The Atg12–Atg5 conjugate was prepared by in vivo reconstitution and was crystallized with Atg16 using the free-interface diffusion method.
Keywords: autophagy, ubiquitin-like conjugation, crystallization, free-interface diffusion method
Abstract
Autophagy mediates the bulk degradation of cytoplasmic components in lysosomes/vacuoles. Five autophagy-related (Atg) proteins are involved in a ubiquitin-like protein conjugation system. Atg12 is conjugated to its sole target, Atg5, by two enzymes, Atg7 and Atg10. The Atg12–Atg5 conjugates form a multimeric complex with Atg16. Formation of the Atg12–Atg5–Atg16 ternary complex is crucial for the functions of these proteins on autophagy. Here, the expression, purification and crystallization of the Atg12–Atg5 conjugate bound to the N-terminal region of Atg16 (Atg16N) are reported. The Atg12–Atg5 conjugates were formed by co-expressing Atg5, Atg7, Atg10 and Atg12 in Eschericia coli. The Atg12–Atg5–Atg16N ternary complex was formed by mixing purified Atg12–Atg5 conjugates and Atg16N, and was further purified by gel-filtration chromatography. Crystallization screening was performed by the free-interface diffusion method. Using obtained microcrystals as seeds, large crystals for diffraction data collection were obtained by the sitting-drop vapour-diffusion method. The crystal contained one ternary complex per asymmetric unit, and diffracted to 2.6 Å resolution.
1. Introduction
Autophagy is a starvation-induced response that mediates the bulk degradation of cytoplasmic components in lysosomes/vacuoles (Seglen & Bohley, 1992 ▶; Takeshige et al., 1992 ▶) and plays a critical role in numerous biological processes (Mizushima, 2005 ▶). In autophagy, a double-membrane structure called an autophagosome sequesters a portion of cytoplasm and fuses with the lysosome/vacuole to deliver its contents into the organelle lumen.
Eighteen autophagy genes involved in autophagosome formation, named ATG genes, have been identified using genetic approaches in Saccharomyces cerevisiae (Klionsky et al., 2003 ▶). Among these, five Atg proteins were shown to be involved in a novel ubiquitin-like conjugation system named the Atg12 system (Mizushima et al., 1998 ▶). In the Atg12 system the carboxyl-terminal glycine of Atg12 is activated by Atg7, an E1-like enzyme (Tanida et al., 1999 ▶), and is then transferred to Atg10, an E2-like enzyme (Shintani et al., 1999 ▶). Finally, Atg12 is conjugated to its sole target, Atg5 (Mizushima et al., 1998 ▶). In addition to the covalent interaction with Atg12, Atg5 interacts with the N-terminal region of a multimeric protein, Atg16, non-covalently (Mizushima et al., 1999 ▶). Using this interaction, the Atg12–Atg5 conjugate forms a multimeric complex with Atg16 (Kuma et al., 2002 ▶; Mizushima et al., 1999 ▶). The Atg12–Atg5–Atg16 ternary complex localizes to autophagosome precursors, and plays essential roles in autophagosome formation (Kim et al., 2001 ▶; Suzuki et al., 2001 ▶).
We previously reported the structures of the Atg5–Atg16 complex (Matsushita et al., 2007 ▶) and plant Atg12 (Suzuki et al., 2005 ▶), and showed that both Atg12 and Atg5 are comprised of ubiquitin fold(s). However, the molecular functions of these proteins could not be elucidated from their structures. In order to obtain further insights into the functions of these proteins, structural information on the functional complex, the Atg12–Atg5–Atg16 ternary complex, is required. In this report we describe the expression, purification and crystallization of the truncated Atg12–Atg5 conjugate bound to the N-terminal region of Atg16.
2. Preparation of the Atg12–Atg5–Atg16 ternary complex
The N-terminal 100 residues of Saccharomyces cerevisiae Atg12 were shown to be dispensable for autophagy (Hanada & Ohsumi, 2005 ▶) and the C-terminal ten residues of Saccharomyces cerevisiae Atg5 were shown to assume a disordered loop (Matsushita et al., 2007 ▶). In order to make the crystallization step easier, the truncated Atg12–Atg5 conjugate, Atg12(100–186)–Atg5(1–284), was used instead of the full-length Atg12–Atg5 for crystallization. The truncated Atg12–Atg5 conjugate was formed by reconstitution of the Atg12 conjugation system in E. coli. Full-length Atg7 and Atg10 were cloned into a pET11a vector and Atg12(100–186) and Atg5(1–284) were cloned into a pACYC184 vector with a T7 promoter. A hexa-histidine tag and a TEV protease cleavage site were attached at the N-terminus of Atg12(100–186). Both vectors were transformed into E. coli BL21 (DE3), and four proteins were simultaneously expressed in E. coli. Atg16 is comprised of the N-terminal domain and the C-terminal coiled-coil domain, and the former domain is necessary and sufficient for binding to Atg5. The N-terminal domain (1–46) of Saccharomyces cerevisiae Atg16 (Atg16N) was used for preparation of the ternary complex. Atg16N was cloned into a pGEX6P-1 vector (GE Healthcare) and was expressed in E. coli BL21 (DE3) as a GST-fusion protein.
After cell lysis, GST-tagged Atg16N was purified by affinity chromatography using a glutathione-Sepharose 4B column (GE Healthcare). After cleaving a GST tag from Atg16N with PreScission protease (GE Healthcare), further purification of Atg16N was performed using a Superdex75 gel filtration column (GE Healthcare) equilibrated with 150 mM NaCl, 20 mM Tris-HCl pH 8.0. Hexa-histidine-tagged Atg12(100–186)–Atg5(1–284) conjugate was purified by affinity chromatography using an Ni-NTA column (QIAGEN), and was mixed with purified Atg16N to form the ternary complex. The hexa-histidine tag was cleaved from the Atg12 moiety of the ternary complex with TEV protease (GE Healthcare) in a solution consisting of 0.5 M NaCl, 2 mM DTT and 20 mM Tris-HCl pH 8.0. The ternary complex was then applied to a Mono S cation-exchange chromatography equilibrated with 20 mM HEPES pH 6.8 and was eluted with a 0–500 mM NaCl gradient in the same buffer. SDS-PAGE of the purified ternary complex is shown in Fig. 1 ▶.
Figure 1.
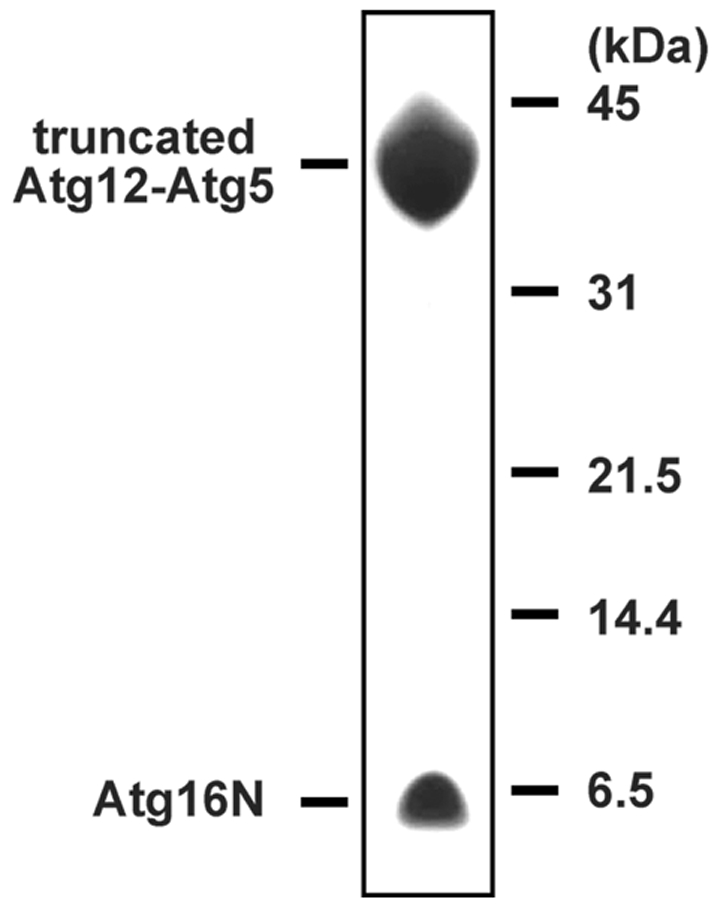
SDS-PAGE of the purified ternary complexes. Proteins were stained with Coomassie Brilliant Blue.
3. Crystallization
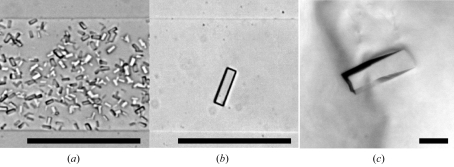
Initial crystallization trials were performed using the free-interface diffusion method using TOPAZ screening chips (Fluidigm). OptiMix-1 and OptiMix-2 (Fluidigm) were used as crystallization reagents. A total of 3 µl of 5.7 mg ml−1 Atg12–Atg5–Atg16N complex in 20 mM HEPES pH 6.8 and 350 mM NaCl was loaded into 192 diffusion chambers within two chips and was mixed with 192 varieties of OptiMix reagents by diffusion at 293 K. Microcrystals of the ternary complex were obtained using a solution consisting of 20% PEG10000, 0.5 M potassium thiocyanate and 0.1 M ADA pH 6.5 within a few hours (Fig. 2a ▶). After optimization of crystallization reagents and protein concentration using TOPAZ chips, a well shaped single crystal was obtained using 8.5 mg ml−1 protein solution and a crystallization solution consisting of 12% PEG10000, 0.5 M potassium thiocyanate and 0.1 M ADA pH 6.5 (Fig. 2b ▶). Using the optimized reagents as reservoir solution, preparation of larger crystals for X-ray diffraction experiments was performed by the sitting-drop vapour-diffusion method. 1.0 µl drops of 8.5 mg ml−1 Atg12–Atg5–Atg16N complex in 20 mM HEPES pH 6.8 and 350 mM NaCl were mixed with equal amounts of reservoir solution and equilibrated against 100 µl of the same reservoir solution by vapour diffusion at 293 K. Since no crystals were obtained using this method, crystals grown in TOPAZ chips were transferred into the sitting drops as crystallization seeds. Crystals with dimensions of 0.3 × 0.1 × 0.1 mm were obtained in a few days after seeding (Fig. 2c ▶). They were shown to be comprised of both the Atg12–Atg5 conjugate and Atg16N by SDS-PAGE analysis (Fig. 3 ▶).
Figure 2.
Crystals of the Atg12–Atg5–Atg16N ternary complex. (a), (b) Microcrystals obtained using the free-interface diffusion method. (c) A crystal obtained using the sitting-drop vapour-diffusion method. The black scale bar is 100 µm in length.
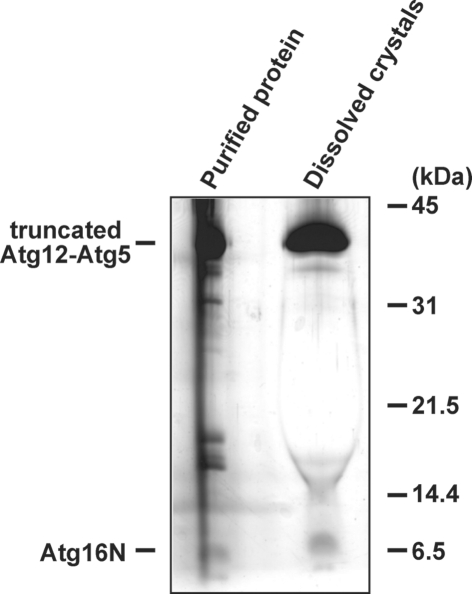
Figure 3.
SDS-PAGE of the dissolved crystals. Before SDS-PAGE, crystals were washed with reservoir solution substantially. Proteins were stained with silver staining.
4. Preliminary crystallographic analysis
Crystals were immersed for several seconds into a reservoir solution supplemented with 20% glycerol as a cryoprotectant and then flash-cooled and kept in a stream of nitrogen gas at 95 K during data collection. Diffraction data were collected at 95 K on an ADSC Quantum 210 charge-coupled device detector on beamline NW12A, KEK, Japan, at a wavelength of 1.00 Å. Diffraction data were indexed, integrated and scaled using HKL2000 (Otwinowski & Minor, 1997 ▶). The data collection statistics are summarized in Table 1 ▶. The crystal belongs to the orthorhombic space group C2221, with unit-cell parameters a = 72.15, b = 106.08, c = 143.07 Å. The acceptable range of volume-to-weight ratio (V M) values (Matthews, 1968 ▶) indicates that the crystal contains one ternary complex per asymmetric unit, with a solvent content of 56.9% (V M = 2.85 Å3 Da−1). Structure determination by the molecular replacement method is now in progress.
Table 1. Data collection statistics.
Values in parentheses indicate those in the resolution range between 2.69 and 2.60 Å.
| Resolution range (Å) | 50.00–2.60 |
| Observed reflections | 125260 |
| Unique reflections | 17173 |
| Completeness (%) | 99.9 (100.0) |
| Rmerge(I) | 0.055 (0.303) |
Acknowledgments
We thank the staff at beamline NW12A, KEK, Japan, for data collection support. This work was supported by Grant-in-Aids for Priority Areas and the National Project on Protein Structural and Functional Analyses from the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was carried out under the NIBB Cooperative Research Program (7-329).
References
- Hanada, T. & Ohsumi, Y. (2005). Autophagy, 1, 110–118. [DOI] [PubMed]
- Kim, J., Huang, W. P. & Klionsky, D. J. (2001). J. Cell Biol.152, 51–64. [DOI] [PMC free article] [PubMed]
- Klionsky, D. J., Cregg, J. M., Dunn, W. A. Jr, Emr, S. D., Sakai, Y., Sandoval, I. V., Sibirny, A., Subramani, S., Thumm, M., Veenhuis, M. & Ohsumi, Y. (2003). Dev. Cell, 5, 539–545. [DOI] [PubMed]
- Kuma, A., Mizushima, N., Ishihara, N. & Ohsumi, Y. (2002). J. Biol. Chem.277, 18619–18625. [DOI] [PubMed]
- Matsushita, M., Suzuki, N. N., Obara, K., Fujioka, Y., Ohsumi, Y. & Inagaki, F. (2007). J. Biol. Chem.282, 6763–6772. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol.33, 491–497. [DOI] [PubMed]
- Mizushima, N. (2005). Cell Death Differ.12, 1535–1541. [DOI] [PubMed]
- Mizushima, N., Noda, T. & Ohsumi, Y. (1999). EMBO J.18, 3888–3896. [DOI] [PMC free article] [PubMed]
- Mizushima, N., Noda, T., Yoshimori, T., Tanaka, Y., Ishii, T., George, M. D., Klionsky, D. J., Ohsumi, M. & Ohsumi, Y. (1998). Nature (London), 395, 395–398. [DOI] [PubMed]
- Otwinowski, Z. & Minor, W. (1997). Methods Enzymol.276, 307–326. [DOI] [PubMed]
- Seglen, P. O. & Bohley, P. (1992). Experientia, 48, 158–172. [DOI] [PubMed]
- Shintani, T., Mizushima, N., Ogawa, Y., Matsuura, A., Noda, T. & Ohsumi, Y. (1999). EMBO J.18, 5234–5241. [DOI] [PMC free article] [PubMed]
- Suzuki, K., Kirisako, T., Kamada, Y., Mizushima, N., Noda, T. & Ohsumi, Y. (2001). EMBO J.20, 5971–5981. [DOI] [PMC free article] [PubMed]
- Suzuki, N. N., Yoshimoto, K., Fujioka, Y., Ohsumi, Y. & Inagaki, F. (2005). Autophagy, 1, 119–126. [DOI] [PubMed]
- Takeshige, K., Baba, M., Tsuboi, S., Noda, T. & Ohsumi, Y. (1992). J. Cell Biol.119, 301–311. [DOI] [PMC free article] [PubMed]
- Tanida, I., Mizushima, N., Kiyooka, M., Ohsumi, M., Ueno, T., Ohsumi, Y. & Kominami, E. (1999). Mol. Biol. Cell, 10, 1367–1379. [DOI] [PMC free article] [PubMed]