Abstract
Estrogen has been shown to attenuate the inflammatory response following injury or lipopolysaccharide treatment in several organ systems. Estrogen's actions are transduced through two estrogen receptor sub-types, estrogen receptor (ER) -alpha and estrogen receptor-beta, whose actions may be overlapping or independent of each other. The present study examined the effects of ERα- and ERβ-specific ligands in regulating the inflammatory response in primary astrocyte cultures. Pre-treatment with 17β-estradiol (ERα/ERβ agonist), HPTE (ERα agonist/ERβ antagonist) and DPN (ERβ agonist) led to attenuation of IL-1β, TNFα, and MMP-9 in astrocyte media derived from young adult (3-4 mos.) and reproductive senescent female (9-11 mos., acyclic) astrocyte cultures, while pretreatment with PPT (ERα agonist) attenuated IL-1β (but not TNFα or MMP-9) in both young and senescent-derived astrocyte cultures. Our previous work determined that 17β-estradiol was unable to attenuate the LPS-induced increase in IL-1β in olfactory bulb primary microglial cultures derived from either young adult or reproductive senescent females. In young adult-derived microglial cultures, the LPS-induced increase in IL-1β was not attenuated by pre-treatment with 17β-estradiol, PPT or HPTE. Interestingly, the ERβ agonist, DPN significantly decreased IL-1β following LPS treatment in young adult-derived microglia. Thus while both microglia and astrocytes synthesize and release inflammatory mediators, the present data shows that compounds which bind ERβ are more effective in attenuating proinflammatory cytokines in both cell types and may therefore be a more effective agent for future therapeutic use.
Keywords: estrogen receptor alpha, estrogen receptor beta, tumor necrosis factor alpha, DPN, MMP-9
1. Introduction
Inflammation contributes to the etiology of many neurodegenerative diseases and in women, the risk for such diseases is further affected by the menopause, where ovarian hormones, which are capable of modulating the inflammatory response, are decreased (Andersen et al., 1999; Brann et al., 2007; Gao et al., 1998). Estrogen has been shown to affect inflammatory mediators such as cytokines in a number of systems and has been shown to decrease inflammation in experimental models of anterior uveitis (Miyamoto et al., 1999), carrageenan-induced pleurisy in the lungs (Cuzzocrea et al., 2001; Cuzzocrea et al., 2000) and adjuvant-induced arthritis (Badger et al., 1999). On the other hand, estrogen promotes prostatitis (Naslund et al., 1988), stimulates edema (Tchernitchin and Galand, 1983) and increases vascular permeability and influx of macrophages in the uterus (De and Wood, 1990; Kaushic et al., 1998). In central nervous system cells, estrogen pretreatment has been shown to attenuate LPS-induced superoxide release, phagocytic activity and an increase in iNOS (Bruce-Keller et al., 2000; Drew and Chavis, 2000; Vegeto et al., 2001; Vegeto et al., 2000) in microglial cells. Estrogen's actions are transduced through two nuclear receptors estrogen receptor alpha (ERα) (Green et al., 1986; Greene et al., 1986) and estrogen receptor beta (ERβ) (Kuiper et al., 1996) and studies with the ER knockout animals indicate that ERα may be important for estrogen's anti-inflammatory actions (Suzuki et al., 2007; Vegeto et al., 2003). However, the role of the receptors using receptor specific agonists is less well understood, especially in neural support cells.
In our previous studies, acyclic older female rats were used to understand neural changes associated with the ovarian aging process. By comparing these animals with their younger normally cycling counterparts, we have shown that the blood brain barrier is compromised in the olfactory bulb and hippocampus of acyclic rats and following estrogen replacement there is increased permeability of the blood-brain barrier in the hippocampus (Bake and Sohrabji, 2004). Moreover, estrogen replacement also increased expression of the pro-inflammatory cytokine interleukin 1-beta (IL-1β) following an excitotoxic injury in the olfactory bulb in ovarian-aged females but attenuated IL-1β expression in young adult females (Nordell et al., 2003). Together these studies show there are constitutive differences between the young and senescent females and that estrogen replacement in ovarian-aged females but not young adult females results in a poor outcome. In order to determine the mechanism underlying these paradoxical age-related actions of estrogen, we adopted a reductionist approach to first determine the cell type most affected by ovarian age. A previous study examined microglial cells in culture, and this study showed that although microglia from both ages of animals responded equally well to an inflammatory stimulus, 17β-estradiol failed to reduce inflammatory mediators in either young or senescent microglial cultures (Johnson and Sohrabji, 2005). The present study focuses on young and senescent astrocytes. Astrocytes are an important neural support cell in the brain, maintaining blood-brain-barrier integrity (for review see (Benarroch, 2005), mediating the brain inflammatory response by secretion of specific cytokines (Kyrkanides et al., 2001) and are estrogen-responsive (Amateau and McCarthy, 2002; Parducz et al., 1993). A microarray analysis indicated that the astrocyte-specific marker, glial fibrillary acidic protein (GFAP) was significantly elevated by excitotoxic injury and further elevated by estrogen, suggesting that astrocytes may be key mediators of the inflammatory response or the injury resolution response in this model.
In the present study, three interrelated issues were examined. First, we addressed whether 17β-estradiol would mediate the inflammatory response in astrocytes derived from young and senescent animals in a manner similar to the in vivo situation. Next, we determined whether ERα- and ERβ-specific agonists would affect the astrocyte inflammatory response differently from 17β-estradiol. Finally, we compared whether estrogen receptor agonists would be effective in mediating the inflammatory response in microglial cells from adult animals. The data indicate that agonists that bind ER-beta (ERβ) or both receptors effectively suppressed all inflammatory mediators measured here in astrocytes from young and senescent animals. Furthermore, in microglial cultures, while 17-β estradiol and the ER-alpha (ERα) agonist were ineffective in reducing LPS-induced cytokine expression, the ERβ agonist DPN effectively reduced cytokine expression in these cultures. Collectively, these data suggest that astrocytes are more broadly responsive to the anti-inflammatory actions of estrogen agonists as compared to microglia. Furthermore, compounds that bind ERβ appear to mediate anti-inflammatory actions in both these neural cells and may therefore be an effective therapeutic target for neuro-inflammatory disease.
2. Materials and Methods
2.1 Animals
All animals were purchased from Harlan Laboratories (IN), as young adults (approx. 250g, 3-4 months). Reproductive senescent animals (9-11 months; 280-350g) were used after they were retired from breeding and had been used solely for breeding purposes. Reproductive senescent animals used in this study met previously established criteria (Jezierski and Sohrabji, 2000; Nordell et al., 2003) to include 4-5 successful prior pregnancies followed by two consecutive failures and were in continuous diestrus as established by daily vaginal smears. All animals were maintained in an AALAC-approved facility on a 12h light:dark cycle with lights on at 06:00 h and with food and water available ad libitum. All procedures were in accordance with NIH and institutional guidelines governing animal welfare. For collection of the olfactory bulb, animals were deeply anesthetized (ketamine: 87 mg/kg; xylazine: 13 mg/kg) and decapitated.
2.2 Surgical procedures & experimental paradigm
Young adult and reproductive senescent female rats were bilaterally ovariectomized as described previously (Jezierski and Sohrabji, 2000; Jezierski and Sohrabji, 2001) at the start of the experiment and assigned to one of four conditions, placebo replaced/sham-injected (OS), placebo replaced/NMDA-lesioned (OL), estrogen treated/sham-injected (ES) and estrogen treated/NMDA-lesioned (EL). Three weeks later, animals were prepared for stereotaxic injections as described previously (Nordell et al., 2005; Nordell et al., 2003) directed toward the olfactory bulbs.
2.3 Tissue Preparation
Twenty-four hours following stereotaxic surgery (1 dpi) all animals were sacrificed by perfusion with cold phosphate buffered saline pH = 7 at a rate of 2 mL/min. The brains were then removed from the cranial vault. Both olfactory bulbs were removed and stored at −80°C. Uterine weight was used to confirm ovariectomy and estrogen replacement. The olfactory bulb was processed for protein extraction.
2.4 RNA extraction and Quantitative Real-Time Polymerase Chain Reaction
RNA was extracted as described previously (Nordell et al., 2005). Reverse transcription of total RNA (2 μg) was performed with the Gibco Superscript™ First Strand Synthesis System. Real-time polymerase chain reactions were analyzed on a BioRad MyIQ™ ICycler Real-time machine using 96-well pcr plates (iCycler 96 well, Biorad, CA). The Real-time cycling parameters were as follows: 95°C, 2:30; 40 cycles of 55°C, 0:30, 72°C, 1:00; 95°C, 1:00, 55°C, 1:00. Reactions consisted of 1X iQ SYBR Green Supermix (BioRad), and a starting primer concentration of 10 μM. RNA was amplified using primers to glial fibrillary acidic protein (5′-3′: GFAP-For: CCG AGA AAC CAG CCT GGA C; GFAP-Rev: GGT GTG GAT GGG AAT TGG G). Samples were normalized to cyclophilin (5′-3′: CP1-1: TGG TCA ACC CCA CCG TGT TCT TCG; CP1-2: TGC CAT CCA GCC ACT CAG TCT TGG). Standard curves were created for each primer set using various concentrations (ranging from 250 ng/μL to 25 ng/μL) of female, Sprague Dawley, whole brain rat RNA. The target gene examined was compared to cyclophilin and “no-template” controls were included for each primer set. The relative expression of each mRNA was calculated by the comparative CT method using the following formula 2-ΔΔCt [where the amount of the target (GFAP) was normalized to the endogenous reference (Cyclophilin) and relative to a calibrator constant (3.317)] according to the protocol described in Bulletin #2 (Applied Biosystems, CA). The tissue used for real time PCR had previously been used for microarray analysis in which RNA from 6 replicate samples were pooled (microarray data not shown). Real time PCR from 3 of the 6 replicates was used for verification of microarray data.
2.5 Tissue Culture – Astrocytes
Astrocyte cultures were established using a modification of methods described by (Guilian and Baker, 1986) and (Lehnardt et al., 2002), expert advice from Dr. E. Tiffany-Castiglioni (Lindahl et al., 1999; Qian et al., 2005) and a previously established laboratory protocol (Johnson and Sohrabji, 2005). The experimental protocol is similar to those used in other published studies using astrocyte cultures (Wu et al., 2004). Briefly, rats were decapitated and the olfactory bulbs removed in a semi-sterile hood. Olfactory bulb tissue was washed with Optimem and N2 supplement (99:1, Invitrogen, CA) in a sterile hood. Tissue was mechanically dissociated with a surgical blade and then chemically dissociated with 1X trypsin/EDTA (Invitrogen) for 20 minutes at room temperature. The cell suspension was centrifuged at 700 rpm for 5 min. to pellet the cells. The supernatant was removed and the cells were re-suspended in an astrocyte-specific growth media (DMEM/F12 [1:1], 10% FBS, 4.6g/L glucose) and plated onto poly-d-lysine hydrobromide (0.05 mg/ml. Sigma, MO) coated dishes. Cells were maintained at 37° C with 5% CO2 for 10 days. The cells were cultured with a 1% streptomycin/penicillin antibiotic mixture for the first four days. At day 10, cells were shaken at 400 rpm on a plate shaker for 10 minutes at room temperature to remove microglia and oligodendrocytes. Microglia and oligodendrocytes were removed from the plate by washing the dishes 3 times with DMEM. The astrocytes were trypsinized for 2-3 minutes and re-plated at a density of 150,000 cells for 35 mm culture dishes (immunohistochemistry) or at 50,000 cells for 24-well plates (estrogen pre-treatment experiments). Astrocytes used for immunohistochemistry were plated onto poly-d-lysine coated glass coverslips. The purity of the astrocyte population was determined by the ratio of the astrocyte specific marker, glial fibrillary acidic protein (GFAP), the neuronal marker, microtubule-associated protein (MAP-2, Chemicon, CA) and two microglial markers, ionized-calcium binding adapter molecule 1 (Iba1, Santa Cruz, CA) and the cell surface glycoprotein Mac-1 (CD11b, Serotec, NC) relative to the total number of stained nuclei. To ensure that the primary astrocyte cultures would respond to LPS, the cells were assayed for the toll-like receptor 4 (TLR4), the receptor known to activate LPS-induced signaling pathways (Chow et al., 1999).
2.6 Tissue Culture - Microglial cultures
Microglial cultures were established as described in (Johnson and Sohrabji, 2005) using a modification of the procedures described in Giulian and Baker (1986) and Lehnardt et al. (2002). Characterization of these cultures has been reported elsewhere and hence not described here.
2.7 Immunohistochemistry
The purity of the astrocyte population and the presence or absence of the toll-like receptor-4 in these cultures was determined by assaying the astrocytes for GFAP, MAP-2, Iba1, CD11b or TLR4. Cultures were washed briefly in 1X phosphate-buffered saline (dPBS, Invitrogen, CA) and fixed for one hour in 2% paraformaldehyde. Astrocytes were washed and incubated in block solution for 1h followed by incubation with the primary for 2h at room temperature (GFAP, 1:80, Chemicon, CA) or 24 hr at 4° C (MAP-2, 1:500; Iba1, 1:50; CD11b, 1:100; TLR4, 1:200). The primary was diluted in dPBS, 1% normal goat serum or fetal bovine serum (TLR4 only), and 0.3% Triton. Controls were incubated in diluent only. Following washes, astrocytes were labeled with a fluorescent secondary Alexa Fluor 594 (GFAP & Iba1, 1:2000, Molecular Probes, CA), Texas Red (TLR4, 1:500, Santa Cruz), Oregon Green 488 (Map-2, 1:500, Molecular Probes, CA) or Fluorescein (CD11b, 1:500, Vector Laboratories, CA) to visualize the labeling for 1 or 2 hours (Texas Red) at room temperature. Astrocytes were counterstained with the nuclear stain, Hoechst 33258 (Polysciences Inc., PA) for 1h at room temperature and cover-slipped with ProLong® Antifade kit (Molecular Probes). Slides were visualized by epifluorescence and photographed using an Olympus BX2 microscope and QCapture imaging software (v2.7.3, QImaging Corporation, Canada).
2.8 Reverse transcription-polymerase chain reaction (RT-PCR) for ERα and ERβ
To verify that the ERα and ERβ receptors were present on astrocytes in vitro, gene expression of both receptors was determined by reverse transcription-polymerase chain reaction (RT-PCR). RNA was extracted and reverse transcription of total RNA was performed as described above. RNA was amplified using primers to estrogen receptor-alpha (5′-3′: ERα-For: TTC AGT GAA GCC TCA ATG ATG GGC; ERα-Rev: GCG GAA TCG ACT TGA CGT AG) and estrogen receptor-beta (5′-3′: ERβ-For: GAC AAG AAC CGG CGT AAA AG; ERβ-Rev: CAC CAG TTG CTC TGG ACT CA). Samples were normalized to cyclophilin (described above). The PCR cycles used for ER-α and ER-β are as follows: 95°C 2 min; 30 cycles of 95° C 30 sec., 60° C 1 min., 72°C 2min. The PCR cycles for cyclophilin are as follows: 95°C 2 min; 20 cycles of 95° C 30 sec., 62° C 1 min., 72°C 2 min. Initial analyses were performed to ensure that both estrogen receptors and cyclophilin amplified in the linear range. The PCR reaction was separated on a 1.5% agarose gel and visualized using Molecular Analyst™ (BioRad, CA). The ERα-specific primers span Exon 4 to Exon 6 while the ERβ-specific primers span from Exon 4 to Exon 5 and the sequence of both gene products was verified by gene sequencing (Gene Technologies Laboratory, Texas A & M University, College Station, TX).
2.9 Lipopolysaccharide Treatments- Estrogen agonist/antagonist treatments
Astrocytes and microglia were re-plated into 24-well tissue culture plates (astrocytes) or 4 chamber slides (microglia) and at confluence were switched to non-serum containing medium. Coincident with the change to non-serum containing medium, astrocytes/microglia were pre-treated with an estrogen agonist or saline for 4 hours. The following compounds and doses were used for the astrocytes: 17β-estradiol (17bE, 20 nM, Sigma, St. Louis; activates both estrogen receptor alpha and estrogen receptor beta), propyl pyrazole triol (PPT, 2 nM, alpha agonist, Tocris), diarylpropionitrile (DPN, 10 nM, beta agonist, Tocris, MO), 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE, 1μM, alpha agonist/beta antagonist, kind gift from Dr. K. Jefferson, NIEHS). All compounds used in the astrocyte cultures were tested at two different concentrations (data not shown) with the exception of HPTE in which the first dose used was effective in attenuating the LPS-induced increase in cytokines. The following compounds and doses were used for microglia: 17β-estradiol (2nM), HPTE (2μM), PPT (2nM), DPN (10nM). Since only a limited number of microglia can be harvested from the adult olfactory bulbs, only one treatment dose was selected for each agonist/antagonist based on either binding affinity/EC50 (Gaido et al., 1999; Kuiper et al., 1996) and/or effective doses as reported in the literature: 17β-estradiol (Zhao et al., 2004), HPTE (Hodges et al., 2000), PPT (Zhao et al., 2004) and DPN (Zhao et al., 2004). Following the 4 hour pre-treatment, astrocytes or microglia were treated with LPS (10μg/mL) in the presence or absence of the selective estrogen receptor sub-type ligand for 24 hours.
2.10 Examination of Inflammatory Markers
Three measures were used to examine the effect of acute estrogen treatment following an LPS challenge: protein expression of IL-1β (BioRad Rat IL-1β X-plex assay system, BioRad, CA), protein expression of tumor necrosis factor-alpha (TNFα ELISA, BioSource, CA) and activity of matrix metalloproteinase-9 (MMP-9, gelatin zymography). IL-1β Cytokine Bioplex Assay: Protein expression of IL-1β was determined using the Bio-Plex Suspension Array Systems with High-Throughput Fluidics in glia-conditioned media. Calibration of the Bio-Plex Suspension System was performed using CAL2 (High PMT, Bio-Plex Calibration Kit). Standard and sample preparation was performed according to manufacturer's directions. Briefly, the 96-well filter plate was pre-wet with Bio-Plex assay buffer and removed by calibrated vacuum filtration prior to application of the IL-1β conjugated beads (1X). After addition of the beads, the filter plate was washed 2 times and then a total of 50 μL of sample or standard was added to each well in duplicate and incubated for 30 min. at room temperature with agitation. The filter plate was washed preceding addition of 25 μL of 1X Bio-Plex detection antibody and again incubated for another 30 minutes at room temperature. Detection of IL-1β occurred following incubation with 1X BioRad Streptavidin-PE for 10 min. at room temperature. Following 3 washes, the beads were resuspended in 125 μL of BioReady assay buffer and read by the BioPlex Suspension System. TNF-α: Manufacturer's instructions were used to determine the expression of TNF-α in astrocyte media. Briefly, standards, controls, samples, and biotinylated TNF-α were pipetted into a 96-well plate pre-coated with antibodies specific for rat TNF-α and incubated at room temperature for 1.5h. Following washes, plates were sequentially incubated with 100 μL streptavidin peroxidase for 45 min. Following the next set of washes, 100 μL of stabilized chromogen was added to each well and incubated for an additional 30 min. The color reaction was stopped by an equal volume of stop solution and read at 450 nm in a microplate reader (Bio-Tek, VT). Standard curves were established from optical densities of wells containing known dilutions of standard, using KC3 software (Bio-Tek, VT) and sample measurements were interpolated from standard curves. Gelatin zymography: Procedures used here are consistent with our previous protocol (Johnson and Sohrabji, 2005; Nordell et al., 2005) except astrocyte media was used. An equal volume of cell culture media (40 μL) from each treatment group were size fractionated on a 10% polyacrylamide gel containing 0.01% gelatin, along with pre-stained protein size markers. Each gel contained a positive control of conditioned media from human umbilical vein endothelial cells (kind gift from G.E. Davis, Department of Medical Pharmacology and Physiology, University of Missouri, Columbia). After electrophoresis, the gels were rinsed with water and incubated with renaturing buffer (2% Triton X-100) at room temperature for 1h with 3 buffer changes. Gels were then rinsed 3 times with water and incubated with developing buffer (50mM Tris, 0.2M NaCl, 5mM CaCl2, 0.02% Brij 35) overnight at 25° C with gentle shaking. After a brief rinse with water, gels were stained with a 0.25% Coomassie blue solution (50% methanol, 20% acetic acid) for 30 minutes and destained in 20% methanol: 10% acetic acid. Gels were dried at 55° C for 2 h, and later digitized. A standard densitometric program (Molecular Analyst, BioRad, CA) was used to calculate the density of the lytic area.
2.11 Statistical Analysis
Statistical analysis was performed using a statistical software package (SPSS Inc., IL), and group differences were considered significant at p≤0.05. The astrocyte data was analyzed using a three-way analysis of variance (ANOVA) with age (young adult versus reproductive senescent), hormone (saline-treated versus estrogen-treatment) and treatment (saline versus LPS) as independent variables. The microglial data was analyzed using a two-way ANOVA with hormone and treatment as independent variables. For astrocyte and microglial cultures, all experiments were performed at least twice and a total of 6 replicates were used for each iteration.
3. Results
3.1 Effect of an Excitotoxic Injury to the Olfactory Bulb on GFAP Expression
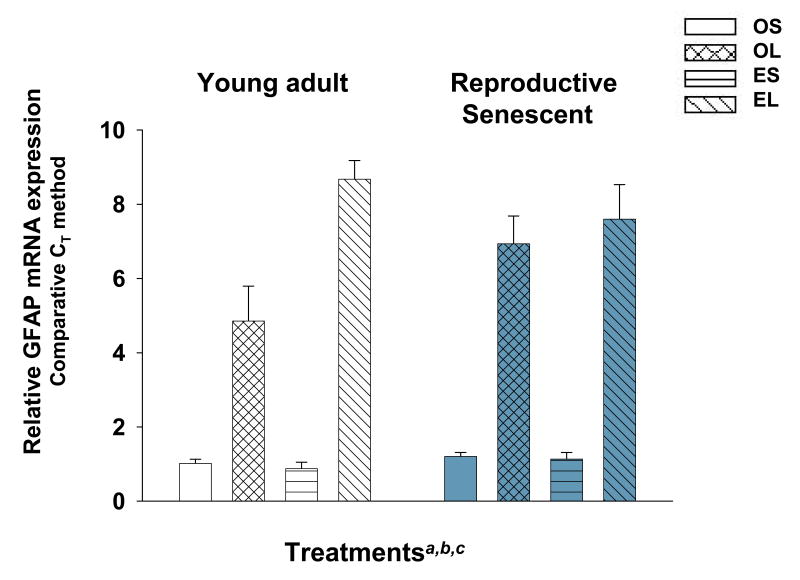
Following an N-methyl-d-aspartic acid (NMDA)-lesion to the olfactory bulb, GFAP relative mRNA expression was increased significantly in young adults and reproductive senescent females (Fig. 1). GFAP expression increased 4.8-fold in lesioned young adults in the absence of estrogen and doubled in the presence of estrogen (F(1,16): 8.31, p≤0.011, hormone × injury interaction). In lesioned reproductive senescent females, GFAP expression increased 5.7-fold in the absence and 6.7-fold in the presence of estrogen. Thus, in both young and ovarian-aged females the astrocytic marker is elevated by injury and estrogen.
Figure 1.
Glial fibrillary acidic protein (GFAP) mRNA expression levels increase in the olfactory bulb following an excitotoxic injury. Ovariectomized, placebo-replaced or estrogen-replaced female rats were subjected to a 24 hour NMDA lesion directed to the olfactory bulb. RNA was extracted and subjected to real time PCR. The relative expression of each mRNA was calculated by the comparative CT method using the following formula 2-ΔΔCt [where the amount of the target (GFAP) was normalized to the endogenous reference (Cyclophilin) and relative to a calibrator constant; ABI methods]. Main effect of hormone (a), main effect of injury (b), interaction effect (c) was significant at p≤0.05. OS: ovariectomized, placebo-replaced, sham-lesioned; OL: ovariectomized, NMDA-lesioned; ES: ovariectomized, estrogen-replaced, sham-lesioned; EL: ovariectomized, estrogen-replaced, NMDA-lesioned.
3.2 Characterization of Primary Astrocyte Cultures
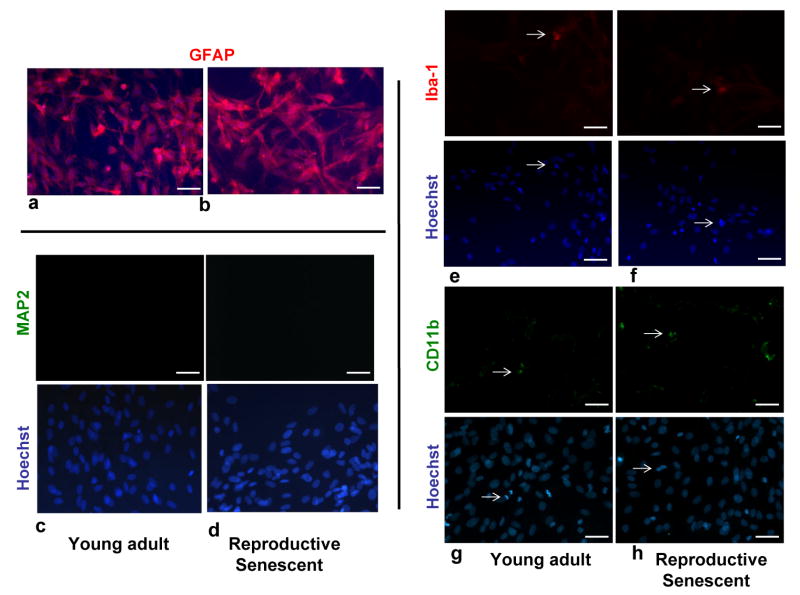
Since astrocytes are known to mediate inflammatory and neurodegenerative actions following injury, an in vitro model was established to determine if estrogen and reproductive age altered these properties of astrocytes. Primary adult astrocytes were harvested from the olfactory bulb of young adult and reproductive senescent females and the purity of these cultures was determined using immunohistochemistry for an astrocyte marker (GFAP, Fig. 2a, 2b) (Cohen et al., 1979), a neuronal marker (MAP2, Fig. 2c, 2d) (Bernhardt and Matus, 1884; Caceres et al., 1984) and two microglial markers, Iba-1 (Ito et al., 1998) (Fig. 2e, 2f) and CD11b (Fig. 2g, 2h) (Akiyama and McGeer, 1990). Virtually all cells in these cultures were immunoreactive for GFAP indicating that these cultures are mainly composed of astrocytes (young adult: 94.44±2.99%, Fig. 2a; reproductive senescent: 93.88±3.37%, Fig. 2b). A small proportion of the cells were immunoreactive for the microglial markers Iba1 and CD11b (Iba1: young adult: 4.80±1.32%, Fig. 2e; reproductive senescent: 5.48±2.02%, Fig. 2f; CD11b: young adult: 5.46±3.2% Fig. 2g; reproductive senescent: 8.5±7.17%, Fig. 2h) and differences in microglial contamination was not significantly different between young adult-derived astrocytes and reproductive senescent-derived astrocytes. No MAP2 staining was found in astrocyte cultures derived from either young adults (Fig. 2c) or reproductive senescent females (Fig. 2d).
Figure 2.
Characterization of primary astrocyte cultures derived from the olfactory bulb of young adult and reproductive senescent female rats. Primary astrocytes cultures were immunoreactive for GFAP (red: a,b) and were counter-stained with the nuclear dye, Hoechst (blue). Primary astrocyte cultures derived from young adult (c) and reproductive senescent females (d) were not immunoreactive for the neuronal marker, microtubule-associated protein-2 (MAP2). A small proportion (5-8%) of cells in these primary cultures derived from young adults (e,g) and reproductive senescent females (f,h) were immunopositive for the microglial markers, ionized-calcium binding adapter molecule 1 (Iba1) and the cell surface glycoprotein Mac-1 (CD11b). Iba-1 and CD11b immunopositive cells and their associated nuclei stained with Hoechst are indicated by white arrows. Immunohistochemistry with the nuclear dye Hoechst is shown in the bottom panel (c-h). Bar: 50 μm.
To determine whether estrogen receptors were present in the young adult-derived and reproductive senescent-derived astrocyte cultures, RT-PCR was used to detect the ERα and ERβ transcripts using receptor-specific primers. Two gene-specific products approximately 300 base pairs long (ERα) and 200 bases long (ERβ) were transcribed and sequenced. As shown in Figure 3, the appropriate gene product was present in astrocytes from young and ovarian-aged animals.
Figure 3.
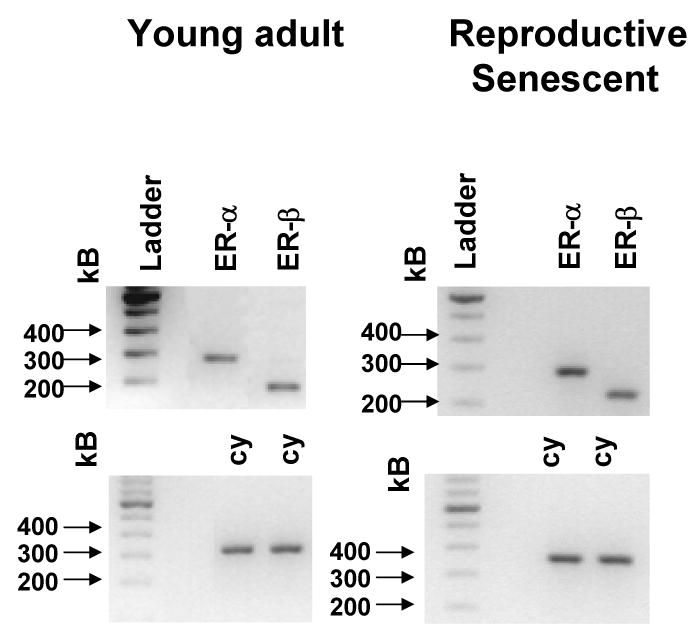
Gene expression of estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) in primary astrocyte cultures derived from young adult and reproductive senescent females as determined by RT-PCR. The ERα-specific primers span Exon 4 to Exon 6 while the ERβ-specific primers span from Exon 4 to Exon 5. Two gene-specific products approximately 300 base pairs long (ERα) and 200 bases long (ERβ) were present in both young and senescent astrocyte cultures. Cyclophilin was used as a loading control (cy) for the transcription of each gene. The bands were visualized on a 1.5% agarose gel and verified by sequencing.
3.3 Treatment Paradigm and Astrocyte Response to an LPS Challenge
Primary astrocytes were treated with 17β-estradiol, PPT, HPTE or DPN 4h prior to LPS and several LPS-induced inflammatory markers (IL-1β, TNFα and MMP-9) were examined in culture media 24 hours later. In all primary astrocyte cultures, the 24 hour LPS treatment was effective in stimulating an increase in all three inflammatory mediators examined and the cultures were immunoreactive for the toll-like receptor 4 (TLR4 receptor), a receptor known to transduce LPS-mediated signaling (Chow et al., 1999). As shown in Figure 4, TLR4 receptors were present on astrocytes derived from young adult and reproductive senescent females (4a).
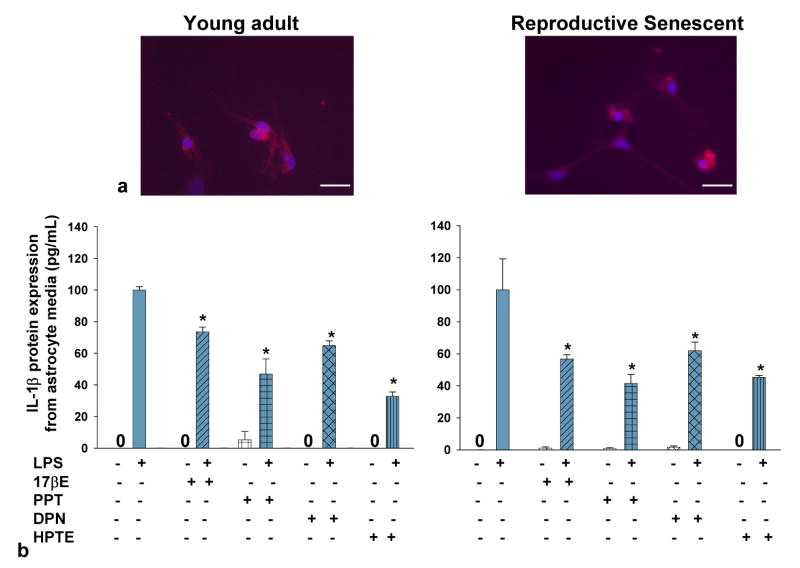
Figure 4.
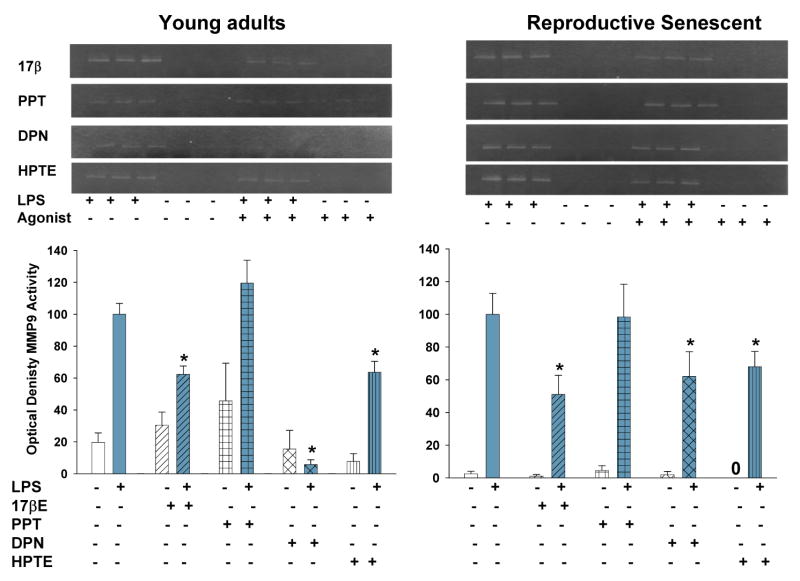
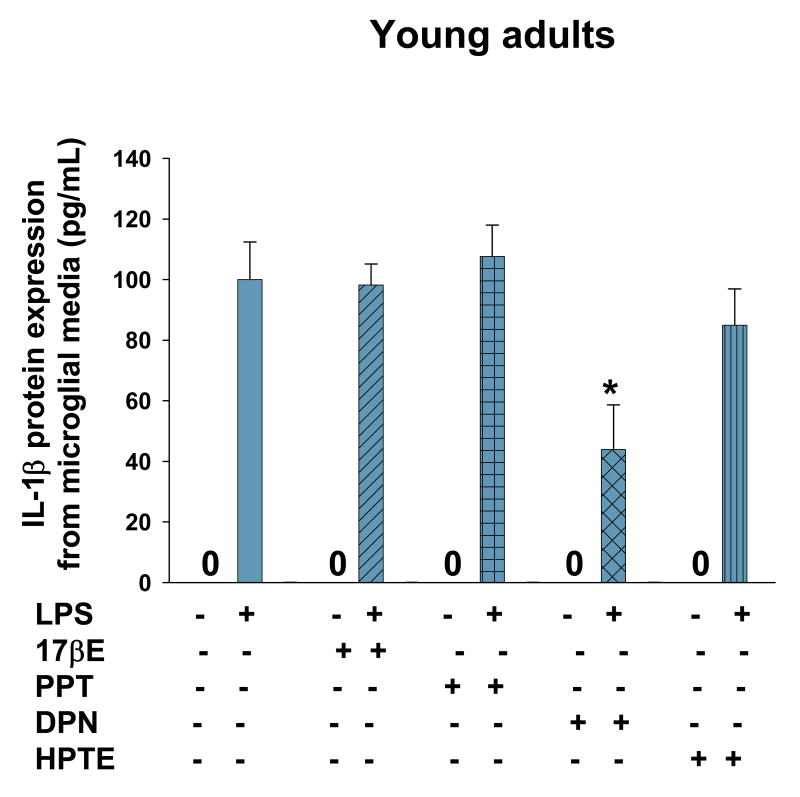
Primary astrocyte cultures derived from young adult and reproductive senescent females express the toll like recepter-4 (TLR-4). TLR4 (a, red) was observed in astrocytes derived from young adult and reproductive senescent females. Cells were counter-stained with the nuclear dye Hoechst (a, blue). Bar: 50 μm. IL-1β protein expression in astrocyte media from young adults (left panel, b) and reproductive senescent females (right panel,b) was determined following a 4-hour pre-treatment with 17β-estradiol (17bE, 20 nM), PPT (2 nM), HPTE (1μM) and DPN (10 nM) followed by a 24 hour treatment with LPS (10 μg/mL) and appropriate agonist. Asterisk (*) indicates significance at p≤0.05 relative to the saline/LPS-treated condition. Zero (0) indicates no detectable levels of expression in saline controls.
3.4 Effect of 17β-Estradiol
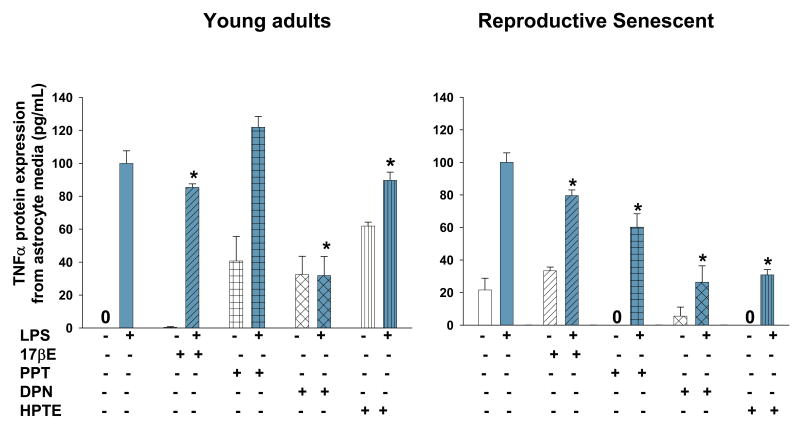
When primary astrocytes from young adults were pre-treated with 17β-estradiol, which has binding affinity for both ERα/ERβ (Kuiper et al., 1997), the LPS-induced increase in IL-β protein expression was attenuated by 27% (F(1,15): 57.423, p≤1.67×10-6, interaction LPS × 17β-estradiol treatment, Fig. 4b) and by 44% (F(1,14): 6.5744, p≤2.249×10-2, interaction LPS × 17β-estradiol treatment, Fig. 4b) in primary astrocyte cultures using astrocytes derived from young and reproductive senescent females, respectively. TNFα protein expression was attenuated by 15% (F(1,14): 4.8924, p≤ 4.4×10-2, interaction LPS × 17β-estradiol treatment, Fig. 5) and by 21% (F(1,12): 10.1202, p≤ 7.903×10-3, interaction LPS × 17β-estradiol treatment, Fig. 5) in astrocytes derived from young adults or senescent females, respectively. Matrix metalloproteinases are enzymes that target the extracellular matrix, chemokines, cytokines and growth factors (for review see Milward et al., 2007). MMP-9 activity was activated only with LPS (Fig. 6). In astrocytes derived from young adults, LPS-induced MMP-9 activity was attenuated by 38% (F(1,20): 13.3092, p≤1.599×10-3, interaction LPS × 17β-estradiol treatment, Fig. 7). When senescent-derived astrocytes were pre-treated with 17β-estradiol the LPS induced increase in MMP-9 activity was attenuated by 49% (F(1,19): 7.1299, p≤1.513×10-2, interaction LPS × 17β-estradiol treatment, Fig. 7).
Figure 5.
TNF-α protein expression in astrocyte media from young adults (left panel) and reproductive senescent females (right panel) was determined following a 4-hour pretreatment with 17β-estradiol (17bE, 20 nM), PPT (2 nM), HPTE (1μM) and DPN (10 nM) followed by a 24 hour treatment with LPS (10 μg/mL) and appropriate agonist. Asterisk (*) indicates significance at p≤0.05 relative to the saline/LPS-treated condition. Zero (0) indicates no detectable levels of expression in saline controls.
Figure 6.
MMP-9 activity was measured by zymography in astrocyte media derived from young adults and reproductive senescent females following a 4-hour pretreatment with 17β-estradiol (17bE, 20 nM), PPT (2 nM), HPTE (1μM) and DPN (10 nM) followed by a 24 hour treatment with LPS (10 μg/mL) and appropriate agonist. Asterisk (*) indicates significance at p≤0.05 relative to the saline/LPS-treated condition. Zero (0) indicates no detectable levels of expression in saline controls.
Figure 7.
IL-1β protein expression in microglial media from young adults was determined following a 4-hour pretreatment with 17β-estradiol (17bE, 2 nM), PPT (2 nM), HPTE (2μM) and DPN (10 nM) followed by a 24 hour treatment with LPS (10 μg/mL) and appropriate agonist. Asterisk (*) indicates significance at p≤0.05 relative to the saline/LPS-treated condition. Zero (0) indicates no detectable levels of expression in saline controls.
3.5 Effect of PPT
When primary astrocytes from young adults or reproductive senescent animals were pre-treated with PPT, an ERα agonist (Stauffer et al., 2000), the LPS-induced increase in IL-1β was attenuated by 54% (F(1,15): 9.2061, p≤8.369×10-3, interaction LPS × PPT treatment, Fig. 4b) and 58% (F(1,16): 60.654, p≤7.84×10-7, interaction LPS × PPT treatment, Fig. 4b), respectively. TNFα protein expression was not attenuated by the ERα agonist, PPT (F(1,10): 48.6532, p≤3.83×10-5, main effect LPS, Fig. 5) in astrocytes derived from young adults but was attenuated by 40% (F(1,14): 17.904, p≤8.38×10-4, interaction LPS × PPT treatment, Fig. 5) in primary cultures of senescent-derived astrocytes. PPT was not effective in attenuating the LPS induced increase in MMP-9 activity in either or young adult- or senescent-derived astrocytes (young adult: F(1,20): 21.5570, p≤1.57×10-4; reproductive senescent: F(1,19): 39.25, p≤5.14×10-6, main effect LPS, Fig. 6).
3.6 Effect of HPTE
HPTE is an ERβ antagonist/ERα agonist (Gaido et al., 1999). Pre-treatment of astrocyte cultures derived from young adults or reproductive senescent females with HPTE resulted in a 68% (F(1,14): 163.52, p≤4.12×10-9, interaction LPS × HPTE treatment, Fig. 4b) and a 55% (F(1,16): 107.5959, p≤1.65×10-8, interaction LPS × HPTE treatment, Fig. 4b) reduction in the LPS-mediated increase in IL-1β, respectively. TNFα protein expression was also attenuated by 11% (F(1,14): 85.6516, p≤ 3.93×10-2, interaction LPS × HPTE treatment, Fig. 5) and 70% (F(1,13): 61.5349, p≤ 2.77×10-6, interaction LPS and HPTE treatment, Fig. 5) in young adult- and reproductive senescent-derived astrocyte cultures, respectively. In astrocytes derived from young adults, the LPS-induced increase in MMP-9 activity was attenuated by 37% (F(1,20): 10.7478, p≤3.758×10-3, interaction LPS × HPTE treatment, Fig. 6) and by 33% (F(1,12): 102.4602, p≤3.14×10-7, interaction LPS × HPTE treatment, Fig. 6) in senescent astrocyte cultures.
3.7 Effect of DPN
Pre-treatment of astrocyte cultures with DPN, the ERβ specific agonist (Meyers et al., 2001), resulted in a 36% decrease in IL-1β protein expression (F(1,11): 32.47619, p≤1.38×10-4, interaction LPS × DPN treatment, Fig. 4b) when the cultures were derived from young adults and a 39% reduction (F(1,13): 21.1127, p≤5.02×10-4, interaction LPS × DPN treatment, Fig. 4b) when applied to astrocyte cultures derived from reproductive senescent females. The LPS-induced increase in TNFα protein expression was attenuated by 69% (F(1,17): 10.0566, p≤ 5.583×10-3, interaction LPS × DPN treatment, Fig. 5) and by 74% (F(1,13): 28.4219, p≤1.36×10-4, interaction LPS × DPN treatment, Fig. 5) in young adult- and reproductive senescent-derived astrocytes. While the LPS-induced increase in MMP-9 activity was attenuated by 95% with DPN pretreatment (F(1,19): 6.0133, p≤2.403×10-2, interaction LPS × DPN treatment, Fig. 6) in young adult-derived astrocytes cultures and by 38% (F(1,19): 6.3871, p≤2.05×10-2, interaction LPS × DPN treatment, Fig. 6) in senescent-derived astrocyte cultures.
3.8 Effect of ER agonists on microglial cultures
Previous work has shown that 17β-estradiol did not suppress IL-1β expression in these cells when obtained from either young or senescent animals (Johnson and Sohrabji, 2005), but the action of other estrogen receptor agonists is not known. In order to determine the effects of estrogen agonists, microglial cells were cultured and exposed to LPS as before. This analysis was limited to cells obtained from young adult animals and IL-1β was assayed as a prototypic inflammatory mediator, shown in a summary histogram in Fig 7. As reported before (Johnson and Sohrabji, 2005), 17β-estradiol failed to suppress IL-1β expression in media from microglia exposed to LPS. Furthermore, unlike the astrocyte cultures, both PPT and HPTE also failed to suppress this cytokine. However, DPN, the ERβ agonist significantly reduced LPS-induced IL-1β expression (F(1,11): 12.6811, p≤4.465×10-3, interaction LPS × DPN treatment, Fig. 7) in microglial cultures indicating that this compound may have a more general anti-inflammatory effect on neural support cells.
4. Discussion
The present study examined the effect of ERα and ERβ agonists on cultured astrocytes obtained from young and senescent females. 17β-estradiol, a compound that has ligand specificity for both receptor sub-types, was able to suppress the LPS-induced increase in IL-1β, TNF-α and MMP-9 activity, which were used as surrogate markers for inflammation. Furthermore this effect was seen uniformly in astrocytes derived from either young adult and reproductive senescent animals. This is in contrast to our findings from microglial cultures where 17β-estradiol did not suppress LPS-induced inflammatory mediators. Moreover, DPN (ERβ agonist) and HPTE (ERα agonist/ERβ antagonist) were also able to suppress LPS-induced IL-1β, TNFα and MMP-9 in both “ages” of astrocytes. PPT, the ERα agonist acted selectively; reducing IL-1β in young and senescent derived astrocytes but not TNFα or MMP-9. These data indicate that ligands for either receptor sub-type (ERα or ERβ) attenuated some aspect of the inflammatory response in astrocytes and that compounds known to bind ERβ effectively suppressed LPS-induced cytokine expression in both cell types. Such overlapping actions of ERα and ERβ agonists have been demonstrated in other systems such as the cardiovasculature (Arias-Loza et al., 2007) and peripheral blood leukocytes (Stygar et al., 2007).
Astrocytes are one of the most abundant cell types in the brain and are critical for neuronal health. Astrocytes support the blood-brain barrier, they regulate neurotransmitter concentrations and modulate osmolarity and pH in the extracellular space surrounding neurons (for review see Benarroch, 2005). Astrocytes respond to and/or produce pro-inflammatory factors and thus have the potential to contribute to increased neurodegeneration in the brain (for review see Chen and Swanson, 2003). Immunohistochemistry of tissue collected from Alzheimer's patients and Down's syndrome patients showed that the astrocyte-specific protein, glial fibrillary acidic protein (GFAP) and the pro-inflammatory cytokines, IL-1β and S-100 were significantly increased in astrocytes from the temporal lobe as compared to tissue collected from age-matched controls (Griffin et al., 1989). In this same study, IL-1β was also increased in microglia and this increase was implicated in increased astrogliosis (Griffin et al., 1989). Moreover, astrocytes have also been targeted as potential contributors to motor neuron death in animal models of amyotrophic lateral sclerosis (ALS) through secretion of astrocyte-specific factor(s) (Nagai et al., 2007) and through a breakdown in the blood-brain barrier due to swelling of astrocyte end foot processes (Garbuzova-Davis et al., 2007). Further, reactive astrocytes have been implicated in the neuropathophysiology observed in Parkinson's disease (for review see Mrak and Griffin, 2005). In the present study, excitotoxic injury increased GFAP mRNA which was further exacerbated by estrogen. This is consistent with our previous observation that the excitotoxic injury increased GFAP immunoreactivity in all cell layers of the olfactory bulb while GFAP immunoreactivity was limited to the needle tract in sham-injected females (Sohrabji et al., 2000). Increased GFAP mRNA expression could be interpreted in two ways: either estrogen increases the reactivity of a stable number of glia, or that estrogen increases the number of glia with no increase in the individual complement of GFAP mRNA per cell. However, while estrogen increases GFAP mRNA in both ages after injury, our previous in vivo study shows that 17β-estradiol suppresses IL-1β in young females but exacerbates cytokine expression in the senescent female (Nordell et al., 2003), indicating that the increase in GFAP is not predictive of cytokine expression. While the precise role of this GFAP increase in not clear, these data unequivocally indicate that astroglial cells are significantly involved in neural injury or the resolution phase of the injury and that these cells are sensitive to 17β-estradiol.
In a series of studies, we have shown that estrogen replacement is beneficial to young adult females and has deleterious actions in the forebrain of acyclic older females (for review see Sohrabji, 2005). For example, in older acyclic females, estrogen decreases growth factor expression in the forebrain (Jezierski and Sohrabji, 2001), increases the permeability of the blood brain barrier in the hippocampus (Bake and Sohrabji, 2004) and increased the pro-inflammatory cytokine IL-1β following an excitotoxic injury (Nordell et al., 2003). Estrogen's modulation of the neural inflammatory response is important to understand since inflammation underlies the etiology of many neurodegenerative diseases. By separately culturing microglia from young and senescent animals, it was noted that microglia from either age group responded appropriately to LPS, however, estrogen failed to decrease IL-1β in either young or senescent-derived microglia (Johnson and Sohrabji, 2005). The present study therefore focused on astrocytes, which are also activated by neural injury and capable of synthesizing inflammatory cytokines when activated, in order to determine if this cell type is affected by ovarian aging.
In the NMDA injury model of the olfactory bulb, GFAP (the putative marker for astrocytes) was significantly up-regulated, indicating that astrocytes are may mediate the tissue response to excitotoxic injury (Burtrum and Silverstein, 1993; Kunkler and Kraig, 1997). Both estrogen receptor alpha and beta were present in these cells, indicating the presence of a substrate that could mediate estrogens effects. 17β-estradiol, which binds both receptors, successfully suppressed several markers of inflammation in these cells. However, paradoxical to the in vivo situation, 17β-estradiol was an effective anti-inflammatory in astrocytes derived from both young and senescent animals. Hence neither microglia (Johnson and Sohrabji, 2005) nor astrocytes are differentially sensitive to estrogen when cultured individually from young and senescent animals. If the in vitro situation is reflective of the in vivo condition, the present data supports our previous hypothesis that the age-dependent alterations in estrogen's actions is likely due to estrogens actions on non-neural immune cells (Johnson and Sohrabji, 2005) or possibly through gating actions at the blood brain barrier (Johnson et al., 2006).
Several studies have argued that ERα is solely responsible for estrogen's anti-inflammatory action. For example, LPS-induced inflammation was not attenuated by estrogen in ERα knock-out (ERKO) animals as compared to wild type animals or ERβ knock out animals (Vegeto et al., 2003). Furthermore, ERα inactivation abolished the protective effects of estrogen in an experimental autoimmune encephalomyelitis model of injury (Morales et al., 2006). ERα-mediated attenuation of 1-methyl-4-phenyl-pyridinium (MPP) toxicity has also been recently reported in mixed cultures containing dopaminergic neurons and astrocytes (Bains et al., 2007). However, other studies show that ERα may be necessary to mount an effective immune response. For example, ERKO mice do not show the expected increase in the LPS receptor TLR-2, after intracerebral LPS injections (Soucy et al., 2005), suggesting that the general innate immune response may be compromised in the absence of ERα.
The role of ERβ, on the other hand, has only recently gained more attention. Although not as potent a transcription factor as ERα, ERβ has several cell-specific functions, including mediating vascular wall relaxation in response to 17β-estradiol in the aorta (Nilsson et al., 2000) and CREB phosphorylation in hypothalamic neurons (Abraham et al., 2003). ERβ has been implicated in cell survival in the developing and aging brain and the ERβ knock-out (BERKO) mouse develops major brain malformations due to neuronal loss as the animal ages (Wang et al., 2003). ERβ has been localized to mitochondria of many cells including neurons and may play a role in neuroprotection (Yang et al., 2004). In the BV-2 cell line, a murine-derived microglial cell line that expresses only ERβ, estrogen attenuates the inflammatory effects of LPS (Baker et al., 2004). In rodent models of stroke, DPN was not effective in reducing the infarct size following permanent middle cerebral artery occlusion (Farr et al., 2007) but it was effective in reducing infarct volume in mice following global transient ischemia involving bilateral occlusion of the carotid artery (Carswell et al., 2004). Our finding that only the ERβ agonist was effective in modulating the inflammatory response in both astrocytes and microglia, has implications for estrogen-replacement therapies. In neural injury a temporal lag exists between microglial and astrocyte activation. Studies have shown that microglial cells proliferate earlier than astrocytes in adult rats following an acute stab wound (Norton, 1999) and the peak concentration of microglia following an acute inflammatory reaction (Lemke et al., 1999; Seeger et al., 1997) occurs almost 1-2 weeks ahead of the peak concentration of astrocytes (Lemke et al., 1998; Lemke et al., 1999). Thus, our data suggests that one strategy for suppressing neural inflammation would be early dosing with an ERβ agonist to attenuate the microglial response and a subsequent dosing with an ERα or pan receptor agonist for the astrocyte wave. Future studies will examine the efficacy of this treatment regimen in an in vivo model.
The present study also sheds light on the role of receptor mechanisms involved in the inflammatory pathway, especially due to the paradoxical role of the receptor antagonists. In these astrocyte cultures, HPTE, an ERα agonist/ERβ antagonist, was equally potent at suppressing the inflammatory response as DPN, which is an ER-β agonist. These paradoxical effects suggest that the actions of estrogen agonists may not be due to conventional estrogen receptor activation. The use of receptor antagonists would typically resolve this issue, however, our studies indicate that the ERα antagonist methyl-piperidino-pyrazole (MPP) independently suppressed LPS-induced IL-1β in microglial cultures (unpublished observations). In fact, in primary rat microglial cultures, the non-specific ER antagonists ICI 182,780 and tamoxifen also have been shown to attenuate the expression of IL-6 and NO (Suuronen et al., 2005). Based on the anti-inflammatory actions of these compounds, these authors have speculated that the response is therefore more like a selective estrogen receptor modulator (SERM) rather than a conventional estrogen-receptor mediated event.
One interesting observation from this study is that several of the agonists caused a moderate increase in TNFα and MMP-9 expression in the absence of LPS. It should be recognized that these molecules may serve diverse functions. Besides their well known roles in inflammation, glia-derived TNFα has been shown to modulate synaptic efficacy (Beattie et al., 2002) and transport of low doses of this cytokine across the blood brain barrier may be cytoprotective following stroke (Pan and Kastin, 2007). Further, MMP-9 may play a role in regulating synaptic plasticity in the brain (Dzwonek et al., 2004). Thus, the agonist-induced expression of these factors in the absence of LPS stimulation suggests that these ligands may activate neuroplastic pathways in addition to their roles in inflammation. In the presence of LPS, however, where TNFα and MMP-9 are vastly elevated, these estrogen agonists serve to decrease these inflammatory molecules indicating that these estrogen-receptor specific ligands may regulate the levels of these immune molecules within a narrow homeostatic range.
The interplay between various cells types likely influences the effects of estrogen on attenuating the immune response. For example, in mixed cultures of neurons and astrocytes, the maximal attenuation of N-methyl-4-phenylpyridine (MPP+) toxicity required the presence of both cell types (Bains et al., 2007). In mixed astrocyte/microglial cultures, astrocytes attenuated the microglial-derived increases in IL-12, a cytokine important for regulating the immune response, following LPS treatment (Aloisi et al., 1997). Astrocytes have also been shown to suppress microglia phagocytosis (DeWitt et al., 1998) and astrocyte-derived transforming growth factor beta (TGFb)-1 is responsible for converting activated (phagocytic) microglia to resting microglia (Schilling et al., 2001). Moreover, the effect of cytokines can be differentially regulated depending on the presence or absence of a particular cell type. For example, interleukin 4 enhanced IL-1β production in mixed astrocyte/microglial cultures but inhibited IL-1β production in microglial only cultures (Cao et al., 2007). Hence one approach for future experiments is to address the effects of estrogenic agonists in mixed cultures of astrocytes and microglia derived from young and senescent animals.
The present data suggest that modulation of ERβ may be a viable therapeutic target for neuroinflammatory disease. While estrogen receptors have been detected in astrocytes and microglial cells, injury can lead to a selective up-regulation of these receptors. LPS treatment to astrocytes and microglia resulted in increased expression of ERβ but not ERα (Liu et al., 2005). ERα labeling was identified in reactive astrocytes following brain injury in three Macaca fascicularis (cynomolgous) monkeys following brain injury (Blurton-Jones and Tuszynski, 2001). In humans, both ERα (Lu et al., 2003) and ERβ (Savaskan et al., 2001) immunoreactivity has been reported to be more highly expressed in the astrocytes of patients with Alzheimer's disease when compared to control brains. One possible hypothesis is that this injury-mediated up-regulation of ERβ underlies the effectiveness of ERβ agonist/antagonists in suppressing the inflammatory cascade. In view of the evidence that ERβ agonists are non-uterotropic (Frasor et al., 2003) and the present data where the ERβ specific compound was effective in both young and senescent animals and in both astrocytes and microglial cells indicates that this would be a good candidate for neural anti-inflammatory therapies.
Acknowledgments
This work was supported by NIH AG19515 and AG028303 to FS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, McGeer PL. Brain microglia constitutively express β-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Penna G, Cerase J, Menendez Iglesias B, Adorini L. IL-12 production by central nervous system microglia is inhibited by astrocytes. J Immunol. 1997;159:1604–1612. [PubMed] [Google Scholar]
- Amateau S, McCarthy M. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol. 2002;14:904–910. doi: 10.1046/j.1365-2826.2002.00858.x. [DOI] [PubMed] [Google Scholar]
- Andersen K, Launer LJ, Dewey ME, Letenneur L, Ott A, Copeland JRM, Dartigues JF, Kragh-Sorensen P, Baldereschi M, Brayne C, Lobo A, Martinez-Lage JM, Stijnen T, Hofman A. Gender differences in the incidence of AD and vascular dementia: The EURODEM Studies. Neurol. 1999;53:1992. doi: 10.1212/wnl.53.9.1992. [DOI] [PubMed] [Google Scholar]
- Arias-Loza PA, Hu K, Dienesch C, Mehlich AM, Konig S, Jazbutyte V, Neyses L, Hegele-Hartung C, Heinrich Fritzemeier K, Pelzer T. Both estrogen receptor subtypes, α and β, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension. 2007;50:432–438. doi: 10.1161/HYPERTENSIONAHA.106.084798. [DOI] [PubMed] [Google Scholar]
- Badger AM, Blake SM, Dodds RA, Griswold DE, Swift BA, Rieman DJ, Stroup GB, Hoffman SJ, Gowen M. Idoxifene, a novel selective estrogen receptor modulator, is effective in a rat model of adjuvant-induced arthritis. J Pharmacol Exp Ther. 1999;291:1380–1386. [PubMed] [Google Scholar]
- Bains M, Cousins JC, Roberts JL. Neuroprotection by estrogen against MPP+- induced dopamine neuron death is mediated by ERα in primary cultures of mouse mesencephalon. Exp Neurol. 2007;204:767–776. doi: 10.1016/j.expneurol.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Sohrabji F. 17β-Estradiol differentially regulate blood-brain barrier permeability in young and aging female rats. Endocrinol. 2004;145:5471–5475. doi: 10.1210/en.2004-0984. [DOI] [PubMed] [Google Scholar]
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor {beta} Endocrinol. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- Beattie E, Stellwagen D, Morishita W, Bresnahan J, Ha B, Von Zastrow M, Beattie M, Malenka R. Control of Synaptic Strength by Glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Benarroch E. Neuron-astrocyte interactions: partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Bernhardt R, Matus A. Light and electron microscopic studies of the distribution of microtubule-associated protein 2 in rat brain: a difference between dendritic and axonal cytoskeletons. J Comp Neurol. 1884;226:203–221. doi: 10.1002/cne.902260205. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Reactive astrocytes express estrogen receptors in the injured primate brain. J Comp Neurol. 2001;433:115–123. doi: 10.1002/cne.1129. [DOI] [PubMed] [Google Scholar]
- Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinol. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Burtrum D, Silverstein FS. Excitotoxic injury stimulates glial fibrillary acidic protein mRNA expression in perinatal rat brain. Exp Neurol. 1993;121:127–132. doi: 10.1006/exnr.1993.1078. [DOI] [PubMed] [Google Scholar]
- Caceres A, Banker G, Steward O, Binder L, Payne M. MAP2 is localized to the dendrites of hippocampal neurons which develop in culture. Brain Res. 1984;315:314–318. doi: 10.1016/0165-3806(84)90167-6. [DOI] [PubMed] [Google Scholar]
- Cao L, Fei L, Chang TT, DeLeo JA. Induction of interleukin-1beta by interleukin-4 in lipopolysaccharide-treated mixed glial cultures: microglial-dependent effects. J Neurochem. 2007;102:408–419. doi: 10.1111/j.1471-4159.2007.04588.x. [DOI] [PubMed] [Google Scholar]
- Carswell HVO, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective estrogen receptor β agonist in a mouse model of global ischemia. Am J Physiol Heart Circ Physiol. 2004;287:H1501–1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- Cohen J, Woodhams PL, Balazs R. Preparation of viable astrocytes from the developing cerebellum. Brain Res. 1979;161:503–514. doi: 10.1016/0006-8993(79)90679-6. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Mazzon E, Sautebin L, Serraino I, Dugo L, Calabro G, Caputi AP, Maggi A. The protective role of endogenous estrogens in carrageenan-induced lung injury in the rat. Mol Med. 2001;7:478–487. [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Santagati S, Sautebin L, Mazzon E, Calabro G, Serraino I, Caputi AP, Maggi A. 17beta-estradiol antiinflammatory activity in carrageenan-induced pleurisy. Endocrinol. 2000;141:1455–1463. doi: 10.1210/endo.141.4.7404. [DOI] [PubMed] [Google Scholar]
- De M, Wood GW. Influence of oestrogen and progesterone on macrophage distribution in the mouse uterus. J Endocrinol. 1990;126:417–424. doi: 10.1677/joe.0.1260417. [DOI] [PubMed] [Google Scholar]
- DeWitt DA, Perry G, Cohen M, Doller C, Silver J. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer's disease. Exp Neurol. 1998;149:329–340. doi: 10.1006/exnr.1997.6738. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- Dzwonek J, Rylski M, Kaczmarek L. Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett. 2004;567:129–135. doi: 10.1016/j.febslet.2004.03.070. [DOI] [PubMed] [Google Scholar]
- Farr TD, Carswell HV, Gsell W, Macrae IM. Estrogen receptor beta agonist diarylpropiolnitrile (DPN) does not mediate neuroprotection in a rat model of permanent focal ischemia. Brain Res. 2007 doi: 10.1016/j.brainres.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Frasor J, Barnett DH, Danes JM, Hess R, Parlow AF, Katzenellenbogen BS. Response-specific and ligand dose-dependent modulation of estrogen receptor (ER) α activity by ERβ in the uterus. Endocrinol. 2003;144:3159–3166. doi: 10.1210/en.2002-0143. [DOI] [PubMed] [Google Scholar]
- Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, Safe S. Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors α and β. Endocrinol. 1999;140:5746–5753. doi: 10.1210/endo.140.12.7191. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: A meta-analysis. Arch Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR. Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res. 2007;1157:126–137. doi: 10.1016/j.brainres.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. PNAS. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilian D, Baker T. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges LC, Bergerson JS, Hunter DS, Walker CL. Estrogenic effects of organochlorine pesticides on uterine Leiomyoma cells in vitro. Toxicol Sci. 2000;54:355–364. doi: 10.1093/toxsci/54.2.355. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Jezierski M, Sohrabji F. Region- and peptide-specific regulation of the neurotrophins by estrogen. Mol Brain Res. 2000;85:77–84. doi: 10.1016/s0169-328x(00)00244-8. [DOI] [PubMed] [Google Scholar]
- Jezierski M, Sohrabji F. Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation. Neurobiol Aging. 2001;22:309–319. doi: 10.1016/s0197-4580(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Johnson A, Sohrabji F. Estrogen's effects on central and circulating immune cells vary with reproductive age. Neurobiol Aging. 2005;26:1365–1374. doi: 10.1016/j.neurobiolaging.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Johnson AB, Bake S, Lewis DK, Sohrabji F. Temporal expression of IL-1beta protein and mRNA in the brain after systemic LPS injection is affected by age and estrogen. J Neuroimmunol. 2006;174:82–91. doi: 10.1016/j.jneuroim.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Kaushic C, Frauendorf E, Rossoll RM, Richardson JM, Wira CR. Influence of the estrous cycle on the presence and distribution of immune cells in the rat reproductive tract. Am J Reprod Immunol. 1998;39:209–216. doi: 10.1111/j.1600-0897.1998.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. PNAS. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinol. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kunkler PE, Kraig RP. Reactive astrocytosis from excitotoxic injury in hippocampal organ culture parallels that seen in vivo. J Cereb Blood Flow Metab. 1997;17:26–43. doi: 10.1097/00004647-199701000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrkanides S, O'Banion M, Whiteley P, Daeschner J, Olschowka J. Enhanced glial activation and expression of specific CNS inflammation-related molecules in aged versus young rats following cortical stab injury. J Neuroimmunol. 2001;119:269–277. doi: 10.1016/s0165-5728(01)00404-0. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett P, Jensen F, Rosenberg P, Volpe J, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke R, Hartig W, Rossner S, Bigl V, Schliebs R. Interleukin-6 is not expressed in activated microglia and in reactive astrocytes in response to lesion of rat basal forebrain cholinergic system as demonstrated by combined in situ hybridization and immunocytochemistry. J Neurosci Res. 1998;51:223–236. doi: 10.1002/(SICI)1097-4547(19980115)51:2<223::AID-JNR11>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lemke R, Hartlage-Rubsamen M, Schliebs R. Differential injury-dependent glial expression of interleukins-1 alpha, beta, and interleukin-6 in rat brain. Glia. 1999;27:75–87. [PubMed] [Google Scholar]
- Lindahl LS, Bird L, Legare ME, Mikeska G, Bratton GR, Tiffany-Castiglioni E. Differential ability of astroglia and neuronal cells to accumulate lead: dependence on cell type and on degree of differentiation. Toxicol Sci. 1999;50:236–243. doi: 10.1093/toxsci/50.2.236. [DOI] [PubMed] [Google Scholar]
- Liu X, Fan XL, Zhao Y, Luo GR, Li XP, Li R, Le WD. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia. J Neurosci Res. 2005;81:653–665. doi: 10.1002/jnr.20583. [DOI] [PubMed] [Google Scholar]
- Lu YP, Zeng M, Hu XY, Xu H, Swaab DF, Ravid R, Zhou JN. Estrogen receptor α-immunoreactive astrocytes are increased in the hippocampus in Alzheimer's disease. Exp Neurol. 2003;183:482–488. doi: 10.1016/s0014-4886(03)00205-x. [DOI] [PubMed] [Google Scholar]
- Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- Milward EA, Fitzsimmons C, Szklarczyk A, Conant K. The matrix metalloproteinases and CNS plasticity: An overview. J Neuroimmunol. 2007;187:9–19. doi: 10.1016/j.jneuroim.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Mandai M, Suzuma I, Suzuma K, Kobayashi K, Honda Y. Estrogen protects against cellular infiltration by reducing the expressions of E-Selectin and IL-6 in endotoxin-induced uveitis. J Immunol. 1999;163:374–379. [PubMed] [Google Scholar]
- Morales LBJ, Loo KK, Liu Hb, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an estrogen receptorα ligand is neuroprotective in experimental autoimmune encephalomyelitis. J Neurosci. 2006;26:6823–6833. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak R, Griffin W. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988;140:1049–1053. doi: 10.1016/s0022-5347(17)41924-0. [DOI] [PubMed] [Google Scholar]
- Nilsson BO, Ekblad E, Heine T, Gustafsson JA. Increased magnitude of relaxation to oestrogen in aorta from oestrogen receptor beta knock-out mice. J Endocrinol. 2000;166:R5–9. doi: 10.1677/joe.0.166r005. [DOI] [PubMed] [Google Scholar]
- Nordell V, Lewis D, Bake S, Sohrabji F. The neurotrophin receptor p75NTR mediates early anti-inflammatory effects of estrogen in the forebrain of young adult rats. BMC Neurosci. 2005;6:58. doi: 10.1186/1471-2202-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordell V, Scarborough M, Buchanan A, Sohrabji F. Differential effects of estrogen in the injured forebrain of young adult and reproductive senescent animals. Neurobiol Aging. 2003;5798:1–11. doi: 10.1016/s0197-4580(02)00193-8. [DOI] [PubMed] [Google Scholar]
- Norton WT. Cell reactions following acute brain injury: a review. Neurochem Res. 1999;24:213–218. doi: 10.1023/a:1022505903312. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ. Tumor necrosis factor and stroke: Role of the blood-brain barrier. Prog Neurobiol. 2007;83:363–374. doi: 10.1016/j.pneurobio.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parducz A, Perez J, Garcia-Segura L. Estradiol induces plasticity of GABAergic synapses in the hypothalamus. Neurosci. 1993;53:395–401. doi: 10.1016/0306-4522(93)90203-r. [DOI] [PubMed] [Google Scholar]
- Qian Y, Zheng Y, Ramos KS, Tiffany-Castiglioni E. The involvement of copper transporter in lead-induced oxidative stress in astroglia. Neurochem Res. 2005;30:429–438. doi: 10.1007/s11064-005-2677-1. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Olivieri G, Meier F, Ravid R, Muller-Spahn F. Hippocampal estrogen beta-receptor immunoreactivity is increased in Alzheimer's disease. Brain Res. 2001;908:113–119. doi: 10.1016/s0006-8993(01)02610-5. [DOI] [PubMed] [Google Scholar]
- Schilling T, Nitsch R, Heinemann U, Haas D, Eder C. Astrocyte-released cytokines induce ramification and outward K+ channel expression in microglia via distinct signalling pathways. Eur J Neurosci. 2001;14:463–473. doi: 10.1046/j.0953-816x.2001.01661.x. [DOI] [PubMed] [Google Scholar]
- Seeger G, Hartig W, Rossner S, Schliebs R, Bruckner G, Bigl V, Brauer K. Electron microscopic evidence for microglial phagocytic activity and cholinergic cell death after administration of the immunotoxin 192IgG-saporin in rat. J Neurosci Res. 1997;48:465–476. doi: 10.1002/(sici)1097-4547(19970601)48:5<465::aid-jnr7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Sohrabji F. Estrogen: a neuroprotective or proinflammatory hormone? Emerging evidence from reproductive aging models. Ann N Y Acad Sci. 2005;1052:75–90. doi: 10.1196/annals.1347.006. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Peeples K, Marroquin O. Local and cortical effects of olfactory bulb lesions on trophic support and cholinergic function and their modulation by estrogen. J Neurobiol. 2000;45:61–74. doi: 10.1002/1097-4695(20001105)45:2<61::aid-neu1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Soucy G, Boivin G, Labrie F, Rivest S. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol. 2005;174:6391–6398. doi: 10.4049/jimmunol.174.10.6391. [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- Stygar D, Masironi B, Eriksson H, Sahlin L. Studies on estrogen receptor (ER) α and β responses on gene regulation in peripheral blood leukocytes in vivo using selective ER agonists. J Endocrinol. 2007;194:101–119. doi: 10.1677/JOE-06-0060. [DOI] [PubMed] [Google Scholar]
- Suuronen T, Nuutinen T, Huuskonen J, Ojala J, Thornell A, Salminen A. Antiinflammatory effect of selective estrogen receptor modulators (SERMs) in microglial cells. Inflamm Res. 2005;54:194–203. doi: 10.1007/s00011-005-1343-z. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol. 2007;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Tchernitchin AN, Galand P. Oestrogen levels in the blood, not in the uterus, determine uterine eosinophilia and oedema. J Endocrinol. 1983;99:123–130. doi: 10.1677/joe.0.0990123. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-α mediates the brain antiinflammatory activity of estradiol. PNAS. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Pollio G, Ciana P, Maggi A. Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp Gerontol. 2000;35:1309–1316. doi: 10.1016/s0531-5565(00)00161-3. [DOI] [PubMed] [Google Scholar]
- Wang L, Andersson S, Warner M, Gustafsson JA. Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain. PNAS. 2003;100:703–708. doi: 10.1073/pnas.242735799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Friedman W, Dreyfus C. Differential regulation of neurotrophin expression in basal forebrain astroctyes by neuronal signals. J Neurosci. 2004;76:76–85. doi: 10.1002/jnr.20060. [DOI] [PubMed] [Google Scholar]
- Yang SH, Liu R, Perez EJ, Wen Y, Stevens SM, Jr, Valencia T, Brun-Zinkernagel AM, Prokai L, Will Y, Dykens J, Koulen P, Simpkins JW. Mitochondrial localization of estrogen receptor β. PNAS. 2004;101:4130–4135. doi: 10.1073/pnas.0306948101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]