Abstract
Clostridium perfringens-induced necrotic enteritis (NE) is a widespread disease in chickens that causes high mortality and reduced growth performance. Traditionally, NE was controlled by the routine application of antimicrobials in the feed, a practice that currently is unpopular. Consequently, there has been an increase in the occurrence of NE, and it has become a threat to the current objective of antimicrobial-free farming. The pathogenesis of NE is associated with the proliferation of C. perfringens in the small intestine and the secretion of large amounts of alpha toxin, the major virulence factor. Since there is no vaccine for NE, we have developed a candidate live oral recombinant attenuated Salmonella enterica serovar Typhimurium vaccine (RASV) that delivers a nontoxic fragment of alpha toxin. The 3′ end of the plc gene, encoding the C-terminal domain of alpha toxin (PlcC), was cloned into plasmids that enable the expression and secretion of PlcC fused to a signal peptide. Plasmids were inserted into Salmonella enterica serovar Typhimurium host strain χ8914, which has attenuating pabA and pabB deletion mutations. Three-day-old broiler chicks were orally immunized with 109 CFU of the vaccine strain and developed alpha toxin-neutralizing serum antibodies. When serum from these chickens was added into C. perfringens broth cultures, bacterial growth was suppressed. In addition, immunofluorescent microscopy showed that serum antibodies bind to the bacterial surface. The immunoglobulin G (IgG) and IgA titers in RASV-immunized chickens were low; however, when the chickens were given a parenteral boost injection with a purified recombinant PlcC protein (rPlcC), the RASV-immunized chickens mounted rapid high serum IgG and bile IgA titers exceeding those primed by rPlcC injection. RASV-immunized chickens had reduced intestinal mucosal pathology after challenge with virulent C. perfringens. These results indicate that oral RASV expressing an alpha toxin C-terminal peptide induces protective immunity against NE.
Clostridium perfringens-induced necrotic enteritis (NE) causes high mortality, up to 30% in broiler chickens, and is associated with subclinical chronic intestinal mucosal damage that results in reduced growth and productivity (19, 21, 29, 48). It is a widespread disease in broilers and poses a significant economic impact, with an estimated global loss of more than $2 billion per year (21, 29). Occasionally, NE associated with high mortality occurs in commercial egg-layer chickens (11).
C. perfringens is a gram-positive spore-forming anaerobe that is ubiquitously present in large numbers in the environment (e.g., in soil, litter, and vermin), and it is a constituent of the normal gut flora of animals and humans (7, 28). C. perfringens produces a wide array of enteric toxins, with the major ones being the alpha, beta, epsilon, and iota toxins (37, 42). Based on the major toxin(s) they produce, C. perfringens isolates are grouped into five types, type A to E (37). The differential production of the major toxins is responsible for the differences in disease symptoms and pathogenesis among C. perfringens clinical isolates. Type A strains produce large amounts of alpha toxin but lack the expression of other major toxins (37, 42). Chickens appear to be more susceptible to the alpha toxin than any of the other C. perfringens toxins; hence type A strains are the predominant isolates in NE (7, 10, 14). Type A strains also are the most accountable for C. perfringens-associated enteric diseases in various animal species (42) and humans (28).
There is no vaccine for NE. Historically, NE and C. perfringens infections have been controlled by the addition of antimicrobial growth promoters (AGP) in the animal feed (10, 48). Large quantities of antibiotics are routinely used as AGP and for prophylaxis against enteric bacterial pathogens, including C. perfringens. In the United States, the poultry industry allegedly uses up to 10 million pounds of antibiotics per year, which accounts for 40% of all antibiotics used in livestock (32). The amount of antibiotics (AGP) used in poultry alone is estimated to be threefold greater than the amounts used to treat human infections (32). Such use of antibiotics in poultry and livestock has been condemned due to concerns about the increased antibiotic resistance of human pathogens (50). On the other hand, in recent times there has been an increase in sporadic outbreaks and widespread subclinical NE that is linked to the withdrawal of AGP (10, 19, 48). This had been observed initially in Scandinavian countries following the ban on AGP in the early 1990s (16, 51). Furthermore, the decline in the use of ionophore coccidiostats, which have an inhibitory effect on C. perfringens growth, has exacerbated the resurgence of NE (29, 52). Thus, NE is considered a reemerging disease and a major threat to the current objective of antimicrobial-free poultry farming.
The pathogenesis of NE is complex. The main factors include disturbances of the intestinal floral balance that promote the overgrowth of C. perfringens with the colonization of the small intestine and the subsequent production of a large quantity of the alpha toxin (47). Certain predisposing factors, primarily the composition of diet and coinfection with Eimeria spp., play a part in the initiation of the mucosal pathology (3, 52). Experimentally, NE has been simulated by inoculating young birds with a growing C. perfringens culture or with either a purified alpha toxin or cell-free culture supernatant (2, 47). However, severe NE with high mortality could be induced only in germ-free chickens (12). Neutralizing the alpha toxin in the inoculum (purified toxin or C. perfringens culture supernatant) with a hyperimmune serum against alpha toxin abolishes the induction of NE (12). Successively, several studies have confirmed the role of alpha toxin in the pathogenesis of NE and the protective effect of a toxoid vaccine (inactivated whole toxin) against experimental and field incidences of NE (18, 24, 25, 26).
C. perfringens alpha toxin is a 43-kDa phospholipase C enzyme that degrades membrane phospholipids and has a distinctive hemolytic activity (46). It is the most potent phospholipase of any origin (45). The alpha toxin is the main cause of the acute myonecrosis in C. perfringens-caused gas gangrene in humans (46). Biochemical studies showed that the catalytic effect of alpha toxin on membranes, which causes membrane disruption, depends on the Ca2+-mediated binding of its carboxy-terminal tail to membrane phospholipids (39, 45). The blockage of the C-terminal domain with epitope-specific antibody neutralizes both phospholipase and hemolytic activities (46). In mice, immunization with a subunit vaccine consisting of the alpha toxin C-terminal domain protects against C. perfringens-caused gas gangrene (43; B. Zekarias, H. Mo, and R. Curtiss III, unpublished data).
Several licensed live attenuated Salmonella enterica serovar Typhimurium vaccine strains exist for poultry (4, 8, 20). These strains are valuable as antigen delivery vectors to induce a systemic and mucosal immunity against recombinant antigen while producing protection against salmonellosis (9, 17, 27). In the present study, we investigate the effectiveness of the recombinant attenuated Salmonella-based delivery of the C-terminal domain of alpha toxin to induce protective immunity against NE and the associated subclinical mucosal lesion development in chickens. The results demonstrate that an oral live recombinant attenuated S. enterica serovar Typhimurium vaccine (RASV) vectoring the C-terminal fragment of the alpha toxin of C. perfringens induces protective immunity that reduces intestinal pathology and growth depression in chickens challenged with C. perfringens.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. Clostridium perfringens CP995 and JGS4143 strains are type A strains originally isolated from the intestines of NE-affected chickens. CP995 was used for cloning the alpha toxin C-terminal domain, and JGS4143, a hypervirulent strain (5), was used in the challenge experiments. Bacteria were grown in chopped meat broth (CMM) or brain heart infusion (BHI) medium with 0.04% d-cycloserine (BBL, Franklin Lakes, NJ). Tryptose-sulfate- cycloserine (TSC) agar plates with 5% egg yolk (TSC-EY) or 5% sheep blood agar were used for colony differentiation based on lecithinase or hemolytic activity, respectively. Fluid thioglycolate medium (FTG) was used to cultivate large quantities of bacteria for animal inoculation and for bacterial resuscitation from tissue samples. All C. perfringens cultures were grown at 37°C under anaerobic condition using BBL GasPak systems. Both CP995 and JGS4143 were confirmed to be negative for β2 toxin by PCR verification (31).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Genotype and/or characteristics | Source or reference |
|---|---|---|
| Strains | ||
| S. enterica serovar Typhimurium χ8914 | ΔpabA1516 ΔpabB232 ΔasdA16; vaccine vector | Laboratory collection |
| E. coli χ6097 | F−araD139 Δ(proAB-lac) λ-Φ80dlacZΔM15 rpsL ΔasdA4 Δ(zhf-2::Tn10) thi-1; contains pYA232 (Tcr, lacIq); used for cloning and propagation of plasmid | 33 |
| C. perfringens | ||
| CP995 | Type A, NE isolate | G. Siragusa, USDA, Athens, GA |
| JGS4143 | Type A, NE isolate, virulent strain | 5 |
| Plasmids | ||
| pYA3493 | Ptrc, asd, pBRori, bla SS; parent plasmid vector | 22 |
| pYA3620 | Ptrc, asd, pBRori, bla SS, bla CT; parent plasmid vector pYA3493 with bla C-terminal fusions | 22 |
| pYA4101 | Plpp, asd, pBRori, ompA SS; parent plasmid vector with OmpA signal peptide fusion | |
| pYA3977 | 375-bp DNA encoding PlcC in pYA3493 PlcC expression plasmid | This study |
| pYA4110 | 375-bp DNA encoding PlcC in pYA4101 PlcC expression plasmid | This study |
| pYA4149 | 375-bp DNA encoding PlcC in pYA3620 PlcC expression plasmid | This study |
| pYA3910 | 375-bp DNA encoding PlcC in pBAD/HisB vector; used for His-tagged PlcC (rPlcC) expression | This study |
S. enterica serovar Typhimurium χ8914, containing defined attenuating deletions of the pabA and pabB genes, was used as the vaccine vector. χ8914 is derived from a highly virulent S. enterica serovar Typhimurium strain (χ3761). χ8914 has an oral 50% lethal dose of greater than 1 × 109 CFU for 1-day-old chicks, whereas the 50% lethal dose for the wild-type χ3761 is 3 × 103 CFU (17). χ8914 also has a deletion in the gene encoding aspartate β-semialdehyde dehydrogenase (asd), which renders it deficient in the synthesis of diaminopimelic acid (DAP). Since DAP is an obligate component of the cell wall peptidoglycan, in the absence of exogenously supplied DAP the growth of χ8914 strictly depends on the complementation of the asd mutation with the vaccine antigen expression plasmid that carries the Salmonella asd gene (13). This dependence on Asd+ plasmid complementation is the basis for the balanced lethal host-vector system in RASV, which abrogates the need for antibiotic resistance markers (13, 33). Bacteria were grown at 37°C in Luria-Bertani (LB) culture medium containing 0.1% dextrose. When required, 50 μg/ml DAP was added.
Cloning of the C. perfringens plcC gene.
C. perfringens DNA was isolated from a bacterial lysate prepared from colonies grown overnight on TSC agar plates. Bacteria were suspended in 150 μl 0.5 M NaOH, pH 8.5, incubated for 30 min at room temperature, and diluted with 25 μl Tris-Cl, pH 7.4, and 425 μl water. A 375-bp region of the alpha toxin gene (plc) encoding the carboxy-terminal end of the alpha toxin (amino acids 248 to 370) was PCR amplified from the CP995 lysate using standard PCR conditions with the following primer sequences: 5′-CGGAATTCGATCCATCAGTTGGAAAGAATGTA-3′ and 5′-CCGAAGCTTATTATTTTATATTATAAGTTGAATTTCC-3′.
For the sequence analysis of the complete alpha toxin gene from CP995, primers binding to flanking regions of plc, 5′ AGTTTAACAATTTAGAGTGGGTAAGGTTAGATGTG 3′ and 5′ GCCAGCTCCTAGGAATCCTGAAATTATATCTAC 3′, were used.
rPlcC protein preparation.
The PCR product of plcC, the alpha toxin C-terminal domain-encoding sequence, was first cloned into the pBAD/HisB plasmid vector (Invitrogen, Carlsbad, CA) for the expression of a His-tagged recombinant PlcC (rPlcC) protein. The plasmid pYA3910 (pBADHisB containing plcC) (Table 1) was electroporated into competent Escherichia coli Top10 cells (Invitrogen, Carlsbad, CA). The expression of His-tagged rPlcC in the Top10 cells (pYA3910) was induced by adding 0.02% l-arabinose into early-log-phase growing bacteria. Bacteria were harvested from a 250-ml culture at an optical density at 600 nm (OD600) of 1.2 by centrifugation at 5,000 × g for 15 min. The cell pellet was resuspended in 40 ml cell lysis solution (Sigma, St. Louis, MO), which contains lysozyme (0.2 mg/ml), benzonase (50 U/ml), and protease inhibitors. Following 15 min of incubation at room temperature in the lytic solution, the bacterial suspension was briefly sonicated (2 min) to ensure cell disruption. Insoluble material was removed by centrifugation at 16,000 × g for 10 min. The supernatant containing His-tagged protein was loaded onto 0.8- by 4-cm chromatography columns (Bio-Rad, Hercules, CA) packed with nickel-Sepharose gel (6%) (Sigma, St. Louis, MO). The affinity gel matrix was washed with 50 mM NaH2PO4, pH 8.0, 0.3 M NaCl solution before and after the bacterial lysate was loaded. Proteins were eluted with 200 mM C3H4N2 (imidazole) in the washing solution. The elute was desalted and concentrated by a Centricon filtration system using 5,000 (5K) and 50K nominal molecular weight membranes (Millipore, Billerica, MA). Protein was analyzed by electrophoresis on a 12% Tris-Bis gel (see Fig. 3) and by Western blotting using 6-HisG antibody (Invitrogen, Carlsbad, CA). The protein concentration was determined by a Bradford assay using bovine serum albumin as the standard.
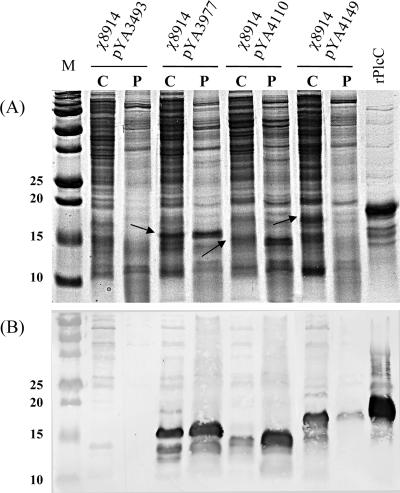
FIG. 3.
Expression of the PlcC antigen by the S. enterica serovar Typhimurium vaccine strain harboring different PlcC expression plasmids. (A) Coomassie blue-stained SDS-PAGE. Lanes: M, molecular mass marker (in kilodaltons) (Bio-Rad, Hercules, CA); C, cytoplasmic fraction; P, periplasmic fraction. (B) Western blot of an analogous gel with rabbit anti-PlcC hyperimmune serum. Arrows show PlcC protein bands that also correspond to bands detected by Western blotting.
To produce rabbit anti-PlcC antibody, two rabbits were injected subcutaneously (s.c.) with 100 μg rPlcC protein emulsified in Freund's adjuvant. The rabbits were immunized three times, with 2-week intervals between injections, and the antiserum was tested for specific reactivity against rPlcC by immunoblot analysis (see Fig. 3).
Plasmids for PlcC expression in the S. enterica serovar Typhimurium vaccine strain.
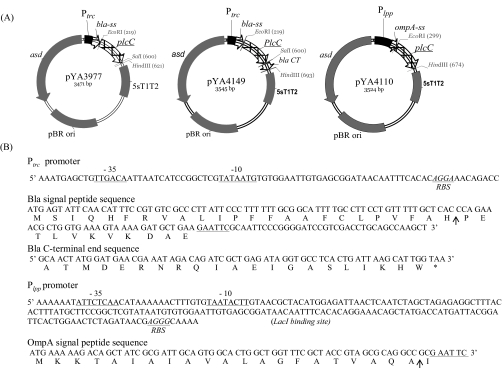
Three different recombinant gene expression plasmids, pYA3493, pYA3620, and pYA4101, were used for the expression of PlcC by the S. enterica serovar Typhimurium vaccine strain (Table 1). The plasmids contain a modified Ptrc or Plpp promoter and a signal peptide sequence from E. coli class A β-lactamase (bla) or the outer membrane protein A (ompA) at the translation start site for the cloned antigen (Fig. 1). The Ptrc or Plpp promoter directs the constitutive expression of the recombinant PlcC in Salmonella. The signal peptides target the protein for secretion by the type II secretion pathway into the periplasm, with subsequent release into the culture supernatant (22, 53). In addition to the β-lactamase signal peptide sequence, pYA3620 also has the β-lactamase C-terminal protein-coding sequence at the 3′ end of the recombinant gene. Such a fusion of the C-terminal peptide sequence of β-lactamase to the recombinant protein has been reported to facilitate the transport of recombinant protein across membranes (9). PlcC expression plasmids pYA3977, pYA4110, and pYA4149 (Table 1) were propagated, and PlcC expression was confirmed first in E. coli strain χ6097. Protein expression in χ6097 was induced by 0.5 mM isopropyl-β-d-thiogalactopyranoside. Once PlcC expression was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis, the plasmids were recovered from E. coli and introduced into competent χ8914 by electroporation. As a vaccine control strain (designated the RAS control), the parent plasmid pYA3493 was similarly introduced into χ8914. To verify plasmid stability, the vaccine strain harboring the expression plasmid was grown for more than 50 generations under nonselective conditions (i.e., in the presence of DAP) and then was tested for growth on medium without DAP.
FIG. 1.
PlcC vaccine antigen expression plasmids. (A) Plasmid maps for the pYA3977 Ptrc bla signal peptide sequence (ss) vector, pYA4149 Ptrc bla ss and bla C-terminal sequence (CT) fusion vector, and pYA4110 Plpp ompA ss vector. (B) The sequences of trc, lpp, bla ss, ompA ss, and bla CT. RBS, ribosome binding site (Shine-Dalgarno sequence). Arrows show predicted signal peptide cleavage sites.
Expression of PlcC protein by the S. enterica serovar Typhimurium vaccine vector.
RASV strains were grown overnight in 5 ml LB broth. The next day, cultures were inoculated into 100 ml LB broth and grown with aeration at 200 rpm until the culture reached an OD600 of 0.8. To test the expression of rPlcC as a cytoplasmic soluble protein, 1 ml of the culture was transferred to a microcentrifuge tube and centrifuged for 3 min at 14,000 × g, and the pellet was resuspended in 500 μl Tris-HCl buffer (50 mM Tris-HCl, 100 mM NaCl, pH 7.2, 10 mM β-mercaptoethanol, and 2 mM phenylmethylsulfonyl fluoride). Bacteria were lysed by sonication, and insoluble protein was removed by centrifugation at 14,000 × g for 2 min. The supernatant was tested for rPlcC. To evaluate PlcC protein localization in the periplasm and secretion into culture supernatant, bacteria were harvested from a 100-ml culture by centrifugation at 4,000 × g for 15 min at 4°C. Subcellular fractionation for periplasm contents was performed using the lysozyme digestion of the bacterial pellet by osmotic shock with sucrose as previously described (22). The culture supernatant was filtered through a 0.22-μm filter and concentrated by precipitation overnight at 4°C in a 10% trichloroacetic acid (TCA) solution. The protein samples were analyzed by SDS-PAGE and Western blotting. Specific protein bands with the predicted molecular masses were distinguished by the Coomassie blue staining of the SDS-PAGE and the immunoblots using rabbit anti-PlcC hyperimmune serum. The amount of PlcC expression was estimated from the SDS-PAGE bands based on comparisons of their densitometry data to those of a known concentration of bovine serum albumin. To ensure the absence of cell lysis that could confound the secreted proteins recovered from culture supernatants and to control the preparation of periplasmic protein, β-galactosidase was used as a cytoplasm protein marker. The MudJ allele (atrB13::MudJ) was inserted into the S. enterica serovar Typhimurium vaccine strain [χ8914(pYA3977)] chromosome by transduction (22). β-Galactosidase production by the χ8914(pYA3977) atrB13::MudJ construct was used as a cytoplasm protein marker. The culture supernatant, periplasm, and cytoplasm fractions from this construct were analyzed by Western blotting using anti-β-galactosidase antibody (Sigma, St. Louis, MO).
RASV inoculum preparation.
RASV strains from −80°C stock were spread on LB agar. Five colonies were inoculated into 5 ml broth and grown statically overnight at 37°C. The following day, the whole culture was inoculated into 100 ml prewarmed LB broth in a 500-ml culture flask and grown with constant shaking at 200 rpm to an OD600 of 0.8 (about 5 h of culture). Bacteria were harvested by centrifugation at 4,000 × g for 15 min at room temperature, and the pellet was resuspended in buffered saline with 1% gelatin (BSG) solution. The volume of the bacterial culture and BSG for resuspension was calculated to yield a bacterial concentration of 2 × 109 CFU/ml. Chickens were inoculated orally with 0.5 ml of the suspension, which contained 1 × 109 CFU.
Chicken immunization.
One-day-old Cornish × Rock chicks were purchased from McMurray Hatchery (Webster City, IA). On arrival three chicks were killed, and samples of internal organs were aseptically collected to test for S. enterica serovar Typhimurium infection by bacterial culture on MacConkey agar plates. Chicks were divided into separate Horsfall isolators, with 10 chicks per Horsfall. On the second day (3 days of age), all chicks, except for those of one group, were orally inoculated (using an oral gavage needle) with 0.5 ml bacterial suspension containing 109 CFU of either RASVs or the RAS control strain [χ8914(pYA3493)]. To facilitate the passage of the inoculum to the intestines, chickens were deprived of feed and water for 8 h prior to inoculation, and feed and water were returned 1 h after the RASV inoculation. Ten days later, a similar second dose was given as a boost immunization. One group of chickens was injected s.c. in the neck with 50 μg rPlcC protein in a 100-μl suspension of complete Freund's adjuvant. These chickens received a boost immunization 14 days later with the same dose but with incomplete Freund's adjuvant.
In a prior experiment, chickens were immunized with two doses of RASV χ8914(pYA3977), χ8914(pYA4110), or χ8914(pYA3493) and by s.c. rPlcC injection, and 5 weeks later (7 weeks of age) all the chickens were given a late boost immunization with rPlcC by s.c. injection. The chickens were administered 50 μg protein in a 100-μl volume suspension emulsified in incomplete Freund's adjuvant. This experiment, oral RASV priming followed by a parenteral protein boost immunization, was carried out to evaluate the effect of primary immunization with RASV or a subunit vaccine on antibody titers. RASV-3 was not included in this experiment.
Chickens were fed an antibiotic-free corn-based starter diet or a wheat/barley-based grower's diet (Purina Mills, St. Louis, MO). Feed and water were provided ad libitum. In the first week, chicks were reared at a brooding temperature of 32°C with 24 h of light. Subsequently, the cage temperature was kept at 25°C and the light schedule modified to 16 h of light and 8 h of dark.
All of the animal experiments in this study were conducted with the permission and under the guidelines of the Arizona State University Institutional Animal Care and Use Committee.
Clostridium perfringens challenge.
Two weeks after RASV immunization, the chicken feed was replaced by a wheat- and barley-based formulated growers' diet, which has higher crude protein and neutral fiber content. After 1 week on the grower diet, chickens were challenged by oral inoculation and repeated infection through contaminated feed with strain JGS4143. On the first day of the challenge, chickens were orally gavaged with 2 ml of an overnight culture of C. perfringens in CMM. Contaminated feed was prepared by mixing an overnight culture with feed as formerly described (44). Briefly, C. perfringens was grown in 10 ml CMM broth for 18 h at 37°C, which then was inoculated into 1 liter of FTG and grown for 18 h. The FTG culture was mixed with feed at a wt/vol ratio of 1:1. The bacterial feed mix was freshly prepared twice per day and was provided to the chickens for four consecutive days. The average number of bacteria in the 18-h FTG culture was 1 × 109 CFU/ml, and shortly after being mixed with feed, 107 CFU/g feed was recovered, which declined to 102 to 103 CFU/g feed after 10 h on the feeder. One day after the end of the challenge infection, five birds were euthanized by CO2 inhalation for postmortem examination. The remaining five chickens were used for delayed-type hypersensitivity (DTH) assays and euthanized five days later. Individual body weights were measured before and after challenge infections.
S. enterica serovar Typhimurium vaccine strain and C. perfringens isolation from chickens.
One week after RASV boost immunization, cloacal swabs were collected for bacteriology to assess RASV shedding. Dilutions of cloacal swabs were spread on MacConkey agar plates with 1% lactose. The RASV strain was identified by PCR on randomly selected S. enterica serovar Typhimurium colonies using primers that anneal to plcC and the promoter region of the expression plasmid. The cloacal swab preparations also were spread on TSC-EY agar plates and incubated in an anaerobic jar to identify lecithinase-positive (C. perfringens) colonies. After challenge infection with C. perfringens, segments of the ileum and cecum with the intestinal contents were aseptically collected and homogenized in BSG. Tissue homogenates were diluted in BSG and spread on MacConkey agar plates for S. enterica serovar Typhimurium detection and on TSC agar plates for C. perfringens detection.
Measuring antibody responses.
Blood samples were collected weekly by wing vein puncture, and bile samples were collected during autopsy. Serum immunoglobulin G (IgG) and bile IgA responses were measured by indirect enzyme-linked immunosorbent assay (ELISA). Briefly, microtiter plates (Nunc, Roskilde, Denmark) were coated with 10 μg/ml (1 μg/well) of purified rPlcC protein diluted in coating solution (Na2CO3, 1.6 g; NaHCO3, 2.9 g; NaN3, 0.2 g; all in 1 liter distilled H2O). Plates were incubated overnight at 4°C, dried, and washed in phosphate-buffered saline (PBS)-0.2% Tween 20 (washing solution). Nonspecific binding was blocked by using Sea Block blocking buffer (Pierce, Rockford, IL) for 1 h at 37°C. Test samples (serum or bile) were twofold diluted in blocking buffer, added in duplicate, and incubated at 37°C with agitation. After repeat washes, biotinylated goat anti-chicken IgG or IgA heavy plus light chain antibody (Bethyl Laboratories, Montgomery, TX) was added (1:5,000 dilution) and incubated for 1 h at 37°C. Plates were washed, streptavidin-horseradish peroxidase solution (Southern Biotech, Birmingham, AL) (1:5,000 dilution) was added, and the plates were incubated for an extra 1 h at 37°C. Peroxidase activity and color development were detected by 2-2′-azino-di-(3-ethylbenzthiazoline sulfuric acid) (ABTS) substrate (Sigma, St. Louis, MO) containing 0.03% H2O2 in citrate buffer, pH 4.35. Plates were incubated for 10 min at 37°C for color development, and the reaction was stopped with 1% SDS solution. The OD of each well's contents was measured at 405 nm using a microplate reader (Molecular Devices, Sunnyvale, CA). A volume of 100 μl/well of the test samples, antibodies, or washing solutions was used in each steps.
The antibody response in the serum also was tested by the immunoblot analysis of immunized chicken sera against alpha toxin obtained from concentrated culture supernatant of C. perfringens and rPlcC proteins.
Alpha toxin neutralization test.
The neutralization of alpha toxin by serum antibody was determined by the inhibition of red blood cell (RBC) hemolysis by alpha toxin. RBCs were prepared by washing samples of freshly collected rabbit blood twice in PBS and diluting them to 2% (vol/vol) in PBS containing 3 mM CaCl2. Alpha toxin was obtained from the supernatant of a C. perfringens culture grown overnight by sequential filtration through 100- and 10-kDa Amicon Ultra filters (Millipore, Billerica, MA). The enzymatic activity of the culture supernatant concentrate was evaluated and quantified using a phosphatidylcholine-phospholipase C assay kit (Invitrogen, Carlsbad, CA). The concentrated culture supernatant was diluted in PBS containing 0.1 mM CaCl2, and 100-μl aliquots containing an estimated 250 ng protein (about 200 U) were distributed into a 96-well dilution plate. Purified recombinant alpha toxin (250 ng) (Sigma, St. Louis, MO) was included as a control. Test sera (pooled serum samples) were twofold diluted in PBS (1:10, 1:20, and 1:40), and 100 μl was added into wells. Control wells contained either serum with no toxin or alpha toxin alone. Well contents were mixed and incubated for 1 h at 37°C with slow agitation. After 1 h, 100 μl of the 2% RBC solution was added into each well. After incubation for 2 h at 37°C, plates were chilled for 15 min at 4°C and briefly centrifuged at 500 × g to sediment intact cells (i.e., RBCs). The absorbance of well contents was measured at 540 nm using a microtiter plate reader. As a reference, 100% RBC lysis was obtained by adding 50 μl of 1% Triton X-100 to wells containing serum and RBCs, while 100% lysis inhibition was recorded from wells without alpha toxin. The hemolysis or inhibition of cell lysis was expressed as the percent difference of the absorbance of wells with test sample, and control wells with half-diluted alpha toxin without a test sample.
DTH assay.
Four weeks after the RASV boost immunization and 3 days after the challenge infection, five chickens per group were injected intradermally with 20 μg of the rPlcC protein in 50 μl saline into the left footpad at the toe web between the first and second digits. The right foot was injected with sterile saline as a negative control. The thickness of the toe web was measured with a digital micrometric caliper at 48 and 72 h after antigen or saline injection. Data were expressed as the difference between results for the left foot and the right foot (control).
Serum bacteriostatic (growth inhibition) assay.
Sera from immunized or control chickens were filtered through 0.45-μm pore filters, decomplemented by being heated at 56°C in a water bath for 30 min, and added into the CMM broth culture at 1:20 and 1:40 final dilutions. A volume of 100 μl overnight culture of C. perfringens diluted to a concentration of 105 CFU per ml was added into 1 ml of culture medium containing serum, and the cultures were incubated anaerobically at 37°C. The growth of bacteria was monitored by the culture OD, and the number of CFU was monitored by plating serial dilutions on TSC-EY agar plates at 12 and 18 h.
Immunofluorescence microscopy.
An indirect immunofluorescence test was performed to determine if antibody against PlcC binds to the bacterial cell surface. Formalin-inactivated bacteria or a fresh colony of C. perfringens from an overnight TSC-EY plate was spread on microscope slides. The formalin inactivation of bacteria was carried out by incubating washed bacterial cells (from a 24-h culture of JGS4143 in BHI broth) in 3 mM formaldehyde solution in PBS for 2 h at 37°C, followed by overnight incubation at room temperature with slow agitation. The suspension (10 ml) was centrifuged at 5,000 × g for 15 min, and the pellet was washed twice and resuspended in PBS. Live cells were spread on slides from colonies and were killed and fixed by being heated. Slides were dried and covered with PBS containing 1% bovine serum albumin and incubated for 1 h at room temperature in a humidified box. The blocking solution was removed, and 200 μl of the test serum from immunized or control chickens diluted 1:50 in PBS was spread onto the slide and covered with a coverslip. Slides were incubated overnight at 4°C. After being washed with PBS-0.1% Tween 20, goat anti-chicken IgG antibody conjugated with fluorescein isothiocyanate (Southern Biotech, Birmingham, AL) was added, and the solution was incubated for an extra 2 h at room temperature. Slides then were washed three times in PBS-0.1% Tween 20, dried, and observed under a fluorescence microscope.
Pathological examination.
Autopsies were performed 24 h after the last day (i.e., the fourth day) of the challenge infections. The whole intestines were examined for gross lesions such as hemorrhagic spots, mucosal paleness or discoloration, swelling, feebleness of the intestinal wall, and the nature of the contents. Tissue samples of the duodenum, midjejunum, and ileum from similar segments (i.e., located at identical distances from the Meckel diverticulum mark) were collected in buffered 4% formaldehyde for histopathology. Sections of the intestinal tissues were stained with hematoxylin and eosin and were examined for microscopic lesions of enteritis and tissue morphological alterations. Villus epithelial degeneration, sloughing, and inflammatory leukocyte infiltration into lamina propria and submucosa were examined, as was villus shortening. The severity and distribution of lesions were graded semiquantitatively by using a scale (0 to 5) that considers the severity and distribution of the lesions based on prior knowledge of normal histology and severe NE lesions. The scores were given as follows: 0, no lesion; 1, scattered mild degeneration of villus tip epithelium; 2, mild degeneration detected uniformly in several microscopic fields; 3, moderate necrotic lesions with epithelial sloughing, degeneration, and leukocyte infiltrations; 4, severe epithelial degeneration and necrosis dispersed in several microscopic fields; and 5, severe and frequent necrotic lesions, often with villus shortening or atrophy.
Statistical analysis.
The differences in body weights were evaluated by two-way analysis of variance with the Bonferroni posttest method. One-way analysis of variance and the Bonferroni posttest were used to compare the differences in ELISA (absorbance) and DTH data. A P value of less than 0.05 was considered significant. The analyses were performed using the GraphPad Prism program.
RESULTS
Amino acid sequence of alpha toxin.
The primary structure of alpha toxin from NE isolate CP995 shows 98% sequence identity to the type A reference strain ATCC 13124 (GenBank accession no. M24904), with a few amino acid substitutions (Leu16 to Thr, Lys17 to Gln, Ala174 to Asp, Thr177 to Pro, Ser335 to Pro, Gly363 to Arg, and Asn364 to Lys) and a deletion of Ile21 (Fig. 2). Except for the absence of Ile21, the amino acid substitutions at the specific sites are common variations among type A isolates from humans and animals (40). These variations do not affect alpha toxin's phospholipase C and hemolytic activities or hinder the neutralizing ability of C terminus-specific antibody against alpha toxin (15).
FIG. 2.
Predicted amino acid sequence and alignment of alpha toxin (PLC) mature protein sequences of strain CP995, an NE isolate, and ATCC 13124. Residues in CP995 that are different from those of ATCC 13124 are indicated. The underlined fragment, amino acids 248 to 370, represents the C-terminal domain (PlcC) of the recombinant vaccine antigen.
PlcC expression in S. enterica serovar Typhimurium vaccine vector.
The expression of the PlcC protein in the S. enterica serovar Typhimurium vaccine strain was detected on the SDS-PAGE gel stained with Coomassie blue (Fig. 3). High levels of PlcC expression were obtained with all three plasmid constructs. The PlcC protein concentration was estimated at roughly 100 ng/μl bacterial lysate, as measured by a densitometry analysis that compared the results to those for a standard concentration of bovine serum albumin (ChemiDoc XRS; Bio-Rad, Hercules, CA). PlcC was effectively secreted by the S. enterica serovar Typhimurium vaccine strains through a type II secretion system. The fusion with the OmpA or Bla signal peptide sufficiently enabled the transport of PlcC into the periplasm, which then was secreted across the outer membrane into the culture supernatant. No β-galactosidase was detected in the culture supernatant of the RASV χ8914 (pYA9977) atrB13::MudJ strain, thus indicating that bacterial lysis had not occurred (data not shown).
The molecular masses of PlcC expressed from the three constructs appear different due to differences in the sizes of the signal peptides, the linker amino acids, and the β-lactamase C-terminal fusion in pYA4149 constructs (Fig. 1). Accordingly, the molecular mass of PlcC was 16 kDa in χ8914(pYA3977), 14 kDa in χ8914(pYA4110), and 18 kDa in χ8914(pYA4149). The molecular mass of the His-tagged rPlcC was 19 kDa.
Antibody responses in immunized chickens.
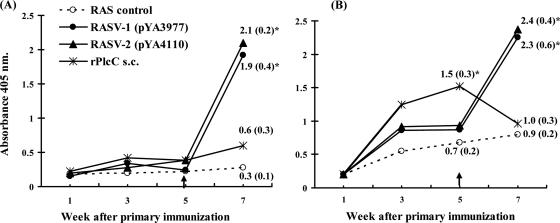
RASV-immunized chickens generally had low serum antibody responses, as measured by ELISA, at serum dilutions as low as 1:40. Unexpectedly, chickens that received s.c. immunization with rPlcC also showed a low serum IgG response similar to that of RASV-immunized chickens. Formerly, we tested whether RASV or rPlcC immunization gives some priming effect when followed by a boost protein immunization at a later time point. When the chickens were injected with 50 μg of purified rPlcC protein 5 weeks after the primary immunization (i.e., at 7 weeks of age), chickens that first were immunized with RASV developed a significant and rapid increase in antibody titers (i.e., in comparison to the typical response pattern), whereas those immunized with rPlcC injection had low titers compared to those of the RASV groups (Fig. 4A). The rPlcC-immunized chickens had a higher initial bile IgA titer, which abruptly declined after the final boost with rPlcC. In the RASV-immunized chickens, the IgA titers increased significantly after the rPlcC boost (Fig. 4B). This strong antibody response in the RASV prime and protein boost approach shows that although there were low levels of serum antibody titers generated by the primary immunization, RASV stimulates an immune reaction that produces a better memory response than repeated immunization with protein alone.
FIG. 4.
ELISA results of serum IgG (A) and bile IgA (B) responses in chickens immunized with oral RASV or s.c. rPlcC. Five weeks after the primary immunization with either RASV or s.c. rPlcC, all of the chickens were given an s.c. boost injection with 50 μg purified rPlcC protein emulsified in 100 μl incomplete Freund's adjuvant. Arrows point to the time of injection with rPlcC protein. Sera were tested at 1:80 dilutions, and bile samples were tested at 1:100 dilutions. The average absorbance of serum or bile samples from five chickens per group at each time point is shown. Values are indicated, with standard deviations in parentheses, for points that show significant mean differences from results for the RAS control group [χ8914(pYA3493)]. *, P < 0.05.
Because our goal is to develop an oral RASV, we immunized a second group of birds with RASV and performed a more complete evaluation of immune responses and C. perfringens challenge to assess the protective efficacy. Sera from the RASV-immunized chickens were tested by Western blotting on SDS-PAGE-separated rPlcC and the whole alpha toxin, and both PlcC protein and the complete alpha toxin obtained from the culture supernatant of C. perfringens were detected (Fig. 5). This confirmed the presence of serum antibody responses in RASV-immunized chickens. Sera from the RASV- or rPlcC-vaccinated chickens reacted positively with the whole alpha toxin, whether it was denatured by TCA precipitation or not (i.e., concentrated by filtration) prior to separation on SDS-PAGE.
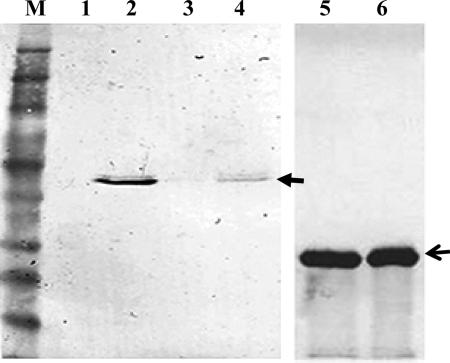
FIG. 5.
Immunoblot of C. perfringens culture supernatant and rPlcC with χ8914(pYA3977)-immunized chicken serum. Sera collected at 2 weeks after the boost immunization were pooled and used at a 1:40 dilution. C. perfringens culture supernatant was concentrated in 10% TCA (denaturing condition) or concentrated by filtration to retain the native structure. Protein bands at about 43 kDa (closed arrow) in lanes 2 and 4 show the reactivity of serum antibody with alpha toxin, and lanes 5 and 6 show the reactivity with His-tagged rPlcC (19 kDa). M, molecular mass marker (Invitrogen, Carlsbad, CA). Lanes 1 and 3, C. perfringens culture supernatant (without concentration); lane 2, C. perfringens culture supernatant concentrated by 10% TCA precipitation; lane 4, C. perfringens culture supernatant concentrated by the Centricon filtration system; lanes 5 and 6, purified rPlcC protein (open arrow).
DTH reaction.
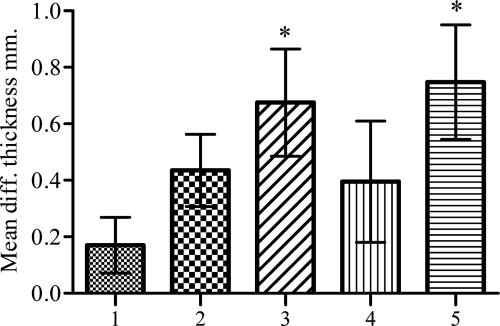
RASV-immunized chickens showed an increase in DTH responses compared to those of the chickens inoculated with the control S. enterica serovar Typhimurium vector strain (Fig. 6). The highest DTH reaction level (P < 0.05) was induced in chickens immunized with χ8914(pYA4110) or s.c. rPlcC at 48 h after PlcC injection. At 72 h after PlcC injection, the DTH responses in all immunized chickens were similar to each other and were higher than those of the control chickens (P > 0.05) (data not shown). The role of cell-mediated immunity in NE is not clear; however, since S. enterica serovar Typhimurium replicates intracellularly within antigen-presenting cells, it presumably facilitates the delivery of recombinant antigens by the endogenous antigen presentation pathway to prime CD4+ T cells and the cell-mediated response (27). DTH is commonly used as an index for cellular responses.
FIG. 6.
DTH in oral RASV- or s.c. rPlcC-immunized chickens. Four weeks after immunization, chickens were injected with 20 μg purified rPlcC protein in the right leg toe web and with saline in the left leg toe web, and the swellings were measured 48 h later. The difference (diff.) in thickness between the left and right toe webs was calculated. Values are averages from five chickens per group. Bars: 1, RAS control [χ8914(pYA3493)]; 2, RASV-1 [χ8914(pYA3977)]; 3, RASV-2 [χ8914 (pYA4110)]; 4, RASV-3 [χ8914(pYA4149)]; and 5, s.c. rPlcC. An asterisk indicates significant mean differences from results for the control group (P < 0.05).
Alpha toxin neutralization.
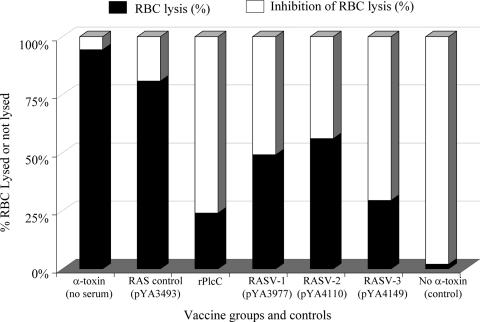
Toxin neutralization by serum antibody was measured as a function of alpha toxin-induced RBC lysis. Pooled sera from chickens immunized with RASV or rPlcC inhibited the hemolytic effect of the alpha toxin in a C. perfringens culture supernatant (Fig. 7). The highest level of RBC lysis inhibition was observed with sera from birds vaccinated with rPlcC and RASV-3 [χ8914(pYA4149)], with inhibitions of 76 and 71%, respectively. The sera from chickens immunized with the other RASVs (RASV-1 and RASV-2) also showed more than 50% reductions in RBC lysis compared to that of the RAS-immunized (control) chicken serum. These results indicate the presence of alpha toxin-neutralizing antibodies in RASV-immunized chickens.
FIG. 7.
Neutralization of alpha toxin hemolytic activity by serum antibody. Pooled sera from oral RASV- or s.c. rPlcC protein-immunized chickens or control chickens orally immunized with RASV χ8914(pYA3493), collected at 2 weeks after the boost immunization, were tested for the neutralization of alpha toxin in culture supernatant of C. perfringens by measuring the inhibition of hemolysis of rabbit RBCs. The test serum final dilution was 1:20. Alpha toxin was obtained by concentrating the culture supernatant of an overnight culture of C. perfringens. The bars represent averages from five chickens per group.
C. perfringens growth inhibition by immunized chicken serum.
Decomplemented sera from immunized chickens inhibited the proliferation of C. perfringens growth in culture (Table 2). Bacterial growth in cultures containing serum from either RASV- or rPlcC-immunized chickens was suppressed by up to 1,000-fold compared to that of cultures with serum from control nonimmunized chickens.
TABLE 2.
Bacterial growth inhibition by serum from RASV- or rPlcC-immunized chickensa
| Exptl group (source of serum) | CFU/ml at start of culture | CFU/ml after 12 h of culture |
|---|---|---|
| Culture with no serum | 2 × 102 | 2.0 × 108 |
| RAS control [χ8914(pYA3493)] | 2 × 102 | 1.6 × 108 |
| RASV-1 [χ8914(pYA3977)] | 2 × 102 | 7.8 × 105 |
| RASV-2 [χ8914(pYA4110)] | 2 × 102 | 3.0 × 106 |
| RASV-3 [χ8914(pYA4149)] | 2 × 102 | 8.0 × 105 |
| rPlcC s.c. injection | 2 × 102 | 5.2 × 106 |
Sera collected at 3 weeks after boost immunization were decomplemented, pooled, and inoculated into C. perfringens cultures containing 2 × 102 CFU at a final serum/culture dilution of 1:20.
Immunofluorescence microscopy.
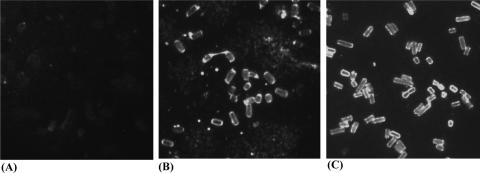
Antibodies binding to bacterial surface proteins or capsules are known to inhibit bacterial growth and to facilitate opsonophagocytosis. To investigate the inhibitory effects of the sera from immunized chickens, we evaluated the possibility of surface binding by serum antibody by using an indirect immunofluorescence assay. Remarkably, serum antibody against PlcC from immunized chickens showed binding to the surface of whole bacteria (Fig. 8). Higher levels of fluorescence were frequently detected at bacterial poles.
FIG. 8.
Indirect immunofluorescence detection of serum antibody binding to the bacterium surface. C. perfringens smears were stained with nonimmunized control chicken serum (A), rPlcC protein injection immunized-chicken serum (B), and RASV-1-primed and rPlcC parenteral boost-immunized chicken serum (C).
Isolation of the RASV strain and C. perfringens from chickens.
The S. enterica serovar Typhimurium vaccine strain was readily detected in cloacal swabs collected 1 week after the boost immunization and in the intestinal segments (from the ileum and cecum) collected during autopsy at 3 weeks after immunization. The average S. enterica serovar Typhimurium numbers in cloacal swabs were 1 × 104 CFU/g of fecal material, and 3 weeks later the numbers in the ileum and in the cecum were, on average, 1 × 102 and 1 × 104 CFU per gram of tissue, respectively. No S. enterica serovar Typhimurium was detected in the liver or spleen at 3 weeks after immunization.
C. perfringens could not be detected from cloacal swab samples collected prior to the challenge infection. Usually, a small number of C. perfringens (resident flora) cells are identified by culture from healthy young birds, especially in birds reared under experimental conditions (3, 5, 47). After the challenge infection, generally smaller numbers of C. perfringens were detected from intestines, even at 1 day after the challenge infection. The average numbers of C. perfringens cells detected in the intestines were less than 1 × 103 and 1 × 105 CFU/g in the ileum and cecum, respectively. Five days after the end of the challenge infection, the numbers in cecum declined to less than 103 CFU/g, and none could be recovered from the ileum. The numbers of C. perfringens cells recovered were highly variable among chickens in all groups, and there was no correlation between the number of bacteria isolated from an intestinal segment and lesion development.
Intestinal mucosal lesion development after challenge infection.
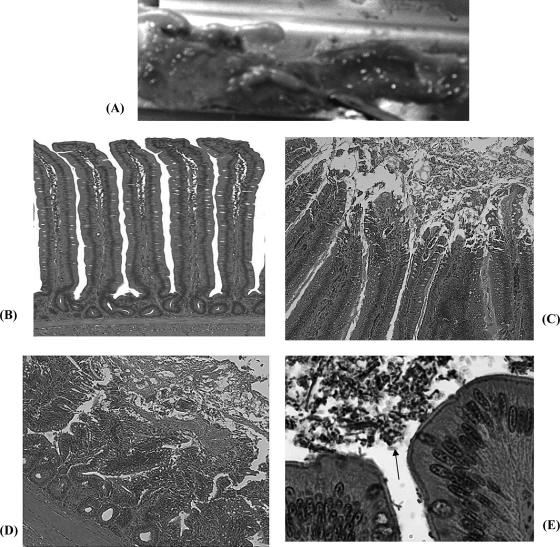
Gross lesions with mucoid intestinal luminal content, feeble intestinal walls, mucosal edema, and hemorrhagic spots were found in the duodenum and proximal jejunum of nonimmunized chickens (Fig. 9A). Overall, few chickens showed overt gross lesions and diarrhea. Histopathology lesions such as hyperemia of the lamina propria, villus tip epithelium degeneration, villus shortening, disruption of the structural integrity of enterocytes with the basement membrane, and inflammatory leukocyte infiltration were observed mainly in the jejunum. These lesions were observed 1 day after challenge predominantly in nonimmunized chickens. The hematoxylin- and eosin-stained sections also revealed clusters of C. perfringens in the intestinal lumen and mucosal surfaces that were seldom attached to epithelial surfaces (Fig. 9E). Sites with bacterial clumps often depicted a mild defect of the epithelial surface and microvilli mostly in nonimmunized or control RAS-immunized chickens. The lesions were severe and more frequent in nonimmunized (control) chickens, while immunized chickens had minimal lesions in a few chickens (Table 3). The histopathological lesions had receded by 4 days after challenge.
FIG. 9.
Micrographs of gross lesions and lesion histopathology of challenged chickens. (A) Gross lesions with hemorrhagic spots in the jejunum in control RAS-immunized chicken. (B) Jejunum (distal region) showing normal elongated villi. (C) Degeneration and sloughing of apical villus epithelium. (D) Severe necrotic lesion on villi and inflammatory cell infiltration (i.e., widened lamina propria). (E) Higher magnification of villus tip showing clumps of bacteria (arrow) in gut lumen and attached to villus tips, often without a major lesion on the surface epithelium in immunized chickens.
TABLE 3.
Gross pathology, histopathology scores, and body weights of immunized and control chickens after challenge with virulent C. perfringens strain JGS4143a
| Exptl group | No. of chickens with gross lesionsb | Histopathologyc
|
BWd (g)
|
% BW gain | ||
|---|---|---|---|---|---|---|
| Jejunum | Ileum | Prechallenge | Postchallenge | |||
| RAS control [χ8914(pYA3493)] | 3 | 3.0 | 3.3 | 365 ± 23 | 484 ± 14a | 32a |
| RASV-1 [χ8914(pYA3977)] | 1 | 2.4 | 1.7 | 331 ± 13 | 550 ± 16b | 66b |
| RASV-2 [χ8914(pYA4110)] | 1 | 1.5 | 1.0 | 330 ± 11 | 543 ± 22b,c | 65b |
| RASV-3 [χ8914(pYA4149)] | 1 | 1.6 | 1.8 | 369 ± 21 | 525 ± 12b,c | 42c |
| rPlcC s.c. injection | 1 | 2.2 | 2.1 | 358 ± 18 | 571 ± 25b | 60b |
All values are average results from five chickens per group. BW, body weight.
Macroscopic lesions in the duodenum and jejunum.
The frequency and severity of lesions were graded semiquantitatively on a scale of 0 (no lesion) to 5 (severe and frequent lesions) for tissues collected 1 day after challenge.
The body weight was measured before the C. perfringens challenge infection, at day 32 of age, and at 1 week after the end of challenge infection (42 days of age). The body weight gain is expressed as the percent difference from the weight before challenge. Different letters designate significant differences (P < 0.01).
RASV-immunized chickens showed significantly higher percentages of body weight gain at 10 days after challenge with C. perfringens compared to the average body weight gain for nonimmunized chickens (Table 3).
DISCUSSION
A subunit vaccine consisting of the C-terminal domain of alpha toxin (PlcC) protects mice against gas gangrene (43). However, no injectable PlcC subunit vaccine or live attenuated S. enterica serovar Typhimurium-delivered PlcC vaccine to induce protective immunity against C. perfringens type A strain-caused enteric disease has been evaluated before. The current study showed that an RASV expressing PlcC or an rPlcC subunit vaccine induced toxin-neutralizing antibodies and reduced intestinal lesion development and body weight loss in chickens challenged with virulent C. perfringens. Formerly, many investigators showed protection against NE by vaccination with a toxoid vaccine that consisted of an inactivated whole alpha toxin (12, 18, 24, 26). The delivery of such a vaccine by repeated parenteral injections is not a feasible approach in commercial broilers and layers.
Attenuated S. enterica serovar Typhimurium is a recognized antigen delivery vector that has a prime advantage in eliciting cellular and mucosal immune responses against recombinant antigens while also inducing immunity against multiple Salmonella enterica serotypes (1, 9, 17). Several different live, attenuated S. enterica serovar Typhimurium vaccine strains that contain defined gene mutations targeting virulence or global regulator genes such as crp/cya, galE, aro, and phoP/phoQ were developed previously (27), and several of these are widely used in chickens (1, 4, 20). The present work also demonstrates that S. enterica serovar Typhimurium with pabA and pabB deletion mutations (χ8914) is sufficiently attenuated and suitable for recombinant antigen delivery in chickens. Live attenuated oral S. enterica serovar Typhimurium vaccines are effective in inducing antibody- and cell-mediated immune responses that significantly reduce intestinal, visceral, reproductive tract, and egg colonization by virulent homologous and heterologous serovars (1, 4, 17). In a comparative study, Babu and colleagues (4) showed that live attenuated oral vaccines give a superior cell-mediated immunity compared to that of killed or bacterin vaccines. Furthermore, live attenuated S. enterica serovar Typhimurium vaccines are effective in reducing the colonization, shedding, horizontal spread, and egg contamination of Salmonella enteritidis in hens stressed by induced molting (20).
Oral RASV vectoring the C-terminal domain of alpha toxin induced neutralizing antibody against alpha toxin and reduced NE lesions. Protection against NE appears to be mediated mainly by alpha toxin neutralization and the effect of antibody on bacterial growth. The circulating IgG and bile IgA antibody titers against PlcC were low in RASV-immunized chickens. Such a weak systemic antibody response (measured in titers) to live bacterial vaccines is a common phenomenon in chickens and often does not indicate susceptibility to the pathogen (34). On the other hand, RASV immunization showed a stronger priming effect or memory response upon parenteral injection with protein than primary vaccination by s.c. injection with protein. Similar results for mice and humans also indicated that live attenuated S. enterica serovar Typhimurium-based vaccines trigger long-lasting humoral, mucosal, and cellular responses upon a boost vaccination with subunit protein without the need for repeated immunization (49). Such a RASV priming and parenteral boost strategy to induce robust antibody responses could be valuable in breeding flocks for which the target is to develop a higher maternally transferable antibody titer to protect young chicks.
Remarkably, serum from RASV-immunized chickens binds to the C. perfringens cell surface and also inhibits bacterial growth in vitro, indicating that the neutralization of virulence-associated secreted proteins such as the alpha toxins impedes bacterial proliferation. Though the level of recovery of C. perfringens from immunized and nonimmunized chicken intestines after the challenge infection generally was low and was further confounded by resident C. perfringens flora, it appeared that immunization reduced colonization by the C. perfringens challenge strain. We found that mice orally immunized with the same PlcC-expressing RASV that we used here have greatly reduced numbers of C. perfringens cells recovered from their muscles soon after challenge by intramuscular injection with C. perfringens in a gas gangrene model (B. Zekarias et al., unpublished). A rapid clearance of C. perfringens from infected muscle tissue in mice immunized with the PlcC subunit vaccine also was reported previously (35). This suggests a role for alpha toxin in C. perfringens invasion and persistence. It is predictable that alpha toxin is involved in the acquisition or assimilation of nutrients by degrading membrane phospholipids in vivo as well as during in vitro growth in CMM. Among other factors, the production of alpha toxin is regulated by the luxS gene through the common quorum-sensing signal molecule AI-2 (36). This indicates that alpha toxin plays a role in the competitive exclusion of other enteric bacteria and in intestinal colonization. Thus, the interruption of these functions could inhibit C. perfringens growth. In addition, immunofluorescence staining showed that serum antibodies bind directly to the bacterial surface, which by itself could hinder bacterial replication. The antibody seems to bind to the alpha toxin preprotein that is attached to the cell membrane. Unlike gram-negative bacteria that have a periplasm space, secreted proteins and exotoxins of gram-positive bacteria accumulate within the cytoplasm, and most remain adhered to the cell membrane (6). It is conceivable that antibodies binding to the membrane-bound preprotein can block protein transport channels and, thus, inhibit the proliferation of the bacteria (41). The high level of fluorescence concentrated at the bacterial poles that indicates antibody binding seems to be related to the distinct Sec pathway apparatuses that have been described for gram-positive bacteria (6, 38).
The molecular pathogenesis of NE and other type A enteropathologies caused by C. perfringens is not clearly defined. While the significance of alpha toxin in the pathogenesis of NE has long been known, experimental infections with C. perfringens often do not reproduce a clinical disease similar to that seen in field cases. Severe NE could be experimentally reproduced only in germ-free (12) or in immunosuppressed (30) chickens by oral inoculation with C. perfringens culture or purified alpha toxin. In contrast, Keyburn and colleagues (23) recently reported the induction of NE in conventional chickens by experimental infection with a plc deletion mutant strain that does not produce alpha toxin. This apparent contradiction to the common theory might be explained by the floral toxigenic C. perfringens strains having inflicted the pathology described in the report by Keyburn et al. (23). A comparison of in vitro toxin secretion from clinical isolates also is less relevant, since isolates from healthy and clinical cases often produce similar amounts of alpha toxin depending on the growth conditions (14). So far, many studies about the pathogenesis of NE have focused on predisposing factors such as diet composition (3) or coinfection with other pathogens (52). More studies are needed on the pathogenomics of NE-associated type A isolates and on the pathogenesis of the disease in distinct geographic locations. It is possible that NE clinical isolates contain some unidentified virulence factors that maximize colonization or displace (non-NE) C. perfringens strains of the normal flora (5).
In conclusion, a candidate oral RASV vectoring the C-terminal fragment of alpha toxin induces antibody responses that neutralize alpha toxin, suppress the replication of C. perfringens, and reduce intestinal lesion development in experimental NE in broilers. It showed that an alpha toxin C-terminal domain vaccine is capable of eliciting protective immunity against C. perfringens-associated enteric pathology. Type A C. perfringens strains are the prevalent causes of C. perfringens-associated enteric diseases in various farm animal species (7, 31, 42). Although the type A strains produce the highest levels of alpha toxin and alpha toxin is the only major toxin in these strains (7, 37), alpha toxin is produced by all C. perfringens isolates and is conserved among clinical isolates (15). Thus, an RASV vectoring PlcC could be used to elicit protection against enteric pathology associated with type A strains in other animals. The RASV against NE and C. perfringens infections confers the potential benefit of utilizing the existing technology of recombinant attenuated S. enterica serovar Typhimurium vaccine strains for the delivery of antigen from pathogens of significant importance to poultry health and food safety.
Acknowledgments
We acknowledge Glenn Songer for constructive discussions and kindly supplying C. perfringens strain JGS4143, Gregory Siragusa for providing strain CP995, Bronwyn Gunn and Jenny Maurer for the construction of S. enterica serovar Typhimurium vaccine strain χ8914, and Soo-Young Wanda for her help in plasmid constructions. We thank Kenneth Roland and Shelley Haydel for their critical reading of the manuscript.
This work was supported by Ellison Medical Foundation grant ID-SS-0520-03.
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Abd El Ghany, M., A. Jansen, S. Clare, L. Hall, D. Pickard, R. A. Kingsley, and G. Dougan. 2007. Candidate live, attenuated Salmonella enterica serotype Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect. Immun. 75:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Sheikhly, F., and R. B. Truscott. 1977. The pathology of necrotic enteritis of chickens following infusion of broth cultures of Clostridium perfringens into the duodenum. Avian Dis. 21:230-240. [PubMed] [Google Scholar]
- 3.Annett, C. B., J. R. Viste, M. Chirino-Trejo, H. L. Classen, D. M. Middleton, and E. Simko. 2002. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 31:598-601. [DOI] [PubMed] [Google Scholar]
- 4.Babu, U., M. Scott, M. J. Myers, M. Okamura, D. Gaines, H. F. Yancy, H. Lillehoj, R. A. Heckert, and R. B. Raybourne. 2003. Effects of live attenuated and killed Salmonella vaccine on T-lymphocyte mediated immunity in laying hens. Vet. Immunol. Immunopathol. 91:39-44. [DOI] [PubMed] [Google Scholar]
- 5.Barbara, A. J., H. T. Trinh, R. D. Glock, and J. G. Songer. 2008. Necrotic enteritis-producing strains of Clostridium perfringens displace non-necrotic enteritis strains from the gut of chicks. Vet. Microbiol. 126:377-382. [DOI] [PubMed] [Google Scholar]
- 6.Buist, G., A. N. Ridder, J. Kok, and O. P. Kuipers. 2006. Different subcellular locations of secretome components of gram-positive bacteria. Microbiology 152:2867-2874. [DOI] [PubMed] [Google Scholar]
- 7.Crespo, R., D. J. Fisher, H. L. Shivaprasad, M. E. Fernandez-Miyakawa, and F. A. Uzal. 2007. Toxinotypes of Clostridium perfringens isolated from sick and healthy avian species. J. Vet. Diagn. Investig. 19:329-333. [DOI] [PubMed] [Google Scholar]
- 8.Curtiss, R., III, and J. O. Hassan. 1996. Nonrecombinant and recombinant avirulent Salmonella vaccines for poultry. Vet. Immunol. Immunopathol. 54:365-372. [DOI] [PubMed] [Google Scholar]
- 9.Curtiss, R., III, X. Zhang, S. Y. Wanda, H. Y. Kang, V. Konjufca, Y. Li, B. Gunn, S. Wang, G. Scarpellini, and I. S. Lee. 2007. Induction of host immune responses using Salmonella-vectored vaccines, p. 297-313. In K. A. Brogden, F. C. Minion, N. Cornick, T. B. Stanton, Q. Zhang, L. Nolan, and M. J. Wannemuehler (ed.), Virulence mechanisms of bacterial pathogens. ASM Press, Washington DC.
- 10.Dahiya, J. P., D. C. Wilkie, A. G. Van Kessela, and M. D. Drew. 2006. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. Anim. Feed Sci. Technol. 129:60-88. [Google Scholar]
- 11.Dhillon, A. S., P. Roy, L. Lauerman, D. Schaberg, S. Weber, D. Bandli, and F. Wier. 2004. High mortality in egg layers as a result of necrotic enteritis. Avian Dis. 48:675-680. [DOI] [PubMed] [Google Scholar]
- 12.Fukata, T., Y. Hadate, E. Baba, T. Uemura, and A. Arakawa. 1988. Influence of Clostridium perfringens and its toxin in germ-free chickens. Res. Vet. Sci. 44:68-70. [PubMed] [Google Scholar]
- 13.Galán, J. E., K. Nakayama, and R. Curtiss III. 1990. Cloning and characterization of the asd gene of Salmonella Typhimurium: use in stable maintenance of recombinant plasmids in Salmonella vaccine strains. Gene 94: 29-35. [DOI] [PubMed] [Google Scholar]
- 14.Gholamiandekhordi, A. R., R. Ducatelle, M. Heyndrickx, F. Haesebrouck, and F. Van Immerseel. 2006. Molecular and phenotypical characterization of Clostridium perfringens isolates from poultry flocks with different disease status. Vet. Microbiol. 113:143-152. [DOI] [PubMed] [Google Scholar]
- 15.Ginter, A., E. D. Williamson, F. Dessy, P. Coppe, H. Bullifent, A. Howells, and R. W. Titball. 1996. Molecular variation between the alpha-toxins from the type strain (NCTC 8237) and clinical isolates of Clostridium perfringens associated with disease in man and animals. Microbiology 142:191-198. [DOI] [PubMed] [Google Scholar]
- 16.Grave, K., M. C. Kaldhusdal, H. Kruse, L. M. Harr, and K. Flatlandsmo. 2004. What has happened in Norway after the ban of avoparcin? Consumption of antimicrobials by poultry. Prev. Vet. Med. 62:59-72. [DOI] [PubMed] [Google Scholar]
- 17.Hassan, J. O., and R. Curtiss III. 1994. Development and evaluation of an experimental vaccination program using a live avirulent Salmonella typhimurium strain to protect immunized chickens against challenge with homologous and heterologous Salmonella serotypes. Infect. Immun. 62:5519-5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heier, B. T., A. Lovland, K. B. Soleim, M. Kaldhusdal, and J. Jarp. 2001. A field study of naturally occurring specific antibodies against Clostridium perfringens alpha toxin in Norwegian broiler flocks. Avian Dis. 45:724-732. [PubMed] [Google Scholar]
- 19.Hermans, P. G., and K. L. Morgan. 2007. Prevalence and associated risk factors of necrotic enteritis on broiler farms in the United Kingdom; a cross-sectional survey. Avian Pathol. 36:43-51. [DOI] [PubMed] [Google Scholar]
- 20.Holt, P. S., R. K. Gast, and S. Kelly-Aehle. 2003. Use of a live attenuated Salmonella typhimurium vaccine to protect hens against Salmonella enteritidis infection while undergoing molt. Avian Dis. 47:656-661. [DOI] [PubMed] [Google Scholar]
- 21.Kaldhusdal, M., and A. Lovland. 2000. Necrotic enteritis (4): the economical impact of Clostridium perfringens is greater than anticipated. World Poult. 16:50-51. [Google Scholar]
- 22.Kang, H. Y., and R. Curtiss III. 2003. Immune responses dependent on antigen location in recombinant attenuated Salmonella Typhimurium vaccines following oral immunization. FEMS Immunol. Med. Microbiol. 37:99-104. [DOI] [PubMed] [Google Scholar]
- 23.Keyburn, A. L., S. A. Sheedy, M. E. Ford, M. M. Williamson, M. M. Awad, J. I. Rood, and R. J. Moore. 2006. Alpha-toxin of Clostridium perfringens is not an essential virulence factor in necrotic enteritis in chickens. Infect. Immun. 74:6496-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni, R. R., V. R. Parreira, S. Sharif, and J. F. Prescott. 2007. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin. Vaccine Immunol. 14:1070-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovland, A., M. Kaldhusdal, and L. J. Reitan. 2003. Diagnosing Clostridium perfringens-associated necrotic enteritis in broiler flocks by an immunoglobulin G anti-alpha-toxin enzyme-linked immunosorbent assay. Avian Pathol. 32:527-534. [DOI] [PubMed] [Google Scholar]
- 26.Lovland, A., M. Kaldhusdal, K. Redhead, E. Skjerve, and A. Lillehaug. 2004. Maternal vaccination against subclinical necrotic enteritis in broilers. Avian Pathol. 33:83-92. [DOI] [PubMed] [Google Scholar]
- 27.Mastroeni, P., J. A. Chabalgoity, S. J. Dunstan, D. J. Maskell, and G. Dougan. 2001. Salmonella: immune responses and vaccines. Vet. J. 161:132-164. [DOI] [PubMed] [Google Scholar]
- 28.McClane, B. A. 2001. Clostridium perfringens. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers, 2nd ed. ASM Press, Washington, DC.
- 29.McDevitt, R. M., J. D. Brooker, T. Acamovic, and N. H. C. Sparks. 2006. Necrotic enteritis; a continuing challenge for the poultry industry. World Poult. Sci. J. 62:221-247. [Google Scholar]
- 30.McReynolds, J. L., J. A. Byrd, R. C. Anderson, R. W. Moore, T. S. Edrington, K. J. Genovese, T. L. Poole, L. F. Kubena, and D. J. Nisbet. 2004. Evaluation of immunosuppressants and dietary mechanisms in an experimental disease model for necrotic enteritis. Poult. Sci. 83:1948-1952. [DOI] [PubMed] [Google Scholar]
- 31.Meer, R. R., and J. G. Songer. 1997. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am. J. Vet. Res. 58:702-705. [PubMed] [Google Scholar]
- 32.Mellon, M., S. Fondriest, et al. 2001. Hogging it! Estimates of antimicrobial abuse in livestock. Nucleus 23:1-3. http://www.ucsusa.org/food_and_environment/antibiotics_and_food/hogging-it-estimates-of-antimicrobial-abuse-in-livestock.html. [Google Scholar]
- 33.Nakayama, K., S. M. Kelly, and R. Curtiss III. 1988. Construction of an asd+ expression-cloning vector: stable maintenance and high level expression of cloned genes in a Salmonella vaccine strain. Bio/Technology 6:693-697. [Google Scholar]
- 34.Noormohammadi, A. H., J. F. Jones, G. Underwood, and K. G. Whithear. 2001. Poor systemic antibody response after vaccination of commercial broiler breeders with Mycoplasma gallisepticum vaccine ts-11 not associated with susceptibility to challenge. Avian Dis. 46:623-628. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien, D. K., and S. B. Melville. 2004. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infect. Immun. 72:5204-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohtani, K., H. Hayashi, and T. Shimizu. 2002. The luxS gene is involved in cell-cell signalling for toxin production in Clostridium perfringens. Mol. Microbiol. 44:171-179. [DOI] [PubMed] [Google Scholar]
- 37.Petit, L., M. Gibert, and M. R. Popoff. 1999. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 7:104-710. [DOI] [PubMed] [Google Scholar]
- 38.Rosch, J., and M. Caparon. 2004. A microdomain for protein secretion in gram-positive bacteria. Science 304:1513-1515. [DOI] [PubMed] [Google Scholar]
- 39.Sakurai, J., M. Nagahama, and M. Oda. 2004. Clostridium perfringens alpha-toxin: characterization and mode of action. J. Biochem. (Tokyo) 136:569-574. [DOI] [PubMed] [Google Scholar]
- 40.Schoepe, H., A. Neubauer, T. Schlapp, L. H. Wieler, and G. Baljer. 2006. Immunization with an alpha toxin variant 121A/91-R212H protects mice against Clostridium perfringens alpha toxin. Anaerobe 12:44-48. [DOI] [PubMed] [Google Scholar]
- 41.Sharipova, M. R. 2002. Late stages of protein secretion in bacilli. Biochemistry (Moscow) 67:1207-1216. [DOI] [PubMed] [Google Scholar]
- 42.Songer, J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens, D. L., R. W. Titball, M. Jepson, C. R. Bayer, S. M. Hayes-Schroer, and A. E. Bryant. 2004. Immunization with the C-domain of alpha-toxin prevents lethal infection, localizes tissue injury, and promotes host response to challenge with Clostridium perfringens. J. Infect. Dis. 190:767-773. [DOI] [PubMed] [Google Scholar]
- 44.Thompson, D. R., V. R. Parreira, R. R. Kulkarni, and J. F. Prescott. 2006. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet. Microbiol. 113:25-34. [DOI] [PubMed] [Google Scholar]
- 45.Titball, R. W., A. M. Fearn, and E. D. Williamson. 1993. Biochemical and immunological properties of the C-terminal domain of the alpha-toxin of Clostridium perfringens. FEMS Microbiol. Lett. 110:45-50. [DOI] [PubMed] [Google Scholar]
- 46.Titball, R. W., C. E. Naylor, and A. K. Basak. 1999. The Clostridium perfringens alpha-toxin. Anaerobe 5:51-64. [DOI] [PubMed] [Google Scholar]
- 47.Truscott, R. B., and F. Al-Sheikhly. 1977. Reproduction and treatment of necrotic enteritis in broilers. Am. J. Vet. Res. 38:857-861. [PubMed] [Google Scholar]
- 48.Van Immerseel, F., J. De Buck, F. Pasmans, G. Huyghebaert, F. Haesebrouck, and R. Ducatelle. 2004. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 33:537-549. [DOI] [PubMed] [Google Scholar]
- 49.Vindurampulle, C. J., L. F. Cuberos, E. M. Barry, M. F. Pasetti, and M. M. Levine. 2004. Recombinant Salmonella enterica serovar Typhi in a prime-boost strategy. Vaccine 22:3744-3750. [DOI] [PubMed] [Google Scholar]
- 50.Wegener, H. C., F. M. Aarestrup, L. B. Jensen, A. M. Hammerum, and F. Bager. 1999. Use of antimicrobial growth promoters in food animals and Enterococcus faecium resistance to therapeutic antimicrobial drugs in Europe. Emerg. Infect. Dis. 5:329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wierup, M. 2001. The Swedish experience of the 1986 year ban of antimicrobial growth promoters, with special reference to animal health, disease prevention, productivity, and usage of antimicrobials. Microb. Drug Resist. 7:183-190. [DOI] [PubMed] [Google Scholar]
- 52.Williams, R. B., R. N. Marshall, R. M. La Ragione, and J. Catchpole. 2003. A new method for the experimental production of necrotic enteritis and its use for studies on the relationships between necrotic enteritis, coccidiosis and anticoccidial vaccination of chickens. Parasitol. Res. 90:19-26. [DOI] [PubMed] [Google Scholar]
- 53.Xin, W., S. Y. Wanda, Y. Li, S. Wang, H. Mo, and R. Curtiss III. 2007. Analysis of type II secretion of recombinant pneumococcal PspA in a Salmonella Typhimurium vaccine with regulated delayed antigen expression, abstr. E-083, p. 283. Abstr. 107th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.