A new detector system for protein crystallography based on an X-ray HARP–FEA is presented.
Keywords: HARP, semiconductor, amorphous selenium, avalanche multiplication, imaging device, protein crystallography
Abstract
A new detector system for protein crystallography is now being developed based on an X-ray HARP–FEA (high-gain avalanche rushing amorphous photoconductor–field emitter array), which consists of an amorphous selenium membrane and a matrix field emitter array. The combination of the membrane avalanche effect with a single driven FEA has several advantages over currently available area detectors, including higher sensitivity, higher spatial resolution and a higher frame rate. Preliminary evaluation of the detector has been carried out and its effectiveness has been confirmed. Next, diffraction images were measured with continuous rotation of a protein crystal, and the images were compared with those measured by the existing CCD detector; the system successfully obtained high-spatial-resolution images. Using shutterless measurement, the total measurement time can be reduced significantly, making the method appropriate for high-throughput protein crystallography. The X-ray HARP–FEA detector is an attractive candidate for the next generation of X-ray area detectors.
1. Introduction
Thousands of protein structures have been solved by crystallographic structure analysis using synchrotron radiation. Many protein structures remain unsolved, often because the protein in question is difficult to crystallize, or forms very small crystals. Protein crystallographers wish to collect data quickly and routinely from even micrometre-size protein crystals, requiring high-intensity and micro-focus X-ray beams. Additionally, crystallographers require a high-throughput data collection system in which beamline equipment can automatically handle large numbers of protein crystals and rapidly and routinely record higher-resolution images.
A new short-gap undulator was recently installed at the short straight section of the Photon Factory. At the same time, a new project was initiated, aiming at the construction of a new measurement system for protein crystallography optimized for micrometre-size protein crystals and high-throughput data collection. A core goal of the project is the development of a next-generation X-ray detector. To date, imaging plates and X-ray CCD detectors have been most widely used for protein crystallography. Imaging plates have a wide dynamic range and large area, but they take a great deal of time to convert and clear image data. CCD detectors have low noise and high sensitivity and can handle a large number of images rapidly and automatically; however, these detectors lack the wider dynamic range of imaging plates and require a reset process between image recordings, making measurement times longer. Furthermore, the detectors use an indirect method for X-ray detection, using scintillators to convert X-rays into visible light, which is then received directly by the CCD. Because the scintillation light diffuses inside the scintillator, there is a trade-off between sensitivity and resolution (thicker scintillators are more sensitive but have lower spatial resolution, and vice versa). The other main requirement for a next-generation X-ray area detector is to increase the framing rate (e.g. to 120 frames s−1) in order to match next-generation light sources such as the energy recovery linac. To meet these needs, an X-ray detector with a direct detection method and higher framing rate would be ideal. NHK Science and Technical Research Laboratories have developed a HARP (high-gain avalanche rushing amorphous photoconductor) target (Tanioka et al., 1987 ▶, 1992 ▶), and adapted it for applications such as TV camera broadcasting at night and in other low-light conditions. In this project, we have applied the HARP technique to a next-generation X-ray area detector. Here, we discuss the development of a new measurement system with the HARP–FEA (HARP–field emitter array) detector.
2. X-ray HARP–FEA detector system
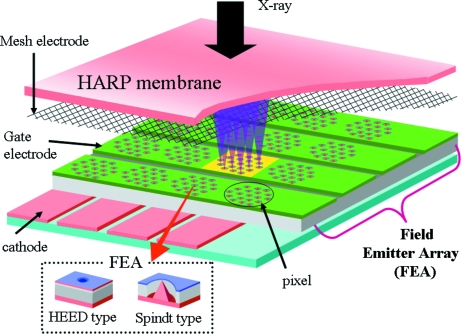
Fig. 1 ▶ shows a schematic diagram of the HARP–FEA detector; Fig. 2 ▶ is a photograph of a prototype. A HARP membrane on the original HARP-TV is formed on a glass plate. Since low-energy X-rays cannot penetrate a thick glass plate, a thin beryllium membrane is used for the formation of the HARP membrane. When high voltage is applied to the HARP, electron–hole (e–h) pairs generated by the X-rays produce additional e–h pair(s), and avalanche multiplication occurs. The accumulated charge is detected by electron beams from the field emitter array. There are two types of FEA, Spindt and HEED (high-efficiency electron emission device), whose difference is mainly in the shape of the array. A group of arrays forms a pixel, multiple pixels are gathered in an aligned signal circuit, and signals are sent from every circuit in series. A mesh electrode is used for focusing each pixel; this adjustment influences spatial resolution. The HARP–FEA detector is assembled in a camera box with readout electronics. On the camera, the HARP–FEA detector is surrounded by a large permanent magnet to focus all of the readout electron beams from the FEA. Because of the space required for the magnet, the distance between the surface of the camera and the beryllium membrane is large, about 30 mm.
Figure 1.
Schematic diagram of the HARP–FEA detector.
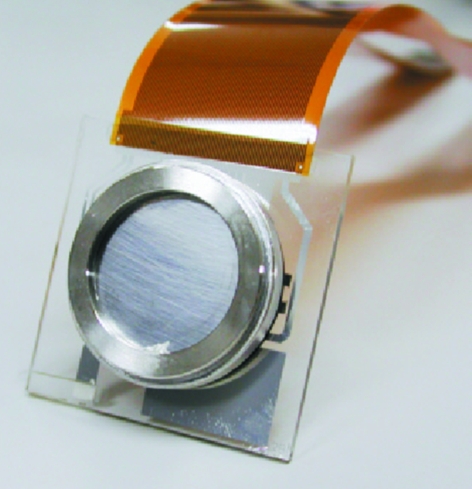
Figure 2.
Prototype of the HARP–FEA detector.
The first prototype of the HARP–FEA detector was completed in June 2006. The detector is assembled into the original camera (the HARP–FEA camera). Table 1 ▶ shows the specifications of the camera. Images from the camera are sent to the camera control unit, where analog images are converted into digital images. Then, images are recorded and displayed by custom-designed recording software. The recording control is synchronized to the beamline devices. The avalanche multiplication ratio (gain) could be greater than 10; in the prototype, however, higher gain can cause an increase in electrical noise and a concomitant decrease in image quality; also, permanent charge concentration spots (called white damage) may be newly generated. Therefore, the gain is limited at 10 in the experiment.
Table 1. Specifications of the first prototype HARP–FEA camera.
| Pixel size (µm) | 20 × 20 |
| Number of pixels | 640 × 480 |
| Frame rate (frames s−1) | 30 |
| Membrane thickness (µm) | 15 |
| Maximum avalanche voltage (V) | 1600 |
| Effective area (mm) | 12.8 × 9.6 |
| Digital data range | 12 bit |
| Image format | 16 bit TIF |
| Readout method | HEED/Spindt-type FEA |
| Output | VGA |
3. Evaluation of the X-ray HARP–FEA detector system
3.1. Overview of evaluation experiment
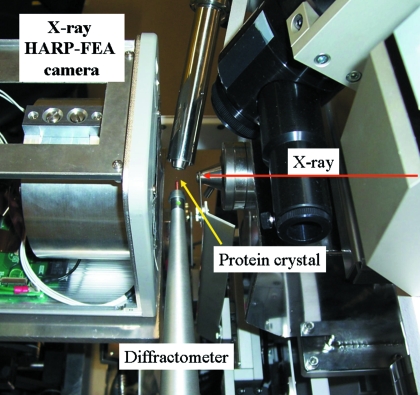
We performed evaluation experiments on a structural biology beamline at the KEK Photon Factory, BL-17A (Igarashi et al., 2007 ▶; Hiraki et al., 2007 ▶). The experiment was conducted using both the Spindt and HEED types of HARP–FEA detector. The goals of the experiments were as follows: (i) to record the dark images to evaluate uniformity; (ii) to measure the sensitivity and dynamic range of the HARP–FEA detector; and (iii) to apply the HARP–FEA to diffraction image measurement with a protein crystal. The HARP detector was also compared with commercial CCD detectors. Fig. 3 ▶ shows the experimental set-up for goal (iii). Since the effective area of the HARP detector is small, the camera was put as close as possible to the protein crystal. In this case, the equipped beam stopper cannot be used, so pieces of a lead plate were used as beam stoppers and attached to the surface of the camera.
Figure 3.
Experimental set-up in the BL-17A experimental hutch.
3.2. Uniformity
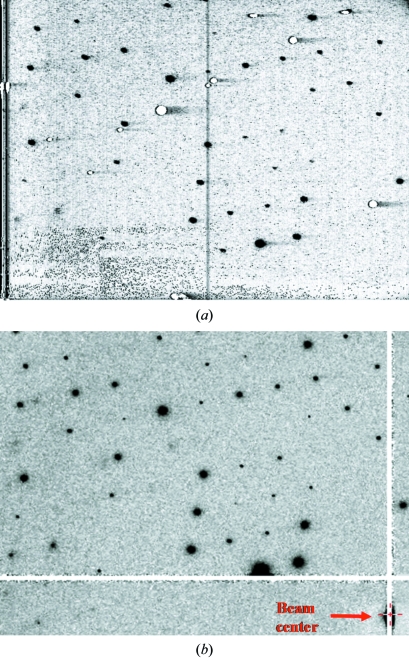
First of all, dark images were measured for preliminary evaluation. It is very difficult to generate a HARP membrane on a beryllium surface. Some white damage is probably formed simply owing to the non-uniformity of the beryllium surface. The dark images derived from Spindt-type and HEED-type HARP–FEA detectors are shown in Figs. 4(a) and 4(b) ▶, respectively. Images of the Spindt type have only a few spots of white damage, whereas in the HEED type more defects and white damage are seen. This is probably due to differences in quality of the beryllium membrane and success ratio at the time of construction of the FEA. This is a known problem and could be improved by the optimization of the HARP formation method. Even given these concerns about image quality, the detector is appropriate for use in X-ray measurements.
Figure 4.
Example of dark images of Spindt-type (a) and HEED-type (b) HARP–FEA detectors.
3.3. Sensitivity and dynamic range
When measuring the sensitivity and dynamic range of the detectors, beam intensity was measured using a PIN photodiode detector and adjusted by aluminium absorbers. For comparison, the CCD detector, ADSC Quantum 270, was also examined. As of 2007, the Q270 is the most current and most high-sensitivity ADSC CCD, and is suitable for use in protein crystallography. To save measurement time, the CCD exposure time was also changed. Since the most sensitive area of the detector is a function of beam intensity, the integrated area was set larger with higher intensity or smaller with lower intensity in order to evaluate statistical error correctly. The result is shown in Fig. 5 ▶. With a gain of 1, the sensitivity of the CCD is better than that of the HARP–FEA detector. With a gain of 10, the sensitivity of the HARP detector is the same or a few times higher than that of the CCD. The dynamic range of the HARP detector is about 10 bit and narrower than that of the CCD; therefore, the range must be improved in order to apply the technique to protein crystallography. Even at this level, it is possible to study the application of the HARP–FEA detector to protein crystallography.
Figure 5.
Beam intensity dependence of digital output for the HARP–FEA and X-ray CCD detector. The X-ray wavelength is 1 Å and the beam size is 0.1 mm × 0.1 mm.
3.4. Measurement of diffraction images
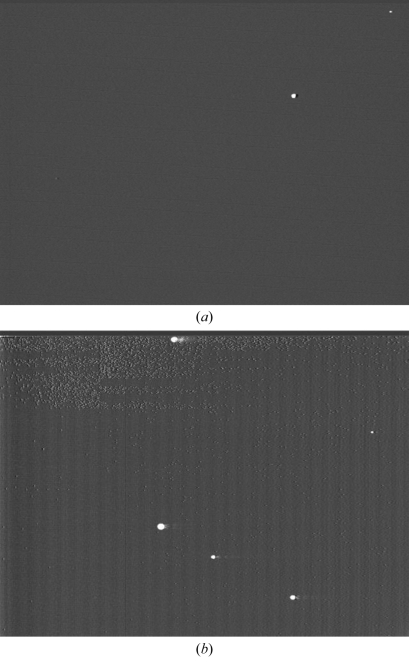
To compare our detector with the existing CCD system for use in protein crystallography, diffraction images were measured by the prototype HARP–FEA detector (HEED type) and an X-ray CCD detector (ADSC Quantum 4R). To make the exposure time the same, the CCD exposure time was set to 334 ms (minimum), and ten images were averaged for the HARP–FEA detector (30 frames s−1 of the fixed frame rate). The distance between the CCD and crystal was set to about three times longer than for the HARP, because the pixel size of the CCD is about four times larger (81.6 µm). The results are shown in Fig. 6 ▶, along with the experimental conditions, and indicate that the number of recognized diffraction spots is almost the same (40 spots) within the area shown. In the HARP image, a high-spatial-resolution spot pattern can be seen, and each spot has a smooth shape because of small pixel size (20 µm). The spot width of the HARP is 7–8 pixels (full width at half-maximum), which is smaller than that of the CCD (2–3 pixels). In the CCD, stronger signals generate intensity leaks, and the number of pixels to be integrated in a spot increases because of photon diffusion at the coupling site between the scintillator and fibres. This is indispensable in indirectly sensing X-ray detectors. However, half the spots are in overflow on the HARP detector, owing to its narrower dynamic range.
Figure 6.
Diffraction images (4.4 Å resolution at the top of images) at 1 Å X-ray with 1° oscillation. The crystal is egg-white lysozyme. (a) Image collected using the HARP detector (average of ten images). The area is 12.8 mm × 9.6 mm. (b) Image collected using the CCD (ADSC Q4R). The exposure time is 334 ms. The zoomed area is ∼33 mm × 27 mm.
Next, we attempted continuous measurement of diffraction images with crystal rotation over 90°. An important feature of the HARP–FEA detector is its ability to perform shutterless measurements. With 3° s−1 sample rotation speed, it takes only 30 s to completely record images through 90° of rotation. Even with a current high-speed CCD area detector, it takes more than a few seconds for the series of required operations: exposure, shutter operation, image flush, image readout and communications. In this case, the ADSC Q4R system takes typically 10 s for the overhead, and the total experimental time was thus ∼900 s. The HARP detector is very useful from the point of view that the measurement does not require the synchronization of the beam shutter or the precision of the rotation axis for the protein crystal. Additionally, the higher frame rate makes the HARP system more suitable for use in high-throughput protein crystallography. To utilize this system most effectively, some improvements are required: wider dynamic range, larger effective area, and clearer images with no white damage.
4. Conclusions
We have completed the first prototype of a HARP–FEA detector and commenced evaluation experiments. First, we found that sensitivity of the HARP–FEA detector with high gain (×10) is better than that of an X-ray CCD detector in a structural biology beamline. Next, we applied the HARP to measurement of diffraction images with a protein crystal. Protein crystal diffraction images of the HARP were obtained for the first time, and the image showed a high-spatial-resolution diffraction pattern. In measurement with continuous rotation over 90°, data acquisition was completed in 30 s, a clear advantage of shutterless measurement. Even if the HARP–FEA detector requires a larger effective area, wider dynamic range and better image quality with no white damage, we demonstrated that the HARP–FEA detector is a potentially effective tool for high-throughput protein crystallography. In addition to data collection, the detector could be useful for screening of diffraction or crystallization. We will continue improvement and develop a new measurement system that will be useful alongside the next generation of synchrotron light sources.
Acknowledgments
This work was performed under the approval of the Photon Factory Program Advisory Committee (proposal no. 2006G393). We wish to thank all of our collaborators. The evaluation experiment was carried out with the help of many colleagues at the Photon Factory. NHK Engineering Services and NHK Science and Technical Research Laboratories helped a great deal with operation of the HARP–FEA detector. This work was funded by the program ‘Development of Systems and Technology for Advanced Measurement and Analysis’ of the Japan Science and Technology Agency.
References
- Hiraki, M., Watanabe, S., Yamada, Y., Matsugaki, N., Igarashi, N., Gaponov, Y. & Wakatsuki, S. (2007). AIP Conf. Proc.879, 1924–1927.
- Igarashi, N., Matsugaki, N., Yamada, Y., Hiraki, M., Koyama, A., Hirano, K., Miyoshi, T. & Wakatsuki, S. (2007). AIP Conf. Proc.879, 812–815.
- Tanioka, K., Shidara, K. & Harai, T. (1992). Proc. SPIE, 1656, 2–12.
- Tanioka, K., Yamazaki, J., Shidara, K., Taketoshi, K., Kawamura, T., Ishioka, S. & Takasaki, Y. (1987). IEEE Electron Device Lett.8, 392–394.