Abstract
Antidepressant treatments have been proposed to produce their therapeutic effects, in part, through increasing neurotrophin levels in the brain. The current experiments investigated the effects of acute and chronic treatment with different pharmacologic and somatic antidepressant treatments on protein levels of BDNF in several brain regions associated with depression in the rat. Repeated applications (10 days) of electroconvulsive shock (ECS), but not a single treatment (1 day), produced 40-100% increases of BDNF protein in the hippocampus, frontal cortex, amygdala, and brainstem. Chronic (21 days), but not acute (1 day), treatment with the tricyclic antidepressant (TCA) desipramine (10 mg/kg), the selective serotonin reuptake inhibitor (SSRI) fluoxetine (10 mg/kg), and the monoamine oxidase inhibitor (MAOI) phenelzine (10 mg/kg) increased BDNF protein levels in the frontal cortex (10-30%), but not in the hippocampus, amygdala, olfactory bulb, and brain stem. To determine whether the regulation of BDNF was unique to antidepressant treatments, drugs used to treat schizophrenia and anxiety were also studied. Chronic administration of the typical antipsychotic haloperidol (1 mg/kg) and the atypical antipsychotic clozapine (20 mg/kg) increased BDNF levels by only 8-10% in the frontal cortex. Haloperidol also elevated BDNF levels in the amygdala, while clozapine decreased BDNF in the olfactory bulb. Acute or chronic treatment with the benzodiazepine chlordiazepoxide (10 mg/kg) did not alter BDNF levels. These results suggest that diverse pharmacologic and somatic antidepressant treatments, as well as antipsychotics, increase levels of BDNF protein in the frontal cortex, even though they have different mechanisms of action at neurotransmitter systems.
Keywords: brain-derived neurotrophic factor (BDNF), antidepressants, antipsychotics, anxiolytic, depression, schizophrenia
1. Introduction
Neurotrophic factors play roles in both the developing and adult central nervous system. Brain derived neurotrophic factor (BDNF) belongs to the family of related proteins called neurotrophins. BDNF is well known for its involvement in the survival and guidance of neurons during development (Segal, 2003). It is essential to the function and survival of neurons in the adult brain (McAllister et al., 1999; Thoenen, 1995). BDNF is involved in synapse formation and has profound effects on the growth, remodeling, and stability of dendrites and axons in hippocampal and cortical neurons (Elmariah et al., 2005). BDNF also regulates adult hippocampal neurogenesis (Lee et al., 2002; Sairanen et al., 2005; Scharfman et al., 2005). The key role BDNF plays in long-term potentiation (LTP) (Figurov et al., 1996; Korte et al., 1995) and its ability to affect sprouting of serotonergic neurons after injury (Mamounas et al., 1995) further demonstrates its influence on neuronal plasticity.
The neurotrophic hypothesis of depression postulates the etiology of this disease and the action of antidepressants is due, in part, to the regulation of central neurotrophin signaling, notably BDNF (Duman and Monteggia, 2006). Stress is known to precipitate or exacerbate depression in susceptible individuals (Gold and Chrousos, 2002). Moreover, depressed patients show atrophy in several brain regions, including the hippocampus, frontal cortex, and amygdala (McEwen, 2001). These anatomical changes caused by stress are paralleled by reductions in BDNF expression (Duman and Monteggia, 2006). On the other hand, chronic administration of antidepressant treatments from different classes have been reported to commonly increase the expression of BDNF mRNA in the hippocampus (Coppell et al., 2003; Dias et al., 2003; Fujimaki et al., 2000; Molteni et al., 2005; Nibuya et al., 1995; Nibuya et al., 1996). Changes in BDNF expression emerge from chronic antidepressant treatment and parallel the time course of clinical response to these drugs. This could indicate that antidepressants regulate BDNF to oppose the effects of chronic stress and may be critical for therapeutic recovery.
The neurotrophic hypothesis, however, has not been supported by other studies that failed to show increases in hippocampal BDNF mRNA expression after chronic treatment with a TCA (Coppell et al., 2003) or SSRI (Altieri et al., 2004; Dias et al., 2003; Jacobsen and Mork, 2004). Moreover, few studies have measured BDNF protein levels following chronic antidepressant treatments. Chronic ECS increased BDNF protein in the hippocampus and frontal cortex (Altar et al., 2003; Jacobsen and Mork, 2004) and chronic treatment with the MAOI tranylcypramine increased protein in the frontal cortex but not the hippocampus (Altar et al., 2003).
The purpose of the following studies was to systematically measure the effects of acute and chronic treatment with pharmacologically distinct antidepressant drugs on BDNF protein levels in various brain regions associated with depression. The SSRI fluoxetine, the TCA desipramine, and the MAOI phenelzine were examined. In addition, these studies examined effects of acute and repeated administration of electroconvulsive shock, a somatic treatment for depression. Moreover, the abilities of drugs used in the treatment of other psychiatric disorders, antipsychotics and the anxiolytic chlordiazepoxide, to alter levels of BDNF protein were also examined. Doses and treatment durations were selected based on studies that had examined the effects of these classes of drugs on BDNF mRNA or on behavioral measures.
2. Results
2.1. Baseline levels of BDNF
Levels of BDNF measured in different brain regions are given in Table 1. BDNF levels varied significantly across regions. The highest levels of BDNF were obtained in the hippocampus, when compared with the brainstem, frontal cortex, amygdala and olfactory bulb. The brainstem contained significantly more BDNF protein than the olfactory bulb, amygdala, and frontal cortex. There was no significant difference in BDNF levels between the other brain regions.
Table 1.
Regional Levels of BDNF in Rat Brain
| Region | N | BDNF levels ng / g tissue |
|---|---|---|
| Hippocampus | 66 | 3.4 ± 0.6 |
| Cortex | 67 | 1.4 ± 0.5 |
| Amygdala | 65 | 1.3 ± 0.4 |
| Olfactory bulb | 68 | 1.4 ± 0.3 |
| Brainstem | 67 | 2.2 ± 0.4 |
Values represent baseline BDNF levels (mean ± SEM) obtained from all rats treated with saline combined from the different experimental control groups.
2.2. Effects of electroconvulsive shock (ECS) on BDNF protein levels
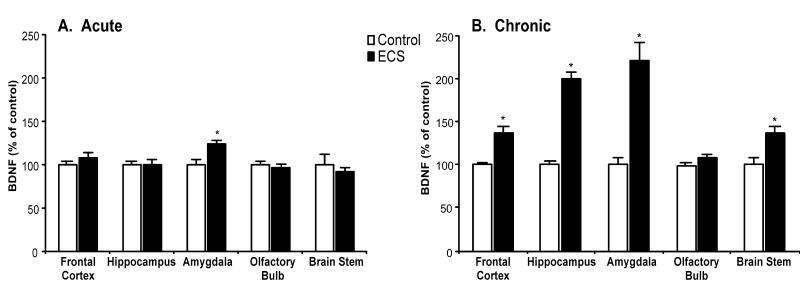
The effects of ECS on BDNF levels were examined in multiple brain regions. A single ECS treatment (Fig. 1, panel A) was sufficient to cause a 25% increase of BDNF in the amygdala. However, it did not affect this neurotrophin in any other brain region examined. Ten days of ECS treatments (Fig. 1, panel B) resulted in robust increases of BDNF protein levels in the hippocampus (100%) and amygdala (100%), moderate but significant elevations in the frontal cortex (40%) and brain stem (40%), but no significant change in the olfactory bulb.
Fig. 1.
Chronic administration of electroconvulsive shock (ECS) elevated BDNF protein levels. Adult rats were administered sham or ECS, either for 1 day (panel A; acute; n = 6) or 10 days (panel B; chronic; n = 10). Values were expressed as a percentage of absolute values in the sham control group. Bars represent mean values ± s.e.m. Asterisk (*) indicates that groups differed significantly (p < 0.05) from the control according to Student’s t-test.
2.3. Effects of pharmacologic antidepressant treatments on BDNF protein levels
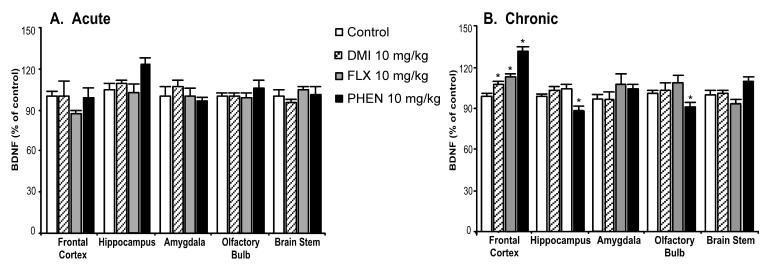
Acute treatment with these antidepressant drugs failed to change BDNF protein levels significantly in all examined brain regions (Fig. 2, panel A). However, chronic administration (Fig. 2, panel B) of all three antidepressants significantly elevated BDNF in the frontal cortex (desipramine: 10%, fluoxetine: 15%, and phenelzine: 30%). Phenelzine was the only antidepressant that caused small but significant decreases (10%) of BDNF levels in the hippocampus and olfactory bulb.
Fig. 2.
Chronic treatment with pharmacologic antidepressants regulated BDNF protein levels. Adult rats were administered saline, desipramine (DMI, 10 mg/kg), fluoxetine (FLX, 10 mg/kg), or phenelzine (PHEN, 10 mg/kg) for either 1 day (panel A; acute; n = 6) or 21 days (panel B; chronic; saline: n = 30, DMI: n = 20, FLX: n = 20, PHEN: n = 10). Values were expressed as a percentage of absolute values in the saline-treated control group. Bars represent mean values ± s.e.m. Asterisk (*) indicates groups that differed significantly from control (p < 0.05) according to Dunnett’s post-hoc analysis.
2.4. Effects of non-antidepressant drug treatments on BDNF levels
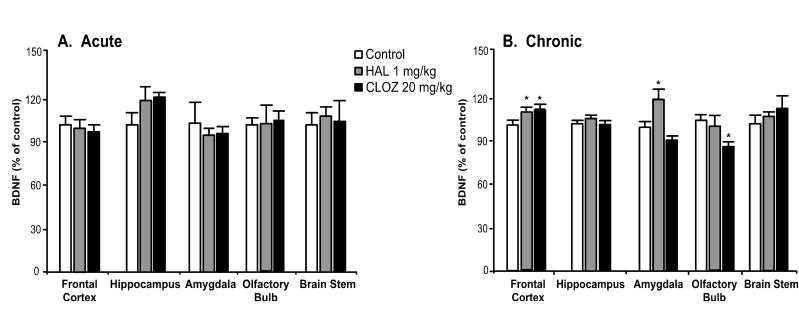
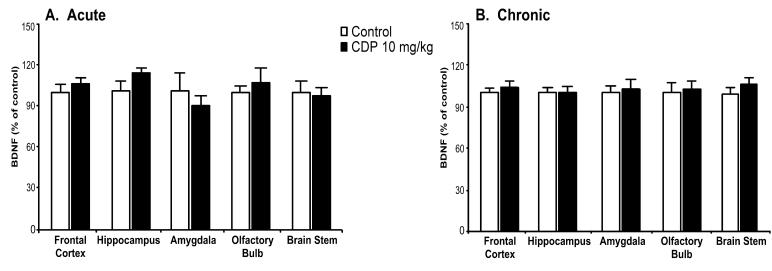
The typical antipsychotic haloperidol, the atypical antipsychotic clozapine, and the benzodiazepine chlordiazepoxide were administered in order to investigate the effects of other classes of psychotherapeutic drugs on BDNF protein levels. Acute administration of haloperidol (1 mg/kg) or clozapine (20 mg/kg) did not regulate BDNF protein levels (Fig. 3, panel A). In contrast, chronic administration (Fig. 3, panel B) of these drugs resulted in small but significant elevations of BDNF levels in the frontal cortex (haloperidol: 8%, clozapine: 10%). In addition to increasing BDNF levels in the frontal cortex, haloperidol elevated BDNF levels in the amygdala (20%), while clozapine decreased BDNF levels in the olfactory bulb (18%). Neither acute (Fig. 4, panel A) nor chronic (Fig. 4, panel B) treatment with chlordiazepoxide regulated BDNF protein levels in the examined brain regions.
Fig. 3.
Differential regulation of BDNF protein by chronic administration of pharmacologically distinct antipsychotics. Adult rats were administered saline, haloperidol (HAL, 1 mg/kg), or clozapine (CLOZ, 20 mg/kg) for either 1 day (panel A; acute; n = 6) or 21 days (panel B; chronic; saline: n = 20, HAL: n = 10, CLOZ: n = 10). Values were expressed as a percentage of absolute values in the saline-treated control group. Bars represent mean values ± s.e.m. Asterisk (*) indicates groups that differed significantly from control (p < 0.05) according to Dunnett’s post-hoc analysis.
Fig. 4.
Acute or chronic benzodiazepine treatment does not alter BDNF protein. Adult rats were administered saline or chlordiazepoxide (CDP, 10 mg/kg) for 1 day (panel A; acute; n = 6) or 21 days (panel B; chronic; n = 10). Values were expressed as a percentage of absolute values in the saline control group. Bars represent mean values ± s.e.m.
3. Discussion
Although a number of studies have been published concerning the effects of psychotropic drugs on BDNF, most of them have been limited in scope, examining a single drug class or brain region. This study compared the effects of acute and chronic administration of antidepressants with treatment by antipsychotic and anxiolytic drugs. BDNF protein levels were compared between five different brain regions. The results of this study demonstrated that chronic treatment with pharmacologically diverse antidepressants commonly resulted in the elevation of BDNF protein levels in the frontal cortex, but not in any other region investigated. Moreover, chronic application of ECS, an effective somatic treatment for depression, caused a more widespread and robust up-regulation of BDNF levels than the pharmacologic treatments. The regulation of BDNF levels by the pharmacological antidepressants required chronic treatment because acute treatment produced no effect, while acute ECS produced an increase only in the amygdala. Although regulation of BDNF levels has most commonly been studied with antidepressant drugs, chronic treatment with the typical antipsychotic haloperidol or the atypical antipsychotic clozapine also resulted in elevations of BDNF protein in the frontal cortex. The effects of antipsychotic drugs, though, were less in magnitude compared to the antidepressant-induced increase. In contrast, the anxiolytic chlordiazepoxide did not regulate BDNF levels.
According to the neurotrophic hypothesis of depression, stress and antidepressant treatment exert opposing effects on the regulation BDNF gene expression when studied alone and antidepressants can block or reverse stress-induced decreases in BDNF (Duman and Monteggia, 2006). Administration of BDNF centrally produces antidepressant-like effects in the rat forced swim test and learned helplessness (Hoshaw et al., 2005; Shirayama et al., 2002). The regulation of BDNF signaling could be involved in causing adaptive changes in neural plasticity changes caused by antidepressants following stress. Hippocampal neurogenesis is one of these plastic processes that are regulated bi-directionally in response to either stress or antidepressant treatment (Dranovsky and Hen, 2006). Elevations in neurogenesis are produced by different classes of antidepressant drugs, including the SSRI fluoxetine, the selective norepinephrine reuptake inhibitor (SNRI) reboxetine, and the MAOI tranylcypramine (Malberg et al., 2000; Manev et al., 2001). In addition, two examples of somatic treatments with antidepressant effects, ECS (Madsen et al., 2000; Malberg et al., 2000) and exercise (Trejo et al., 2001; van Praag et al., 1999) increase hippocampal neurogenesis.
In addition, BDNF has been shown to regulate adult hippocampal neurogenesis. The role of BDNF in regulating hippocampal cell proliferation is still unclear. BDNF heterozygous knockout (+/-) mice have been reported to have increased (Sairanen et al., 2005) or reduced (Lee et al., 2002) levels of proliferation, while transgenic mice overexpressing a dominant negative form of the TrkB receptor displayed increased proliferative activity (Sairanen et al., 2005). The ability of BDNF to enhance the survival of adult born hippocampal neurons is well established. Chronic intrahippocampal infusion of BDNF increased the survival of newly born neurons (Scharfman et al., 2005), while BDNF ±(Lee et al., 2002; Sairanen et al., 2005) and TrkB transgenic mice (Sairanen et al., 2005) had reduced levels of cell survival in the hippocampus.
Since there is evidence that antidepressants elevate hippocampal BDNF mRNA, antidepressants might elevate neurogenesis via a hippocampal-dependent BDNF mechanism. At the mRNA level, however, antidepressant treatments have shown mixed results in elevations of hippocampal BDNF gene expression. The present results that chronic antidepressant treatments did not elevate BDNF protein levels in the whole hippocampus, agrees with previous findings (Altar et al., 2003; De Foubert et al., 2004). It is possible that the inability to detect an increase in total hippocampal BDNF protein could be due to a lack of spatial resolution with the ELISA method, and that BDNF levels could be increased in subregions of the hippocampus. For example, chronic treatment with fluoxetine (10 mg/kg for 21 days) increased BDNF immunoreactivity in the CA2 and CA3 sub-regions of the hippocampus, but not in the dentate gyrus, the area of the hippocampus where the neural progenitors are found (De Foubert et al., 2004). It has also been shown that chronic administration of amitriptyline and venlafaxine at (5 mg/kg for 21 days) elevated BDNF protein immunoreactivity in all hippocampal sub-regions, except the dentate gyrus. In contrast, a higher treatment dose (10 mg/kg) of both drugs reduced BDNF in all hippocampal subareas (Xu et al., 2003). The doses and treatment duration of antidepressants used in the current study were based on previous studies showing increases in BDNF mRNA (Nibuya et al., 1995). Although increases of BDNF protein might not have been detected because only a single treatment dose was used, the doses of each drug were sufficient to cause changes in other brain regions while the hippocampus showed no change. Nevertheless, as a caveat, additional doses of drugs should be studied to obtain a complete dose-response curve of changes in BDNF protein levels.
This study found that four different antidepressant treatments increased BDNF protein levels in the frontal cortex. The TCA and norepinephrine reuptake inhibitor desipramine and the SSRI fluoxetine increased BDNF protein level in the frontal cortex following their chronic administration for 3 weeks. Chronic treatment with the MAOI phenelzine, increased BDNF levels in the frontal cortex to a greater extent than either desipramine or fluoxetine given alone. It is possible that phenelzine, which inhibits the metabolism of all monoamines, might have greater effects on BDNF protein levels than drugs that are more selective for specific monoamines. ECS is effective in the treatment of drug-resistant depression (Taylor, 2007). ECS robustly elevated BDNF protein levels in multiple brain regions, including the hippocampus and frontal cortex. The ability of ECS to be efficacious in drug-resistant depressed patients might, in part, be linked to its more robust effects on BDNF protein levels.
The frontal cortex is also sensitive to effects of stress and antidepressant treatments and is likely to be involved in depression. In postmortem studies of depressed patients, cellular and morphological changes reported in cortical brain regions include reductions in the number of glia and neuronal size of cortical structures (Cotter et al., 2001; Ongur et al., 1998; Rajkowska and Miguel-Hildalgo, 2007). In rodents, chronic stress decreases cell proliferation and the production of glia in the cerebral cortex, and this effect was reversed by chronic fluoxetine treatment (Banasr et al., 2007). Moreover, chronic antidepressant treatments elevate BDNF mRNA in the frontal cortex (Nibuya et al., 1995). These findings favor the existence of decreased cortical neurotrophic support in depression, and suggest that the increased levels of BDNF in the frontal cortex following chronic antidepressant treatment could be important in reversing pathogenic deficits in this region. The promotion of hippocampal neurogenesis by chronic antidepressant treatments might also be linked to the ability of these drugs to increase BDNF protein levels in the frontal cortex. Increased levels of BDNF could influence hippocampal function by direct connections from the frontal cortex to the hippocampus (Zhong et al., 2006), or by a network of indirect connections to hippocampal afferents (Fuchs et al., 2006). Further studies measuring neurogenesis, long-term potentiation or electrical activity of the hippocampus following the infusion of BDNF into the frontal cortex would directly test this hypothesis.
Although anxiety can be effectively treated with SSRI antidepressants such as fluoxetine (Davidson, 2006), the converse does not hold true; patients with depression are generally not treated effectively with most benzodiazepine anxiolytics. In the current study, neither acute nor chronic treatment with chlordiazepoxide elevated BDNF protein levels. Since the current clinically effective antidepressants modulate BDNF, this finding suggests that the inability of benzodiazepines to treat depression may, in part, be due to their inability to modulate BDNF.
Antipsychotic drugs have been divided into two broad classes, typical and atypical, based on their propensity for producing extrapyramidal side effects (De Oliveira and Juruena, 2006). The ability of antipsychotic drugs to regulate central BDNF levels has not been extensively examined. The typical antipsychotic haloperidol has produced mixed effects on BDNF mRNA levels, with some studies reporting reductions in various hippocampal subfields with 1mg/kg given for 28 days (Bai et al., 2003) while others reported no change after 2 mg/kg given for 21 days (Nibuya et al., 1995). At the protein level, administration of haloperidol in chow for 29 days was reported to reduce BDNF levels in the hippocampus and frontal cortex (Angelucci et al., 2000). Clozapine (10 mg/kg for 28 days), an atypical antipsychotic, was shown to elevate BDNF mRNA in sub-regions of the hippocampus (Bai et al., 2003). However, the effects of clozapine were not examined in any other brain regions. The present study found that both haloperidol and clozapine elevated BDNF protein in the frontal cortex, while haloperidol also elevated BDNF levels in the amygdala. The discrepancy between our findings and those of Angelucci et al. (2000) could be due to differences in the duration of treatment, route of drug administration, and dosing regimen. The increase in BDNF protein levels in the frontal cortex by haloperidol and clozapine was less than that produced by the antidepressant drugs and could be related to their efficacy in treating depression. Moreover, additional clinical benefits from drug regimens that combine antidepressant treatments with antipsychotic drugs for the treatment of bipolar and treatment-resistant depressions (Nemeroff, 2005; Thase et al., 2007) could be due, in part, to potential synergistic increases in BDNF protein in the frontal cortex.
In summary, chronic administration of diverse pharmacologic antidepressant treatments produced a common increase in BDNF protein levels specifically in the frontal cortex. Although repeated electroconvulsive shock shared increasing BDNF protein in the frontal cortex with other antidepressants, the pattern and magnitude of its effects suggest additional mechanisms of action. The ability of other psychotropic agents to regulate BDNF protein levels in the frontal cortex following chronic treatment might be indicative of their antidepressant effects.
4. Experimental Procedures
4.1. Animals
Male Sprague-Dawley rats (Charles River, Wilmington, MA, USA) weighing 250-275 g at the beginning of antidepressant treatments were used in these studies. The animals were housed in pairs in polycarbonate cages and maintained on a 12-h light/dark cycle (lights on at 07:00 A.M.) in a temperature (22°C)-and humidity-controlled colony at the University of Pennsylvania. The animals were given free access to food and water. Animal procedures were conducted in accordance with the guidelines published in the NIH Guide for Care and Use of Laboratory Animals and all protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee. The studies involving electroconvulsive shock were conducted at Wyeth Laboratories under similar conditions.
4.2. Drug Treatments
Rats were administered once daily s.c. injections of sterile saline (0.9%) or drug for either 1 day (n = 6/group) or 21 days (n = 10-20/group). The drugs were dissolved in sterile water and given at the following doses: fluoxetine HCl (Anawa, Zurich Switzerland; 10 mg/kg), desipramine HCl (Sigma, St. Louis MO; 10 mg/kg), phenelzine sulfate (Sigma, St. Louis MO; 10 mg/kg), chlordiazepoxide HCl (Sigma, St. Louis MO; 10 mg/kg), haloperidol HCl (Tocris, Ellisville MO; 1 mg/kg). Clozapine (Tocris, Ellisville MO; 20 mg/kg) was dissolved in a small volume of acetic acid, adjusted to pH 5.2 with 10 N NaOH, and brought up to final volume with sterile water. The doses were calculated according to the base weight of each drug in a volume of 2 ml/kg.
4.3. Electroconvulsive Shock
Rats were administered sham or ECS treatment either for 1 day (n = 6/group) or 10 days (n = 10/group). To administer the ECS, the rat was lightly restrained by being wrapped in a paper towel with its head exposed. Conducting jelly was applied to the ears and electric current was administered between two earclip electrodes (50 mA, 0.5 sec) using an electroshock generator (Ugo Basile, Italy). Sham-stimulated animals received the same treatment, except that no current was administered. This level and duration of shock produces a seizure that lasts less than 1 min and is characterized by full extension of the hind limbs (tonic phase) for 10-15 sec, followed by repetitive flexion-extension of the forelimbs for 10-15 sec (clonic phase). After the cessation of the shock, each rat was placed in a plastic cage where it remained singly housed for one hour, at which point it was returned to its home cage.
4.4. BDNF Quantification
Twenty-four hours following the last antidepressant treatment, rats were decapitated and their brains quickly removed for dissection into the following regions: olfactory bulb, hippocampus, frontal cortex, amygdala, and brain stem. Each region was flash frozen in isopentane and placed in -80° C until analysis. BDNF protein levels were quantified using a commercially available sandwich ELISA kit (Millipore, Billerica, MA). The tissue was homogenized in lysis buffer (100 mM Tris pH 7.0, 1M NaCl, 4mM EDTA, 0.1% sodium azide, 2% bovine serum albumin, 2% Triton-X100, 5 μg/ml aprotinin, 0.1μg/ml pepstatin A, 0.5 μg/ml antipain) at 20 volumes of the wet tissue weight (mg). The homogenate was centrifuged at 14,000 ×g for 30 minutes at 4°C. The supernatant was removed and the amount of BDNF protein in each sample was analyzed in duplicate by ELISA. BDNF levels were normalized to wet tissue weight.
4.5. Statistical Analysis
BDNF levels were expressed as a percentage of values obtained for the saline-treatment (control) group included for each experiment. Absolute BDNF levels in each brain region combined across experiments are shown in Table 1 and were compared using analysis of variance followed by Scheffe post-hoc analysis. The effects of fluoxetine and desipramine, and those of phenelzine, were conducted as separate experiments and statistical analysis was performed on the pooled data. There were no significant differences between any of the control groups. Similarly, the effects of clozapine and haloperidol were studied in separate experiments and statistical analysis was performed on the pooled data. There were no significant differences in BDNF levels between control groups between the different experiments. BDNF levels between drugtreatment groups and saline-injected controls from the same study were compared using one-way analysis of variance. Dunnett’s post-hoc analysis was used to compare individual treatment groups to the common control group. Unpaired two-tailed Student’s t-test was used to analyze results for the ECS and chlordiazepoxide treatments. * p < .05 was considered significant.
Acknowledgements
The study was funded by NIH grant MH72832 for a National Center for Drug Discovery Group in Mood Disorders established between the University of Pennsylvania and Wyeth Research. We thank other principal members of the Group, Dr. Robert Ring at Wyeth Research and Dr. Julie Blendy at the University of Pennsylvania, for their generous advice.
Footnotes
Section: Neurophysiology, Neuropharmacology and other forms of Intercellular Communication
References
- Altar CA, Whitehead RE, Chen R, Wortwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–709. doi: 10.1016/s0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Altieri M, Marini F, Arban R, Vitulli G, Jansson BO. Expression analysis of brain- derived neurotrophic factor (BDNF) mRNA isoforms after chronic and acute antidepressant treatment. Brain Res. 2004;1000:148–155. doi: 10.1016/j.brainres.2003.12.028. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Mathe AA, Aloe L. Brain-derived neurotrophic factor and tyrosine kinase receptor TrkB in rat brain are significantly altered after haloperidol and risperidone administration. J Neurosci Res. 2000;60:783–794. doi: 10.1002/1097-4547(20000615)60:6<783::AID-JNR11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Bai O, Chlan-Fourney J, Bowen R, Keegan D, Li XM. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res. 2003;71:127–131. doi: 10.1002/jnr.10440. [DOI] [PubMed] [Google Scholar]
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Coppell AL, Pei Q, Zetterstrom TS. Bi-phasic change in BDNF gene expression following antidepressant drug treatment. Neuropharmacology. 2003;44:903–910. doi: 10.1016/s0028-3908(03)00077-7. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Davidson JR. Pharmacotherapy of social anxiety disorder: what does the evidencetell us? J Clin Psychiatry. 2006;67(Suppl 12):20–26. [PubMed] [Google Scholar]
- De Foubert G, Carney SL, Robinson CS, Destexhe EJ, Tomlinson R, Hicks CA, Murray TK, Gaillard JP, Deville C, Xhenseval V, Thomas CE, O'Neill MJ, Zetterstrom TS. Fluoxetine-induced change in rat brain expression of brain- derived neurotrophic factor varies depending on length of treatment. Neuroscience. 2004;128:597–604. doi: 10.1016/j.neuroscience.2004.06.054. [DOI] [PubMed] [Google Scholar]
- De Oliveira IR, Juruena MF. Treatment of psychosis: 30 years of progress. JClin Pharm Ther. 2006;31:523–534. doi: 10.1111/j.1365-2710.2006.00784.x. [DOI] [PubMed] [Google Scholar]
- Dias BG, Banerjee SB, Duman RS, Vaidya VA. Differential regulation of brain derived neurotrophic factor transcripts by antidepressant treatments in the adult rat brain. Neuropharmacology. 2003;45:553–563. doi: 10.1016/s0028-3908(03)00198-9. [DOI] [PubMed] [Google Scholar]
- Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Elmariah SB, Hughes EG, Oh EJ, Balice-Gordon RJ. Neurotrophin signaling among neurons and glia during formation of tripartite synapses. Neuron Glia Biol. 2005;1:1–11. doi: 10.1017/S1740925X05000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Front Biosci. 2006;11:2746–2758. doi: 10.2741/2004. [DOI] [PubMed] [Google Scholar]
- Fujimaki K, Morinobu S, Duman RS. Administration of a cAMP phosphodiesterase 4 inhibitor enhances antidepressant-induction of BDNF mRNA in rat hippocampus. Neuropsychopharmacology. 2000;22:42–51. doi: 10.1016/S0893-133X(99)00084-6. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Jacobsen JP, Mork A. The effect of escitalopram, desipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res. 2004;1024:183–192. doi: 10.1016/j.brainres.2004.07.065. [DOI] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamounas LA, Blue ME, Siuciak JA, Altar CA. Brain-derived neurotrophic factor promotes the survival and sprouting of serotonergic axons in rat brain. J Neurosci. 1995;15:7929–7939. doi: 10.1523/JNEUROSCI.15-12-07929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manev H, Uz T, Smalheiser NR, Manev R. Antidepressants alter cell proliferation in the adult brain in vivo and in neural cultures in vitro. Eur J Pharmacol. 2001;411:67–70. doi: 10.1016/s0014-2999(00)00904-3. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Bedogni F, Tongiorgi E, Fumagalli F, Racagni G, Andrea Riva M. Chronic treatment with fluoxetine up-regulates cellular BDNF mRNA expression in rat dopaminergic regions. Int J Neuropsychopharmacol. 2005:1–11. doi: 10.1017/S1461145705005766. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Use of atypical antipsychotics in refractory depression and anxiety. J Clin Psychiatry. 2005;66(Suppl 8):13–21. [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, MiguelHidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu RevNeurosci. 2003;26:299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. Electroconvulsive therapy: a review of history, patient selection, technique, and medication management. South Med J. 2007;100:494–498. doi: 10.1097/SMJ.0b013e318038fce0. [DOI] [PubMed] [Google Scholar]
- Thase ME, Corya SA, Osuntokun O, Case M, Henley DB, Sanger TM, Watson SB, Dube S. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, and fluoxetine in treatment-resistant major depressive disorder. J Clin Psychiatry. 2007;68:224–236. doi: 10.4088/jcp.v68n0207. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Xu H, Steven Richardson J, Li XM. Dose-related effects of chronic antidepressants on neuroprotective proteins BDNF, Bcl-2 and Cu/Zn-SOD in rat hippocampus. Neuropsychopharmacology. 28:53–62. doi: 10.1038/sj.npp.1300009. [DOI] [PubMed] [Google Scholar]
- Zhong YM, Yukie M, Rockland KS. Distinctive morphology of hippocampal CA1terminations in orbital and medial frontal cortex in macaque monkeys. Exp Brain Res. 2006;169:549–553. doi: 10.1007/s00221-005-0187-7. [DOI] [PubMed] [Google Scholar]