Abstract
Immunoassay interference causing unexpected reactive results in magnetic-microparticle-based assays was detected. A systematic evaluation of Liaison Epstein-Barr virus immunoglobulin M showed that 5% of the positive results (0.4% of tested samples) could be explained by such interference. Adding chemical blocking reagents (polyvinylpyrrolidone and polyvinyl alcohol) to the assay buffers partially prevented this phenomenon.
The Liaison diagnostic system (DiaSorin, Saluggia, Italy) is a convenient, automated immunoassay platform based on chemiluminescence and antigen/antibody-coated magnetic microparticles (4, 8). Immunoassay interference was suspected when, in a patient suffering from chronic fatigue, positive results were found for Borrelia burgdorferi sensu lato immunoglobulin M (IgM) (index, 7.0; cutoff, 1.1), cytomegalovirus IgM (70 mU/liter; cutoff, 30 mU/liter), Epstein-Barr virus (EBV) IgM (1,620 mU/liter; cutoff, 40 mU/liter), and herpes simplex virus IgM (index, 32; cutoff, 1.1). None of these positive results could be confirmed, either with other immunoassays or with immunoblotting. The patient serum contained neither rheumatoid factor nor paraprotein. We further thoroughly investigated the serum from this patient; we estimated the frequency of this type of interference, and we demonstrated a simple and inexpensive solution to partially prevent the problem.
In the experimental methods described below, appropriate positive and negative control samples were used to detect any undesirable effects of the procedures.
The index sample was pretreated with RF-Absorbent (Dade Behring, Marburg, Germany), which contains sheep IgM antibodies targeted against human IgG Fc fragments: 250 μl of RF-Absorbent was added to 250 μl of serum, and the mixture was briefly vortexed and incubated for 1 h at room temperature. This procedure, which precipitates IgG along with rheumatoid factor, did not affect the results and excluded insufficient sample pretreatment in the Liaison assays.
Heterophilic antibody interference was excluded by treating the sample, according to the manufacturer's instructions, with a nonspecific antibody-blocking tube (Scantibodies Laboratory, Santee, CA). We also treated the sample by adding 40 μg of PolyMAK-33 (MAK33-IgG1/IgG1 Poly; Roche Diagnostics, Mannheim, Germany) to 250 μl of serum and incubated this mixture for 1 h at room temperature. PolyMAK-33 is a polymerized murine IgG1 preparation, superior to polyclonal mouse immunoglobulins in blocking heterophilic antibody activity (9). This procedure did not affect the results.
However, incubating 250 μl of sample with 75 μl unlabeled beads (kindly provided by DiaSorin) at room temperature for 15 min and centrifuging this mixture for 5 min at 2,000 × g completely eliminated the interference. Apparently, IgM antibodies from the patient reacted with the solid phase in the assays. To test whether this reactivity was restricted to one specific type of microparticle, we evaluated seven different types of microparticles (Dynabeads; Dynal Biotech, Oslo, Norway): M-270 amine, M-270 carboxylic acid, M-270 epoxy, M-280 sheep anti-mouse IgG, M-270 streptavidin, M-280 streptavidin, and M-280 tosyl activated. Two hundred fifty microliters of serum was added to approximately 0.4 × 109 beads, briefly vortexed, and incubated for 15 min at room temperature. After centrifugation (5 min; 2,000 × g), the supernatant was analyzed. The interference could be completely eliminated by using M-280 tosyl-activated beads or M-270 epoxy beads and partially eliminated by using M-270 amine beads, M-280 streptavidin beads, or M-280 sheep anti-mouse IgG beads. No effect was seen when the serum was incubated with M-270 streptavidin beads or with M-270 carboxylic acid beads.
From these experiments, we concluded that (i) since the M-270 streptavidin beads are based on carboxylic acid beads, the described interference would probably not occur in assays applying carboxylic acid-based beads, and (ii) the interference could be completely eliminated with unlabeled tosyl-activated beads but only partially with tosyl-activated beads already covered with an antigen (M-280 streptavidin or M-280 sheep anti-mouse IgG). Increasing the antigen density on the beads might prevent contact of IgM with the bead surface, avoiding the bead-linked interference. However, increasing the antigen density is not easily done; additional chemical blocking could be an alternative solution for the problem. To test this hypothesis, polyvinylpyrrolidone (PVP-360; Sigma-Aldrich) and polyvinyl alcohol (P8136; Sigma-Aldrich) were added to the sample diluents used in the assays (11, 12). This modification indeed strongly reduced or completely eliminated the interference effect. Polyvinylpyrrolidone and polyvinyl alcohol, both water-soluble polymers, can minimize background signals, mainly by competing with nonspecific adsorption of proteins to the solid phase (1, 6, 10).
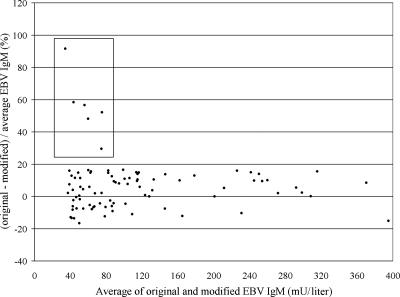
Since interference was most prominent in the EBV IgM assay, we focused on this assay and found that adding polyvinylpyrrolidone and polyvinyl alcohol to the EBV IgM dilution buffer (buffer A) at final concentrations of 0.1% and 0.005%, respectively, was optimal in reducing the interference. Although this procedure did not completely abolish the strong interference in the index patient, the effect was significantly reduced: the EBV IgM concentration fell from 1,620 mU/liter in the original assay to 320 mU/liter in the modified assay. This easy, inexpensive modification allowed us to estimate the frequency at which this interference occurs by using the modified assay on 120 consecutive samples with a positive result for EBV IgM (>40 mU/liter). The results from this comparative study are shown in Fig. 1 as a Bland-Altman plot. Discrepant results were defined as differing more than 24% (three times the interassay coefficient of variation) between the original and modified assay. Six discrepant results were found (5% of EBV IgM-positive samples; 0.4% of all EBV IgM requests). Interpreting the results as suggested by the manufacturer (<20 mU/liter, negative; 20 to 39 mU/liter, equivocal; ≥40 mU/liter, positive), one sample became negative and three became equivocal by using this modified assay.
FIG. 1.
Bland-Altman plot comparing results obtained with the modified and original EBV IgM assays on the Liaison platform. The six discrepant results are marked (box). Only results of <400 mU/liter are shown (n = 89).
Determination of EBV IgM in these six samples using an enzyme-linked immunosorbent assay (Enzygnost anti-EBV/IgM II; Dade Behring) and an immunoblot (Euroline anti-EBV-profile 2; Euroimmun, Lübeck, Germany) showed them to be EBV IgM negative. These six samples were also positive for herpes simplex virus IgM by the Liaison system (index range, 1.9 to 2.8) but negative using an enzyme-linked immunosorbent assay (Enzygnost anti-HSV/IgM; Dade Behring). One sample was also positive on Liaison testing for B. burgdorferi sensu lato IgM (index, 1.5) but negative on immunoblotting (Borrelia afzelii Western blot; Euroimmun).
Immunoassay interference through endogenous antibodies, such as rheumatoid factor or heterophilic antibodies, remains a continuing challenge (2, 3, 7). The interference we described here was apparently caused by the direct binding of IgM antibodies to surface-modified polystyrene microparticles. These “solid-phase reactive antibodies” have also been described in flow cytometry-based multiplex bead array assays (5, 12). In this technology, the use of reagent blank beads can detect high-level background signals caused by polyreactive antibodies or nonspecific binding antibodies. However, this important advantage is not present in the Liaison assay and many other immunoassay platforms. Since this type of assay interference cannot be predicted or easily recognized in the Liaison assay, the inexpensive preventive measure we propose can reduce the number of false-positive results.
Acknowledgments
We are grateful to the DiaSorin Belgian customer support team for providing us with necessary information on Liaison assay applications and for providing the unlabeled beads we used in our initial experiments. We also thank A. Vereecken and G. Salembier for their support of this study.
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Barrett, D. A., M. S. Hartshome, M. A. Hussain, P. N. Shaw, and M. C. Davies. 2001. Resistance to nonspecific protein adsorption by poly(vinyl alcohol) thin films adsorbed to a poly(styrene) support matrix using surface plasmon resonance. Anal. Chem. 73:5232-5239. [DOI] [PubMed] [Google Scholar]
- 2.Berth, M., E. Bosmans, J. Everaert, J. Dierick, J. Schiettecatte, E. Anckaert, and J. Delanghe. 2006. Rheumatoid factor interference in the determination of carbohydrate antigen 19-9 (CA 19-9). Clin. Chem. Lab. Med. 44:1137-1139. [DOI] [PubMed] [Google Scholar]
- 3.Cavalier, E., A. Carlisi, J. P. Chapelle, and P. Delanaye. 2008. False positive PTH results: an easy strategy to test and detect analytical interferences in routine practice. Clin. Chim. Acta 387:150-152. [DOI] [PubMed] [Google Scholar]
- 4.Feng, Z., Z. Li, B. Sui, G. Xu, and T. Xia. 2005. Serological diagnosis of infectious mononucleosis by chemiluminescent immunoassay using capsid antigen p18 of Epstein-Barr virus. Clin. Chim. Acta 354:77-82. [DOI] [PubMed] [Google Scholar]
- 5.Fritzler, M. J., F. Behmanesh, and M. L. Fritzler. 2006. Analysis of human sera that are polyreactive in an addressable laser bead immunoassay. Clin. Immunol. 120:349-356. [DOI] [PubMed] [Google Scholar]
- 6.Haycock, J. W. 1993. Polyvinylpyrrolidone as a blocking agent in immunochemical studies. Anal. Biochem. 208:397-399. [DOI] [PubMed] [Google Scholar]
- 7.Ismail, A. A. 2005. A radical approach is needed to eliminate interference from endogenous antibodies in immunoassays. Clin. Chem. 51:25-26. [DOI] [PubMed] [Google Scholar]
- 8.Petersen, E., M. V. Borobio, E. Guy, O. Liesenfeld, V. Meroni, A. Naessens, E. Spranzi, and P. Thulliez. 2005. European multicenter study of the LIAISON automated diagnostic system for determination of Toxoplasma gondii-specific immunoglobulin G (IgG) and IgM and the IgG avidity index. J. Clin. Microbiol. 43:1570-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinsberg, J. 1998. Interferences with two-site immunoassays by human anti-mouse antibodies formed by patients treated with monoclonal antibodies: comparison of different blocking reagents. Clin. Chem. 44:1742-1744. [PubMed] [Google Scholar]
- 10.Rodda, D. J., and H. Yamazaki. 1994. Poly(vinyl alcohol) as a blocking agent in enzyme immunoassays. Immunol. Investig. 23:421-428. [DOI] [PubMed] [Google Scholar]
- 11.Studentsov, Y. Y., M. Schiffman, H. D. Strickler, G. Y. Ho, Y. Y. Pang, J. Schiller, R. Herrero, and R. D. Burk. 2002. Enhanced enzyme-linked immunosorbent assay for detection of antibodies to virus-like particles of human papillomavirus. J. Clin. Microbiol. 40:1755-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waterboer, T., P. Sehr, and M. Pawlita. 2006. Suppression of non-specific binding in serological Luminex assays. J. Immunol. Methods 309:200-204. [DOI] [PubMed] [Google Scholar]