the postcentral gyrus such as Brodmann areas (BA) 1 and 2 since earlier SEP components such as the P14/N20, N20/P25 and N18/N22 were not affected by tDCS (Matsunaga et al., 2004). On the other hand, Dieckhofer and colleagues reported a longer lasting reduction of the N20 component after tDCScathodal centered over SI with no effect after tDCSanodal (Dieckhofer et al., 2006). The results from Dieckhofer and colleagues are in accordance with a recent psychophysical study (Rogalewski et al., 2004) indicating that tDCS-induced excitability changes within SI may be associated with a modulation of tactile performance in human subjects: 7 min of tDCScathodal over S1 induced a prolonged decrease of tactile frequency discrimination while tDCSanodal or sham had no effect (Rogalewski et al., 2004).
Possible reasons for the different effects of tDCSanodal reported across these different studies might be the different target area of tDCS (M1 vs. S1) and the different size of the stimulation electrodes used. The electrode size in the present study was smaller than electrodes used in previous investigations (25 vs. 35 mm2). Consequently the current density in the present study was likely higher as well as relatively more focal perhaps leading to more overt beneficial effects of tDCSanodal on tactile spatial acuity. Additionally, the application of tDCS for 7 min in previous studies (as compared to 20 min in the present study) may have been insufficient to elicit performance improvements. Finally, the use of different behavioral outcome measures might also account for some of these differences.
The purpose of our investigation was to determine the effects of tDCSanodal applied to the human S1 on tactile spatial acuity in the contralateral hand, as measured by the GOT task (Van Boven and Johnson, 1994; Craig, 1999). Afferent information required to accurately perform this task derives predominantly from rapidly adapting (RA) and slowly adapting type 1 (SA) afferents (for review see (Johnson, 2001)). The task has been extensively used as psychophysical measurement of spatial tactile acuity and in clinical settings (Van Boven and Johnson, 1994; Van Boven et al., 2000; Goldreich and Kanics, 2003; Molloy et al., 2003) and is sensitive enough to unveil changes in performance in healthy subjects (Tremblay et al., 2002; Werhahn et al., 2002) and in patients with stroke (Voller et al., 2006).
The stimulation paradigm utilized in the present study involved application of tDCS at intensities and durations that are safe (Nitsche et al., 2003; Poreisz et al., 2007) and that consistently induced changes in excitability in the underlying cortical regions, as well as changes in performance in different sensory and motor modalities (Matsunaga et al., 2004; Rogalewski et al., 2004; Hummel et al., 2005; Nitsche et al., 2005).
When compared to other brain stimulation protocols like TMS, tDCS application may have a relatively lower focality because of the relatively larger stimulation electrodes (Gandiga et al., 2006) and the electrode montage. As a consequence, a relatively more widespread current flow is expected with tDCS than with TMS in the respective projection areas (Lang et al., 2005; Wagner et al., 2007). Despite this apparent drawback, tDCS is a promising tool in neurorehabilitation since it is easy to apply, easier to sham than TMS, and performance changes can be documented more easily during the time of stimulation (Hummel and Cohen, 2006).
The magnitude of tDCS-induced improvements in our study (29.65 ± 5.01 %) as well as its duration (≥ 40 min) compares favorably with effects reported on performance of motor tasks such as the Jebson-Taylor Test (JTT; improvements of approximately 11.75 ± 3.61 % in chronic stroke patients or 10.96 ± 3.61 % in healthy controls) when applied over M1 (Hummel et al., 2005; Hummel and Cohen, 2005). Therefore, effects of tDCS appear to elicit qualitatively similar effects on different modalities (somatosensory and motor domain).
The mechanisms underlying these behavioral gains in tactile spatial acuity are still prospective. On one hand, it is possible that tDCS-induced excitability changes within S1 contributed to this effect (Matsunaga et al., 2004). On the other hand, it is possible, while formal evidence to this effect is lacking, that tDCS application contributed to improve the signal-to-noise ratio for grating detection in somatosensory cortices, as previously reported for the effects of dopaminergic agents on motor performance (Floel et al., 2005). Resolution of this issue would require additional experiments.
An alternative explanation that could explain the described behavioral gains is that tDCS affected directly attentional processes. It could be argued that the location of the cathode electrode over the contralateral forehead could have influenced attentional processing, contributing to the described behavioral gains, since attention is able to modify cortical finger representations within S1 (Buchner et al., 1999). This is however unlikely for different reasons. First, previous investigations using a frontal cathode electrode failed to detect attentional effects (Gandiga et al., 2006). Second, consistent with these results, reports of attention before and after tDCSanodal in our study were comparable to sham. Third, changes in attention, if present, should have impacted to relatively similar extents tactile performance in both hands, and not only the hand contralateral to the stimulated S1, as we found. Finally, it should be kept in mind that, given the size of electrodes, the areas polarized might have expanded beyond the targeted S1.
In summary, these results provide novel evidence that tDCSanodal delivered over S1 results in improvement in tactile spatial acuity in the contralateral hand, an effect of potential application in future studies geared to improve somatosensory function in patients with diminished tactile perception.
Acknowledgments
This research was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) to P.R. (Ra 1391/1-1) and by the Intramural Research Program of the NIH, NINDS. Y.V. was supported by a NINDS Competitive Fellowship grant and a FSR Post-Doctoral Grant from the Université catholique de Louvain (Belgium).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Buchner H, Reinartz U, Waberski TD, Gobbele R, Noppeney U, Scherg M. Sustained attention modulates the immediate effect of de-afferentation on the cortical representation of the digits: source localization of somatosensory evoked potentials in humans. Neurosci Lett. 1999;260:57–60. doi: 10.1016/s0304-3940(98)00948-3. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Cohen LG, dos Santos RL, Scaff M, Marie SK. Effects of somatosensory stimulation on motor function in chronic cortico-subcortical strokes. J Neurol. 2007;254:333–339. doi: 10.1007/s00415-006-0364-z. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain. 2006;129:1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Craig JC. Grating orientation as a measure of tactile spatial acuity. Somatosens Mot Res. 1999;16:197–206. doi: 10.1080/08990229970456. [DOI] [PubMed] [Google Scholar]
- Dieckhofer A, Waberski TD, Nitsche M, Paulus W, Buchner H, Gobbele R. Transcranial direct current stimulation applied over the somatosensory cortex - differential effect on low and high frequency SEPs. Clin Neurophysiol. 2006;117:2221–2227. doi: 10.1016/j.clinph.2006.07.136. [DOI] [PubMed] [Google Scholar]
- Duque J, Vandermeeren Y, Lejeune TM, Thonnard JL, Smith AM, Olivier E. Paradoxical effect of digital anaesthesia on force and corticospinal excitability. Neuroreport. 2005;16:259–262. doi: 10.1097/00001756-200502280-00011. [DOI] [PubMed] [Google Scholar]
- Floel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG. Dopaminergic influences on formation of a motor memory. Ann Neurol. 2005;58:121–130. doi: 10.1002/ana.20536. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Goldreich D, Kanics IM. Tactile acuity is enhanced in blindness. J Neurosci. 2003;23:3439–3445. doi: 10.1523/JNEUROSCI.23-08-03439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsdorfer J, Hagl E, Nowak DA. Deficits of anticipatory grip force control after damage to peripheral and central sensorimotor systems. Hum Mov Sci. 2004;23:643–662. doi: 10.1016/j.humov.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen LG. Improvement of Motor Function with Noninvasive Cortical Stimulation in a Patient with Chronic Stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005 doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–712. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56:550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Johnson KO. The roles and functions of cutaneous mechanoreceptors. Curr Opin Neurobiol. 2001;11:455–461. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- Johnson KO, Phillips JR. Tactile spatial resolution. I. Two-point discrimination, gap detection, grating resolution, and letter recognition. J Neurophysiol. 1981;46:1177–1192. doi: 10.1152/jn.1981.46.6.1177. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, Rothwell JC, Lemon RN, Frackowiak RS. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22:495–504. doi: 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Zeller D, Oostenveld R, Maris E, Cohen A, Schramm A, Gentner R, Zaaroor M, Pratt H, Classen J. LTP-like changes induced by paired associative stimulation of the primary somatosensory cortex in humans: source analysis and associated changes in behaviour. Eur J Neurosci. 2007;25:2862–2874. doi: 10.1111/j.1460-9568.2007.05531.x. [DOI] [PubMed] [Google Scholar]
- Matsunaga K, Nitsche MA, Tsuji S, Rothwell JC. Effect of transcranial DC sensorimotor cortex stimulation on somatosensory evoked potentials in humans. Clin Neurophysiol. 2004;115:456–460. doi: 10.1016/s1388-2457(03)00362-6. [DOI] [PubMed] [Google Scholar]
- Molloy FM, Carr TD, Zeuner KE, Dambrosia JM, Hallett M. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain. 2003;126:2175–2182. doi: 10.1093/brain/awg219. [DOI] [PubMed] [Google Scholar]
- Monzee J, Lamarre Y, Smith AM. The effects of digital anesthesia on force control using a precision grip. J Neurophysiol. 2003;89:672–683. doi: 10.1152/jn.00434.2001. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527 Pt. 2000;3:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114:2220–2222. doi: 10.1016/s1388-2457(03)00235-9. author reply 2222-2223. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, Fricke K, Liebetanz D, Lang N, Antal A, Paulus W, Tergau F. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. 2005 doi: 10.1113/jphysiol.2005.092429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pause M, Kunesch E, Binkofski F, Freund HJ. Sensorimotor disturbances in patients with lesions of the parietal cortex. Brain. 1989;112(Pt 6):1599–1625. doi: 10.1093/brain/112.6.1599. [DOI] [PubMed] [Google Scholar]
- Pleger B, Blankenburg F, Bestmann S, Ruff CC, Wiech K, Stephan KE, Friston KJ, Dolan RJ. Repetitive transcranial magnetic stimulation-induced changes in sensorimotor coupling parallel improvements of somatosensation in humans. J Neurosci. 2006;26:1945–1952. doi: 10.1523/JNEUROSCI.4097-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Ragert P, Franzkowiak S, Schwenkreis P, Tegenthoff M, Dinse HR. Improvement of tactile perception and enhancement of cortical excitability through intermittent theta burst rTMS over human primary somatosensory cortex. Exp Brain Res. 2007 doi: 10.1007/s00221-007-1073-2. [DOI] [PubMed] [Google Scholar]
- Ragert P, Dinse HR, Pleger B, Wilimzig C, Frombach E, Schwenkreis P, Tegenthoff M. Combination of 5 Hz repetitive transcranial magnetic stimulation (rTMS) and tactile coactivation boosts tactile discrimination in humans. Neurosci Lett. 2003;348:105–108. doi: 10.1016/s0304-3940(03)00745-6. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res. 2000;131:135–143. doi: 10.1007/s002219900269. [DOI] [PubMed] [Google Scholar]
- Rogalewski A, Breitenstein C, Nitsche MA, Paulus W, Knecht S. Transcranial direct current stimulation disrupts tactile perception. Eur J Neurosci. 2004;20:313–316. doi: 10.1111/j.0953-816X.2004.03450.x. [DOI] [PubMed] [Google Scholar]
- Taub E. Movement in nonhuman primates deprived of somatosensory feedback. Excercise and Sports Reviews Santa Barbara: Journal Publishing Affiliates. 1977:335–374. [PubMed] [Google Scholar]
- Tegenthoff M, Ragert P, Pleger B, Schwenkreis P, Forster AF, Nicolas V, Dinse HR. Improvement of Tactile Discrimination Performance and Enlargement of Cortical Somatosensory Maps after 5 Hz rTMS. PLoS Biol. 2005;3:e362. doi: 10.1371/journal.pbio.0030362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Mireault AC, Letourneau J, Pierrat A, Bourrassa S. Tactile perception and manual dexterity in computer users. Somatosens Mot Res. 2002;19:101–108. doi: 10.1080/08990220120113066. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Johnson KO. The limit of tactile spatial resolution in humans: grating orientation discrimination at the lip, tongue, and finger. Neurology. 1994;44:2361–2366. doi: 10.1212/wnl.44.12.2361. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Hamilton RH, Kauffman T, Keenan JP, Pascual-Leone A. Tactile spatial resolution in blind braille readers. Neurology. 2000;54:2230–2236. doi: 10.1212/wnl.54.12.2230. [DOI] [PubMed] [Google Scholar]
- Voller B, Floel A, Werhahn KJ, Ravindran S, Wu CW, Cohen LG. Contralateral hand anesthesia transiently improves poststroke sensory deficits. Ann Neurol. 2006;59:385–388. doi: 10.1002/ana.20689. [DOI] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. Neuroimage. 2007;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61:1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Grafman J. Recharging cognition with DC brain polarization. Trends Cogn Sci. 2005;9:503–505. doi: 10.1016/j.tics.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Mortensen J, Van Boven RW, Zeuner KE, Cohen LG. Enhanced tactile spatial acuity and cortical processing during acute hand deafferentation. Nat Neurosci. 2002;5:936–938. doi: 10.1038/nn917. [DOI] [PubMed] [Google Scholar]


