Abstract
Phytochemicals have shown promise in inhibiting UV-induced oxidative stress, and therefore considered as potent inhibitors of UV-induced oxidative stress-mediated skin diseases. We have shown previously that topical treatment of silymarin, a flavonoid from milk thistle (Silibum marianum), inhibits UV-induced oxidative stress in mouse skin. However, the cellular targets responsible for the inhibition of UV-induced oxidative stress by silymarin are not clearly defined. To address this issue, C3H/HeN mice were UV irradiated (90 mJ/cm2) with or without topical treatment with silymarin (1mg/cm2 skin area). Mice were sacrificed 48 h later and skin samples collected. Flow cytometric analysis of viable dermal cells revealed that the number of infiltrating CD11b+ cells were the major source of oxidative stress (31.8%) in UV irradiated skin compared to non-UV exposed skin (0.4%). Treatment of silymarin inhibited UV-induced oxidative stress through inhibition of infiltrating CD11b+ cells. The analysis of myeloperoxidase also indicated that silymarin significantly (p<0.001) decreased UV-induced infiltration of leukocytes, and this effect of silymarin was similar to that of intraperitoneal treatment of mice with monoclonal antibodies to CD11b. The inhibitory effect of silymarin, whether it is topically treated before or after UV irradiation, was of similar magnitude. Intraperitoneal administration of monoclonal antibodies to CD11b (rat IgG2b) to C3H/HeN mice inhibited UVB-induced oxidative stress generated by both epidermal and dermal cells as is evident by relative fluorescence intensity of oxidized rhodamine. Similar to the effect of anti-CD11b, silymarin also inhibited UV-induced oxidative stress in both epidermal and dermal cells. Further, CD11b+ and CD11b− cell subsets from UV-treated or silymarin+UV-treated mice were separated by immunomagnetic cell isolation technique from total epidermal and dermal single cell suspensions and analyzed for ROS/H2O2 production. Analytical data revealed that CD11b+ cell population from UV irradiated skin resulted in significantly higher production of ROS in both epidermis and dermis than CD11b− cell population, and that silymarin inhibited UV-induced oxidative stress through targeting infiltrating CD11b+ cell type in the skin.
Keywords: Silymarin, ultraviolet radiation, oxidative stress
INTRODUCTION
The exposure of the skin to solar ultraviolet (UV) radiation generates reactive oxygen species (ROS) or oxidative stress, which is capable of oxidizing macromolecules, like lipids, proteins and DNA. UV-induced oxidative stress has been associated with several cutaneous disorders including photoaging and photocarcinogenesis (1–2). UV induces ROS in the skin through two mechanisms: (i) through the direct interaction of photons and cellular molecules, and (ii) through infiltrating leukocytes in the skin after several hours of UV irradiation (1). Infiltrating leukocytes start appearing in the dermis at 6-h after UV exposure, but maximum infiltration was noticed in between 48–72 h post-UV exposure both in dermis and epidermis (3,4). We have shown that UV-induced infiltrating cells are the major source of reactive oxygen species generation both in animal and human skin (4,5), and that Class II MHC+ CD11b+ cells are the major infiltrating cells in UV irradiated skin, and this cell subset is a potent source of oxidative stress in UV-exposed skin sites (4,5).
Silymarin, a flavonoid, obtained from the fruits and seeds of the milk thistle (Silybum marianum L. Gaertn.), and is composed of mainly silibinin (≈90%) together with small amounts of other silibinin stereoisomers, such as isosilybin, dihydrosilybin, silydianin, and silychristin (6). Silymarin has been shown to possess antioxidant effect against UV radiation in mouse skin model (7), and therefore has been tested against photocarcinogenic effect of UV radiation. It was observed that treatment of mouse skin with silymarin inhibited UV-induced skin carcinogenesis in terms of tumor incidence, tumor multiplicity and growth of the tumors (8). In earlier studies, we showed that topical application of silymarin decreases UV-induced oxidative stress in the mouse skin (7); however, it is not clearly understood that which type of UV-induced infiltrating cell subset is the major target of silymarin through which it inhibits oxidative stress in the UV-exposed skin. To understand and define whether inhibition of UV-induced oxidative stress by topical application of silymarin is mediated through the inhibition of infiltrating CD11b+ cell subset, we conducted experiments using C3H/HeN mice. Shaved dorsal skin of the mice was exposed to an acute UV dose of 90 mJ/cm2 which is sufficient to induce significant infiltration of leukocytes, and because 48 h time point after UV irradiation is the peak time of infiltrating leukocytes, mice were sacrificed at this time point and skin biopsies were collected for experimental purposes. In other treatment groups, mice were topically treated with silymarin (1mg/cm2 skin area) either at least 30 min before UV irradiation or just after UV-irradiation, and sacrificed 48 h after irradiation.
To further understand whether silymarin inhibits UV-induced oxidative stress primarily through the inhibition of infiltrating CD11b+ cells, mice were treated in vivo with an anti-CD11b mAb (clone 5C6). This mAb inhibits extravasation of myelomonocytic cells in response to inflammatory stimuli without affecting the egression of myelomonocytic cells from the bone marrow into the blood (9). Since anti-CD11b mAb has been used to examine the effect of CD11b+ infiltrating leukocytes in diverse murine models (9–12), we used this mAb in the present study.
MATERIALS AND METHODS
Chemicals and antibodies
Silymarin was purchased from Sigma Chemical Co. (St. Louis, MO). Dihydrorhodamine 123 (DHR) and ethidium monoazide were obtained from Molecular Probes (Eugene, OR). Monoclonal antibodies to anti-CD11b (rat anti-mouse, clone 5C6) were purchased from Serotec Ltd. (Oxford, UK). Phycoerythrin-conjugated rat anti-CD11b (clone, M1/70), anti-CD16/32 and rat IgG2b isotype were purchased from PharMingen (San Diego, CA). Rat anti-mouse CD11b antibody coated magnetic beads were purchased from Dynal Inc. (Lake Success, NY).
Animals and administration of anti-CD11b (5C6) antibody to UV-exposed mice
Pathogen-free female C3H/HeN (6–7 weeks old) mice were purchased from Charles River Laboratories (Wilmington, MA). Mice were kept five per cage and were acclimatized for one week before use, and subjected to a 12 h-light/12 h-dark cycle, 24±2°C and 50±10% relative humidity. Mice were housed in Animal Resource Facility in accordance with current US Department of Agriculture, Department of Health and Human Services regulations and standards. Endotoxin-free rat anti-mouse CD11b monoclonal antibody or rat IgG2b isotype control was administered intraperitoneally through injection for three consecutive days, i.e., one day before UV exposure, same day of UV exposure and one day after UV exposure of the animals. In each injection, 50 ig of anti-CD11b or rat IgG2b isotype was administered in each animal. Control mice were injected PBS intraperitoneally to maintain same treatment protocol.
UV irradiation
Dorsal skin of the mice was shaved with electric clipper and chemically depilated at least 48 h before UVB exposure. Mice were UVB-irradiated as described previously (13). Briefly, the clipper-shaved dorsal skin was exposed to UV radiation from a band of four FS24T1 UVB lamps (Daavlin, UVA/UVB Research Irradiation Unit, Bryan, OH) equipped with an electronic controller to regulate UV dosage. The UV lamps emit UVB (290–320 nm; ≈80% of total energy) and UVA (320–375 nm; ≈20% of total energy), with UVC emission being insignificant. The majority of the resulting wavelengths of UV radiation are in the UVB (290–320 nm) range with peak emission at 313 nm. The UV irradiation is controlled using Daavlin Flex Control Integrating Dosimeters. In this system, the UVB dose can be entered as mJ/cm2 and the unit automatically compensates for variations in energy output such that the desired UV dose is delivered. Mice were subjected to acute UVB exposure of 90 mJ/cm2. Non-UVB exposed group of mice were also shaved, depilatory lotion was applied to maintain same treatment protocol. This study and animal protocol was approved by the Institutional Animal Care and Use Committee.
Isolation of epidermal and dermal single cell suspension
Skin samples were obtained from normal (non-UV exposed) and 48 h post UV exposed mice and single cell suspensions from the epidermis and dermis were prepared as described previously (14). Briefly, skin samples were placed into a solution of 50U/ml dispase (Collaborative Research, Bedford, MA) overnight at 4°C. Epidermis and dermis were separated from each other. Epidermis was incubated with 0.25% trypsin (Sigma Chemical Co.) for 60 min at 37°C and dispersed into cell suspension buffer containing 0.025% DNase. Cell suspension was filtered through 50μm nylon mesh to obtain epidermal single cell suspension. Similarly, dermis was placed into a digestion buffer of RPMI 1640 (Gibco BRL, Grand Island, NY) containing 10mM HEPES (Irvine Scientific, Santa Ana, CA), 0.01% DNase, 1000 U/ml of hyaluronidase and collagenase (Sigma Chemical Co.), and digested at 37°C for 1 h. The digested dermis was filtered through 100- and 50- μm nylon mesh to collect single cell suspension from the dermis.
Flow cytometric analysis of CD11b+ cells as a source of ROS/H2O2 production
As UV-induced infiltrating leukocytes highly accumulated in the dermis we used dermal single cell suspension for analyzing CD11b+ cells as a major source of H2O2 production. CD11b+ cells represent a heterogeneous cell population in UV irradiated skin, and mainly constitute activated monocytes/macrophages and neutrophils. CD11b-positive hydrogen peroxide producing cells were analyzed using flow cytometry as described previously (15). One million FcIgG receptor-blocked (anti-CD16/32, 2.4G2, Pharmingen, San Diego, CA) dermal cells from the skin of normal (non-UV exposed) mice, UV irradiated mice or silymarin-treated UVB-exposed mice obtained 48 h after UV exposure were incubated with the following combination: phycoerythrin-conjugated rat anti-CD11b (M1/70), and dihydrorhodamine 123 (DHR). Control cells were stained with identically conjugated isotype control, rat IgG2b. Dead cells were identified by addition of ethidium monoazide to freshly stained cells for 10 min on ice under visible light for cross-linkage to DNA (16). Stained cells were fixed in 1% paraformaldehyde before flow cytometric analysis using an Epics Elite Cytometer (Coulter Electronics, Hialeah, FL). Gating of quadrant to identify CD11b+H2O2+ cell subset and their analysis was performed on the basis of its isotype control and using ListMode software program. Further, low and high expressions of CD11b on cells were distinguished based on the intensity of mean channel fluorescence and distinct cell population. Positive cell percentages were determined by calculating the percentage of a given positive cell population in terms of total viable cells and subtracting the percentage of cells found within the same positive gate in isotype control-stained dermal cell samples.
Myeloperoxidase (MPO) activity
Myeloperoxidase was determined as a marker of cellular infiltration in the skin using the cytosolic fractions obtained from epidermis and dermis following the procedure of Bradley (17). Briefly, skin samples were collected from non-UVB exposed as well as 48 h after UVB exposed mouse skin. Epidermal and dermal layers of the skin were separated using dispase enzyme as described previously (14). The epidermis and dermis separately were homogenized in 50 mM potassium phosphate buffer, pH 6.0, containing 0.5% hexadecyltrimethyl-ammonium bromide followed by sonication of the homogenate at 4° C for three 10 s bursts with a heat system sonicator equipped with a microprobe. For complete extraction of the enzyme from the cells, the tissue homogenates were frozen and thawed three times, and each time sonication was repeated. The tissue homogenate thus obtained was centrifuged at 40,000 g for 15 min at 4° C, and the resulting supernatants were used for the analysis of MPO. MPO activity in supernatant (0.1 ml) was assayed by mixing with 50 mM phosphate buffer (2.9 ml), pH 6.0, containing 0.167 mg/ml ortho-dianisidine dihydrochloride and H2O2 (0.0005%). The change in absorbance resulting from decomposition of H2O2 in the presence of ortho-dianisidine was measured at 460 nm using Beckman DU 640 spectrophotometer. The results are expressed as the mean OD/min/mg protein.
Quantitative assay of intracellular release of ROS/H2O2
Intracellular level of ROS including the levels of H2O2 in epidermal and dermal cells was determined using dihydrorhodamine 123 (DHR) as a specific fluorescent dye probe as described previously (5,18). Briefly, one million epidermal or dermal cells from each treatment group were taken in each well of a 24 well tissue culture plate in triplicate. Cells were treated with dihydrorhodamine 123 (5 μM) for 45 min. Reduced DHR is irreversibly oxidized and converted to the fluorescent compound rhodamine 123 by UVB-induced intracellular release of reactive oxygen species. Fluorescence intensity was measured on a Synergy HT (Bio-TEK Instruments, Inc.) fluorescence plate reader with an excitation wavelength of 485 nm and an emission wavelength of 530 nm.
Isolation of CD11b+ and CD11b− cells using immunomagnetic separation technique
Immunomagnetic cell separation using Dynabeads mouse CD11b provides a fast and reliable method for isolation of CD11-positive cells. Dynabeads are uniform magnetizable polystyrene beads coated with a rat monoclonal antibody specific for the CD11b membrane antigen which is expressed on cell surface of monocytes/macrophages and neutrophils. CD11b is expressed on the cells surfaces of heterogeneous population of cells like monocytes/macrophages and neutrophils and they may have different functional activities. CD11b+ and CD11b− cells were separated following the manufacturer’s protocol. Briefly, positive selection of CD11b+ cells was performed from the total epidermal or dermal single cell suspensions from UV irradiated skin using monoclonal antibodies and magnetic device (Dynal MPC-Magnetic Particle Concentrator). Cell supernatants collected after separating CD11b+ cells contained approximately 95% CD11b−cells. By using manufacturer’s protocol, the purity of CD11b+ cell population was >98% and viability of the cells was >95%. To compare the relative production of ROS in both populations, one million cells from each population in triplicate were placed in 24 well culture plates. Thus, both cell populations, i.e., CD11b+ and CD11b−, were employed to determine separately intracellular release of ROS including H2O2 using DHR 123 as a fluorescent dye probe as described previously (5,18).
Statistical analysis
The statistical significance of difference between control and treated groups was determined by Student’s t test. P<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
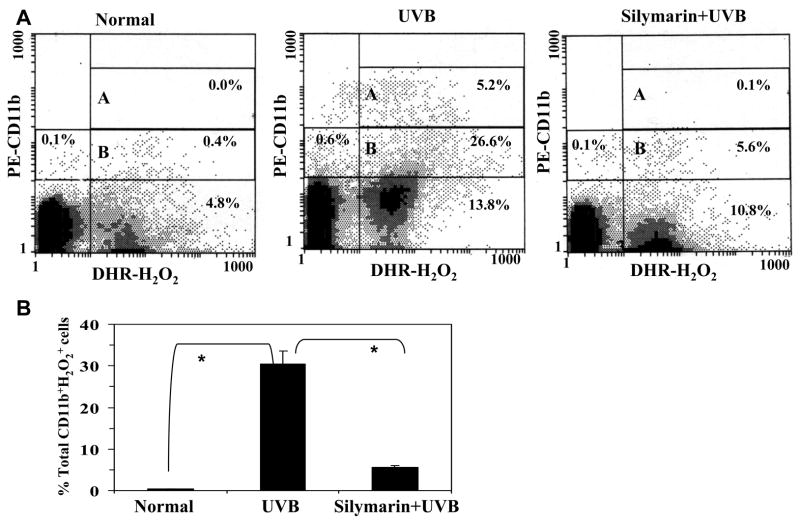
We have shown previously that skin exposure to the UV radiation induced infiltration of leukocytes and these infiltrating leukocytes are the major source of reactive oxygen species generation, including the production of hydrogen peroxide and nitric oxide (4,5). It has been shown that the number of CD11b+ cells were higher in UV-irradiated skin sites than non-UV irradiated skin (4,5,15). The UV-induced oxidative stress has been implicated in the development of various skin disorders, including premature aging of the skin and skin cancers. We have shown previously that several phytochemicals, including silymarin, significantly inhibits UV-induced oxidative stress in the mouse skin through the inhibition of UV-induced infiltration of leukocytes. However, it was not clearly understood which type of cell subset is inhibited from infiltrating the skin. As UV-induced infiltrating CD11b+ cells are major source of oxidative stress, we determined whether treatment of the skin with silymarin inhibits the infiltration of CD11b+ cells in skin and thus results in the reduction of oxidative stress. To address this issue, we conducted three color flow cytometric analysis of dermal cells obtained from UV-irradiated skin and silymarin+UV treated skin and the resultant data were compared with the non-UVB-irradiated control skin. Because infiltrating cells highly accumulated in the dermis, we preferentially used dermal single cell suspension for this purpose. ROS including H2O2 were analyzed as marker of oxidative stress. As shown in Fig. 1A, UV-irradiated skin contained 31.8% CD11b+ H2O2 + cells (double positive) in terms of viable cells while non-UV exposed normal skin contained only 0.4% CD11b+ H2O2 + cell population. Further, 26.6% H2O2 producing cell population induced low expression of CD11b (quadrant B) while 5.2% H2O2 producing cells induced higher expression of CD11b on infiltrating cells (quadrant A). Pre-treatment of the skin with silymarin resulted in only 5.7% infiltration of CD11b+ H2O2 + cell population. These data suggest that silymarin inhibits UV-induced oxidative stress primarily through the inhibition of infiltrating CD11b+ cell type and this inhibition is about 80% (p<0.001). Data were summarized from three independent experiments and presented as means± SD of total CD11b+ H2O2 + cells in different treatment groups (Fig. 1B).
Figure 1.
Silymarin inhibits the percentage of hydrogen peroxide producing CD11b+ (CD11b+ H2O2 +) cells in the UV-exposed mouse skin compared to those mice which were not treated with silymarin but exposed to UVB. Silymarin was dissolved in 200 μl of acetone and topically applied (1mg/cm2 skin area) on the skin 30 min before UV exposure. Mice were sacrificed 48 h later and skin samples were collected. Panel A, Quantitative analysis of CD11b+ H2O2 + cell subset was performed in dermal single cell suspension using flow cytometry. Dermal single cell suspension was prepared from the dorsal skin of each mouse (4cm2/mouse) of each treatment group, and subjected to three-color flow cytometric analysis, as detailed in Materials and Methods, n=5. A representative analytical FACS histogram from three independent experiments is shown. Quadrant B and A represents respectively the cells expressing low and high expression of CD11b. Panel B, Total percent CD11b+ H2O2+ cells are presented as mean± SD from three independent experiments. *Significant difference between UVB-exposed mice versus normal (non-UV-exposed), and UVB versus silymarin +UVB-treated mice, p<0.001.
As we have described previously the effect of UVA and UVB on oxidative stress (1), the studies have classified the role of UVB and UVA wavelengths on the skin. These studies suggested that UVB, not UVA, inducing infiltration of macrophages to the skin while UVA, not UVB, generates ROS or oxidative stress (1,19). The present study suggest that UV (which contained major part of UVB)-induced infiltration of activated macrophages (CD11b+ cells) are the major source of H2O2 production, and thus UVB exposure to the skin is also responsible for the generation of oxidative stress at the UVB-irradiated skin site. As UV-induced oxidative stress has been implicated in several skin diseases, including photoaging and photocarcinogenesis, the inhibition of infiltrating CD11b+ cells in UV exposed skin by silymarin may be a possible mechanism through which it prevents photocarcinogenesis in mice (8).
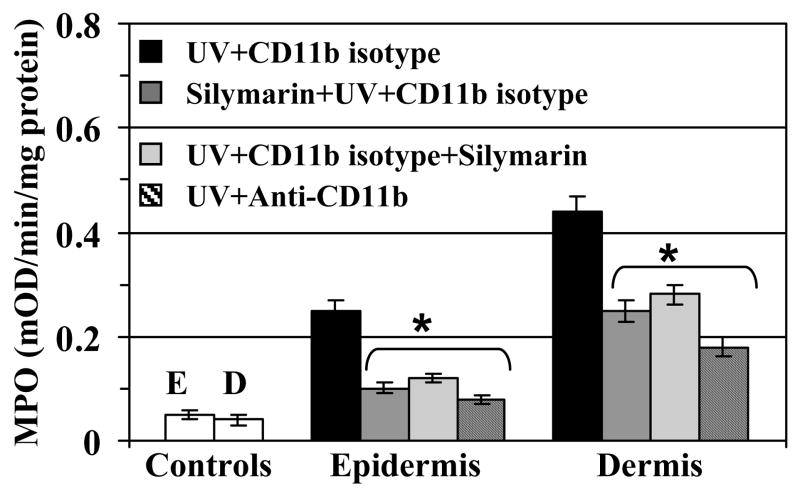
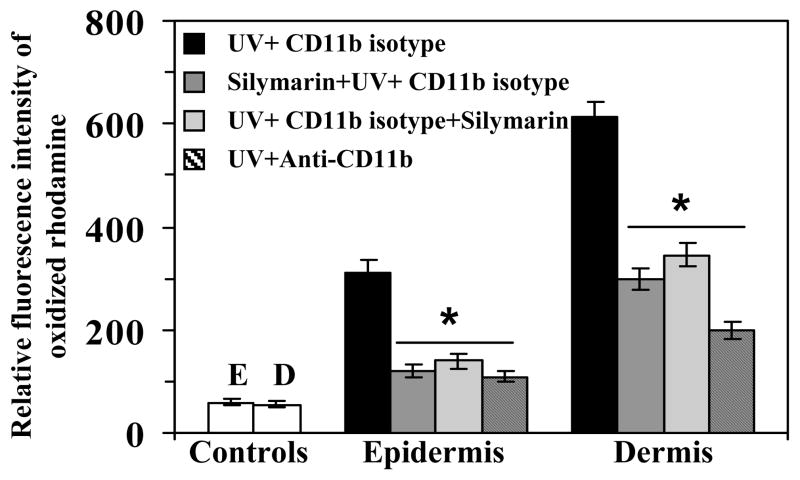
Comparative infiltration of leukocytes in different treatment groups was also confirmed by measuring MPO activity in cytosolic fraction of the epidermis and dermis (Fig. 2). Mice were treated with silymarin before and immediate after UV exposure of the skin with the aim to compare the protective effect of silymarin in both systems. Further, UV-exposed mice were treated either with anti-CD11b or isotype of the CD11b to compare with the effect of silymarin-treated mice to confirm the observation that the effect of silymarin is similar to that of anti-CD11b mAb treatment of the mice. The level of MPO was determined as a marker of tissue infiltration. MPO data clearly demonstrated that infiltration in the epidermis and dermis was higher in UV-exposed mice than non-UV-exposed mice. However, the treatment of mice with silymarin before UV exposure significantly inhibited the levels of MPO in both epidermis and dermis. Identical results were observed when silymarin was treated on the mouse skin just after UV-irradiation. These observations suggest that topical application of silymarin, either before or after UV irradiation, has the ability to protect the skin from UV-induced infiltration of inflammatory leukocytes. Similar to silymarin, the treatment of mice with anti-CD11b mAb also resulted in significant inhibition (p<0.001) of UV-induced infiltration of leukocytes as evident from the MPO data. The use of rat IgG2b isotype in UV-exposed mice served as a control. These data suggest that silymarin reduced UV-induced infiltration similar to the effect of anti-CD11b mAb (Fig. 2). Further to confirm that treatment of silymarin and anti-CD11b mAb inhibited UV-induced oxidative stress in UV irradiated skin through the inhibition of CD11b+ cells, quantitative analysis of intracellular release of ROS/H2O2 in epidermal and dermal cells was performed. For this purpose equal area of non-UV-exposed and UV-exposed dorsal skin from each treatment was used for the isolation of epidermal and dermal cells. As shown in Fig. 3, treatment of CD11b mAb to mice significantly inhibited UV-induced ROS/H2O2 both in epidermis and dermis (64–67%, p<0.001) compared to the mice which were not treated with the anti-CD11b mAb but treated with its isotype control (rat IgG2b). Similarly, treatment of mice with silymarin either before or after UV-irradiation resulted in significant inhibition of UV-induced oxidative stress in epidermis (55–61%, p<0.001) and dermis (45–51%, p<0.001) which was determined in terms of relative fluorescence intensity of oxidized rhodamine. Experiments were also performed to determine whether treatment of the skin with silymarin protects multiple UV-irradiated mouse skin from UV-induced oxidative stress and the infiltration of leukocytes. It was found that treatment of silymarin resulted in inhibition of multiple UV irradiation-induced oxidative stress and MPO level in mouse skin (data not shown).
Figure 2.
In vivo intraperitoneal treatment of anti-CD11b or topical treatment of silymarin (1mg/cm2 skin) inhibits UVB-induced myeloperoxidase (MPO) both in the epidermis and dermis. Mice were treated with silymarin either 30 min before UV irradiation or just after UV irradiation, sacrificed 48 h later and skin samples (4 cm2) were collected. Epidermal or dermal cytosolic fractions were prepared and used to analyze MPO activity, as detailed in Materials and Methods, n=5. MPO was determined as a marker of tissue infiltration of leukocytes. Experiments were repeated twice and resultant data are presented as means ± SD. E= epidermis; D= dermis.
*Significant inhibition of MPO in anti-CD11b or silymarin treated groups versus UV+CD11b−treated isotype controls, p<0.001.
Figure 3.
Topical treatment of silymarin or in vivo intraperitoneal treatment of anti-CD11b mAb inhibits UV-induced intracellular production of ROS in epidermal or dermal cells. Animals were treated as detailed in Figure 2. Quantitative analysis of UVB-induced intracellular production of ROS including H2O2 was performed in single cell suspensions from epidermis and dermis separately collected from the skin samples of five mice in each treatment group. Total ROS level was determined using dihydrorhodamine 123 as a fluorescent dye probe as described in Materials and Methods. Experiments were repeated twice and resultant data are presented as means ± SD in terms of relative fluorescence intensity of oxidized rhodamine. The mice in control group were not UV irradiated. E= epidermis; D= dermis in control group.
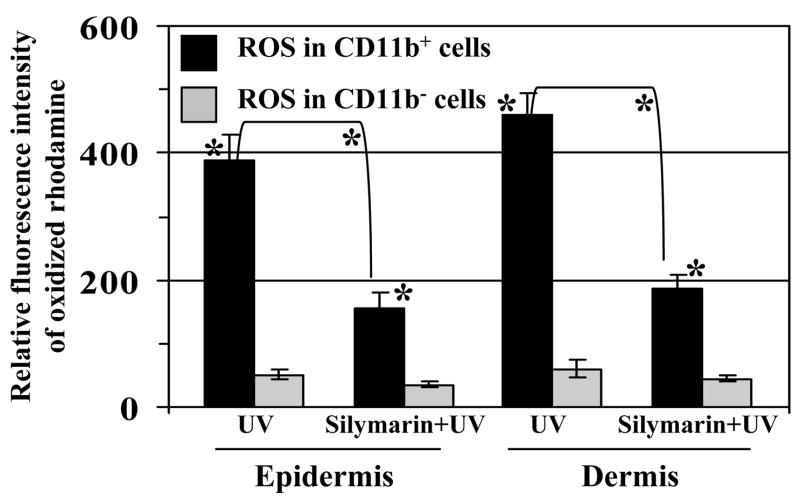
Further to confirm our observation that silymarin decreased UV-induced oxidative stress in the mouse skin primarily through the inhibition of infiltrating CD11b-positive cells; mice were exposed to acute UV dose of 90mJ/cm2 with and without pre-treatment of silymarin. Mice were sacrificed at 48 h after UV exposure, epidermal and dermal single cell suspensions were prepared from the equal area of dorsal skin (4 cm2/mouse) of each mouse from each treatment group. CD11b+ and CD11b− cell populations from the epidermis and dermis were separated using Dynabeads, as detailed in materials and methods, and intracellular ROS/H2O2 was determined as a marker of oxidative stress in both populations using DHR as a fluorescent dye probe. As shown in Fig. 4, CD11b+ cell population in UV alone-exposed epidermal cells are the major source of oxidative stress compared to CD11b− cell population. CD11b+ cell subset produced 88% of total ROS in comparison to 12% ROS produced by CD11b− cell population. Similarly, CD11b+ cell subset from dermal cells also produced significantly higher amount of ROS/H2O2 (88%, p<0.001) in comparison to CD11b− cell population (12%). The amount of ROS production by CD11b+ cells from silymarin treated UV-exposed mice was significantly reduced in epidermis (60%, p<0.001) as well as in dermis (59%, p<0.001) compared to those UV-exposed mice which were not treated with silymarin. Additionally, it is evident that CD11b+ cells are the major source of ROS production in each treatment group compared to CD11b− cells.
Figure 4.
Silymarin inhibits UVB-induced ROS production in UV-exposed mouse skin through targeting the inhibition of infiltrating CD11b−positive cells. Mice were treated with silymarin either 30 min before UV irradiation or just after UV irradiation, sacrificed 48 h later and skin samples (4 cm2) were collected. Epidermal and dermal single cell suspensions were subjected to analysis of intracellular ROS production using dihydrorhodamine 123 as described in Materials and Methods. Experiments were performed twice and resultant data are presented as means ± SD in terms of relative fluorescence intensity of oxidized rhodamine, n=5.
*Significantly higher amount of ROS is produced by CD11b+ cells versus CD11b− cells, p<0.001
*Significant difference of ROS level between UV and silymarin+UV-treated groups, p<0.001
It has been reported that in vivo anti-CD11b treatment through osmotic pumps in peritoneal cavity of C3H/HeN mice blocked infiltration of MHC+ CD11b+ cells into UV-irradiated skin (8). Blocking of CD11b+ cells was found to be associated with the reversal of UV-induced suppression of contact hypersensitivity response to contact allergens and also blocked tolerance induction (8). We also have shown that UV-induced infiltrating cells were the major source of IL-10 in UV exposed mouse skin (14). It has been documented that UV-induced immune suppression is mediated, at least in part, through IL-10 (20). This notion is confirmed by the fact that UV-induced suppression can be largely blocked by administration of neutralizing antibodies to IL-10 (21,22). Thus, it can be suggested that reversal of UV-induced suppression of contact hypersensitivity by in vivo anti-CD11b treatment may be associated with the reduction in IL-10 production. Further, UV-induced immunosuppression has been considered as a risk factor for the skin cancer development (23,24). UV-induced oxidative stress in the skin has also been implicated in greater risk of skin cancer (1,2). Based on the fact that UV-induced infiltrating cells are the major source of IL-10 production (14) as well as oxidative stress (4,5), it can be speculated that UV-induced oxidative stress may have association with the immune suppression. Sluyter et al. (25,26) have reported that mouse skin exposed to UV radiation significantly increased numbers of inflammatory infiltrates including CD11b+ cells, and observed that tumor growth was increased with the increase in infiltration of CD11b+ cells. Infiltrating cells may contribute to tumor growth either by providing paracrine growth factors, suppressing local effector immune responses or by activating suppressor and/or regulatory T cells (25,26). On the basis of our observations along with the observations of Hammerberg et al. (9), it can be suggested that reduction in oxidative stress through reduction in infiltration of CD11b+ cells may be associated with the reversal of UV-induced suppression of contact hypersensitivity response and tolerance induction. In continuation with these observations, we have found that topical treatment of silymarin significantly inhibited photocarcinogenesis in mice in terms of tumor incidence, tumor multiplicity and tumor size (7). Thus it seems that inhibition of photocarcinogenesis by silymarin may be associated with the inhibition of UVB-induced infiltration of CD11b+ cell population and CD11b+ cells-mediated oxidative stress. Similar to photocarcinogenesis, UV-induced oxidative stress has also been shown to be associated with photoaging of the skin (27,28). In this context, the use of silymarin which has the ability to reduce UV-induced oxidative stress could be a promising chemopreventive candidate against solar UV radiation-induced oxidative stress-mediated skin disorders including photoaging and photocarcinogenesis in human population.
Acknowledgments
I feel honored to dedicate this review article to Hasan Mukhtar, Ph.D., Professor of Dermatology, University of Wisconsin, Madison, WI, on his 60th birthday. This work was supported in part by the funds from the National Cancer Institute (CA 105368) and the funds from Veterans Administration Merit Review Award (S.K.K.). The content of this publication does not necessarily reflect the views or policies of the funding sources.
Abbreviations used
- H2O2
hydrogen peroxide
- MPO
myeloperoxidase
- DHR
dihydrorhodamine
- mAb
monoclonal antibody
- ROS
reactive oxygen species
References
- 1.Katiyar SK. Oxidative stress and photocarcinogenesis: Strategies for prevention. In: Singh KK, editor. Oxidative Stress, Disease and Cancer. Imperial College Press; London: 2006. pp. 933–964. [Google Scholar]
- 2.Mukhtar H, Elmets CA. Photocarcinogenesis: mechanisms, models and human health implications. Photochem Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 3.Hammerberg C, Duraiswamy N, Cooper KD. Temporal correlation between UV radiation locally-inducible tolerance and the sequential appearance of dermal, then epidermal, class II MHC+ CD11b+ monocytic/macrophagic cells. J Invest Dermatol. 1996;107:755–763. doi: 10.1111/1523-1747.ep12365802. [DOI] [PubMed] [Google Scholar]
- 4.Katiyar SK, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J Leukoc Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- 5.Katiyar SK, Afaq F, Perez A, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis. 2001;22:287–294. doi: 10.1093/carcin/22.2.287. [DOI] [PubMed] [Google Scholar]
- 6.Wagner H, Horhammer L, Munster R. Chemistry of silymarin (silibinin), the active principle of the fruits of Silybum marianum L. Gaertn. (Carduus marianus L.) Arzneimittelforschung. 1968;18:688–696. [PubMed] [Google Scholar]
- 7.Katiyar SK. Treatment of silymarin, a plant flavonoid, prevents ultraviolet light-induced immune suppression and oxidative stress in mouse skin. Int J Oncol. 2002;21:1213–1222. [PubMed] [Google Scholar]
- 8.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of Silymarin against photocarcinogenesis in a mouse skin model. J Natl Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 9.Hammerberg C, Duraiswamy N, Cooper KD. Reversal of immunosuppression inducible through ultraviolet-exposed skin by in vivo anti-CD11b treatment. J Immunol. 1996;157:5254–5261. [PubMed] [Google Scholar]
- 10.Rosen H, Gordon S. Monoclonal antibody to the murine type 3 complement receptor inhibits adhesion of myelomonocytic cells in vitro and inflammatory cell recruitment in vivo. J Exp Med. 1987;166:1685–1701. doi: 10.1084/jem.166.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen HV, Christensen JP, Anderson EC, Marker O, Thomsen AR. Expression of type 3 complement receptor on activated CD8+ T cells facilitates homing to inflammatory sites. J Immunol. 1994;153:2021–2028. [PubMed] [Google Scholar]
- 12.Hutchings P, Rosen H, O’Reilly L, Simpson E, Gordon S, Cooke A. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1990;348:639–642. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- 13.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 14.Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Prevention of UVB-induced immunosuppression in mice by green tea polyphenol (−)-epigallocatechin-3-gallate may be associated with alterations in IL-10 and IL-12 production. Carcinogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- 15.Mittal A, Elmets CA, Katiyar SK. CD11b+ cells are the major source of oxidative stress in UV radiation-irradiated skin: Possible role in photoaging and photocarcinogenesis. Photochem Photobiol. 2003;77:259–264. doi: 10.1562/0031-8655(2003)077<0259:ccatms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Cooper KD, Duraiswamy N, Hammerberg C, Allen E, Kimbrough-Green C, Dillon W, Thomas D. Neutrophils, differentiated macrophages and monocyte/macrophage antigen presenting cells infiltrate murine epidermis after UV injury. J Invest Dermatol. 1993;101:155–163. doi: 10.1111/1523-1747.ep12363639. [DOI] [PubMed] [Google Scholar]
- 17.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 18.Mantena SK, Katiyar SK. Grape seed proanthocyanidins inhibit UV radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Rad Biol Med. 2006;40:1603–1614. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Godar DE, Thomas DP, Miller SA, Lee W. Long-wavelength UVA radiation induces oxidative stress, cytoskeletal damage and hemolysis. Photochem Photobiol. 1993;57:1018–1026. doi: 10.1111/j.1751-1097.1993.tb02965.x. [DOI] [PubMed] [Google Scholar]
- 20.Granstein RD. Cytokines and photocarcinogenesis. Photochem Photobiol. 1996;63:390–394. doi: 10.1111/j.1751-1097.1996.tb03052.x. [DOI] [PubMed] [Google Scholar]
- 21.Niizeki H, Streilein JW. Hapten-specific tolerance induced by acute, low-dose ultraviolet B radiation of skin is mediated via interleukin-10. J Invest Dermatol. 1997;109:25–30. doi: 10.1111/1523-1747.ep12276415. [DOI] [PubMed] [Google Scholar]
- 22.Rivas JM, Ullrich SE. The role of IL-4, IL-10 and TNFαin the immune suppression induced by ultraviolet radiation. J Leukocyte Biol. 1994;56:769–775. doi: 10.1002/jlb.56.6.769. [DOI] [PubMed] [Google Scholar]
- 23.Streilein JW, Taylor JR, Vincek V, Kurimoto I, Richardson J, Tie C, Medema JP, Golomb C. Relationship between ultraviolet radiation-induced immunosuppression and carcinogenesis. J Invest Dermatol. 1994;103:107S–111S. doi: 10.1111/1523-1747.ep12399400. [DOI] [PubMed] [Google Scholar]
- 24.Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 25.Sluyter R, Halliday GM. Infiltration of inflammatory cells required for solar-simulated ultraviolet radiation enhancement of skin tumor growth. Cancer Immunol Immunother. 2001;50:151–156. doi: 10.1007/PL00006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sluyter R, Halliday GM. Enhanced tumor growth in UV-irradiated skin is associated with an influx of inflammatory cells into the epidermis. Carcinogenesis. 2000;21:1801–1807. doi: 10.1093/carcin/21.10.1801. [DOI] [PubMed] [Google Scholar]
- 27.Sander CS, Chang H, Salzmann S, Muller CSL, Ekanayake-Mudiyanselage S, Elsner P, Thiele JJ. Photoaging is associated with protein oxidation in human skin in vivo. J Invest Dermatol. 2002;118:618–625. doi: 10.1046/j.1523-1747.2002.01708.x. [DOI] [PubMed] [Google Scholar]
- 28.Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce AP-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Invest Dermatol Symp Proceed. 1998;3:61–68. [PubMed] [Google Scholar]