Abstract
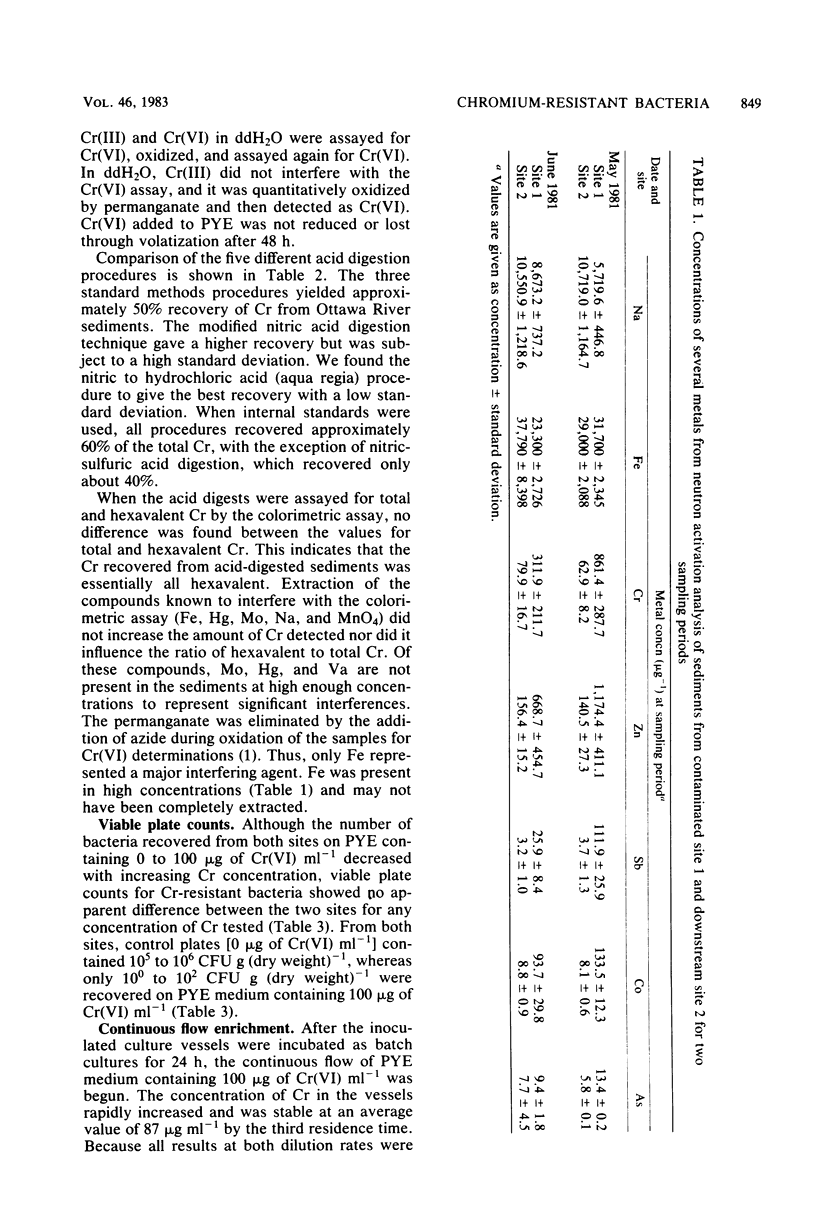
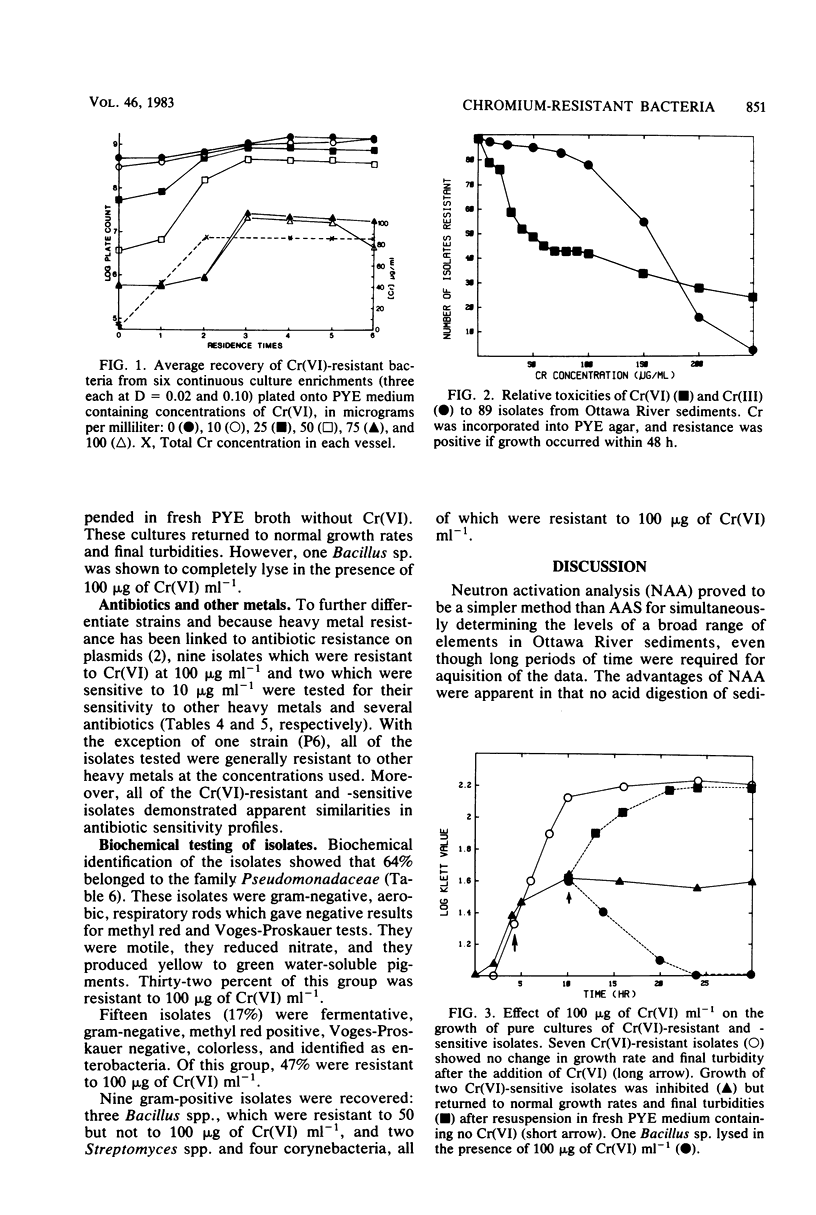
Hexavalent chromium [Cr(VI)] is a known carcinogen and mutagen; however, the actual mechanisms of Cr toxicity are unknown. Two approaches were used to isolate Cr(VI)-resistant bacteria from metal-contaminated river sediments. Diluted sediments were plated directly onto a peptone-yeast extract (PYE) medium containing 0 to 100 micrograms of Cr(VI) ml-1. Approximately 8.4 x 10(5) CFU g-1 were recovered on 0 microgram of Cr(VI) ml-1, whereas 4.0 x 10(2) CFU g-1 were recovered on PYE plus 100 micrograms of Cr(VI) ml-1. Alternatively, continuous culture enrichment techniques were employed using PYE and 100 micrograms Cr(VI) ml-1 input at dilution rates of 0.02 and 0.10 h-1. After six residence periods, 10(9) CFU were recovered on PYE agar containing 0 microgram of Cr(VI) ml-1 and 10(7) CFU on PYE agar plus 100 micrograms of Cr(VI) ml-1. Of 89 isolates obtained by direct plating onto PYE, 47% were resistant to 100 micrograms of Cr(VI) ml-1, and 29% were resistant to 250 micrograms of Cr(VI) ml-1. When the same isolates were plated onto PYE containing Cr(III), 88% were resistant to 100 micrograms ml-1 but only 2% were resistant to 250 micrograms ml-1. Cr, Co, Sb, and Zn were found in significantly higher concentrations at an industry-related contaminated site than at a site 11 km downstream. Total Cr in the sediments at the contaminated site averaged 586 micrograms (dry weight) g-1, and the downstream site averaged 71 micrograms (dry weight) g-1. The Cr recovered from acid-digested Ottawa River sediment samples was predominantly hexavalent. Five acid digestion procedures followed by atomic absorption spectroscopy were compared and found to be 30 to 70% efficient for recovery of Cr relative to neutron activation analysis. A population of aerobic, heterotrophic bacteria was recovered from sediments containing elevated levels of Cr.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. A., Austin B., Colwell R. R. Antibiotic resistance patterns of metal-tolerant bacteria isolated from an estuary. Antimicrob Agents Chemother. 1977 Oct;12(4):545–547. doi: 10.1128/aac.12.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINTON H. P., FRASIER E. S., KOVEN A. L. Morbidity and mortality experience among chromate workers. Public Health Rep. 1952 Sep;67(9):835–847. [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. D., McKay L. L. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J Bacteriol. 1977 Apr;130(1):257–265. doi: 10.1128/jb.130.1.257-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale T. F. Embryotoxic effects of chromium trioxide in hamsters. Environ Res. 1978 Jul;16(1-3):101–109. doi: 10.1016/0013-9351(78)90147-0. [DOI] [PubMed] [Google Scholar]
- HUFF J. W., SASTRY K. S., GORDON M. P., WACKER W. E. THE ACTION OF METAL IONS ON TOBACCO MOSAIC VIRUS RIBONUCLEIC ACID. Biochemistry. 1964 Apr;3:501–506. doi: 10.1021/bi00892a006. [DOI] [PubMed] [Google Scholar]
- Mertz W. Chromium occurrence and function in biological systems. Physiol Rev. 1969 Apr;49(2):163–239. doi: 10.1152/physrev.1969.49.2.163. [DOI] [PubMed] [Google Scholar]
- Nishioka H. Mutagenic activities of metal compounds in bacteria. Mutat Res. 1975 Jun;31(3):185–189. doi: 10.1016/0165-1161(75)90088-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Roth C. Plasmid-linked resistance to inorganic salts in Staphylococcus aureus. J Bacteriol. 1968 Apr;95(4):1335–1342. doi: 10.1128/jb.95.4.1335-1342.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli F. L., De Flora S. Metabolic deactivation of hexavalent chromium mutagenicity. Mutat Res. 1978 Oct;54(2):139–147. doi: 10.1016/0165-1161(78)90034-1. [DOI] [PubMed] [Google Scholar]
- Petrilli F. L., De Flora S. Toxicity and mutagenicity of hexavalent chromium on Salmonella typhimurium. Appl Environ Microbiol. 1977 Apr;33(4):805–809. doi: 10.1128/aem.33.4.805-809.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli F. L., de Flora S. Oxidation of inactive trivalent chromium to the mutagenic hexavalent form. Mutat Res. 1978 Nov;58(2-3):167–173. doi: 10.1016/0165-1218(78)90006-x. [DOI] [PubMed] [Google Scholar]
- Rades-Rohkohl E., Hirsch P., Fränzle O. Neutron activation analysis for the demonstration of amphibolite rock-weathering activity of a yeast. Appl Environ Microbiol. 1979 Dec;38(6):1061–1068. doi: 10.1128/aem.38.6.1061-1068.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe F. J., Carter R. L. Chromium carcinogenesis: calcium chromate as a potent carcinogen for the subcutaneous tissues of the rat. Br J Cancer. 1969 Mar;23(1):172–176. doi: 10.1038/bjc.1969.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Jacoby G. A. Plasmid-determined resistance to boron and chromium compounds in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1978 Apr;13(4):637–640. doi: 10.1128/aac.13.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers R. J., Boudreaux D. P., Srinivasan V. R. Continuous Cultivation for Apparent Optimization of Defined Media for Cellulomonas sp. and Bacillus cereus. Appl Environ Microbiol. 1979 Jul;38(1):66–71. doi: 10.1128/aem.38.1.66-71.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venitt S., Levy L. S. Mutagenicity of chromates in bacteria and its relevance to chromate carcinogenesis. Nature. 1974 Aug 9;250(5466):493–495. doi: 10.1038/250493a0. [DOI] [PubMed] [Google Scholar]



