Abstract
Plant ribosome-inactivating proteins (RIPs) are RNA N-glycosidases that inhibit protein synthesis in cells. Abrin, a type II RIP, is an AB type toxin, which is one of the most lethal types of toxin known. The B chain facilitates the entry of the molecule into the cell, whereas the A chain exerts the toxic effect. We have generated hybridomas secreting antibodies of the immunoglobulin G class specific to the recombinant A chain of abrin. One monoclonal antibody, namely, D6F10, rescued cells from abrin toxicity. Importantly, the antibody also protected mice from lethal doses of the toxin. The neutralizing effect of the antibody was shown to be due to interference with abrin attachment to the cell surface.
Ribosome-inactivating proteins (RIPs) are a family of protein toxins that bring about the inhibition of protein synthesis either directly by inactivating the ribosomes or indirectly by modifying factors involved in translation (27). They are distributed widely in nature and are found in bacteria, fungi, and plants (34). One of the most potent members of the RIP family is abrin produced by the subtropical climber Abrus precatorius (2). Abrin is an AB type toxin with a 30-kDa A chain, an RNA N-glycosidase that irreversibly inactivates the 28S rRNA of the mammalian 60S ribosomal subunit. Once in the cytosol, the A chain depurinates the adenine of the alpha-sarcin-ricin loop and thereby arrests host cell protein synthesis (7, 28). The B chain is a galactose-specific lectin and hence binds to cell surface glycosylated receptors, which allows the toxin entry (31, 16). Apart from inhibiting protein synthesis, RIPs induce apoptosis (23). Abrin shows significant similarities to ricin at the sequence level as well as the structural level, but abrin is several times more potent than ricin (28). There is much interest in understanding the bioactivities of these proteins, owing to their extreme toxicity (the 50% lethal dose [LD50] of abrin for mice is 0.04 μg/kg of body weight, and the LD50 of ricin is 3 μg/kg) (35), stability, and easy availability. Both proteins cause pulmonary edema, with acute destructive alveolitis and apoptosis and necrosis in the lower respiratory tract epithelium (10, 41). RIPs from different plants are potent elicitors of sensitization and immunoglobulin E (IgE) production (36). The use of these proteins as bioterrorist weapons is also of considerable concern (3, 4, 17). The passive administration of antibodies has proven to be a specific and effective mode of defense against poisoning by various biological toxins (29). Although both anti-A chain and anti-B chain antibodies are able to neutralize toxins in vitro and in vivo, antibodies against the A chain of ricin have better protective efficacy than anti-B chain antibodies (14, 19).
While many detection and treatment modalities for ricin toxicity have been developed previously, few methods for the treatment of abrin toxicity are known (3, 40, 15). No antidote or vaccine for abrin has been described before now. The aim of the present study was to identify high-affinity neutralizing antibodies capable of inhibiting the toxicity of abrin. Toward this end, a panel of monoclonal antibodies (MAbs) against the recombinant abrin A chain (rABA) were raised and the neutralizing potentials of the antibodies were tested. One MAb, D6F10, completely inhibited abrin toxicity when tested in a cell culture system as well as when tested in mice.
MATERIALS AND METHODS
Dulbecco's modified Eagle's medium, RPMI 1640, Iscove's modified Dulbecco's medium (IMDM), bovine serum albumin (BSA), acridine orange, ethidium bromide, fetal bovine serum (FBS), propidium iodide, RNase A, and proteinase K were purchased from Sigma (St. Louis, MO), Ni-nitrilotriacetic acid (NTA) beads were purchased from Qiagen (Germany), and Sephadex G-100 was purchased from Amersham Pharmacia Biotech (Sweden).
Cell lines.
MCF-7 (human breast cancer cell line) and OVCAR-3 (human ovarian cancer cell line) cells were cultured in Dulbecco's modified Eagle's medium, JR4 Jurkat (human T-cell line) cells were cultured in RPMI 1640, and Sp2/0 myeloma and hybridoma cells were cultured in IMDM at 37°C in a humidified air-CO2 (19:1) atmosphere. The cell lines were obtained from the National Centre for Cell Sciences, Pune, India. All media were supplemented with 10% (vol/vol) FBS, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml.
Purification of abrin and A. precatorius agglutinin I.
Abrin and A. precatorius agglutinin I were purified from the seeds of A. precatorius as described previously (11). The seed kernels were soaked in 5% acetic acid overnight and homogenized. The crude extract was subjected to 30% ammonium sulfate precipitation, and the supernatant was subsequently subjected to 90% ammonium sulfate precipitation. The precipitate was dissolved in 20 mM phosphate buffer, pH 7.4, containing 150 mM NaCl (PBS) and dialyzed extensively against the same buffer. The dialysate was centrifuged at 10,000 rpm at 4°C for 15 min (Beckman Coulter Avanti JE), and the supernatant was analyzed by chromatography on a lactamyl-Sepharose affinity column preequilibrated with PBS. The bound proteins (abrin and A. precatorius agglutinin I) were then eluted with 0.4 M lactose and dialyzed against PBS. The dialysate was then loaded onto a Sephadex G-100 gel filtration column equilibrated with PBS. The fractions corresponding to peak I (molecular mass of 120 kDa) and peak II (molecular mass of 60 kDa) were pooled separately, dialyzed extensively against water, and lyophilized.
Ricin and Ricinus communis agglutinin I were purified from the seeds of R. communis earlier in this laboratory, using procedures reported elsewhere (2; S. Bagaria and A. A. Karande, unpublished data).
Purification of rABA and native A chain of abrin.
The rABA clone was a kind gift from J. Y. Lin, National Taiwan University, Taiwan, Republic of China. The rABA was subcloned in the pRSETA vector and expressed in Escherichia coli BL21/pLysS cells upon induction with 400 μM IPTG (isopropyl-β-d-thiogalactopyranoside) for 4 h. The cells were then pelletted, sonicated, and centrifuged at 14,000 rpm for 30 min at 4°C (Beckman Coulter Avanti JE). The recombinant protein was purified from the cell lysate on Ni-NTA beads per the manufacturer's protocol. The native A chain of abrin was prepared by subjecting abrin to reduction with 50 mM dithiothreitol in PBS for 1.5 h. Upon the dissociation of the disulfide bond, the B chain precipitated and the supernatant containing the A chain was subjected to lactamyl-Sepharose affinity chromatography to remove the unreduced abrin (26, 32).
Establishment of hybridomas.
The protocol followed for the generation of hybridomas was essentially that reported by Kohler and Milstein (12) with a few modifications (6, 9). Twenty micrograms of rABA emulsified in Freund's adjuvant was administered subcutaneously to 6- to 8-week-old female BALB/c mice. After two booster doses of rABA of 10 μg each, mice were rested for a month. Subsequently, mice were injected intraperitoneally with 100 μg of the antigen in saline, and on the fourth day after injection, the animals were sacrificed, the spleens were excised, and single-cell suspensions were prepared. Splenocytes were mixed with Sp2/0 mouse myeloma cells at a ratio of 5:1 and fused using polyethylene glycol 4000 (Merck, Rahway, NJ). The cells were then aliquoted into 96-well plates in IMDM supplemented with 20% FBS, 50 μM β-mercaptoethanol, and HAT (10 mM hypoxanthine, 40 μM aminopterin, 1.6 mM thymidine). After 12 to 14 days, when the hybridoma cells attained >50% confluence, the supernatant from each well was tested for the presence of antibodies by an enzyme-linked immunosorbent assay (ELISA). Clones secreting antibodies were selected based on their reactivities to rABA, the native A chain of abrin, and abrin; expanded; and subsequently subcloned to obtain monoclonality by the method of limiting dilution. The antibody class and isotype were determined by ELISA. MAbs were purified by affinity chromatography on a protein A-Sepharose column by following standard procedures (9).
Immunoassays. (i) ELISAs.
The wells of ELISA plates were coated with rABA or other RIPs (10 μg/ml in PBS), and unoccupied sites were blocked with 0.5% gelatin in PBS, after which the plates were incubated with either the MAbs or polyclonal antibodies (serum from test bleed samples). After washes with PBS containing 0.05% Tween 20, rabbit antibodies to mouse Ig conjugated to horseradish peroxidase (rabbit α-mIg-HRP; DAKO, Glostrup, Denmark) were added and incubation was continued. The bound-peroxidase activity was detected using tetramethylbenzidine at a concentration of 60 μg/ml in citrate phosphate buffer, pH 5.5, containing 0.03% H2O2. The reaction was stopped with 1 M H2SO4, and absorption at 450 nm was measured in an ELISA reader (Spectramax; Molecular Devices).
(ii) Western blotting.
Two-microgram samples of abrin, rABA, ricin, A. precatorius agglutinin I, and R. communis agglutinin I were electrophoresed on polyacrylamide gels under reducing conditions and transferred onto nitrocellulose membranes. After blocking of the unoccupied sites with 0.5% BSA, the membranes were incubated with mouse antibodies specific to the abrin A chain, then with rabbit α-mIg-HRP conjugate, and subsequently with the substrate diaminobenzidine (1 mg/ml in citrate buffer, pH 5.0, containing 0.05% H2O2).
Neutralization of abrin-mediated cytotoxicity.
The abilities of the MAbs to protect against the cytotoxic effect of abrin were determined by a protein synthesis assay. As described earlier (32), cells (0.2 × 106/well) were cultured for 8 h either in the absence or in the presence of different concentrations of abrin or abrin preincubated for 30 min with the purified antibodies. After leucine starvation for 2 h, 20 μCi of [3H]leucine was added and the cells were incubated for 1 h. The total cell protein was precipitated with 5% trichloroacetic acid, washed twice with 20% ice-cold ethanol, dried, and dissolved in 200 μl of 1% sodium dodecyl sulfate in 0.1 N NaOH. The incorporated radioactivity was measured in a scintillation counter (Beckman Coulter).
Inhibition of abrin-induced apoptosis.
Cells (106/ml) were cultured with abrin in the presence or absence of the MAbs for 12 h, harvested, fixed with 70% ethanol overnight at −20°C, and then centrifuged at 3,000 rpm for 5 min at 4°C (Hettich Universal R). After a wash with PBS, the cells were suspended in the staining solution (50 μg of ethidium bromide/ml, 0.1 μg of RNase A/ml, and 100 μM EDTA in PBS) and the suspension was incubated for 2 h at 42°C. Analysis was carried out by flow cytometry using FACScan (Becton Dickinson). Blue light with a wavelength of 510 nm was used for excitation, and the emission wavelength was 590 nm. The extent of apoptosis was quantified as the percentage of cells in the sub-G1 area, i.e., the percentage of cells with fragmented nuclei. The cell population in the sub-G1 area before the G1 peak was measured and calculated as the percentage of the total cell population (1). Morphological observations of apoptosis were carried out by dual staining of cells with ethidium bromide (100 μg/ml in PBS) and acridine orange (100 μg/ml in PBS). The cells were visualized under a fluorescence microscope (Leica, Germany) using a blue filter. While acridine orange can permeate the cells, ethidium bromide enters the cells only when the membrane integrity is lost. Healthy nuclei appeared green and round, while cells undergoing apoptosis showed fragmented green and red nuclei at early and late stages of apoptosis, respectively.
Abrin-FITC binding assay.
Abrin was labeled with fluorescein isothiocyanate (FITC) by a method described earlier (38). Briefly, to 3 mg of abrin in sodium bicarbonate buffer, pH 9.0, 150 μg of FITC in dry dimethyl sulfoxide was added dropwise and the mixture was incubated for 8 h at 4°C. The conjugate was separated from free FITC on a desalting column. Cells (0.5 × 106) were incubated with 0.5 μg of abrin-FITC/ml in the presence or absence of an ∼100-fold molar excess of either anti-rABA MAbs or unlabeled abrin for 60 min on ice. After a brief spin at 5,000 rpm for 5 min, cells were washed twice with ice-cold Hanks balanced salt solution, resuspended in 200 μl of ice-cold PBS, and either analyzed by fluorescence-activated cell sorting (FACS) or visualized under a fluorescence microscope (Leica, Germany). A total of 10,000 events were analyzed, and the mean fluorescence was calculated. The FITC-labeled abrin retained 80% of its activity, as determined by protein synthesis inhibition assays.
In vivo protective effect of MAb D6F10.
Experiments were carried out with mice per the guidelines laid down by the Indian Institute of Science Animal Ethics Committee after approval for the experimental protocol was obtained. Three groups of adult BALB/c mice (seven mice per group) were injected intraperitoneally with 250 μg of any one of the following: MAb D6F10 or F5B10 or normal mouse IgG. The fourth group received 250 μl of PBS. One hour later, 1.25 μg of abrin in 100 μl of PBS was injected via the same route. The mice were observed for up to 10 weeks after injection.
RESULTS
All the experiments, except the in vivo toxicity experiments with mice, were carried out at least three times. Representative data are presented. Statistical analyses of data were carried out by using the Student t test.
Purification of toxins, abrin A chain, and rABA.
Abrin and A. precatorius agglutinin I were purified as described above. The native A chain of abrin was prepared by subjecting the protein to reduction by 50 mM dithiothreitol. Owing to the presence of five intramolecular disulfide bonds, the B chain precipitates upon reduction, while the A chain remains in solution. The A chain was purified by passing through a lactamyl-Sepharose affinity column to remove any unreduced abrin. rABA was expressed as a His-tagged fusion protein in E. coli BL21/pLysS cells and purified by Ni-NTA affinity column chromatography. The Coomassie blue-stained-gel profile of the purified proteins is shown in Fig. 1. The larger size of the rABA than of the native A chain is because of 32 additional amino acids derived from the pRSETA vector during cloning. The native abrin A chain is 251 amino acids in length, and the rABA contains 283 amino acids. The A. precatorius agglutinin I A chain is 10 residues longer at the C terminus than the abrin A chain and contains a potential N glycosylation site at Asn250, which is not present in the abrin A chain (Fig. 1).
FIG. 1.
Gel profiles of purified proteins. Two micrograms each of abrin and A. precatorius agglutinin I and 1 μg of rABA were electrophoresed under reducing conditions on a 12.5% polyacrylamide gel and stained with Coomassie brilliant blue. Lanes: M, size markers; 1, rABA; 2, abrin; 3, A. precatorius agglutinin I.
Reactivities of anti-rABA MAbs to the abrin A chain and other RIPs.
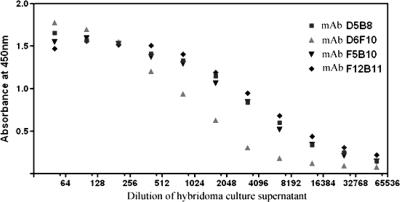
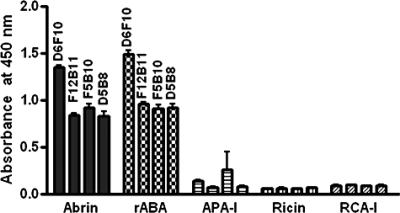
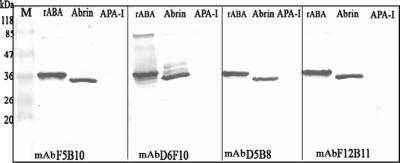
Hybridomas were established from splenocytes of mice immunized with rABA. Hybridomas were selected based on the abilities of the secreted antibodies to bind both the native A chain of abrin and rABA. All the antibodies were found to be of the IgG1 class as determined by isotyping. Studies pertaining to four of the MAbs, D6F10, F5B10, D5B8, and F12B11, are described in this report. The dilution curves for the binding of MAbs to immobilized rABA are shown in Fig. 2. It is clear from the figure that dilution curves for the MAbs F5B10, D5B8, and F12B11 exhibited identical slopes, while that of MAb D6F10 showed a different profile. All the MAbs also bound to the A chain in the whole abrin molecule. However, the level of binding of MAb D6F10 was significantly higher than those of the other antibodies (Fig. 3). Abrin shows a good level of sequence homology to A. precatorius agglutinin I and, to some extent, also to the closely related type II RIPs ricin and R. communis agglutinin (1, 13, 28). When tested for binding in an ELISA (Fig. 3) or a Western blot analysis (Fig. 4; data not shown for ricin and R. communis agglutinin), none of the MAbs bound to the other RIPs, demonstrating that the MAbs recognize epitopes unique to abrin. All the MAbs bound to the A chain of abrin and the rABA in Western blots, showing that the MAbs recognize sequential epitopes (Fig. 4).
FIG. 2.
Dilution curve for anti-rABA MAb binding to the rABA. The wells of 96-well ELISA plates were coated with 1 μg of rABA, and the protein was incubated with 100-μl aliquots of various dilutions of the hybridoma culture supernatants for 3 h at room temperature. The extent of binding of the antibodies to the immobilized antigens was determined by the addition of rabbit α-mIg-HRP, followed by the addition of tetramethylbenzidine-H2O2 and the measurement of the absorbance at 450 nm.
FIG. 3.
Reactivities of anti-rABA MAbs to abrin, rABA, A. precatorius agglutinin I (APA-I), and ricin. The wells of 96-well microtiter plates were coated with 1-μg samples of the proteins, and the plates were blocked with 0.2% BSA in PBS and then incubated with 100-μl aliquots of the hybridoma culture supernatants. After incubation for 3 h, the rest of the procedure was carried out as described in the legend to Fig. 2. RCA-I, R. communis agglutinin I.
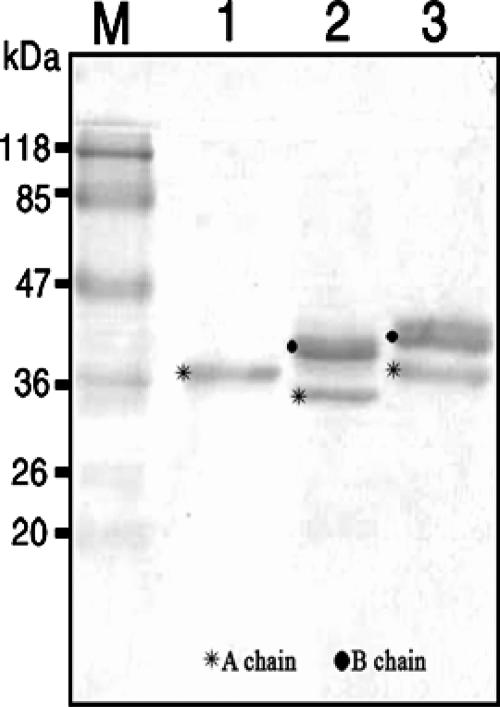
FIG. 4.
Western blotting analysis. Two micrograms of each of the proteins (rABA, abrin, and A. precatorius agglutinin I [APA-I]) was electrophoresed on a 12.5% sodium dodecyl sulfate gel under reducing conditions and then transferred electrophoretically onto a nitrocellulose membrane. After the plates were blocked with 0.2% BSA, the blots were probed with A-chain-specific MAbs, followed by rabbit α-mIg-HRP, and subsequently visualized with the substrate diaminobenzidine-H2O2. Lane M, molecular size markers.
Screening of rABA MAbs for neutralization of abrin cytotoxicity.
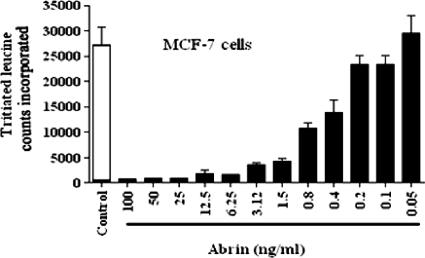
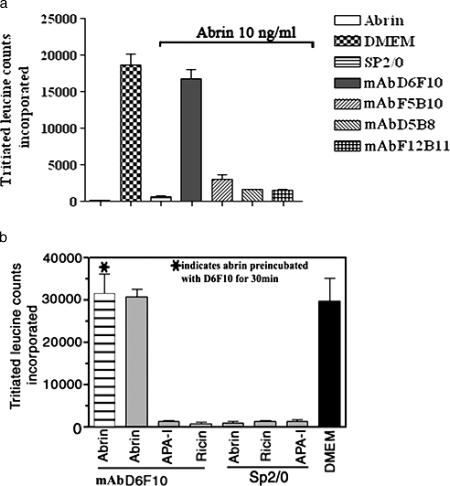
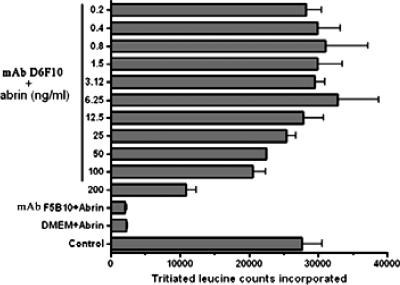
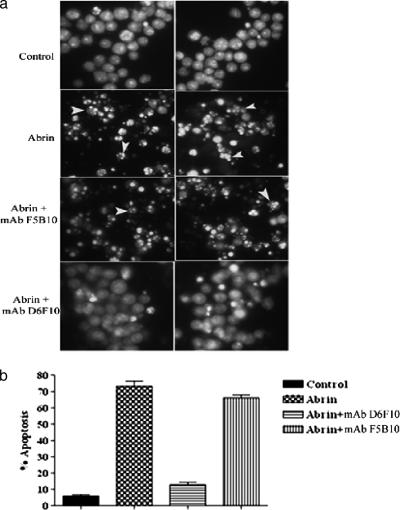
The abilities of the MAbs to neutralize abrin toxicity were tested by a protein synthesis assay. The 50% inhibitory concentrations (IC50s) for the inhibition of protein synthesis by abrin on MCF-7 cells (Fig. 5) and OVCAR-3 cells (data not shown) were estimated to be ∼0.4 to 0.8 ng/ml. Abrin at concentrations >10-fold higher than the IC50, 10 ng/ml, was mixed with 25 μg of each of the purified MAbs/ml, and the mixtures were added to MCF-7 cells grown in cell culture plates. Only the MAb D6F10 showed complete rescue of the cells from abrin-mediated inhibition of protein synthesis, while none of the other MAbs showed any detectable neutralization activity (Fig. 6a). In agreement with the observation that the MAb D6F10 did not bind RIPs other than abrin under native conditions (Fig. 3), we found no neutralizing activity of MAb D6F10 toward ricin (50 ng/ml) and A. precatorius agglutinin I (1 μg/ml) (Fig. 6b). MAb D6F10 at 2.5 μg was able to neutralize 100% of the toxicity induced by 12.5 ng of abrin/ml (Fig. 7). As abrin also induces apoptosis (22), cells cultured with abrin (10 ng/ml) in the presence of MAb D6F10 (25 μg/ml) were analyzed for apoptosis. There was complete inhibition of abrin-induced apoptosis of Jurkat cells (Fig. 8a). The apoptotic population was also quantified by using FACScan (Fig. 8b).
FIG. 5.
Cells were cultured in the absence or presence of various concentrations of abrin for a period of 8 h. After leucine starvation for 2 h, the cells were pulsed with [3H]leucine for 1 h. The total cell protein was precipitated, and the incorporated radioactivity was measured. The experiment was carried out at least three times. Representative data are presented.
FIG. 6.
MCF-7 cells were cultured with 10 ng of abrin/ml (a and b) or 100 ng of ricin/ml or 1 μg of A. precatorius agglutinin I (APA-I)/ml (b) in the presence of A-chain-specific MAbs (25 μg/ml) for a period of 8 h. After the treatment, the cells were subjected to 2 h of leucine starvation, followed by [3H]leucine incorporation for 1 h. The total cellular protein was precipitated, and the incorporated radioactivity was measured. Sp2/0 culture supernatant was included as a negative control. The experiment was carried out at least three times. Representative data are presented. DMEM, Dulbecco's modified Eagle's medium.
FIG. 7.
OVCAR-3 cells were cultured with increasing concentrations of abrin (0.2 to 200 ng/ml) in the presence of 2.5 μg of MAb D6F10 or MAb F5B10/ml for a period of 8 h and monitored for protein synthesis as described in the legend to Fig. 5. “Control” denotes cells cultured without abrin or MAbs. In the case of MAb F5B10, data for 0.2 ng of abrin/ml alone are shown here. The experiment was carried out at least three times. Representative data are presented. DMEM, Dulbecco's modified Eagle's medium.
FIG. 8.
(a) Jurkat cells were cultured with abrin (10 ng/ml) in the presence or absence of antibodies (25 μg/ml) for a period of 12 h, stained with acridine orange-ethidium bromide, and analyzed by fluorescence microscopy. Arrowheads point to apoptotic nuclei. (b) In another experiment, the cells were incubated with abrin, fixed in 70% ethanol, and stained with ethidium bromide. The percentage of apoptosis was quantified by using FACScan. The experiment was carried out at least three times. Representative data are presented.
MAb D6F10 prevents abrin binding on cells.
The antibody D6F10 was raised against the A chain of abrin. The mode of inhibition of the cytotoxic effect by many anti-A chain antibodies described earlier is still not clear (18). The binding of toxin on the cell surface is the first event in the intoxication process, and therefore, we analyzed the binding of abrin-FITC on Jurkat cells in the presence and absence of MAb D6F10. Results obtained from FACS analysis (Fig. 9a) and fluorescence microscopy (Fig. 9b) indicated that MAb D6F10 inhibits the binding of abrin on the cell surface. Hence, it appears that the binding of the antibody to the toxic subunit prevents the binding of the toxin to the receptors and brings about the neutralization effect. Interestingly, cells incubated with only abrin-FITC separated into two peaks (Fig. 9a); the smaller one represents the population of cells with low-level abrin binding. The observation of only one peak when the cells were incubated with the labeled toxin in the presence of MAb F5B10 can be explained as follows: MAb F5B10 binding to abrin does not inhibit the binding of the toxin to the cell surface. As antibodies are bivalent and two molecules of abrin-FITC would be bound to each molecule of the MAb, there would be two molecules of abrin-FITC at the surfaces of the cells for every one molecule of cell-bound abrin-FITC. In such a case, the population of cells with fewer molecules bound to the galactose receptors would also acquire more fluorescence due to the recruitment of more abrin molecules through the bound antibody.
FIG. 9.
Cells were incubated with 0.5 μg of FITC-labeled toxin/ml in the presence (+) of 250 μg of MAb D6F10 or F5B10/ml or 50 μg of unlabeled toxin/ml for a period of 30 min at 4°C. After brief washes with ice-cold PBS, the cells were either analyzed by FACS (a) or observed under a fluorescence microscope (b). FL1-H, fluorescence intensity (green channel).
In vivo protection by MAb D6F10.
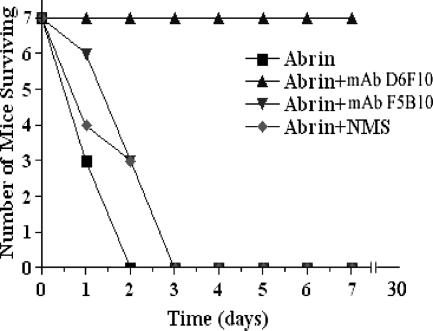
We studied the in vivo protective efficacy of MAb D6F10. Mice (∼6 months old) were injected intraperitoneally with either MAb D6F10 or MAb F5B10, and 60 min later, abrin was administered via the same route. Mice were observed for more than 2 months. A concentration of 250 μg of the D6F10 antibody provided complete protection against the lethality of 1.25 μg of abrin (Fig. 10), while no such effect was observed with either the MAb F5B10 or normal mouse IgG.
FIG. 10.
Mice were given 250 μg of MAb D6F10, MAb F5B10, or normal mouse IgG (NMS) in PBS intraperitoneally. After 60 min of administration of the antibodies, 1.25 μg of abrin was injected intraperitoneally, and mice were observed for 10 weeks.
DISCUSSION
Abrin is one of the most powerful plant toxins known (28, 25). The reported IC50 for protein synthesis by abrin in cultured cell lines is ∼0.4 ng/ml (23), and the LD50 for mice is ∼0.04 μg/kg (35). Although not infectious, it is a probable agent for use as a bioweapon (4) because of its stability (it is partially active at temperatures up to 50°C [data not shown]) and the relatively easy purification procedure that yields milligram amounts of the toxin from the seeds. Though the mechanisms of toxicity of some RIPs at the cellular and molecular levels have been delineated previously, the development of an antidote has proven elusive (21). Chemical inhibitors targeting the active site in the A chain or the lectin pockets of the B chain have been identified previously but have had limited application due to a lack of cell specificity and general toxicity (3, 30). Antibody-based therapies, on the other hand, for the treatment of poisoning by various toxins are more promising. Several neutralizing antibodies for biological toxins have been described earlier (5, 20, 24, 39). Antibodies to the galactose binding domain of the B chain have been shown to inhibit ricin binding to cells, thereby inhibiting toxin activity (19, 20). Antibodies to the A chain of ricin also protect cells effectively from ricin toxicity (18). However, there are no reports on neutralizing antibodies to the A chain or B chain of abrin.
Toward the objective of establishing antibodies that would neutralize abrin, a panel of MAbs against the rABA was established and characterized extensively. The MAbs recognized specifically the rABA as well as the abrin A chain under native as well as denaturing conditions. There is a divergence of more than 75% between plant and bacterial RIPs in primary structures, even though they have identical mechanisms of action. This large amount of sequence variability may result in the plant and bacterial RIPs' having distinct neutralizing epitopes (13). In agreement with the earlier predictions by other groups (13), in spite of the high level of sequence similarity observed among different RIPs (abrin, ricin, R. communis agglutinin I, and A. precatorius agglutinin I), mostly unique epitopes were recognized by the anti-rABA antibodies. The inhibition of protein synthesis and apoptosis are the two major effects of RIPs on cells (8, 33). MAb D6F10 but none of the other MAbs rescued MCF-7 cells from abrin-mediated inhibition of protein synthesis (Fig. 6a). We also observed that, in the presence of this antibody, there was a significant reduction in apoptosis induced by abrin among Jurkat cells (Fig. 8) and that a 200-fold molar excess of antibody was required to inhibit the abrin toxicity in OVCAR-3 cells (Fig. 7). It is pertinent to mention here that all the toxin binding and neutralization assays were carried out with all three cell lines, Jurkat, MCF-7, and OVCAR-3, and we obtained very comparable data.
Studies with mice were also initiated to validate the protective effect of the MAb D6F10 as determined by in vitro cell culture assays. A level of protection of 100% was seen in the mice that received this antibody either along with, or 1 h prior to, the administration of a lethal dose of the toxin, and the mice appeared to be normal as observed for up to 10 weeks. Further studies are in progress to understand the effects of the long-term administration of this MAb to mice and to identify the time spans within which the antibody would be effective if administered after abrin poisoning through various routes. Antibodies neutralize the cytotoxicity of toxins by different mechanisms: blocking the initial stages of toxin trafficking, like binding and internalization, or inhibiting the intracellular processing or inducing the degradation of the toxins (34). Binding assays performed with FITC-labeled abrin indicated that the neutralization effect exhibited by D6F10 is probably due to the prevention of toxin attachment on the cell surface by steric hindrance of the B chain binding. In conclusion, we have obtained a MAb, namely, D6F10, to the abrin A chain that neutralizes the toxicity of abrin both in vitro and in vivo. Experiments are under way in our laboratory to identify the epitopes of the anti-A chain antibodies and to characterize the mode of inhibition of toxin binding by MAb D6F10.
Acknowledgments
We thank Suvarna Krishnappa and Jaikumar Thimma for their assistance in the production and characterization of MAbs and S. K. Podder for useful discussions. The assistance of Omana Joy of our institute FACS facility, funded by the Department of Biotechnology, Government of India, is acknowledged.
Footnotes
Published ahead of print on 19 March 2008.
REFERENCES
- 1.Bagaria, A., K. Surendranath, U. A. Ramagopal, S. Ramakumar, and A. A. Karande. 2006. Structure-function analysis and insights into the reduced toxicity of Abrus precatorius agglutinin I in relation to abrin. J. Biol. Chem. 281:34465-34474. [DOI] [PubMed] [Google Scholar]
- 2.Barbieri, L., M. G. Battelli, and F. Stirpe. 1993. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta 1154:237-282. [DOI] [PubMed] [Google Scholar]
- 3.Burnett, J. C., E. A. Henchal, A. L. Schmaljohn, and S. Bavari. 2005. The evolving field of biodefence: therapeutic developments and diagnostics. Nat. Rev. Drug Discov. 4:281-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2003. Investigation of a ricin-containing envelope at a postal facility—South Carolina, 2003. Morb. Mortal. Wkly. Rep. 52:1129-1131. [PubMed] [Google Scholar]
- 5.Colombatti, M., V. G. Johnson, H. A. Skopicki, B. Fendley, M. S. Lewis, and R. J. Youle. 1987. Identification and characterization of a monoclonal antibody recognizing a galactose-binding domain of the toxin ricin. J. Immunol. 138:3339-3344. [PubMed] [Google Scholar]
- 6.Dighe, R. R., G. S. Murthy, and N. R. Moudgal. 1990. Two simple and rapid methods to detect monoclonal antibodies with identical epitope specificities in a large population of monoclonal antibodies. J. Immunol. Methods 131:229-236. [DOI] [PubMed] [Google Scholar]
- 7.Endo, Y., K. Mitsui, K. Motizuki, and K. Tsurugi. 1987. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 262:5908-5912. [PubMed] [Google Scholar]
- 8.Gan, Y. H., S. Q. Peng, and H. Y. Liu. 2000. Molecular mechanisms of apoptosis induced by ricin in HeLa cells. Acta Pharmacol. Sin. 21:243-253. [PubMed] [Google Scholar]
- 9.Gangatirkar, P., S. Gangadharan, A. Narendranath, S. Nagpal, D. M. Salunke, and A. A. Karande. 2002. Monoclonal antibodies to gonadotropin-releasing hormone (GnRH) inhibit binding of the hormone to its receptor. Hybrid. Hybridomics 21:281-286. [DOI] [PubMed] [Google Scholar]
- 10.Gareth, D., G. D. Griffiths, P. Rice, A. C. Allenby, S. C. Bailey, and D. G. Upshall. 1995. Inhalation toxicology and histopathology of ricin and abrin toxins. Inhal. Toxicol. 7:269-288. [Google Scholar]
- 11.Hegde, R., T. K. Maiti, and S. K. Podder. 1991. Purification and characterization of three toxins and two agglutinins from Abrus precatorius seed by using lactamyl-Sepharose affinity chromatography. Anal. Biochem. 194:101-109. [DOI] [PubMed] [Google Scholar]
- 12.Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495-497. [DOI] [PubMed] [Google Scholar]
- 13.Lebeda, F. J., and M. A. Olson. 1999. Prediction of a conserved, neutralizing epitope in ribosome-inactivating proteins. Int. J. Biol. Macromol. 24:19-26. [DOI] [PubMed] [Google Scholar]
- 14.Lemley, P. V., and D. C. Wright. 1992. Mice are actively immunized after passive monoclonal antibody prophylaxis and ricin toxin challenge. Immunology 76:511-513. [PMC free article] [PubMed] [Google Scholar]
- 15.Ligler, F. S., C. R. Taitt, L. C. Shriver-Lake, K. E. Sapsford, Y. Shubin, and J. P. Golden. 2003. Array biosensor for detection of toxins. Anal. Bioanal. Chem. 377:469-477. [DOI] [PubMed] [Google Scholar]
- 16.Lord, J. M., E. Deeks, C. J. Marsden, K. Moore, C. Pateman, D. C. Smith, R. A. Spooner, P. Watson, and L. M. Roberts. 2003. Retrograde transport of toxins across the endoplasmic reticulum membrane. Biochem. Soc. Trans. 31:1260-1262. [DOI] [PubMed] [Google Scholar]
- 17.Lord, J. M., L. M. Roberts, and J. D. Robertus. 1994. Ricin: structure, mode of action, and some current applications. FASEB J. 8:201-208. [PubMed] [Google Scholar]
- 18.Maddaloni, M. C., R. Cooke, A. Wilkinson, V. Stout, L. Eng, and S. H. Pincus. 2004. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J. Immunol. 172:6221-6228. [DOI] [PubMed] [Google Scholar]
- 19.Mantis, N. J., C. R. McGuinness, O. Sonuyi, G. Edwards, and S. A. Farrant. 2006. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect. Immun. 74:3455-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGuinness, C. R., and N. J. Mantis. 2006. Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect. Immun. 74:3463-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, D. J., K. Ravikumar, H. Shen, J. K. Suh, S. M. Kerwin, and J. D. Robertus. 2002. Structure-based design and characterization of novel platforms for ricin and Shiga toxin inhibition. J. Med. Chem. 45:90-98. [DOI] [PubMed] [Google Scholar]
- 22.Narayanan, S., A. Surolia, and A. A. Karande. 2004. Ribosome-inactivating protein and apoptosis: abrin causes cell death via mitochondrial pathway in Jurkat cells. Biochem. J. 377:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narayanan, S., K. Surendranath, N. Bora, A. Surolia, and A. A. Karande. 2005. Ribosome inactivating proteins and apoptosis. FEBS Lett. 579:1324-1331. [DOI] [PubMed] [Google Scholar]
- 24.Nowakowski, A. C., D. B. Wang, P. Powers, T. J. Amersdorfer, V. A. Smith, R. Montgomery, R. Sheridan, L. Blake, A. Smith, and J. D. Marks. 2002. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. USA 99:11346-11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olsnes, S. 2004. The history of ricin, abrin and related toxins. Toxicon 44:361-370. [DOI] [PubMed] [Google Scholar]
- 26.Olsnes, S., A. M. Pappenheimer, Jr., and R. Meren. 1974. Lectins from Abrus precatorius and Ricinus communis. II. Hybrid toxins and their interaction with chain-specific antibodies. J. Immunol. 113:842-847. [PubMed] [Google Scholar]
- 27.Olsnes, S., and A. Pihl. 1973. Different biological properties of the two constituent peptide chains of ricin, a toxic protein inhibiting protein synthesis. Biochemistry 12:3121-3126. [DOI] [PubMed] [Google Scholar]
- 28.Olsnes, S., K. Sandvig, K. Eiklid, and A. Pihl. 1978. Properties and action mechanism of the toxic lectin modeccin: interaction with cell lines resistant to modeccin, abrin, and ricin. J. Supramol. Struct. 9:15-25. [DOI] [PubMed] [Google Scholar]
- 29.Pappenheimer, A., M. T. Uchida, and A. A. Harper. 1972. An immunological study of the diphtheria toxin molecule. Immunochemistry 9:891-906. [DOI] [PubMed] [Google Scholar]
- 30.Rainey, G. J., and J. A. Young. 2004. Antitoxins: novel strategies to target agents of bioterrorism. Nat. Rev. Microbiol. 2:721-726. [DOI] [PubMed] [Google Scholar]
- 31.Sandvig, K., and B. van Deurs. 2002. Transport of protein toxins into cells: pathways used by ricin, cholera toxin and Shiga toxin. FEBS Lett. 529:49-53. [DOI] [PubMed] [Google Scholar]
- 32.Sharma, S., S. K. Podder, and A. A. Karande. 1999. Comparative studies on kinetics of inhibition of protein synthesis in intact cells by ricin and a conjugate of ricin B chain with momordin. Mol. Cell. Biochem. 200:133-141. [DOI] [PubMed] [Google Scholar]
- 33.Shih, S. F., Y. H. Wu, C. H. Hung, H. Y. Yang, and J. Y. Lin. 2001. Abrin triggers cell death by inactivating a thiol-specific antioxidant protein. J. Biol. Chem. 276:21870-21877. [DOI] [PubMed] [Google Scholar]
- 34.Stirpe, F. 2004. Ribosome-inactivating proteins. Toxicon 44:371-383. [DOI] [PubMed] [Google Scholar]
- 35.Stirpe, F., L. Barbieri, M. G. Battelli, M. Soria, and D. A. Lappi. 1992. Ribosome inactivating proteins from plants: present status and future prospects. Bio/Technology 10:405-412. [DOI] [PubMed] [Google Scholar]
- 36.Szalai, K., I. Scholl, E. Forster-Waldlw, L. Politoz, A. Bolognesiz, E. Untersmayr, A. B. Riemer, G. Boltz-Nitulescu, F. Stirpez, and E. Jensen-Jarolim. 2005. Occupational sensitization to ribosome-inactivating proteins in researchers. Clin. Exp. Allergy 34:1354-1360. [DOI] [PubMed] [Google Scholar]
- 37.Tahirov, T. H., T. H. Lu, Y. C. Liaw, Y. L. Chen, and J. Y. Lin. 1995. Crystal structure of abrin-A at 2.14 Å. J. Mol. Biol. 250:354-367. [DOI] [PubMed] [Google Scholar]
- 38.Talian, J. C., J. B. Olmsted, and R. D. Goldman. 1983. A rapid procedure for preparing fluorescein-labeled specific antibodies from whole antiserum: its use in analyzing cytoskeletal architecture. J. Cell Biol. 97:1277-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tzipori, S., A. Sheoran, D. Akiyoshi, A. Donohue-Rolfe, and H. Trachtman. 2004. Antibody therapy in the management of Shiga toxin-induced hemolytic uremic syndrome. Clin. Microbiol. Rev. 17:926-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitetta, E. S., J. E. Smallshaw, E. Coleman, H. Jafri, C. Foster, R. Munford, and J. Schindler. 2006. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc. Natl. Acad. Sci. USA 103:2268-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoder, J. M., R. U. Aslam, and N. J. Mantis. 2007. Evidence for widespread epithelial damage and coincident production of monocyte chemotactic protein 1 in a murine model of intestinal ricin intoxication. Infect. Immun. 75:1745-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]