Abstract
To test the hypothesis that activation of the transient receptor potential vanilloid type 1 (TRPV1) channels leads to natriuresis and diuresis via an increase in glomerular filtration rate (GFR), recirculating Krebs-Henseleit buffer added with inulin was perfused in the isolated perfused kidney of male Wistar rat at a constant flow, and perfusion pressures (PP) were pre-adjusted to three different levels (~100, ~150, and ~190 mmHg) with phenylephrine. Capsaicin (Cap), a selective TRPV1 agonist, was perfused in the presence or absence of capsazepine (Capz), a selective TRPV1 antagonist, CGRP8–37, a selective calcitonin gene-related peptide (CGRP) receptor antagonist, or spantide II (Spa), a selective substance P (SP) receptor antagonist. At the higher (150 and 190 mmHg) but not baseline (100 mmHg) PP levels, Cap at 10µM significantly decreased PP and increased GFR, urine flow rate (UFR) and Na+ excretion (UNaV). At the highest (190 mmHg) PP level, Cap (2, 10, 30 µM) dose-dependently decreased PP and increased GFR, UFR, UNaV, and the release of CGRP and SP. Capz or CGRP8–37 combined with Spa fully blocked the effect of Cap on PP, GFR, UFR, UNaV, and the release of CGRP and SP. In conclusion, activation of TRPV1 in the isolated kidney decreases renal PP and increases GFR and water/sodium excretion possibly via simultaneous activation of CGRP and SP receptors upon their enhanced release, suggesting that TRPV1 plays a key role in modulating renal hemodynamics and excretory function.
Keywords: transient receptor potential vanilloid type 1, glomerular filtration rate, isolated perfused kidney, capsaicin
Introduction
Transient receptor potential vanilloid type 1 (TRPV1) receptors, also known as capsaicin receptors, are nonselective cation channels mainly expressed in a subpopulation of primary sensory nerves. TRPV1 can be activated not only by capsaicin but also by a variety of physical and chemical stimuli that present challenges to homeostasis (1–3). It has been shown that TRPV1 plays an important role in regulating cardiovascular effects of capsaicin, anandamide, and other vanilloid compounds. These effects include vasodilatation in a variety of vascular beds (4,5). hypotension in anesthetized rats (6,7) and protection of the heart from ischemia-reperfusion injury (8).
The kidney, a key organ in regulating body ion and fluid homeostasis and blood pressure, is densely innervated by capsaicin-sensitive sensory nerves (9,10). Our previous studies showed that 1) TRPV1 in the kidney and mesenteric resistance arteries was upregulated by high salt intake in rats, which played a counter-regulatory role in preventing salt-induced increase in blood pressure (11), 2) Degeneration of capsaicin-sensitive sensory nerves led to impairment of renal excretory function and increases in blood pressure in the face of salt load (12,13) and 3) high salt intake impaired TRPV1 function and expression in the kidney and mesenteric arteries in Dahl salt sensitive but not Dahl salt resistant rats, which might contribute to the development of hypertension in this genetic hypertension model (14). These findings suggest that TRPV1 in the kidney may play an antihypertensive role via regulating renal function. However, no direct evidence is available which demonstrates that TRPV1 plays a role in regulating renal hemodynamics and excretory function. Given that administration of calcitonin gene-related peptide (CGRP) or substance P (SP) resulted in a marked increase in glomerular filtration rate (GFR) and diuresis (15,16) and activation of TRPV1 expressed in capsaicin-sensitive sensory nerves resulted in increased release of CGRP and substance P (4,5), we hypothesized that activation of TRPV1 in the kidney increases GFR, and sodium and water excretion. To test the hypothesis, the isolated perfused kidney was used to avoid the confounding systemic effect of TRPV1 activation.
Methods
Isolated perfused rat kidney preparation
Male Wistar rats, weight 250 to 300 g, were used for these studies approved by the Michigan State University Animal Care and Use Committee. Rats were anesthetized with inactin (80 mg/kg i.p.) plus ketamine (30 mg/kg i.m.). The right jugular vein was cannulated for intravenous infusion of 0.9% sodium chloride during surgery. Following a midline incision of abdomen, the left ureter was cannulated (PE-10) for collection of urine. The abdominal aorta and inferior vena cava were isolated 0.3–0.5 cm proximally and 1.5 cm distally to the left renal artery and vein. All visible branches from the isolated aorta and vena cava were ligated except for the left renal artery and vein. After heparinization (1000 U/kg IV), a PE-90 catheter was inserted retrogradely into the distal vena cava toward the left renal vein and a PE-90 catheter connected to the perfusion setup was inserted retrogradely into the distal aorta toward the left renal artery. The left kidney was perfused with warmed (37°C), oxygenated Krebs-Henseleit solution of the following composition (in mmol/L): NaCl 118, KCl 4.7, KH2PO4 1.19, MgSO4 1.19, CaCl2 1.9, NaHCO3 25, and glucose 5.5(17). Inulin (60 mg/dL) was added in the perfusion solution for measurement of GFR (18). Indomethacin (10 µmol/L) was added in perfusate to inhibit cyclooxygenase. Immediately afterward, the aorta and vena cava were tied off just above the left renal artery and vein, and the kidney was removed from the animal and transferred to 37°C chamber containing the Krebs-Henseleit solution. Kidneys were perfused for 5 to 8 minutes on an open circuit to remove all traces of blood before being transferred to a recirculating unit with a capacity of 110 ml. Immediately after recirculation of perfusate had been established, the perfusion flow rate was adjusted to achieve a basal perfusion pressure of 95–100 mmHg. The established perfusion flow rate was then maintained constant throughout the subsequent procedure. After 10 minutes of equilibration, the perfusion pressure was increased to 145–150 or 185–190 mmHg by addition of phenylephrine (17). The effects of capsaicin on renal function were determined at these different levels of perfusion pressure. The perfusion pressure was continuously measured with a pressure transducer and workbench software (Kent Scientific Corporation, Torrington, CT, USA).
Experiment 1. Pressure-related activation of TRPV1 by capsaicin
Activation of TRPV1 by capsaicin in the isolated kidney was performed at three levels of perfusion pressure. The basal perfusion pressure of 95–100 mmHg (n=6) was established by adjusting the perfusion flow rate, and two higher levels of perfusion pressures of 145–150 (n=6) and 185–190 (n=6) mmHg were established by addition of phenylephrine into the perfusion solution without changing the perfusion flow rate. Each kidney was used for only one perfusion pressure. Once perfusion pressures were stable, vehicle or capsaicin (10 µmol/L) was perfused. Our preliminary data showed that capsaicin induced immediate decreases in perfusion pressure and increases in the urine flow rate, which reached the peak at about 1 minute and recovered at about 5 minutes after capsaicin perfusion. Thus, the urine samples were collected for 2 minutes starting thirty seconds after capsaicin or vehicle administration when pre-existing urine in the tube was emptied. The perfusate sample was collected from the renal vein for 1 minute starting thirty seconds after capsaicin or vehicle perfusion. The perfusion pressure was continuously monitored.
Experiment 2. Dose related activation of TRPV1 by capsaicin
The concentration-dependent responses to different doses of capsaicin were examined at the perfusion pressure of 185–190 mmHg established as above. Once perfusion pressures were stable, vehicle or capsaicin was perfused. The urine samples were collected for 2 minutes while the perfusate samples collected from the renal vein for 1 minute starting thirty seconds after capsaicin or vehicle administration. Capsaicin was added into the perfusate at three concentrations: 2 (n=5), 10 (n=6) and 30 (n=5) µmol/L. Each kidney was perfused with only one concentration of capsaicin. The perfusion pressure was continuously monitored.
Experiment 3: Blockade of TRPV1 with capsazepine
The effects of TRPV1 blockade by capsazepine were examined at perfusion pressure of 185–190 mmHg established as above. Once perfusion pressures were stable, vehicle, capsazepine (Calbiochem, San Diego, CA), or combination of capsazepine and capsaicin was perfused. The urine samples were collected for 2 minutes while the perfusate samples collected from the renal vein for 1 minute starting thirty seconds after vehicle, capsazepine, or combination of capsazepine and capsaicin administration. Capsazepine, a selective antagonist of TRPV1, was added (30 µmol/L) alone (n=5) or in combination with capsaicin (10 µmol/L) (n=5) that was added 2 minutes after capsazepine administration. Preliminary experiments showed that capsazepine (30 µmol/L) caused a slight increase in perfusion pressure, which returned to the baseline by the end of 2 minutes. The perfusion pressure was continuously monitored.
Experiment 4: Blockade of the CGRP receptor with CGRP8–37
Blockade of the CGRP receptor with CGRP8–37 was performed at the perfusion pressure of 185–190 mmHg established as above. Once perfusion pressures were stable, vehicle, CGRP8–37 (Sigma), or combination of CGRP8–37 and capsaicin was perfused. The urine samples were collected for 2 minutes while the perfusate samples collected from the renal vein for 1 minute starting thirty seconds after perfusion of vehicles, CGRP8–37, or CGRP8–37 plus capsaicin. CGRP8–37, a selective CGRP receptor antagonist, was added to the perfusate (0.01, 0.1, 0.5 µmol/L, n=5) alone or in combination with capsaicin (10 µmol/L, n=5) that was added thirty seconds after CGRP8–37 administration. The perfusion pressure was continuously monitored.
Experiment 5: Blockade of the substance P receptor with spantide II
Blockade of the substance P receptor with spantide II was performed at the perfusion pressure of 185–190 mmHg established as above. Once perfusion pressures were stable, vehicle, spantide II (Phoenix Pharmaceuticals, Inc., Belmont, CA), or combination of spantide II and capsaicin was perfused. The urine samples were collected for 2 minutes while the perfusate samples collected from the renal vein for 1 minute starting thirty seconds after perfusion of vehicles, spantide II, or spantide II plus capsaicin. Spantide II, a selective antagonist of substance P receptor, was added to the perfusate (0.01, 0.1, 0.5 µmol/L) alone or in combination with capsaicin (10 µmol/L) that was added thirty seconds after Spantide II administration. The perfusion pressure was continuously monitored.
Experiment 6: Simultaneous blockade of the CGRP and substance P receptors
Blockade of both CGRP and substance P receptors was performed at the perfusion pressure of 185–190 mmHg established as above. Once perfusion pressures were stable, vehicle or CGRP8–37 and spantide II combined with capsaicin was perfused. The urine samples were collected for 2 minutes while the perfusate samples collected from the renal vein for 1 minute starting thirty seconds after perfusion of vehicles or CGRP8–37 and spantide II combined with capsaicin. CGRP8–37 and spantide II (0.1 µmol/L for each) were simultaneously added to perfusate thirty seconds before capsaicin administration (10 µmol/L, n=5). The perfusion pressure was continuously monitored.
Measurement of sodium concentrations in urine and perfusate
Urine and perfusate samples were centrifuged at 5,500rpm for 5 minutes. Fifty µL of urine or perfusate were diluted with deionized water (1:4) and the sodium concentrations were determined by the use of a flame photometer (IL943, Instrumentation Laboratories, Lexington, MA, USA).
Colorimetric assay of inulin
Inulin was determined in perfusate and urine samples by colorimetric analysis using anthrone complexation as described previously (18). Briefly, 30 µL of perfusate samples were added to 30 µL of glucose oxidase (2U/µL) in 1.5 mL micro-centrifuge tubes (for urine analysis, 10µL of samples were added to 10µL of glucose oxidase). The tubes were vortexed for 5 seconds and placed in a shaker water bath at 37°C for 15 minutes. The tubes were then cooled to ambient temperature (25°C) and the sample volume adjusted to 250µL with deionized water. A total of 250µL of 1% (w/v) aqueous zinc sulfate solution was then added, and the mixture vortexed for 15 seconds. The tubes were then centrifuged for 10 min at 9000x g. Aliquots of 350µL were then transferred to 15-mL test tubes in an ice bath. Four mL of ice-cold anthrone reagent were added and vortex-mixed for 30 seconds. The tubes were then incubated in a shaker water bath at 57°C for 10 minutes. During this period, samples exhibited differences in color (green) as a function of inulin concentrations. The test tubes were then placed in ice-cold water for 5 minutes to stop the reaction. Within 40 minutes after stopping the reaction, samples were analyzed by an UV/Vis spectrophotometer at a wavelength of 620 nm.
Radioimmunoassay of CGRP and SP
CGRP and SP in perfusate were measured by using a rabbit-anti-rat CGRP or SP radioimmunoassay kits (Peninsula Laboratories Inc, San Carlos, CA, USA), respectively. The assay was performed as recommended by the supplier.
Calculation
All values were normalized by the wet weight of the right kidney. Renal clearance (C) was calculated by the standard formulae: C = U V/P Where U is the urine concentration, V is the urine flow rate, P is the plasma concentration.
Statistical analysis
Statistical comparisons were performed by one-way analysis of variance followed by the Tukey-Kramer multiple comparison tests and t-tests. All results were expressed as means ± SE. Differences were considered statistical significant at p<0.05.
Results
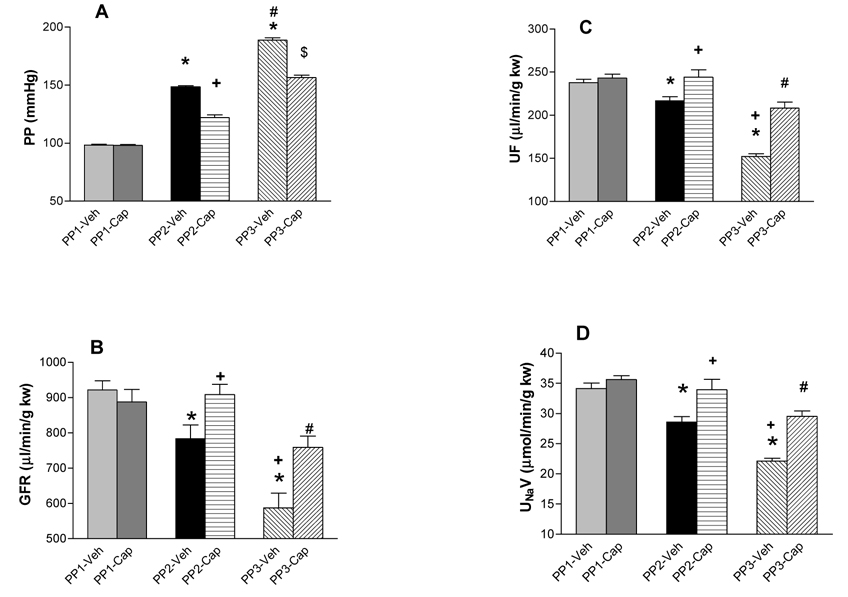
GFR, urine flow (UF), and urine sodium excretion (UNaV) were proportionally decreased following the increases of perfusion pressure induced by phenylephrine in the isolated kidney. Capsaicin did not change perfusion pressure (PP), GFR, UF, and UNaV when the kidney was perfused at the basal PP level of 95–100 mmHg compared to vehicle. In contrast, when PP was increased by phenylephrine to 145–150 mmHg and 185–190 mmHg, capsaicin caused significant decreases in PP (at ~150 mmHg: vehicle, 149 ± 1 vs Cap, 122 ± 3 mmHg; at ~190 mmHg: vehicle, 189 ± 2 vs Cap, 156 ± 2 mmHg, p<0.001), and increases in GFR [at ~150 mmHg: vehicle, 784 ± 39 vs Cap, 909 ± 30 µl/min/g kidney weight (kw); at ~190 mmHg: vehicle, 587 ± 43 vs Cap, 759 ± 32 µl/min/g kw, p<0.05], UF [at ~150 mmHg: vehicle, 217 ± 5 vs Cap, 244 ± 8 µl/min/g kw; at ~190 mmHg: vehicle, 152 ± 3 vs Cap, 208 ± 7 µl/min/g kw, p<0.01], and UNaV (at ~150 mmHg: vehicle, 28.6 ± 0.9 vs Cap, 34.0 ± 1.7 µmol/min/g kw; at ~190 mmHg: vehicle, 22.1 ± 0.5 vs Cap, 29.6 ± 0.9 µmol/min/g kw, p<0.05) (Figure 1). The decrease in PP induced by capsaicin represented the decrease in renal vascular resistance because the perfusion flow rate was constant throughout the experiment, and the results showed that capsaicin was more effective at higher PP indicating that elevated PP sensitizes TRPV1.
Figure 1. Pressure-related effects of capsaicin.
The effects of capsaicin (Cap, 10µM), a selective agonist of transient receptor potential vanilloid type 1 (TRPV1), on renal perfusion pressure (PP), glomerular filtration rate (GFR), urine flow (UF), and sodium excretion (UNaV) in the isolated kidney perfused at different perfusion pressure. Veh means vehicle. PP1 = 95–100 mmHg, PP2 = 145–150 mmHg, PP3 = 185-190 mmHg. N = 6. In panel A: *p<0.05 vs PP1-Veh; +p<0.05 vs PP1-Cap and PP2-Veh; #p<0.05 vs PP2-Veh; $p<0.05 vs PP1-Cap, PP2-Cap and PP3-Veh. In panel B, C and D: * p<0.01 vs PP1-Veh; +p<0.05 vs PP2-Veh; #p<0.05 vs PP1-Cap, PP2-Cap and PP3-Veh.
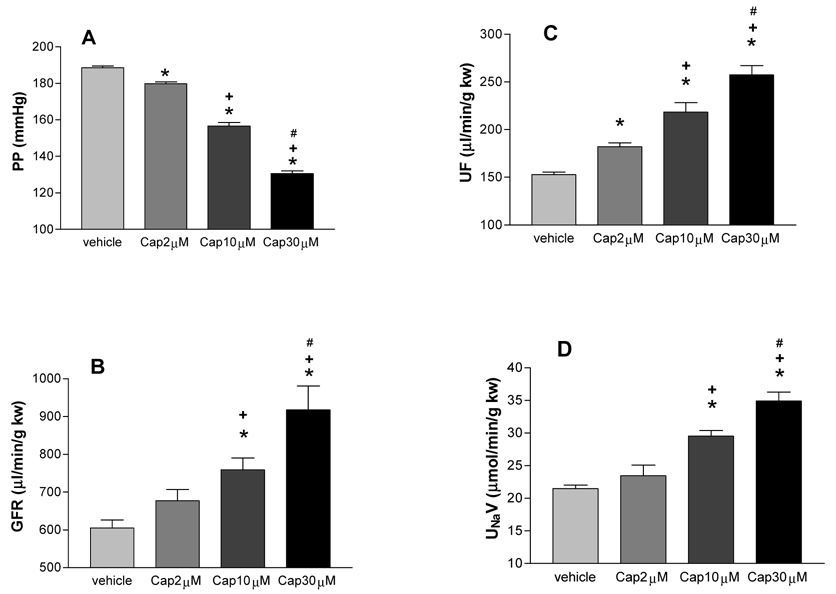
At the PP of 185–190 mmHg, perfusion of capsaicin (2, 10, 30 µmol/L) dose-dependently decreased PP (vehicle: 189 ± 1, Cap2 µmol/L: 179 ± 1, Cap10 µmol/L: 157 ± 2, Cap30 µmol/L: 131 ± 2 mmHg, p<0.001) and increased GFR (605 ± 21, 677 ± 30, 759 ± 32, 918 ± 63 µl/min/g kw, respectively, p<0.05), UF (153 ± 3, 182 ± 4, 218 ± 10, 257 ± 10 µl/min/g kw, respectively, p<0.01), and UNaV (21.5 ± 0.6, 23.5 ± 1.6, 29.6 ± 0.9, 34.9 ± 1.4 µmol/min/g kw, respectively, p<0.05) compared to vehicle (Figure 2).
Figure 2. Dose-related effects of capsaicin.
The effects of capsaicin (Cap, 2, 10, 30 µM), a selective agonist of transient receptor potential vanilloid type 1 (TRPV1) on renal perfusion pressure (PP), glomerular filtration rate (GFR), urine flow (UF), and urine sodium excretion (UNaV) in the isolated kidney perfused at a perfusion pressure of 185–190 mmHg. N=5–6. In panel A: * p<0.001 vs vehicle, +p<0.001 vs Cap2µM, #p<0.001 vs Cap10µM. In panel B and D: * p<0.01 vs vehicle, +p<0.05 vs Cap2µM, #p<0.05 vs Cap10µM. In panel C: * p<0.01 vs vehicle, +p<0.01 vs Cap2µM, #p<0.01 vs Cap10µM.
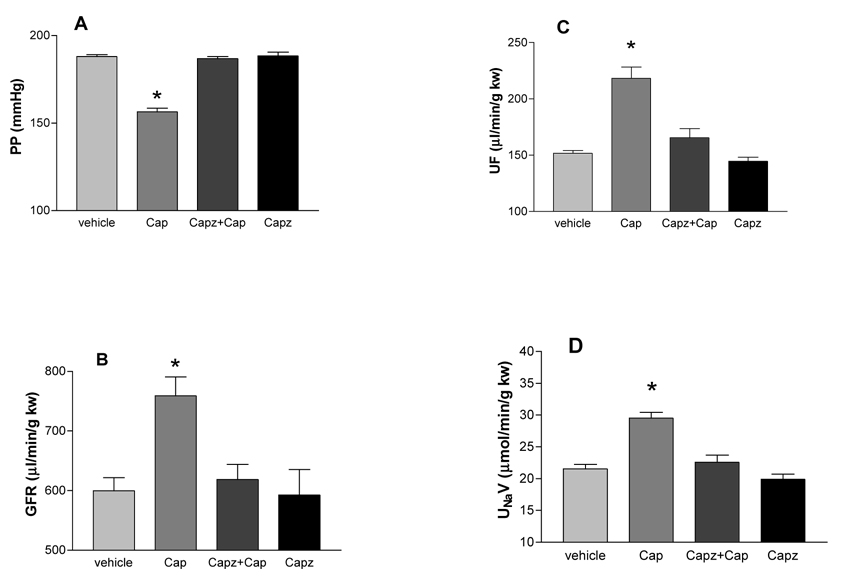
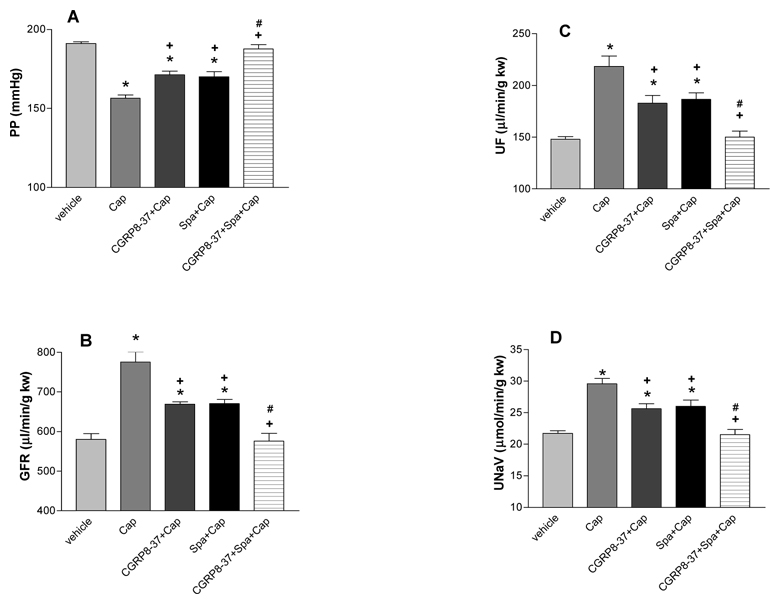
Capsazepine (Capz, 30 µmol/L) fully blocked the effects of capsaicin (Cap, 10 µmol/L) on PP, GFR, UF, and UNaV (Figure 3). CGRP8–37 (0.1 µmol/L) or Spa (0.1 µmol/L) alone attenuated the effects of Cap (10 µmol/L) on PP (39 or 36%), GFR (41 or 33%), UF (53 or 35%), and UNaV (49 or 46%), respectively. Combination of CGRP8–37 (0.1 µmol/L) and Spa (0.1 µmol/L) fully inhibited the effects of Cap (10 µmol/L) on PP, GFR, UF, and UNaV (Figure 4). Lower and higher concentrations of CGRP8–37 (0.01 and 0.5 µmol/L) and Spa (0.01 and 0.5 µmol/L) were also used. CGRP8–37 and Spa at 0.01 µmol/L showed weaker effects than 0.1 µmol/L of CGRP8–37 and Spa. CGRP8–37 and Spa at 0.5 µmol/L showed the similar effects as that of 0.1 µmol/L CGRP8–37 and Spa (data not shown), suggesting that 0.1 µmol/L was a saturated dose for blockade of the effects of Cap at 10 µmol/L. Perfusion of CGRP8–37 or Spa alone had no significant effect on PP, GFR, UF, and UNaV (data not shown).
Figure 3. Effects of capsazepine.
The blockade effects of capsazepine (Capz, 30 µM), a selective antagonist of transient receptor potential vanilloid type 1 (TRPV1) on capsaicin (Cap, 10 µM)-induced changes in renal perfusion pressure (PP), glomerular filtration rate (GFR), urine flow (UF), and sodium excretion (UNaV) in the isolated kidney perfused at a perfusion pressure of 185–190 mmHg. N=5–6. In panel A, C and D: * p<0.001 vs vehicle, Capz+Cap and Capz. In panel B: * p<0.05 vs vehicle, Capz+Cap and Capz.
Figure 4. Effects of CGRP8–37 and spantide II.
The inhibitory effects of CGRP8–37 (0.1 µM), a selective antagonist of calcitonin gene-related peptide (CGRP) receptor, spantide II (Spa, 0.1 µM), a selective antagonist of neurokinin-1 (NK1) receptor on capsaicin (Cap, 10 µM)–induced changes in renal perfusion pressure (PP), glomerular filtration rate (GFR), urine flow (UF), and sodium excretion (UNaV) in the isolated kidney perfused at a perfusion pressure of 185–190 mmHg. N=5–6. In panel A and C: * p<0.01 vs Con, + p<0.01 vs Cap, # p<0.01 vs CGRP8–37+Cap and Spa+Cap. In panel B and D: * p<0.05 vs Con, + p<0.05 vs Cap, # p<0.05 vs CGRP8–37+Cap and Spa+Cap
Perfusion of Cap (2, 10, 30 µmol/L) dose-dependently increased the release of CGRP (vehicle: 4.3 ± 0.1, Cap2 µmol/L: 6.3 ± 0.2, Cap10 µmol/L: 8.8 ± 0.4 and Cap30 µmol/L: 12.4 ± 1.3 pg/min/g kw, p<0.01) and SP (2.2 ± 0.1, 2.7 ± 0.2, 3.3 ± 0.2 and 4.0 ± 0.2 pg/min/g kw, respectively, p<0.01) compared to vehicle. Capz (30 µmol/L) blocked the Cap (10 µmol/L)-induced increases in CGRP (4.4 ± 0.2 pg/min/g kw) and SP (2.2 ± 0.1 pg/min/g kw) release in the isolated perfused kidney (Figure 5).
Figure 5. Dose-related effects of capsaicin on CGRP and SP release.
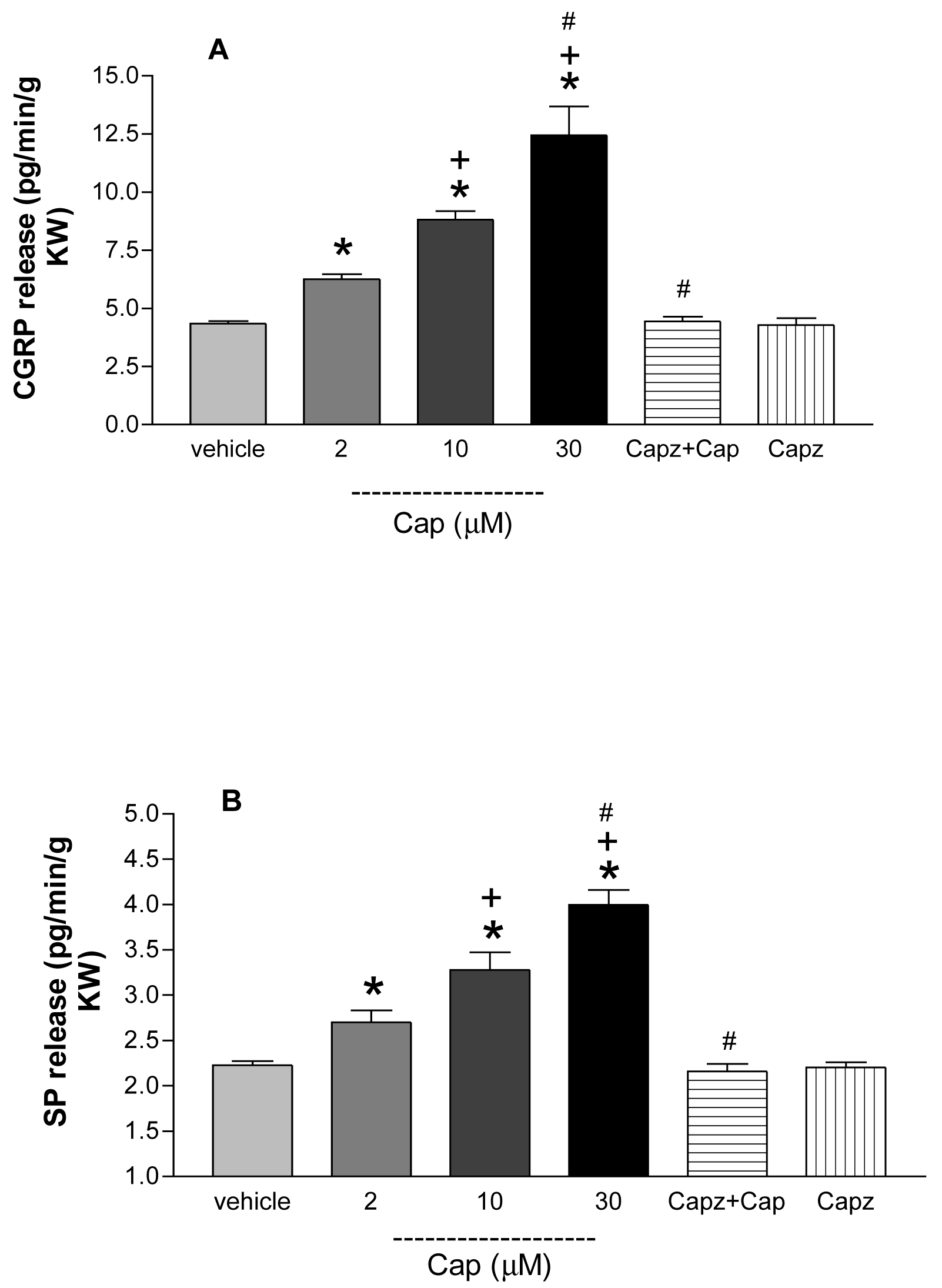
Capsaicin (Cap, 2, 10, 30µM) in the presence or absence of capsazepine (Capz, 30 µM) on calcitonin gene-related peptide (CGRP) and substance P (SP) release in the isolated perfused kidney. N=5–6. In panel A: * p<0.01 vs vehicle, +p<0.01 vs Cap2µM, #p<0.001 vs Cap10 µM. In panel B: * p<0.01 vs vehicle, +p<0.05 vs Cap2µM, #p<0.01 vs Cap10 µM.
Discussion
We have previously shown that 1) TRPV1 expression in the kidney was upregulated by high salt intake in rats (11), 2) degeneration of capsaicin-sensitive sensory nerves led to impairment of renal excretory function and increases in blood pressure in the face of salt load (12,13), and 3) high salt intake impaired TRPV1 function and expression in the kidney and mesenteric arteries in Dahl salt sensitive hypertensive rats but not Dahl salt resistant rats (14). These results suggest that TRPV1 in the kidney may regulate renal function. The present study was designed to determine whether and how TRPV1 in the kidney might play a direct role in regulation of renal function. Our data show that (1) capsaicin, a selective TRPV1 agonist, caused pressure-dependent decreases in perfusion pressure and increases in GFR and sodium/water excretion in the isolated kidney; (2) at a higher perfusion pressure, capsaicin caused concentration-dependent decreases in perfusion pressure and increases in GFR, sodium/water excretion, and release of CGRP and SP in the isolated kidney; (3) the effects of capsaicin were blocked by capsaizepine, a selective antagonist of TRPV1, or by simultaneous administration of CGRP and SP receptor antagonists. The findings indicate that activation of TRPV1 in the kidney induces renal vasodilation and increases GFR leading to increased sodium/water excretion when renal vascular resistance is increased, suggesting a compensatory protective role of TRPV1 against the increase in renal vascular resistance. The effect of TRPV1 is likely mediated by increased release of CGRP and SP from renal capsaicin-sensitive sensory nerves. The results provide direct evidence that TRPV1 in the kidney plays an important role in the regulation of renal excretory function through regulating GFR. Although capsaicin had no effect at lower perfusion pressure, it could not rule out the possibility that TRPV1 might be activated by endogenous TRPV1 agonists that maintain an endovanilloid tone. Ascertaining of this would required further future studies of TRPV1 activity as well as endovanilloid production/release at the lower and higher prefusion pressures as well as pathophysiological conditions such as salt loading.
The fact that activation of TRPV1 decreases perfusion pressure but increases GFR while perfusion flow is constant indicates that activation of TRPV1 causes a greater vasodilation in afferent than efferent arterioles, leading to an increase in GFR despite a fall in renal perfusion pressure. The heterogeneous effects on renal microvasculature induced by TRPV1 activation may play an important role in regulation of sodium and water excretion under pathophysiological conditions. It is known that TRPV1 can be activated by a variety of physical and chemical stimuli that present challenges to homeostasis (1–3). Thus, TRPV1 may function as a molecular sensor to detect alterations in renal micro-environment and respond with modification of GFR via its heterogeneous effects on renal microvasculature. If so, altered TRPV1 expression/activity in the kidney could impact sodium and water excretion and blood pressure. Indeed, our previous studies showed that TRPV1 expression in the kidney was upregulated by high salt intake, which may contribute to the prevention of salt-induced increases in blood pressure (11). In contrast, high salt intake downregulated TRPV1 expression in the kidney of Dahl salt sensitive but not resistant rats, a change that may contribute to the development of hypertension in this genetic model (14). Taken together, our data indicate that altered TRPV1 expression/activity may affect sodium and water excretion and blood pressure via alteration of GFR.
In addition to altered TRPV1 expression, modification of expression or production of endovanilloids in the kidney may affect sodium and water excretion via TRPV1-mediated regulation of GFR. This notion was supported by a previous study showing that anandamide, an endogenous TRPV1 as well as cannabinoid CB1 receptor agonist, was present in the kidney where it exerted significant vasorelaxant and neuromodulatory effects (19, 20). The possibility that endovanilloids and TRPV1 receptors play important pathophysiological roles in the regulation of renal function and blood pressure opens the way and justifies for the future development of synthetic compounds targeting either TRPV1 receptors or the enzymes responsible for endovanilloid biosynthesis and degradation as potential new therapeutic drugs against renal function deterioration and hypertension (20).
To determine the mechanisms underling TRPV1-mediated renal vasodilation and increases in GFR and sodium/water excretion, selective antagonists of CGRP and SP receptors were used in combination with capsaicin. We found that the decrease in perfusion pressure and the increase in GFR and sodium/water excretion induced by capsaicin were partially inhibited either by blockade of the CGRP receptor or the SP receptor, but were abolished by simultaneous blockade of both the CGRP and SP receptors. These results indicate that TRPV1-mediated renal heterogeneous vasodilation and the increase in GFR were the result of activation of the CGRP and SP receptors. These results are consistent with previous studies showing that CGRP-immunoreactive nerve fibers were observed surrounding renal blood vessels and that infusion of either capsaicin or CGRP reduced increases in perfusion pressure induced by norepinephrine in isolated perfused rat kidney (21, 22). Furthermore, the present results are consistent with the previous in vivo studies showing that CGRP and SP exerted a preferential endothelium-dependent vasodilation effect on afferent arterioles and thereby led to an increase in GFR (15, 23–25).
Activation of TRPV1 expressed in a subpopulation of primary sensory nerves induces vasodilation in a variety of vascular beds, possibly via release of CGRP and SP from the sensory nerves (4,5). Although TRPV1 expression has recently been detected in cerebromicrovascular and mesenteric arterial endothelial cells in culture (26, 27), the finding from the present study supports an exclusive neurogenic mechanism, i.e., TRPV1 activation leads to CGRP and SP release from TRPV1-positive sensory nerve endings, which subsequently activate their respective receptors in the kidney to dilate blood vessels and increases GFR.
It is possible that glomerular filteration coefficiency, another important determinant of GFR, was increased by TRPV1 activation when considering increased GFR in the face of decreased perfusion pressure. CGRP immunoreactive nerve fibers have been found in the proximity of renal glomeruli (9), and CGRP causes a cAMP mediated relaxation of renal mesangial cells and an increase in the glomerular filtration fraction (28). Thus, CGRP released from renal TRPV1-positive sensory nerve endings could increase glomerular filtration coefficiency by relaxing mesangial cells, which may contribute to the increase in GFR induced by TRPV1 activation.
In conclusion, our data show that activation of TRPV1 in the isolated perfused kidney decreases perfusion pressure and increases GFR and sodium/water excretion when renal vascular resistance is increased, possibly via increasing release of CGRP and SP from renal TRPV1-positive sensory nerves. These results suggest that TRPV1 expressed in renal sensory nerves plays a key role in modulating renal hemodynamics and excretory function.
Perspective
Given that activation of TRPV1 increases GFR and sodium/water excretion in the present study and lowers blood pressure as shown in the previous studies (6,7,29), it is conceivable that activation of TRPV1 or modulation of TRPV1 expression/activity in the kidney may represent new pharmacological strategies for overcoming renal function deterioration in conditions associated with increased glomerular arteriolar resistance and reduced GFR and for treatment of hypertension. Studies of abnormalities in TRPV1 expression, TRPV1-mediated release of sensory neuropeptides, and TRPV1 signaling pathways in the kidney may improve our understanding of the pathogenesis of hypertension.
Acknowledgments
This study was supported in part by National Institutes of Health (grants HL-57853, HL-73287, and DK67620) and a grant from The Michigan Economic Development Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–542. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Julius D. The vanilloid receptor: a molecular gate-way to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 4.Ralevic V, Kendall DA, Randall MD, Zygmunt PM, Movahed P, Hogestatt ED. Vanilloid receptors on capsaicin-sensitive sensory nerves mediate relaxation to methanadamide in the rat isolated mesenteric arterial bed and small mesenteric arteries. Br J Pharmacol. 2000;130:1483–1488. doi: 10.1038/sj.bjp.0703456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 6.Malinowska B, Kwolek G, Gothert M. Anandamide and methanandamide induce both vanilloid VR1-and cannabinoid CB1 receptor-mediated changes in heart rate and blood pressure in anaesthetized rats. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:562–569. doi: 10.1007/s00210-001-0498-6. [DOI] [PubMed] [Google Scholar]
- 7.Smith PJ, McQueen DS. Anandamide induces cardiovascular and respiratory reflexes via vasosensory nerves in the anaesthetized rat. Br J Pharmacol. 2001;134:655–663. doi: 10.1038/sj.bjp.0704296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Wang DH. TRPV1 gene konckout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005;112:3617–3623. doi: 10.1161/CIRCULATIONAHA.105.556274. [DOI] [PubMed] [Google Scholar]
- 9.Kurtz A, Muff R, Born W, Lundberg JM, Millberg BI, Gnadinger M, Uehlinger D, Weidmann P, Hokfelt T, Fischer JA. Calcitonin gene-related peptide is a stimulator of renin secretion. J Clin Invest. 1988;82:538–543. doi: 10.1172/JCI113629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight DS, Beal JA, Yuan ZP, Fournet TS. Substance P-immunoreactive nerves in the rat kidney. J Auton Nerv Syst. 1987;21(2–3):145–155. doi: 10.1016/0165-1838(87)90017-8. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Wang DH. Function and regulation of the vanilloid receptor in rats fed a high salt diet. J Hypertens. 2003;21(8):1525–1530. doi: 10.1097/00004872-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Wang DH, Li J, Qiu J. Salt-sensitive hypertension induced by sensory denervation: introduction of a new model. Hypertension. 1998;32:649–653. doi: 10.1161/01.hyp.32.4.649. [DOI] [PubMed] [Google Scholar]
- 13.Wang DH, Zhao Y. Increased salt sensitivity induced by impairment of sensory nerves: is the nephropathy the cause? J Hypertens. 2003;21(2):403–409. doi: 10.1097/00004872-200302000-00033. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: Role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47(part 2):609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- 15.Amuchastegui CS, Remuzzi G, Perico N. Calcitonin gene-related peptide reduces renal vascular resistance and modulates ET-1-induced vasoconstriction. AM J Physiol. 1994;267(5 Pt 2):F839–F844. doi: 10.1152/ajprenal.1994.267.5.F839. [DOI] [PubMed] [Google Scholar]
- 16.DeFelice AF, Brousseau A. Natriuretic and vasodilating activities of intrarenally administered atriopeptin II, substance P and bradykinin in the dog. J Pharmacol Exp Ther. 1988;246(1):183–188. [PubMed] [Google Scholar]
- 17.Pomposiello SI, Quilley J, Carroll MA, Falck JR, McGiff JC. 5,6-Epoxyeicosatrienoic Acid Mediates the Enhanced Renal Vasodilation to Arachidonic Acid in the SHR. Hypertension. 2003;42:548–554. doi: 10.1161/01.HYP.0000090095.87899.36. [DOI] [PubMed] [Google Scholar]
- 18.Poola NR, Bhuiyan D, Ortiz S, Savant IA, Sidhom M, Taft DR. A novel HPLC assay for pentamidine: comparative effects of creatinine and inulin on GFR estimation and pentamidine renal excretion in the isolated perfused rat kidney. J Pharm Pharmaceut Sci. 2002;5(2):135–145. [PubMed] [Google Scholar]
- 19.Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HHO, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starowicz K, Nigam S, Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol Ther. 2007;114(1):13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Geppetti P, Baldi E, Castelucci A, DelBianco E, Santicioli P, Maggi CA, Lippe IT, Amann R, Skofitsch G, Theodorsson E. Calcitonin gene-related peptide in the rat kidney: occurrence, sensitivity to capsaicin, and stimulation to adenylate cyclase. Neuroscience. 1989;30(2):503–513. doi: 10.1016/0306-4522(89)90268-6. [DOI] [PubMed] [Google Scholar]
- 22.Geppetti P, Baldi E, Manzini S, Del Bianco E, Maggi CA, Natali A, Mannelli M. Regional differences of adenylate cyclase stimulation by calcitonin and calcitonin gene-related peptide in the human kidney. J Clin Endocrinol Metab. 1989;69(3):491–495. doi: 10.1210/jcem-69-3-491. [DOI] [PubMed] [Google Scholar]
- 23.Cairns HS, Rogerson ME, Westwick J, Neild GH. Regional heterogeneity of endothelium-dependent vasodilatation in the rabbit kidney. J Physiol. 1991;436:421–429. doi: 10.1113/jphysiol.1991.sp018558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seikaly MG, Eisner GM, Jose PA. Contrasting effect of substance P on renal function and dopamine excretion in hydropaenic and volume expanded dogs. J Auton Pharmacol. 1992;12(5):377–387. doi: 10.1111/j.1474-8673.1992.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 25.Elhawary AM, Pang CC. Renal vascular and tubular actions of calcitonin gene-related peptide: effect of NG-nitro-L-arginine methyl ester. J Pharmacol Exp Ther. 1995;273(1):56–63. [PubMed] [Google Scholar]
- 26.Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, Shohami E, Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Brain Res Mol Brain Res. 2004;132(1):87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 27.Poblete IM, Orliac ML, Briones R, Adler-Graschinsky E, Huidobro-Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol. 2005;568(Pt 2):539–551. doi: 10.1113/jphysiol.2005.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtz A, Schurek HJ, Jelkmann W, Muff R, Lipp HP, Heckmann U, Eckardt KU, Scholz H, Fischer JA, Bauer C. Renal mesangium is a target for calcitonin gene-related peptide. Kidney Int. 1989;36(2):222–227. doi: 10.1038/ki.1989.183. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Kaminski NE, Wang DH. Anandamide-induced depressor effect in spontaneously hypertensive rats: role of the vanilloid receptor. Hypertension. 2003;41(3 pt2):757–762. doi: 10.1161/01.HYP.0000051641.58674.F7. [DOI] [PubMed] [Google Scholar]