Abstract
T-cell-restricted intracellular antigen-1 (TIA-1) regulates alternative pre-mRNA splicing in the nucleus, and mRNA translation in the cytoplasm, by recognizing uridine-rich sequences of RNAs. As a step towards understanding of RNA recognition by this regulatory factor, the X-ray structure of the central RNA recognition motif (RRM2) of human TIA-1 is presented at 1.95Å resolution. Comparison with structurally homologous RRM-RNA complexes identifies residues at the RNA interfaces that are conserved in TIA-1–RRM2. The versatile capability of RNP motifs to interact with either proteins or RNA is reinforced by symmetry-related protein-protein interactions mediated by the RNP motifs of TIA-1–RRM2. Importantly, the TIA-1–RRM2 structure reveals the locations of mutations responsible for inhibiting nuclear import. In contrast with previous assumptions, the mutated residues are buried within the hydrophobic interior of the domain, where they would be likely to destabilize the RRM fold rather than directly inhibit RNA binding.
Keywords: RRM, RNA binding domain, Pre-mRNA splicing, mRNA translation, TIA-1, TIAR
Post-transcriptional mechanisms for regulating gene expression depend on numerous coordinated interactions among RNA sequences and trans-acting protein factors. The RNA binding protein TIA-1 (T-cell-restricted intracellular antigen-1) functions as a post-transcriptional regulator of gene expression by recognizing uridine-rich RNA sites. In the nucleus, TIA-1 regulates alternative splicing of pre-mRNAs, including those encoding fibroblast growth factor receptor-2 (fgfr-2), type II procollagen (col2a1), the cystic fibrosis transmembrane conductance regulator (cftr), and the pro-apoptotic Fas receptor (fas), among others [1; 2; 3; 4; 5; 6]. In the cytoplasm, TIA-1 regulates mRNA translation [7; 8; 9; 10], and suppresses mRNA translation during environmental stress (reviewed in [11]). The functional importance of TIA-1 is shown by its requirement for viability of DT40 cells [12], and the high rates of embryonic lethality among mice lacking TIA-1 [8; 13].
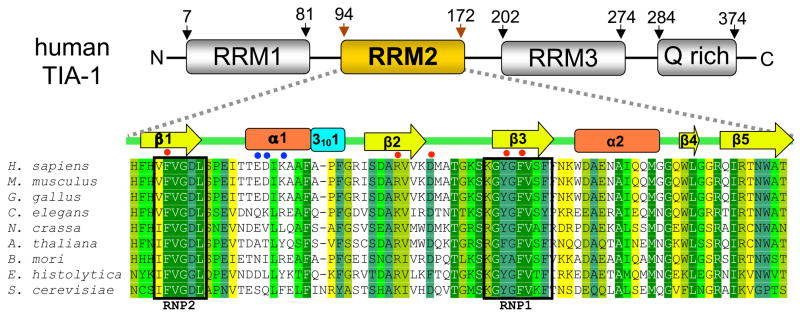
The TIA-1 polypeptide contains three RNA recognition motifs (RRMs, a prevalent class of RNA binding domain) and a C-terminal, Q-rich domain (Fig. 1) [14; 15]. In humans, a second gene encodes the nearly identical TIAR protein [16], and at least two alternative spliceoforms exist for both TIA-1 and TIAR [14; 15; 17]. The Q-rich effecter domain directs TIA-1-associated mRNAs to cytoplasmic stress granules during translational silencing [18], and recruits the U1 spliceosomal component (snRNP) to weak 5′ splice sites during nuclear pre-mRNA splicing [19]. Like the Q-rich domain, the N-terminal RRM (RRM1) participates in U1 snRNP recruitment [19], and additionally associates with single-stranded DNA molecules [20]. The C-terminal RRM (RRM3) co-precipitates with cellular RNAs, however interactions of TIA-1–RRM3 with uridine-rich RNAs are not detected in vitro [21]. The central RRM (RRM2) of TIA-1 is the established, uridine-specific region, as demonstrated by the ability of isolated TIA-1–RRM2 to bind the msl-2 5′ splice site region [19], viral RNAs [22], and uridine-rich SELEX RNAs [21]. Furthermore, the RRM2 region is necessary and sufficient for nuclear import of TIA-1 [23]. To investigate the major RNA binding surface of TIA-1, we have determined the structure of RRM2 in the absence of RNA at 2.0Å resolution.
Fig. 1.
Domain structure of human TIA-1 above a sequence alignment of TIA-1–RRM2 from representative homologs. Sequences are colored by percent identity: 100%=green, <90%=sea green, <80%=lime, <70%=bright green, <60%=yellow, and <50%=white. Secondary structure elements are indicated above the alignment [45]. The RNP1 and RNP2 are boxed and labeled below the alignment. Surface residues of human Tia-1 mutated to facilitate crystallization are highlighted by blue filled circle. Residues that interact with RNA in both PAB and SXL complexes are marked with red filled circle. Accession numbers for aligned sequences include: Homo sapiens (NP_071320), Mus musculus (NP_035715), Gallus gallus (XP_001233215), Caenorhabditis elegans (NP_495121), Neurospora crassa (XP_962723), Arabidopsis thaliana (NP_188026), Bombyx mori (NP_001036895), Entamoeba histolytica (XP_649094), Saccharomyces cerevisiae (CAA46011).
Materials and Methods
Protein purification
The human TIA-1–RRM2 fragment (residues 94-175) was expressed using the pGEX-6p vector (GE Healthcare). Glutathione-S-transferase (GST) fusion proteins were purified by glutathione affinity, and then the TIA-1 fragment was further purified by cation exchange chromatography following removal of the GST tag. The TIA RRM2 and crystallized variant (described below) were concentrated to >15mg/mL in 100mM NaCl, 25mM HEPES pH7.4, 0.2mM TCEP.
Crystallization
Initial crystals of TIA-1–RRM2 grew in clusters of fine needles (<25 × 200 μm) (Supp. Fig. 1A). To improve crystal quality, the surface entropy reduction (SER) server (http://nihserver.mbi.ucla.edu/SER) was used to identify suitable modifications of TIA-1–RRM2 to promote crystallization. The site furthest from the putative RNA binding surface was selected for mutagenesis. Improved crystals of the resulting SER variant (E109A-D110A-K112A) grew in 2–3 days by the hanging drop vapor diffusion method at 22°C, from a 1:1 mixture of protein with 15% polyethylene glycol 3350, 0.5M KI, 0.1M Tris pH8.0 (Supp. Fig. 1B).
Data collection, structure determination, and refinement
X-ray data were collected using a MICROSTAR X-ray source with X8 Proteum detector (Bruker-AXS) (Table 1). A homology model of TIA-1–RRM2 [24] based on the N-terminal RRM of poly-A binding protein (PDB ID=1CVJ) [25] was used for molecular replacement in the program Phaser [26; 27]. Refinement and model building were respectively completed using CNS [28] and Coot [29; 30]. The coordinates and structure factors are available (PDB-code=3BS9).
Table 1.
Crystallographic data and refinement statistics.
| A: Data collection statisticsa | |
| Space group | P61 |
| Unit cell | |
| a=b, c (Å) | 56.50, 76.70 |
| Resolution limit (Å) | 1.95 |
| Rsymb(%) | 7.0 (27.9) |
| I/σ(I) | 15.5 (3.5) |
| Completeness (%) | 99.8 (99.8) |
| Redundancy | 5.2 (3.2) |
| B: Refinement statistics | |
| Resolution range (Å) | 20.0–1.95 |
| No. reflections (working/test set) | 9648/489 |
| Rcryst/Rfreec (%) | 21.6/24.5 |
| No. protein atoms | 1221 |
| No. water molecules | 118 |
| No. iodide ions | 7 |
| RMSD bond lengths (Å) | 0.006 |
| RMSD bond angles (°) | 1.31 |
| Average B-factors (Å2) | |
| all atoms | 15.2 |
| protein atoms | 14.2 |
| water molecules | 23.2 |
Values in parenthesis are for the highest resolution shell (2.04–1.95 Å).
Rsym=ΣhklΣi|Ii − 〈I〉|/ΣhklΣiIi, where Ii is an intersity I for the ith measurement of a reflection with indices hkl and 〈I〉 is the weighted mean of all measurements of I.
Rcryst=ΣhklΣi||Fo(hkl)| − k|Fc(hkl)||/Σhkl|Fo(hkl)| for the working set of reflections; Rfree is Rcryst for 5% of the reflections excluded from the refinement
Fluorescence measurements
The affinities of the wild-type RRM2 and SER-RRM2 domains of TIA-1 were measured for a 3′ fluorescein-labeled 20-uridine RNA (U20) (Dharmacon Research). Proteins were titrated into a thermostatted, 0.5cm quartz cuvette containing U20 in 100mM NaCl, 25mM HEPES pH7.4 at 25°C, and the fluorescence anisotropy values were measured using a Fluoromax-3 fluorimeter (Jobin-Yvon). Excitation and emission wavelengths were typically 490/520nm, respectively. Data were fit by non-linear regression assuming single site binding (Fig. 2) to obtain the apparent KD with the following equation, where x is the total protein concentration, [RNA] is the total RNA concentration, r is the observed anisotropy at the ith titration, rB is the anisotropy at zero protein concentration, and rF is the anisotropy at saturating protein concentration (floated in fit).
Fig. 2.
Overall structure of TIA-1–RRM2. (A) Ribbon diagram of the two TIA-1–RRM2 molecules in the asymmetric unit of the crystal. Molecules ‘A’ and ‘B’ are distinguished by subscripts of the residues labels. Secondary structure elements of molecule ‘A’ are labeled. Composite omit electron density (light blue) at 1σ contour level surrounds key aromatic residues of the RNP motifs. Anomalous difference Fourier maps (magenta) at 3σ contour level indicate the iodide positions. (B) Superposition of the top five related structures identified using DALI [46]: TIA-1–RRM2 (orange), PAB (PDB-code=1CVJ, cyan), SXL (PDB-code=1B7F, magenta), hnRNP A1 (PDB-code=1HA1, green), PRP24 (PDB-code=2GHP, marine), and nuclear cap binding protein (PDB-code=1H6K, red). (C) Noncrystallographic contacts mediated by the SER mutations, viewed 180° about the y-axis relative to (A). Ball-and-stick diagrams are shown for SER residues (green) and other residues of the iodide binding sites (orange). The iodides bound at the interface are shown as magenta spheres, and the water molecules directly coordinated to these iodides are shown as blue spheres. (D) Comparison of TIA-1 RRM and SER TIA-1–RRM2 binding RNA using fluorescence anisotropy. Overlay of nonlinear least square fits of TIA-1–RRM2 (orange) and SER TIA-1–RRM2 (green) binding 5′ fluorescein-labeled 20mer polyuridine. The average apparent equilibrium dissociation constants (KD) and standard deviations of 2–3 experiments are inset.
Results and Discussion
Overall structure and comparison with other RRMs
The structure of TIA-1–RRM2, with a SER modification to improve crystal quality (E109A-D110A-K112A), was determined at 1.95Å resolution (Table 1). Two, nearly identical TIA-1–RRM2 molecules (residues 94-172, 0.4Å RMSD between 79 Cα atoms) in the crystallographic asymmetric unit adopt canonical RRM folds (βαββαβ topology) (Fig. 2A). Accordingly, the TIA-1–RRM2 structure most closely matches the N-terminal RRMs of the RNA binding proteins poly-A binding protein (PAB) ([25]; PDB-code=1CVJ; Z-score=15.5; RMSD=1.1 Å and 37% sequence identity for residues 11-88) and sex-lethal (SXL) ([31]; PDB-code=1B7F; Z-score=14.6; RMSD=1.3Å and 28% identity for residues 125-202) (Fig. 2B). Other close matches identified using the DALI protein structure server include hnRNP A1 ([32]; PDB-code=1HA1; Z-score=13.7; RMSD=1.3Å and 27% identity for residues 13-89), nuclear cap binding protein ([33]; PDB-code=1H6K; Z-score=12.7; RMSD=1.3 and 34% identity for residues 40-117), and PRP24 ([34]; PDB-code=2GHP; Z-score=12.7; RMSD=2.0 and 21% identity for residues 41-115). The β2-β3 loop conformations of TIA-1–RRM2, PRP24, and the nuclear cap binding protein are the most variable regions of the structures, whereas β2-β3 loop conformations are very similar among the structures of TIA-1–RRM2 and the other single-stranded RNA binding proteins PAB, SXL, and hnRNP A1. The β2-β3 loop differences are likely to result from interdomain interactions mediated by the PRP24 β2-β3 loop, and removal of the nuclear cap binding protein β2-β3 loop by limited proteolysis. All of the aligned structures share a common 310-helical extension of the first α-helix, and a β-hairpin (β4-β5) insertion prior to the terminal β-strand (Fig. 2B).
3.2 Influence of the SER mutations on RNA binding and crystallization
As predicted based on homology with other RRMs, the SER-modified residues are located on the exterior of the first α-helix, distant from the putative RNA binding surface presented by the β sheet (Fig. 2C). To ascertain that the SER–RRM2 binds RNA comparably to unmodified RRM2 of TIA-1, fluorescence anisotropy assays were used to compare the affinities of the wild-type and SER–RRM2 for uridine-rich RNA. The SER mutations had no detectable effect on the polyuridine-binding characteristics of TIA-1–RRM2 (Fig. 2D), demonstrating the utility of these mutations for structural investigations of TIA-1–RNA binding.
Rather than mediating crystallographic symmetry-related interactions, the SER residues are located at the interface of two RRM2 molecules in the asymmetric unit (Fig. 2C). Juxtaposition of the SER alanine-replacements for Glu109, Asp110, and Lys112, coupled with surrounding hydrophobic residues, created a nonpolar iodide-binding site. An iodide with a significant anomalous difference Fourier signal (22σ) makes eight contacts ranging from 3.4 Å (H2O---I−) to 4.6 Å (Glu109Ala methyl group---I−) in length, consistent with the experimentally determined 3.6–3.7 Å distances of iodide hydration [35] and coordination distances of iodides bound nonspecifically to proteins [36]. Anti-parallel association of the N-terminal α-helices about the noncrystallographic two-fold axis places the β-sheet surfaces of the two molecules on the equivalent face of the RRM2 dimer. Although the SER mutations promote a noncrystallographic interaction between two TIA-1 RRMs, this interaction in turn positions the molecules appropriately to build the crystal lattice.
3.3 Symmetry-related protein-protein interactions mediated by RNP motifs
Although the level of primary sequence identity among most RRMs is low (<25%), two ribonucleoprotein consensus motifs (RNP1 and RNP2) in the central β-strands commonly display aromatic stacking interactions with bound nucleic acids. Nevertheless, the RNP motifs of certain RRMs engage in protein-protein rather than protein-RNA recognition (reviewed in [37; 38]), including the Y14-mago [39; 40; 41] and Upf3-Upf2 [42] complexes. Remarkably, the TIA-1 RNP1 and RNP2 motifs interact extensively with the α-helices of a symmetry-related molecule (Fig. 3A). The buried solvent-accessible surface area is large for a crystal packing interface (945Å2 compared with 570Å2 average crystal packing interface), but slightly less than expected for an oligomeric protein complex (1200–2000 Å2) [43]. Despite low primary sequence homology (27%), the symmetry-related TIA-1 contacts are qualitatively similar to the Y14 interactions with the α-helices of the mago partner [39; 40; 41] (Fig. 3B). In contrast, the Upf3-Upf2 orientation [42] differs from that of the symmetry-related TIA-1 RRMs. Although the TIA-1–RRM2 domain is likely to bind RNA in a canonical manor given its established role in RNA binding [19; 21; 22], the extensive symmetry-related interface mediated by the TIA-1 RNP motifs illustrates the versatile potential of RNP motifs to engage in protein-protein as well as protein-RNA interactions.
Fig. 3.

Comparisons with the TIA-1 RNP motifs. (A) Symmetry-related interactions between two TIA-1–RRM2 molecules, respectively colored yellow and green. Key interacting residues are shown with ball-and-stick representation, and consensus residues of the RNP motifs responsible for mediating the intermolecular interaction are colored magenta. Primed italics distinguish residue labels of the symmetry-related molecule. (B) View of the Y14 (red)-mago (blue) complex (PDB-code 1OO0). Regions of the mago subunit outside the α-helical interactions with the Y14 RRM are omitted for clarity. (C) Superposition of TIA-1–RRM2 with PAB residues 11-88 (PDB-code 1CVJ). (D) Superposition of TIA-1–RRM2 with SXL residues 125-202 (PDB-code 1B7F). TIA-1 is colored yellow-orange, PAB is blue, and SXL is green. The RNA strands are colored magenta. The view is rotated 90° clockwise about the y-axis relative to (A). Residues of TIA-1 (Phe98, Arg125, Asp129, Tyr138, Phe140, Arg167) are labeled. Corresponding residues of PAB (Tyr14, Arg41, Asp45, Tyr54, Tyr56, Arg83) and SXL (Ile128, Arg155, Asp159, Tyr168, Phe170, Lys197) are shown as ball-and-stick representations.
3.4 Comparison of putative RNA binding surfaces
The close structural matches of TIA-1–RRM2 with the N-terminal RRMs of PAB and SXL provide an opportunity to compare the residues involved in RNA interactions. Six prominent residues responsible for RNA interactions in both the PAB and SXL complexes are also conserved in the TIA-1–RRM2 (Fig. 3C–D), despite slight differences among the RNA sequences preferred by these factors. Three hydrophobic residues of the RNP motifs (RNP2-Phe98; RNP1-Tyr138; RNP1-Phe140) mediate conserved base- and sugar-stacking interactions of PAB and SXL, as well as the majority of other canonical RRMs [38]. Despite amino acid conservation, the remaining three residues are involved in distinct types of RNA interactions in the PAB compared with SXL complexes, due to differences in the local conformations of the RNA ligands. Most prominently, an adenosine in the PAB complex is rotated 180° relative to the SXL-bound uridine counterpart. Altogether, the PAB residues corresponding to Arg125, Asp129, and Arg167 of TIA-1 respectively engage in base stacking interactions, a specific hydrogen bond with an adenosine exocyclic amine, and a phosphate salt bridge [25], whereas the corresponding residues of SXL respectively hydrogen bond with a ribose 2′ hydroxyl, lack direct RNA interactions, or form a specific hydrogen bond with a uracil base [31]. These differences underscore the difficulties confronting accurate prediction of RRM-RNA interactions, despite an expanding number of RRM-nucleic acid structures [44].
3.5 Locations of RNP residues implicated in nuclear import
Previously, the RRM2 RNP motifs of the TIA-1 homologue, TIAR, were mutated to investigate the importance of RNA binding for nuclear import [23]. Given that the RRM2 regions of TIA-1 and TIAR share 93% sequence identity, the availability of the TIA-1–RRM2 structure provides the opportunity to examine the locations of the mutated residues (Fig. 4). The nuclear localization of two mutant proteins was investigated, each with a cluster of mutations in either RNP1 (Gly137Asp/Tyr138Asp/Phe140Asp/Val141Glu, TIA-1 numbering), or RNP2 (Phe98Asp/Gly100Pro/Leu102Asp). Although several of the residues are solvent-exposed and may interact with RNA, both Leu102 (RNP2) and Val141 (RNP1) are buried in the hydrophobic protein interior (Fig. 4), where charged aspartate and glutamate side chains introduced by the site-directed mutagenesis would be likely to interfere with domain folding. Thus, the conclusion that TIAR RNA binding ability is important for nuclear accumulation is premature. Instead, the TIA-1–RRM2 structure presented here supports the conclusion that the folded RRM2 domain is necessary for nuclear accumulation, as shown by deletion analysis of both TIA-1 and TIAR [23].
Fig. 4.

Site-directed mutations used to investigate TIAR nuclear localization mapped on the TIA-1–RRM2 structure. Side chains of solvent-exposed mutated residues are shown with ball-and-stick representation and colored orange. The mutated glycines are indicated by spheres. Leu102 of the RNP2 motif (blue) and Val141 of the RNP1 motif (red) are buried within the RRM interior. Residues that contact Leu102 are shown with ball-and-stick representation, and colored salmon. Residues that contact Val141 are also shown, and colored light blue. Residues that contact both Leu102 and Val141 are colored violet.
Supplementary Material
Supplementary Fig. 1. Crystals of TIA-1–RRM2 in the absence (A) and presence (B) of SER mutations.
Acknowledgments
We thank J. Valcárcel for graciously sharing plasmids, and E.C. Reilly for protein purification. M.C.S. was supported in part by a training grant from the National Cancer Institute (CA009110). The Kimmel Foundation and National Institutes of Health Grant R01-GM070503 to C.L.K supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Izquierdo JM, Majos N, Bonnal S, Martinez C, Castelo R, Guigo R, Bilbao D, Valcarcel J. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol Cell. 2005;19:475–484. doi: 10.1016/j.molcel.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Forch P, Puig O, Kedersha N, Martinez C, Granneman S, Seraphin B, Anderson P, Valcarcel J. The apoptosis-promoting factor TIA-1 is a regulator of alternative pre-mRNA splicing. Mol Cell. 2000;6:1089–1098. doi: 10.1016/s1097-2765(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 3.Del Gatto-Konczak F, Bourgeois CF, Le Guiner C, Kister L, Gesnel MC, Stevenin J, Breathnach R. The RNA-binding protein TIA-1 is a novel mammalian splicing regulator acting through intron sequences adjacent to a 5′ splice site. Mol Cell Biol. 2000;20:6287–6299. doi: 10.1128/mcb.20.17.6287-6299.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shukla S, Del Gatto-Konczak F, Breathnach R, Fisher SA. Competition of PTB with TIA proteins for binding to a U-rich cis-element determines tissue-specific splicing of the myosin phosphatase targeting subunit 1. RNA. 2005;11:1725–1736. doi: 10.1261/rna.7176605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zuccato E, Buratti E, Stuani C, Baralle FE, Pagani F. An intronic polypyrimidine-rich element downstream of the donor site modulates cystic fibrosis transmembrane conductance regulator exon 9 alternative splicing. J Biol Chem. 2004;279:16980–16988. doi: 10.1074/jbc.M313439200. [DOI] [PubMed] [Google Scholar]
- 6.McAlinden A, Liang L, Mukudai Y, Imamura T, Sandell LJ. Nuclear protein TIA-1 regulates COL2A1 alternative splicing and interacts with precursor mRNA and genomic DNA. J Biol Chem. 2007;282:24444–54. doi: 10.1074/jbc.M702717200. [DOI] [PubMed] [Google Scholar]
- 7.Lopez de Silanes I, Galban S, Martindale JL, Yang X, Mazan-Mamczarz K, Indig FE, Falco G, Zhan M, Gorospe M. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol Cell Biol. 2005;25:9520–9521. doi: 10.1128/MCB.25.21.9520-9531.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J. 2000;19:4154–4163. doi: 10.1093/emboj/19.15.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon DA, Balch GC, Kedersha N, Anderson P, Zimmerman GA, Beauchamp RD, Prescott SM. Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1. J Exp Med. 2003;198:475–481. doi: 10.1084/jem.20030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamasaki S, Stoecklin G, Kedersha N, Simarro M, Anderson P. T-cell intracellular antigen-1 (TIA-1)-induced translational silencing promotes the decay of selected mRNAs. J Biol Chem. 2007;282:30070–7. doi: 10.1074/jbc.M706273200. [DOI] [PubMed] [Google Scholar]
- 11.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30:963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- 12.Le Guiner C, Gesnel MC, Breathnach R. TIA-1 or TIAR is required for DT40 cell viability. J Biol Chem. 2003;278:10465–10467. doi: 10.1074/jbc.M212378200. [DOI] [PubMed] [Google Scholar]
- 13.Beck AR, Miller IJ, Anderson P, Streuli M. RNA-binding protein TIAR is essential for primordial germ cell development. Proc Natl Acad Sci U S A. 1998;95:2331–2336. doi: 10.1073/pnas.95.5.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck AR, Medley QG, O’Brien S, Anderson P, Streuli M. Structure, tissue distribution and genomic organization of the murine RRM-type RNA binding proteins TIA-1 and TIAR. Nucleic Acids Res. 1996;24:3829–3825. doi: 10.1093/nar/24.19.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawakami A, Tian Q, Streuli M, Poe M, Edelhoff S, Disteche CM, Anderson P. Intron-exon organization and chromosomal localization of the human TIA-1 gene. J Immunol. 1994;152:4937–4945. [PubMed] [Google Scholar]
- 16.Kawakami A, Tian Q, Duan X, Streuli M, Schlossman SF, Anderson P. Identification and functional characterization of a TIA-1-related nucleolysin. Proc Natl Acad Sci U S A. 1992;89:8681–8685. doi: 10.1073/pnas.89.18.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izquierdo JM, Valcarcel J. Two isoforms of the T-cell intracellular antigen 1 (TIA-1) splicing factor display distinct splicing regulation activities. Control of TIA-1 isoform ratio by TIA-1-related protein. J Biol Chem. 2007;282:19410–7. doi: 10.1074/jbc.M700688200. [DOI] [PubMed] [Google Scholar]
- 18.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell. 2004;15:5383–5398. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forch P, Puig O, Martinez C, Seraphin B, Valcarcel J. The splicing regulator TIA-1 interacts with U1-C to promote U1 snRNP recruitment to 5′ splice sites. EMBO J. 2002;21:6882–6892. doi: 10.1093/emboj/cdf668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suswam EA, Li YY, Mahtani H, King PH. Novel DNA-binding properties of the RNA-binding protein TIAR. Nucleic Acids Res. 2005;33:4507–4518. doi: 10.1093/nar/gki763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dember LM, Kim ND, Liu KQ, Anderson P. Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J Biol Chem. 1996;271:2783–2788. doi: 10.1074/jbc.271.5.2783. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Li Y, Kedersha N, Anderson P, Emara M, Swiderek KM, Moreno GT, Brinton MA. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J Virol. 2002;76:11989–12000. doi: 10.1128/JVI.76.23.11989-12000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang T, Delestienne N, Huez G, Kruys V, Gueydan C. Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J Cell Sci. 2005;118:5453–5463. doi: 10.1242/jcs.02669. [DOI] [PubMed] [Google Scholar]
- 24.Sali A, Blundell TL. Comparative protein modeling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 25.Deo RC, Bonanno JB, Sonenberg N, Burley SK. Recognition of polyadenylate RNA by the poly(A)-binding protein. Cell. 1999;98:835–845. doi: 10.1016/s0092-8674(00)81517-2. [DOI] [PubMed] [Google Scholar]
- 26.Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr. 2004;D60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 27.McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Likelihood-enhanced fast translation functions. Acta Crystallogr. 2005;D61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]
- 28.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. 1998;D54:905–21. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 29.C.C. Project, and N. 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. 1994;D50:760–63. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 30.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. 2004;D60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 31.Handa N, Nureki O, Kurimoto K, Kim I, Sakamoto H, Shimura Y, Muto Y, Yokoyama S. Structural basis for recognition of the tra mRNA precursor by the Sex-lethal protein. Nature. 1999;398:579–585. doi: 10.1038/19242. [DOI] [PubMed] [Google Scholar]
- 32.Shamoo Y, Krueger U, Rice LM, Williams KR, Steitz TA. Crystal structure of the two RNA binding domains of human hnRNP A1 at 1.75 A resolution. Nat Struct Biol. 1997;4:215–22. doi: 10.1038/nsb0397-215. [DOI] [PubMed] [Google Scholar]
- 33.Mazza C, Ohno M, Segref A, Mattaj IW, Cusack S. Crystal structure of the human nuclear cap binding complex. Mol Cell. 2001;8:383–396. doi: 10.1016/s1097-2765(01)00299-4. [DOI] [PubMed] [Google Scholar]
- 34.Bae E, Reiter NJ, Bingman CA, Kwan SS, Lee D, Phillips GN, Jr, Butcher SE, Brow DA. Structure and interactions of the first three RNA recognition motifs of splicing factor prp24. J Mol Biol. 2007;367:1447–58. doi: 10.1016/j.jmb.2007.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtaki H. Ionic solvation in aqueous and nonaqueous solutions. Monatshefte für Chemie. 2001;132:1237–1268. [Google Scholar]
- 36.Sickmier EA, Frato KE, Kielkopf CL. Crystallization and preliminary X-ray analysis of U2AF65 variant in complex with a polypyrimidine tract analogue by use of protein engineering. Acta Crystallogr. 2006;F62:457–459. doi: 10.1107/S1744309106012504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kielkopf CL, Lucke S, Green MR. U2AF homology motifs: protein recognition in the RRM world. Genes Dev. 2004;18:1513–26. doi: 10.1101/gad.1206204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maris C, Dominguez C, Allain FH. The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 2005;272:2118–31. doi: 10.1111/j.1742-4658.2005.04653.x. [DOI] [PubMed] [Google Scholar]
- 39.Fribourg S, Gatfield D, Izaurralde E, Conti E. A novel mode of RBD-protein recognition in the Y14-Mago complex. Nat Struct Biol. 2003;10:433–9. doi: 10.1038/nsb926. [DOI] [PubMed] [Google Scholar]
- 40.Lau CK, Diem MD, Dreyfuss G, Van Duyne GD. Structure of the Y14-Magoh core of the exon junction complex. Curr Biol. 2003;13:933–41. doi: 10.1016/s0960-9822(03)00328-2. [DOI] [PubMed] [Google Scholar]
- 41.Shi H, Xu RM. Crystal structure of the Drosophila Mago nashi-Y14 complex. Genes Dev. 2003;17:971–6. doi: 10.1101/gad.260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kadlec J, Izaurralde E, Cusack S. The structural basis for the interaction between nonsense-mediated mRNA decay factors UPF2 and UPF3. Nat Struct Mol Biol. 2004;11:330–7. doi: 10.1038/nsmb741. [DOI] [PubMed] [Google Scholar]
- 43.Janin J, Rodier F, Chakrabarti P, Bahadur RP. Macromolecular recognition in the Protein Data Bank. Acta Crystallogr D Biol Crystallogr. 2007;63:1–8. doi: 10.1107/S090744490603575X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auweter SD, Oberstrass FC, Allain FH. Sequence-specific binding of single-stranded RNA: is there a code for recognition? Nucleic Acids Res. 2006;34:4943–59. doi: 10.1093/nar/gkl620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laskowski RA, MacArthur MW, Moss DS, Thornton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr. 1993;26:283–291. [Google Scholar]
- 46.Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J Mol Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Crystals of TIA-1–RRM2 in the absence (A) and presence (B) of SER mutations.