Abstract
Current efforts are aimed at optimizing the protective efficacy of Mycobacterium bovis BCG by the use of vaccine combinations. We have recently demonstrated that the protection afforded by BCG alone is enhanced by vaccinating cattle with a combination of vaccines comprising BCG and a protein tuberculosis vaccine, namely, culture filtrate proteins (CFPs) from M. bovis plus an adjuvant. In the current study, three different adjuvant systems were compared. The CFP was formulated with a depot adjuvant, dimethyldioctadecyl ammonium bromide (DDA), together with one of three different immunostimulants: monophosphoryl lipid A (MPL), a synthetic mycobacterial phosphatidylinositol mannoside-2 (PIM2), and a synthetic lipopeptide (Pam3Cys-SKKKK [Pam3CSK4]). Groups of cattle (n = 10/group) were vaccinated with BCG-CFP-DDA-PIM2, BCG-CFP-DDA-MPL, or BCG-CFP-DDA-Pam3CSK4. Two additional groups (n = 10) were vaccinated with BCG alone or BCG-adjuvant (DDA-MPL), and a control group was left unvaccinated. Protection was assessed by challenging the cattle intratracheally with M. bovis. Groups of cattle vaccinated with BCG-CFP-DDA-PIM2, BCG-CFP-DDA-MPL, BCG-CFP-DDA-Pam3CSK4, and BCG alone showed significant reductions in three, three, five, and three pathological and microbiological disease parameters, respectively, compared to the results for the nonvaccinated group. Vaccination with the combination of BCG and the DDA-MPL adjuvant alone abrogated the protection conferred by BCG alone. The profiling of cytokine gene expression following vaccination, prior to challenge, did not illuminate significant differences which could explain the latter result. Vaccination of cattle with a combination of BCG and protein tuberculosis vaccine enhances protection against tuberculosis.
Infection with Mycobacterium bovis is a significant problem for the cattle industry throughout the world and is also a cause of concern for human health, as M. bovis readily infects humans (13). An efficient vaccination strategy would significantly aid in the control of tuberculosis in cattle (11, 40). M. bovis bacillus Calmette-Guérin (BCG) has been used extensively in humans, with variable success (17), and experimental studies with cattle have shown that vaccination of cattle with BCG can induce a significant level of protection against the development of tuberculous lesions in animals challenged with virulent M. bovis isolates (7, 8, 12, 40). However, the same caveats encountered with the use of BCG in humans apply to the vaccination of cattle; namely, the protection afforded by BCG is incomplete and can be compromised by exposure to environmental mycobacteria (10). An area of investigation in tuberculosis vaccination gathering momentum is the development of vaccination strategies that complement the protective efficacy of BCG (35). The concept is to prime and boost the immune system with a combination of vaccine formulations and immunomodulators that provide optimal protection against tuberculosis. The use of BCG as a priming agent would have a number of advantages, most notably, the safety profile of this vaccine (23, 24).
We have recently shown that the vaccination of cattle with a combination of BCG and a mycobacterial protein vaccine prepared from M. bovis culture filtrate (CFP) induced better protection against bovine tuberculosis than the protection induced by vaccination with BCG alone (45). While this protein vaccine, when it is administered to cattle on its own, can induce some protection against tuberculosis, it is likely that better protection can be achieved by formulating the CFP with more potent adjuvants and immunomodulators (46). Combinations of BCG and a DNA vaccine coding for immunogenic mycobacterial components induced levels of protection superior to those obtained with BCG alone (28, 36, 37). Similarly, a combination of BCG and a viral vector expressing key antigens from mycobacteria was shown to be efficient at stimulating a superior immune response in cattle (41). Collectively, these data argue for the validity of using combinations of vaccines to optimize protection against tuberculosis in cattle.
Recent data obtained with mouse models of vaccination against tuberculosis have emphasized the requirements that appropriate adjuvants need to optimize a protective immune response (14, 23). Adjuvants can act as immunomodulators by a variety of immunological avenues (18). A class of adjuvants that is receiving attention are Toll-like receptor (TLR) agonists: Toll receptors constitute a family of gateway receptors which, upon recognition of a “danger signal,” typically a microbial or viral component, initiate the rapid and robust induction of innate immunity (32). In addition, TLR activation also serves to initiate and amplify the development of a specific immune response. Numerous studies have shown that TLR agonists, such as monophosphoryl lipid A (MPL), a TLR4 agonist, are potent adjuvants (4). In the context of tuberculosis, evidence suggesting a role for TLR2 activation in the host resistance of mice and humans to tuberculosis has been presented (15, 38).
In this study, we elected to test combinations of adjuvants and immunostimulators to formulate an optimal CFP-based tuberculosis vaccine for use with BCG. Dimethyldioctadecyl ammonium bromide (DDA) acts via its surfactant properties and produces a depot effect: the antigen and DDA combine to form micromicelles which enhance the bioavailability of the immunizing antigens. DDA is an effective adjuvant for enhancing cellular immune responses to M. tuberculosis proteins in mice (3, 26, 27) and for enhancing protective immunity against Johne's disease in cattle when it is combined with a Mycobacterium avium subsp. paratuberculosis protein (25). MPL was selected as a promising immunostimulant on the basis of its protective efficacy when it is used as an adjuvant in CFP vaccines in experimental models of tuberculosis (5), as well as the fact that it has been shown to be safe for use in cattle (43). The second immunostimulant chosen was a phosphatidylinositol mannoside (PIM), which is a constituent of the cell wall of mycobacteria and other bacterial species. PIMs are a family of glycolipids which can be hyperglycosylated to form complex cell wall molecules which have important biological implications. We have recently reported that PIMs, particularly the dimannoside form of PIMs (PIM2), are efficient activators of the innate and adaptive immune responses (1, 2, 16). In those studies, we showed that PIM2 stimulated the release of the key Th1 cytokine interleukin-12 (IL-12) by dendritic cells (DCs), illustrating the potential of PIMs to act as adjuvants. PIMs appear to exert their proinflammatory activities mainly via the activation of TLR2 (19, 22). One caveat in using purified PIMs from the mycobacterial cell wall is the possibility of contamination with other products or the possibility that mixtures of PIMs with different degrees of mannosylation will be obtained. To that end, we have recently described an approach that can be used to obtain pure synthetic PIM2, which is one of the most active forms of PIMs (1). The third immunostimulant tested was a lipopeptide, Pam3Cys-SKKKK (Pam3CSK4), which is a synthetic triacylated lipopeptide that has adjuvant activity based on its ability to bind to and activate TLR2. Pam3CSK4 has been used in a variety of animal models, including cattle, from which data showing that Pam3CSK4 activates bovine DCs have been presented (20, 47). Veterinary vaccines must be highly cost-effective, and there should be cost advantages in using synthetic forms of immunostimulants compared to the costs of using those purified from natural products (i.e., MPL).
The data obtained in this study suggest that the administration of a vaccine consisting of BCG and CFP combined with an adjuvant formulation that included Pam3CSK4 induced significant levels of protection against challenge with a virulent strain that were superior to those obtained with BCG alone. On the other hand, vaccination with BCG and an adjuvant formulation that included MPL in the absence of CFP had a detrimental impact on the protection afforded by BCG alone. These data provide clues to the means of optimization of vaccination strategies to protect cattle against bovine tuberculosis and highlight the value of including synthetic TLR2 agonists in the adjuvants of vaccines for cattle.
MATERIALS AND METHODS
Animals.
Friesian-cross female calves, approximately 6 months old, were obtained from tuberculosis-free accredited herds from an area of New Zealand where both farmed and feral animals were free of tuberculosis. The animals were grazed on pasture in a high-security containment unit. Prior to the experiments, the cattle tested negative for reactivity to purified protein derivative (PPD) from M. bovis (bovine PPD; CSL Limited, Parkville, Australia) in a whole-blood gamma interferon (IFN-γ) assay (33). Animal ethics approval was granted for all animal experiments by the local ethics committees (Wallaceville and Grasslands AgResearch Animal Ethics Committees).
Bacterial strains.
M. bovis BCG strain Pasteur 1173P2 was used as the vaccine strain; and M. bovis WAg202, originally isolated from a tuberculous possum (Trichosurus vulpecula) in New Zealand, was used as the virulent challenge strain. BCG Pasteur and WAg202 have been used in previous vaccination-challenge studies with cattle (7, 8, 43). Bacteria were grown to mid-log phase in Tween-albumin broth (Dubos broth base; Difco Laboratories, Detroit, MI) supplemented with 0.006% (vol/vol) alkalinized oleic acid, 0.5% (wt/vol) albumin fraction V, and 0.25% (wt/vol) glucose. Dilutions were made in Tween albumin broth to obtain the appropriate doses for inoculation. The number of CFU inoculated was determined retrospectively by plating 10-fold dilutions on Middlebrook 7H11 medium (Difco) supplemented with 0.5% (wt/vol) albumin, 0.2% (wt/vol) glucose, and 1% (wt/vol) sodium pyruvate.
Preparation of vaccines and vaccination of cattle.
The compositions of the different vaccines are shown in Table 1. CFPs from M. bovis AN5 were prepared as described in detail elsewhere (43, 46). MPL from Salmonella enterica was purchased from Sigma Chemicals (St. Louis, MO), and Pam3CSK4 {Pam3C is a palmitoyl-Cys[(RS)-2,3-di(palmitoyloxy)-propyl] residue [37, 39]} was purchased from EMC Microcollections (Tuebingen, Germany). PIM2 was synthesized and characterized as described elsewhere (1, 2). Both Pam3CSK4 and PIM2 contained undetectable levels of contaminating endotoxin, as determined with a commercial assay kit (E-Toxate; Sigma). DDA (Sigma) was prepared by heating a 10-mg/ml solution at 80°C until the formation of micelles. The DDA was cooled to room temperature and added to the rest of the vaccine constituents.
TABLE 1.
Vaccine groups and formulations
| Group | Composition in vaccine
|
|||||
|---|---|---|---|---|---|---|
| BCG (CFU) | M. bovis CFP (mg/dose) | DDA (% [vol/vol]) | MPL (mg/dose) | PIM2 (mg/dose) | Pam3CSK4 (mg/dose) | |
| Nonvaccinated | —a | — | — | — | — | — |
| BCG alone | 106 | — | — | — | — | — |
| BCG-CFP-DDA-PIM2 | 106 | 0.4 | 0.5 | — | 0.25 | — |
| BCG-CFP-DDA-MPL | 106 | 0.4 | 0.5 | 0.2 | — | — |
| BCG-CFP-DDA-Pam3CSK4 | 106 | 0.4 | 0.5 | — | — | 0.25 |
| BCG-DDA-MPL | 106 | — | 0.5 | 0.2 | — | — |
—, the constituent is not part of the vaccine formulation.
The calves were divided into six groups (10 animals per group) by using a randomized stratified sampling system such that all groups contained animals with a similar distribution of IFN-γ responses to PPD prepared from Mycobacterium avium (avian PPD) prior to commencement of the trial. Four groups of 10 calves each were vaccinated subcutaneously in the lateral midregion of the neck on the left side with either the CFP-DDA-PIM, CFP-DDA-MPL, CFP-DDA-Pam3CSK4, or DDA-MPL vaccine and were injected subcutaneously in the neck with 106 CFU of BCG at a site 2 to 3 cm apart from the protein vaccine injection site. The animals were revaccinated with the same protein vaccine 3 and 6 weeks later. A fifth group of calves (n = 10) was vaccinated subcutaneously in the neck with 106 CFU of BCG, and a control group of 10 calves served as nonvaccinated controls.
M. bovis challenge and necropsy procedure.
At 14 weeks after the first vaccination, all calves were challenged intratracheally with 5 × 103 CFU of virulent M. bovis, as described previously (7). All cattle were euthanized by electrical stunning and severance of the carotid artery and were necropsied 15 weeks after the challenge to assess the level of protection against tuberculosis. Samples from four thoracic lymph nodes (the left and right bronchial and anterior lymph nodes and the posterior mediastinal lymph nodes) were collected from all of the animals for bacterial culture and histology. Additional samples were collected from all tuberculous lesions observed in the lungs, other lymph nodes, or organs. Procedures for the identification of macroscopic tuberculous lesions and processing for histopathology and bacterial counts have been described previously (44).
Cytokine protein assays.
Heparinized blood samples (1.5 ml) were dispensed into three wells of a 24-well plate within 4 h of blood collection, and 100 μl of PPD prepared from Mycobacterium bovis PPD (bovine PPD) or avian PPD (final concentrations, 24 μg/ml; Prionics, Switzerland) or phosphate-buffered saline (PBS; negative control) was added. The whole-blood cultures were incubated at 37°C for 20 h, and the IFN-γ levels in the plasma supernatants were measured by use of a sandwich enzyme-linked immunosorbent assay (ELISA) kit (Prionics), as described previously (33, 49). The IFN-γ levels are expressed as ng/ml, as determined by use of a standard curve prepared with recombinant bovine IFN-γ (43). The IL-10 levels in plasma supernatants from whole-blood cultures stimulated with bovine PPD were measured. For the IL-10 measurements, plates were coated with anti-bovine IL-10 (Serotec) and incubated overnight at room temperature. The plates were washed in washing buffer, and blocking buffer was added for 1 h. Following a further washing step, samples were added for 1 h. Dilutions of the samples were added to the plates for 1 h. After the plates were washed, biotin-labeled anti-IL-10 (Serotec) was added for 1 h, followed by washing and addition of streptavidin-horseradish peroxidase (Jackson ImmunoResearch Laboratories) for 45 min. Following the final washing step, tetramethylbenzidine substrate was added, the reaction was stopped by the addition of H2SO4, and the absorbance values were read at 450 mn on a microtiter plate reader. An in-house positive control was used in the assay, and the results were expressed as the antigen-specific responses minus the background medium control.
IFN-γ-producing cells.
The enzyme-linked immunospot (ELISPOT) assay was used to quantify the numbers of IFN-γ-producing cells in four randomly selected animals from each group at 8 weeks after the initial vaccination. The method used was that described previously (36). The results were expressed as the change in the number of IFN-γ spot-forming cells (SFCs), which represent the number of SFCs for the bovine PPD-stimulated peripheral blood mononuclear cells (PBMCs) minus the number of SFCs for the medium control PBMCs/106 cells. The mean number of IFN-γ SFCs for the medium control was 81 ± 19 SFCs/106 PBMCs.
Immune gene expression in vaccinated cattle.
In a subsequent experiment, an additional 10 calves were randomly divided into two groups (5 animals per group) and vaccinated with BCG-CFP-DDA-MPL or BCG-DDA-MPL (adjuvant alone), as described above for the first vaccine trial. This experiment was aimed at determining if there were differences in immune gene expression profiles which could predict protection or lack thereof in vaccinated cattle and shed light on why DDA-MPL adjuvant alone abrogated the protection conferred by BCG. These animals were revaccinated with the adjuvanted vaccines 3 and 6 weeks later, and their immune responses were monitored. Bovine PPD-specific expression of a range of immune genes in PBMCs from the animals was measured by real-time PCR 9 weeks after the initial vaccination, as described previously (44). RNA was treated with DNase I (Invitrogen, Carlsbad, CA) prior to reverse transcription to cDNA with transcriptor reverse transcriptase (Roche Diagnostics NZ Ltd., Auckland, New Zealand). The real-time PCR primers for the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the cytokine IL-4 were described previously (44). Primers for the transcription factor specific for T regulatory cells, forkhead box P3 (FoxP3), were described elsewhere (34). Primers for the other immune genes were as follows: for IL-10, 5′-TGCTGGATGACTTTAAGGGTTACC-3′ (forward) and 5′-TCATTTCCGACAAGGCTTGG-3′ (reverse); for IL-12 p40, 5′-ACCCCGCATTCCTACTTCTC-3′ (forward) and 5′-CTTTCCCTGGACCTGAACAC-3′ (reverse); for IL-13, 5′-AGAACCAGAAGGTGCCGCT-3′ (forward) and 5′-GGTTGAGGCTCCACACCATG-3′ (reverse); and for IL-18, 5′-TTATTGCATCAGCTTTGTGGA-3′ (forward) and 5′-GGTCTTCATCATTTTCAGCTA-3′ (reverse). Gene expression in the PBMCs was reported as the difference 2−ΔΔCT, where ΔΔCT = ΔCT for bovine PPD-stimulated PBMCs − ΔCT for nonstimulated (PBS-treated) PBMCs, where ΔCT is the threshold cycle (CT) for the immune gene minus the CT for GAPDH.
Tuberculin skin test.
A comparative cervical tuberculin skin test was undertaken at 11 weeks after vaccination and 13 weeks after challenge. The animals were inoculated intradermally with 0.1-ml volumes containing either 0.05 mg avian PPD or 0.1 mg bovine PPD (AgriQuality, Upper Hutt, New Zealand) in the right side of the neck, which was the side of the neck opposite from the vaccination site. The skin-fold thickness was measured with calipers prior to injection and 72 h after injection for both bovine and avian PPDs.
Antibody ELISA.
Sera were stored at −20°C until they were tested. The M. bovis AN5 culture filtrate was diluted to 3 μg/ml in carbonate buffer (pH 9.6); 100 μl per well was added to 96-well ELISA plates (Maxisorp; Nunc, Roskilde, Denmark), and the plates were incubated overnight at 4°C. The antibody ELISA was carried out as described previously (45). The results were expressed as “absorbance indexes,” calculated by expressing the values found for the test sera as a fraction of the binding of a strongly positive reference serum multiplied by 100.
Statistical analysis.
Analyses of the IFN-γ protein responses were performed by analysis of variance of log10-transformed data. Analyses of the mean skin tuberculin test responses, the number of IFN-γ-producing cells, the IL-10 responses, cytokine mRNA expression, lesion scores, and the number of M. bovis culture-positive lymph nodes per animal were undertaken by analysis of variance of the raw data. The correlation between IFN-γ-producing cells and IFN-γ protein levels was estimated by using Pearson's correlation coefficient. The proportion of animals with lesions was analyzed by using Fisher's exact test with pairwise comparisons.
RESULTS
IFN-γ responses after vaccination and challenge.
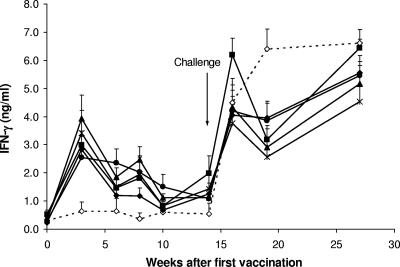
The IFN-γ responses in animals vaccinated and subsequently challenged with virulent M. bovis were measured. Immediately prior to vaccination, the mean IFN-γ response (± standard error) to avian PPD and bovine PPD for all animals were 0.58 ± 0.08 and 0.32 ± 0.04 ng/ml, respectively. Vaccination with BCG or the BCG and adjuvanted vaccines induced a significant enhancement in the IFN-γ responses to bovine PPD. The mean IFN-γ responses to bovine PPD in animals vaccinated with BCG or the four combinations of BCG and adjuvanted vaccines were significantly higher than those in animals in the nonvaccinated group between 3 and 8 weeks after the initial vaccination (P < 0.01) (Fig. 1). At 2 weeks after challenge with M. bovis, the IFN-γ responses to bovine PPD increased markedly in all groups and remained high until the animals were euthanized (Fig. 1). The responses in the nonvaccinated animals were higher than those in the animals vaccinated with the BCG or BCG and protein vaccines at 5 weeks after challenge (P < 0.05).
FIG. 1.
Mean levels of IFN-γ ± SEM released from bovine PPD-stimulated cultures of whole blood from animals vaccinated with BCG alone (▪), BCG-CFP-DDA-PIM (▴), BCG-CFP-DDA-MPL (•), BCG-CFP-DDA-Pam3CSK4 (*), or BCG-DDA-MPL (⧫) and nonvaccinated animals (⋄).
IFN-γ-producing cells.
The results of the IFN-γ ELISPOT assay performed with antigen-stimulated PBMCs from four animals from each of the six groups at 8 weeks after vaccination are shown in Table 2. The four groups which were vaccinated with BCG-adjuvanted vaccines had mean changes in IFN-γ SFC values significantly greater than the mean value for the nonvaccinated group (P < 0.05). There was a strong correlation between the change in the IFN-γ SFC value for individual animals and the corresponding level of IFN-γ released from bovine PPD-stimulated cultures of blood collected at the same time (Pearson's correlation coefficient = 0.506; P = 0.012) (data not shown).
TABLE 2.
IFN-γ ELISPOT assay results at 8 weeks after initial vaccination
| Groupa | Mean change in no. of IFN-γ SFCs/106 PBMCsb |
|---|---|
| Nonvaccinated | 35 ± 13 |
| BCG | 131 ± 37 |
| BCG-CFP-DDA-PIM2 | 205 ± 51c |
| BCG-CFP-DDA-MPL | 212 ± 39c |
| BCG-CFP-DDA-Pam3CSK4 | 213 ± 94c |
| BCG-DDA-MPL | 222 ± 48c |
Four animals per group were randomly selected for the IFN-γ ELISPOT assay.
The change in the numbers of SFCs represents the number of SFCs for the bovine PPD-stimulated PBMCs minus the number of SFCs for the medium control PBMCs/106 cells. The mean number of SFCs for the medium control PBMCs/106 cells was 81 ± 19.
The results are significantly different from the results for the nonvaccinated group (P < 0.05).
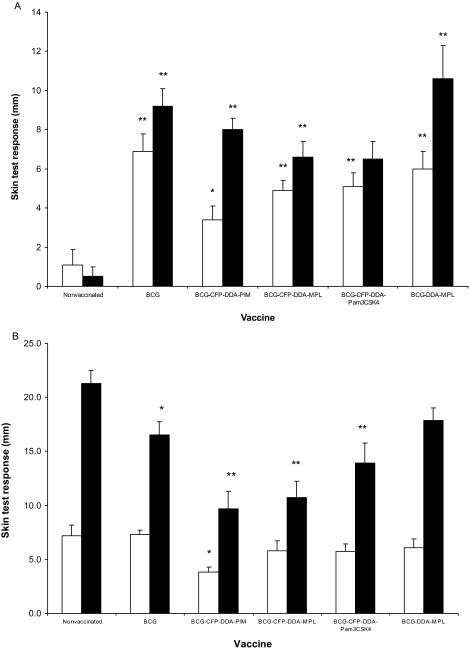
Skin test responses.
At 11 weeks after vaccination and at 13 weeks after challenge, all challenged calves were subjected to a comparative cervical tuberculin skin test. The mean responses for the different groups are shown in Fig. 2. For all BCG-vaccinated groups, the mean responses to bovine PPD after vaccination were greater than those for the nonvaccinated group (P < 0.01) (Fig. 2A). The mean skin test responses to bovine PPD in the animals vaccinated with BCG alone or BCG-CFP-DDA-PIM, BCG-CFP-DDA-MPL, or BCG-CFP-DDA-Pam3CSK4 at 13 weeks after challenge (Fig. 2B) were lower than the mean response in the nonvaccinated animals (P < 0.05) (Fig. 2B).
FIG. 2.
Skin test responses to avian PPD (□) and bovine PPD (▪) at 11 weeks after vaccination (A) and 13 weeks after challenge (B). The data are expressed as the mean increase in skin thickness (mm) ± SEM between the time of inoculation and 72 h later. *, the mean was significantly different compared to that for the nonvaccinated group (P < 0.05); **, the mean was significantly different compared to that for the nonvaccinated group (P < 0.01).
Antibody responses after vaccination.
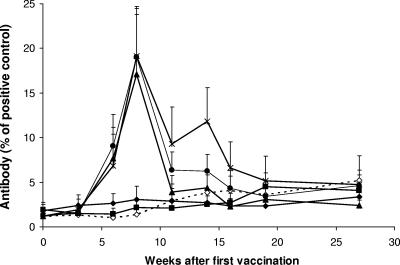
Significant antibody responses to the M. bovis CFP were observed in the animals vaccinated with combinations of BCG and CFP vaccines (Fig. 3). The mean responses in these animals 6 to 8 weeks after the initial vaccination were significantly higher than the mean responses in the animals in the BCG, BCG-DDA-MPL, and the nonvaccinated groups (P < 0.05).
FIG. 3.
Mean antibody responses ± SEM to M. bovis CFP in animals vaccinated with BCG alone (▪), BCG-CFP-DDA-PIM (▴), BCG-CFP-DDA-MPL (•), BCG-CFP-DDA-Pam3CSK4 (*), or BCG-DDA-MPL (⧫) and nonvaccinated animals (⋄). The results are expressed as a percentage in relation to that for a strongly positive control serum.
Pathological and microbiological findings after challenge.
The proportion of animals with tuberculous lesions in the lungs and lymph nodes and the mean lung and lymph node lesion scores are shown in Table 3. There was a significantly lower proportion of animals with lung lesions in the group vaccinated with BCG-CFP-DDA-Pam3CSK4 than in the nonvaccinated group (P < 0.05). Four of the vaccinated groups, i.e., those vaccinated with BCG alone, BCG-CFP-DDA-PIM, BCG-CFP-DDA-MPL, and BCG-CFP-DDA-Pam3CSK4, had significantly lower proportions of animals with lymph node lesions than the nonvaccinated group (P < 0.05). Tuberculous lesions were mostly found only in the thoracic cavity, although one lesion was found in a retropharyngeal lymph node of an animal in the nonvaccinated group. The lung lesions in the challenged animals mainly consisted of a number of small nodular lesions 2 to 5 mm in diameter with yellow caseous centers, whereas the lymph node lesions varied considerably in size, from 1 to 20 mm in diameter.
TABLE 3.
Pathological and microbiological findings following challenge of calves with M. bovis
| Vaccine group | Proportiona of animals with:
|
Mean lesion scores ± SEM in:
|
Mean no. of M. bovis culture-positive LNs/animal ± SEM | ||
|---|---|---|---|---|---|
| Lung lesions | LNb lesions | Lungsc | LNsd | ||
| Nonvaccinated | 7/10 | 10/10 | 3.1 ± 0.7 | 7.8 ± 1.1 | 2.5 ± 0.3 |
| BCG alone | 5/10 | 4/10e | 1.1 ± 0.4e,f | 2.2 ± 1.1e | 1.6 ± 0.4 |
| BCG-CFP-DDA-PIM2 | 3/10 | 2/10e | 0.9 ± 0.5e,f | 0.9 ± 0.6e,f | 1.7 ± 0.4 |
| BCG-CFP-DDA-MPL | 3/10 | 5/10e | 0.6 ± 0.4e,f | 1.3 ± 0.6e | 1.7 ± 0.3 |
| BCG-CFP-DDA-Pam3CSK4 | 1/10e,f | 2/10e | 0.3 ± 0.3e,f | 0.2 ± 0.1e,f | 1.3 ± 0.4e,f |
| BCG-DDA-MPL | 8/10 | 7/10 | 2.5 ± 0.5 | 3.8 ± 1.2e | 2.5 ± 0.3 |
The data represent the number of animals with lung or lymph node lesions/total number of animals in the group.
LN, lymph node.
Lung lesion scores: 0, no lesions; 1, 1 to 9 lesions; 2, 10 to 29 lesions; 3, 30 to 99 lesions; 4, 100 to 199 lesions; 5, ≥200 lesions.
Total lymph node lesion score per animal for individual nodes: 0, no lesions; 1, 1 to 19 small lesions (diameter, 1 to 4 mm); 2, ≥20 small lesions; 3, medium-size lesions (diameter, 5 to 9 mm); 4, large lesions (diameter, ≥10 mm). Scores for the four pulmonary lymph nodes for each animal were pooled.
The results are significantly different from those for the nonvaccinated group (P < 0.05).
The results are significantly different from those for the BCG-DDA-MPL group (P < 0.05).
Animals vaccinated with BCG alone or the three combinations of BCG-CFP vaccines (CFP-DDA-PIM, CFP-DDA-MPL, and CFP-DDA-Pam3CSK4) had significantly lower mean scores for lesions in the lungs and lymph nodes than the nonvaccinated cattle (P < 0.05). The BCG-CFP-DDA-Pam3CSK4 group was the only group with a significantly lower number of M. bovis culture-positive lymph nodes per animal compared to the number in the nonvaccinated group (P < 0.05). Vaccination with BCG-DDA-MPL was shown to confer minimal protection to the animals, and the only significant reduction in a disease parameter compared to the parameters for the nonvaccinated group was for the mean lymph node lesion score (P < 0.05). There were two disease parameters (proportion with lung lesions and mean number of M. bovis culture-positive lymph nodes per animal) for which the BCG-DDA-MPL-vaccinated group had values greater than or the same as those for the nonvaccinated group. Furthermore, there were numerous instances in which the disease parameters for the BCG-DDA-MPL-vaccinated group were significantly higher than those for the other vaccinated groups, and in particular, the mean lung lesion score for this group was significantly higher than that for the BCG-alone group (P < 0.05).
IL-10 responses after vaccination.
To gain possible insights into the differences in the levels of protection between animals vaccinated with BCG-CFP-DDA-MPL and those vaccinated with BCG-DDA-MPL (adjuvant alone), the release of IL-10 from whole blood stimulated with bovine PPD was measured after vaccination. There were no significant differences between the groups at 3, 8, or 14 weeks after the initial vaccination. The mean levels of IL-10 ± standard error of the mean (SEM) for the BCG-CFP-DDA-MPL- and BCG-DDA-MPL-vaccinated animals were 0.41 ± 0.24 and 0.48 ± 0.29 ng/ml, respectively, at 3 weeks; 1.02 ± 0.32 and 0.95 ± 0.27 ng/ml, respectively, at 8 weeks; and 1.62 ± 0.33 and 1.59 ± 0.50 ng/ml, respectively, at 14 weeks.
Immune gene expression in vaccinated cattle.
In a subsequent trial, two groups of cattle were vaccinated with the BCG-CFP-DDA-MPL or the BCG-DDA-MPL combination. The aim of this experiment was to investigate if vaccines that induce high levels of protection (BCG-CFP-DDA-MPL) or low levels of protection (BCG-DDA-MPL) had different biosignatures which could predict protective efficacy. The levels of expression of bovine PPD-specific mRNA of selected cytokines and the T regulatory cell transcription factor FoxP3 in the PBMCs of calves were measured at 9 weeks after vaccination. There were no significant differences observed between the mean responses of the two groups of calves for IL-4, IL-10, IL-12 p40, IL-13, IL-18, or FoxP3. Of these genes, only those for IL-4 and IL-13 showed regulation by bovine PPD. The mean levels of IL-4 ± SEM in PBMCs from BCG-CFP-DDA-MPL- and BCG-DDA-MPL-vaccinated animals were 2.4 ± 1.0 and 2.8 ± 0.7-fold higher, respectively, in cells cultured with bovine PPD than in cells cultured with PBS. Similarly, the levels of IL-13 in these two groups were 7.0 ± 3.4 and 7.3 ± 4.1-fold higher, respectively, in response to bovine PPD.
DISCUSSION
Subunit vaccines, such as those based upon mycobacterial CFPs, have been shown to induce significant levels of protection in mice and stimulate the emergence of antigen-specific protective CD4+ T cells which release IFN-γ (3). A major obstacle in the implementation of vaccine strategies against tuberculosis based on nonliving preparations is the relatively poor immunogenicity of such subunit vaccines compared to that of live vaccines such as BCG. This has led to the search for optimal adjuvants that can augment the response of the immune system to the vaccine formulation. An approach currently pursued is to combine BCG vaccination with vaccination with subunit vaccines. This has the virtue that a safe vaccine (BCG) can be used with additional vaccination formulations consisting of proteins or peptides, which can lead to a more significant level of protection by complementing the impact of BCG used alone. The latter paradigm has received some attention in cattle, with a variety of approaches aimed at using BCG in concert with DNA (28, 37) or protein (45) vaccines.
In the current study, we aimed to evaluate and compare the efficacies of three defined adjuvant systems which enhance immune reactivity by targeting TLRs. Two TLR2 agonists were tested, namely, PIM2 and Pam3CSK4, as well as a TLR4 agonist, MPL. The results concur with previous findings, as overall better protection was achieved when animals were vaccinated with a combination of BCG and a mycobacterial protein vaccine with one adjuvant system than when they were vaccinated with BCG alone (45). The data gathered here suggest a role for TLR2 agonists in enhancing protective immune responses against bovine tuberculosis, in keeping with recent findings with a mouse model of the disease (42). A recent publication has shown a lower relative level of expression of the TLR2 gene in M. bovis-infected cattle and has suggested that the down-regulation of this receptor may play a role in host susceptibility (29).
Vaccination of animals with the preparations used here induced strong antibody responses to mycobacterial antigens, but as was observed previously, the induction of this antibody response was not associated with a poor outcome of the vaccinations (45). In addition, vaccination with BCG and CFP induced positive tuberculin skin test responses in vaccinated animals similar to those induced by BCG vaccination alone. These responses could hamper the potential for discriminating between vaccinated animals and those infected with M. bovis, if a vaccination campaign with such preparations were adopted and conventional diagnostic procedures were used. However, the use of differential diagnostic tests with tuberculosis-specific antigens such as ESAT-6 and CFP-10 in the whole-blood test would distinguish between BCG-vaccinated and infected animals (9). We have previously shown that cattle vaccinated with a combination of BCG and adjuvanted CFP do not develop IFN-γ responses to CFP-10 or ESAT-6 after vaccination (45), suggesting that these antigens could be used to distinguish between animals vaccinated with BCG and CFPs and infected animals. In addition, cattle vaccinated with the CFP vaccine do not produce a skin test response to bovine PPD (45, 46). When a comparative cervical skin test has been used with BCG-vaccinated cattle, the animals were tuberculin negative by 18 months postvaccination (31).
Sensitization to environmental mycobacteria can adversely influence the outcome of vaccination (6, 10). In the current study, we compared prevaccination avian PPD responses in individual animals with the level of pathology (lung lesion and lymph node scores) observed postmortem. There was no association between prior reactivity to avian PPD and the level of protective immunity conferred by BCG or BCG-protein vaccines (data not shown). However, the avian PPD responses in individual animals are often transient and can vary markedly over short periods.
Surprisingly, the protection induced by BCG vaccine was abrogated when the BCG subcutaneous injection was immediately followed by a subcutaneous injection of an adjuvant combination alone (DDA-MPL) at a site 2 to 3 cm from the BCG injection site. It is unclear why vaccination with BCG immediately followed by vaccination with DDA-MPL abrogated the protection conferred by BCG alone, as a BCG injection followed by an injection of CFP-DDA-MPL enhanced protection. In the current study, vaccination with BCG-DDA-MPL induced levels of IFN-γ similar to those induced by BCG-CFP-DDA-MPL. In order to gain insight into this paradox, we measured the IL-10 levels in bovine PPD-stimulated cultures of blood from these animals at 3, 8, and 14 weeks following vaccination. There were no differences in the antigen-specific release of IL-10 between the two groups. In the second vaccination trial, the levels of expression of a range of cytokine and immune genes were measured by real-time PCR from bovine PPD-stimulated PBMCs of cattle vaccinated with BCG-CFP-DDA-MPL or BCG-DDA-MPL, to provide a possible explanation for the differences in protection. At 9 weeks after vaccination, there were no significant differences between the two groups in the antigen-specific expression of the cytokines IL-4, IL-10, IL-12 p40, IL-13, IL-18, and FoxP3, a transcription factor specific for T regulatory cells. These results suggest that there was no evidence of immunosuppression associated with the administration of BCG and adjuvant without the accompanying administration of CFP. In addition, these data suggest that a clear immune signature correlating with protection (or a lack thereof) following vaccination is still lacking (30).
Two recent mouse studies have provided some insights into the mechanisms associated with immunosuppression which could arise following exposure to TLR ligands and the subsequent encounter of antigens. The first study indicated that exposure to TLR ligands induced a maturation of DCs and that the mature DCs had down-regulated their capacity to cross-present newly encountered antigens in vivo (48). In the second study, mice injected intravenously with TLR ligands, which caused systemic DC maturation, could not induce CD4 T-cell responses against subsequently inoculated soluble antigens (51). This immunosuppression could be reversed by the adoptive transfer of DCs preloaded with peptide antigen, showing that the lack of T-cell proliferation in the TLR ligand-treated mice was due to impaired antigen presentation rather than the general suppression of T-cell activation. Other studies have reported that prolonged TLR signaling by certain proteins or lipopeptides could inhibit certain macrophage responses to IFN-γ, particularly those associated with major histocompatibility complex class II antigen presentation (21, 50). Collectively, these studies and the results from the current trial highlight the possible caveats associated with the administration of TLR ligands under certain circumstances.
In conclusion, a vaccination strategy based on the immunization of cattle with a combination of BCG and a protein vaccine may provide protection better than that obtained by the administration of BCG alone, with the best protection achieved by the inclusion of the TLR2 agonist PAM3CSK4 in the protein vaccine formulation. The use of a low dose of BCG and additional proteins to vaccinate cattle is unlikely to cause animal welfare concerns, although all aspects of food safety associated with the use of a live attenuated vaccine would need to be addressed.
Acknowledgments
We thank Keith Hamel, Natalie Parlane, Allison McCarthy, Denise Keen, Gina Pedersen, and Gary Yates for technical assistance and Fred Potter for help with the statistical analyses.
This work was supported financially by Defra (United Kingdom), the New Zealand Ministry of Agriculture and Forestry (Policy Management), and a New Zealand FRST grant.
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Ainge, G. D., N. A. Parlane, M. Denis, C. M. Hayman, D. S. Larsen, and G. F. Painter. 2006. Phosphatidylinositol mannosides: synthesis and adjuvant properties of phosphatidylinositol di- and tetramannosides. Bioorg. Med. Chem. 14:7615-7624. [DOI] [PubMed] [Google Scholar]
- 2.Ainge, G. D., N. A. Parlane, M. Denis, B. S. Dyer, A. Härer, C. M. Hayman, D. S. Larsen, and G. F. Painter. 2007. Phosphatidylinositol mannoside ether analogues: syntheses and interleukin-12-inducing properties. J. Org. Chem. 72:5291-5296. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P. 1994. Effective vaccination of mice against Mycobacterium tuberculosis infection with a soluble mixture of secreted mycobacterial proteins. Infect. Immun. 62:2536-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldridge, J. R., P. McGowan, J. T. Evans, C. Cluff, S. Mossman, D. Johnson, and D. Persing. 2004. Taking a Toll on human disease: Toll-like receptor 4 agonists as vaccine adjuvants and monotherapeutic agents. Expert Opin. Biol. Ther. 4:1129-1138. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. N. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 8.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. de Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 9.Buddle, B. M., N. A. Parlane, D. L. Keen, F. E. Aldwell, J. M. Pollock, K. Lightbody, and P. Andersen. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buddle, B. M., B. J. Wards, F. E. Aldwell, D. M. Collins, and G. W. de Lisle. 2002. Influence of sensitisation to environmental mycobacteria on subsequent vaccination against bovine tuberculosis. Vaccine 20:1126-1133. [DOI] [PubMed] [Google Scholar]
- 11.Buddle, B. M., D. N. Wedlock, M. Denis, and M. A. Skinner. 2005. Identification of immune response correlates for protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 10:45-51. [DOI] [PubMed] [Google Scholar]
- 12.Buddle, B. M., D. N. Wedlock, and M. Denis. 2006. Progress in the development of tuberculosis vaccines for cattle and wildlife. Vet. Microbiol. 112:191-200. [DOI] [PubMed] [Google Scholar]
- 13.Cosivi, O., J. M. Grange, C. J. Daborn, M. C. Raviglione, T. Fujikura, D. Cousins, R. A. Robinson, H. F. Huchzermeyer, I. de Kantor, and F. X. Meslin. 1998. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 4:59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty, T. M., and P. Andersen. 2005. Vaccines for tuberculosis: novel concepts and recent progress. Clin. Microbiol. Rev. 18:687-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drennan, M. B., D. Nicolle, V. J. Quesniaux, M. Jacobs, N. Allie, J. Mpagi, C. Fremond, H. Wagner, C. Kirschning, and B. Ryffel. 2004. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am. J. Pathol. 164:49-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyer, B. S., J. D. Jones, G. D. Ainge, M. Denis, D. S. Larsen, and G. F. Painter. 2007. Synthesis and structure of phosphatidylinositol dimannoside. J. Org. Chem. 72:3282-3288. [DOI] [PubMed] [Google Scholar]
- 17.Fine, P. E. 2001. BCG: the challenge continues. Scand. J. Infect. Dis. 33:243-245. [DOI] [PubMed] [Google Scholar]
- 18.Fraser, C. K., K. R. Diener, M. P. Brown, and J. D. Hayball. 2007. Improving vaccines by incorporating immunological coadjuvants. Expert Rev. Vaccines 6:559-578. [DOI] [PubMed] [Google Scholar]
- 19.Gilleron, M., V. F. Quesniaux, and G. Puzo. 2003. Acylation state of the phosphatidylinositolhexamannosides from Mycobacterium bovis bacillus Calmette Guerin and Mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J. Biol. Chem. 278:29880-29889. [DOI] [PubMed] [Google Scholar]
- 20.Hope, J. C., A. O. Whelan, R. G. Hewinson, H. M. Vordermeier, and C. J. Howard. 2003. Maturation of bovine dendritic cells by lipopeptides. Vet. Immunol. Immunopathol. 95:21-31. [DOI] [PubMed] [Google Scholar]
- 21.Hovav, A. H., J. Mullerad, L. Davidovitch, Y. Fishman, F. Bigi, A. Cataldi, and H. Bercovier. 2003. The Mycobacterium tuberculosis recombinant 27-kilodalton lipoprotein induces a strong Th1-type immune response deleterious to protection. Infect. Immun. 71:3146-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones, B. W., T. K. Means, K. A. Heldwein, M. A. Keen, P. J. Hill, J. T. Belisle, and M. J. Fenton. 2001. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 69:1036-1044. [PubMed] [Google Scholar]
- 23.Kaufmann, S. H. 2006. Envisioning future strategies for vaccination against tuberculosis. Nat. Rev. Immunol. 6:699-704. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann, S. H., S. Baumann, and A. Nasser Eddine. 2006. Exploiting immunology and molecular genetics for rational vaccine design against tuberculosis. Int. J. Tuberc. Lung Dis. 10:1068-1079. [PubMed] [Google Scholar]
- 25.Koets, A., A. Hoek, M. Langelaar, M. Overdijk, W. Santema, P. Franken, W. van Eden, and V. Rutten. 2006. Mycobacterial 70kD heat-shock protein is an effective subunit vaccine against bovine paratuberculosis. Vaccine 24:2550-2559. [DOI] [PubMed] [Google Scholar]
- 26.Lindblad, E. B., M. J. Elhay, R. Silva, R. Appelberg, and P. Andersen. 1997. Adjuvant modulation of immune responses to tuberculosis subunit vaccines. Infect. Immun. 65:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logan, K. E., M. A. Chamber, R. G. Hewinson, and P. J. Hogarth. 2005. Frequency of IFN-gamma producing cells correlates with adjuvant enhancement of bacille Calmette-Guerin induced protection against Mycobacterium bovis. Vaccine 23:5526-5532. [DOI] [PubMed] [Google Scholar]
- 28.Maue, A. C., W. R. Waters, M. V. Palmer, B. J. Nonnecke, F. C. Minion, W. C. Brown, J. Norimine, M. R. Foote, C. F. Scherer, and D. M. Estes. 2007. An ESAT-6:CFP10 DNA vaccine administered in conjunction with Mycobacterium bovis BCG confers protection to cattle challenged with virulent M. bovis. Vaccine 25:4735-4746. [DOI] [PubMed] [Google Scholar]
- 29.Meade, K. G., E. Gormley, M. B. Doyle, T. Fitzsimons, C. O'Farrelly, E. Costello, J. Keane, Y. Zhao, and D. E. Machugh. 2007. Innate gene repression associated with Mycobacterium bovis infection in cattle: toward a gene signature of disease. BMC Genomics 8:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittrucker, H. W., U. Steinhoff, A. Kohler, M. Krause, D. Lazar, P. Mex, D. Miekley, and S. H. Kaufmann. 2007. Poor correlation between BCG vaccination-induced T cell responses and protection against tuberculosis. Proc. Natl. Acad. Sci. USA 104:12434-12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moodie, P. A. 1977. Tuberculin reactions in BCG-vaccinated cattle. Br. Vet. J. 133:642-645. [DOI] [PubMed] [Google Scholar]
- 32.Rezaei, N. 2006. Therapeutic targeting of pattern-recognition receptors. Int. Immunopharmacol. 6:863-869. [DOI] [PubMed] [Google Scholar]
- 33.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1990. A sandwich enzyme immunoassay for bovine interferon-gamma and its use for the detection of tuberculosis in cattle. Aust. Vet. J. 67:134-137. [DOI] [PubMed] [Google Scholar]
- 34.Seo, K. S., S. U. Lee, Y. H. Park, W. C. Davis, W. K. Fox, and G. A. Bohach. 2007. Long-term staphylococcal enterotoxin C1 exposure induces soluble factor-mediated immunosuppression by bovine CD4+ and CD8+ T cells. Infect. Immun. 75:260-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skeiky, Y. A., and J. C. Sadoff. 2006. Advances in tuberculosis vaccine strategies. Nat. Rev. Microbiol. 4:469-476. [DOI] [PubMed] [Google Scholar]
- 36.Skinner, M. A., B. M. Buddle, D. N. Wedlock, D. Keen, G. W. de Lisle, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, P. J. Cockle, H. M. Vordermeier, and R. G. Hewinson. 2003. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect. Immun. 71:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skinner, M. A., D. N. Wedlock, G. W. de Lisle, M. M. Cooke, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, H. M. Vordermeier, R. G. Hewinson, and B. M. Buddle. 2005. The order of prime-boost vaccination of neonatal calves with Mycobacterium bovis BCG and a DNA vaccine encoding mycobacterial proteins Hsp65, Hsp70, and Apa is not critical for enhancing protection against bovine tuberculosis. Infect. Immun. 73:4441-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Texereau, J., J. D. Chiche, W. Taylor, G. Choukroun, G. Comba, and J. P. Mira. 2005. The importance of Toll-like receptor 2 polymorphisms in severe infections. Clin. Infect. Dis. 41(Suppl. 7):S408-S415. [DOI] [PubMed] [Google Scholar]
- 39.von den Esche, U., M. Ayoub, S. D. Pfannes, M. R. Muller, M. Huber, K. H. Wiesmuller, T. Loop, M. Humar, K. F. Fischbach, M. Strunkelnberg, P. Hoffmann, W. G. Bessler, and K. Mittenbuhler. 2000. Immunostimulation by bacterial components. I. Activation of macrophages and enhancement of genetic immunization by the lipopeptide P3CSK4. Int. J. Immunopharmacol. 22:1093-1102. [DOI] [PubMed] [Google Scholar]
- 40.Vordermeier, H. M., M. A. Chambers, B. M. Buddle, J. M. Pollock, and R. G. Hewinson. 2006. Progress in the development of vaccines and diagnostic reagents to control tuberculosis in cattle. Vet. J. 171:229-244. [DOI] [PubMed] [Google Scholar]
- 41.Vordermeier, H. M., K. Huygen, M. Singh, R. G. Hewinson, and Z. Xing. 2006. Immune responses induced in cattle by vaccination with a recombinant adenovirus expressing mycobacterial antigen 85A and Mycobacterium bovis BCG. Infect. Immun. 74:1416-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, B., M. Henao-Tamayo, M. Harton, D. Ordway, C. Shanley, R. J. Basaraba, and I. M. Orme. 2007. A Toll-like receptor-2-directed fusion protein vaccine against tuberculosis. Clin. Vaccine Immunol. 14:902-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wedlock, D. N., B. Vesosky, M. A. Skinner, G. W. de Lisle, I. M. Orme, and B. M. Buddle. 2000. Vaccination of cattle with Mycobacterium bovis culture filtrate proteins and interleukin-2 for protection against bovine tuberculosis. Infect. Immun. 68:5809-5815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wedlock, D. N., M. A. Skinner, N. A. Parlane, H. M. Vordermeier, R. G. Hewinson, G. W. de Lisle, and B. M. Buddle. 2003. Vaccination with DNA vaccines encoding MPB70 or MPB83 or a MPB70 DNA prime-protein boost does not protect cattle against bovine tuberculosis. Tuberculosis (Edinburgh) 83:339-349. [DOI] [PubMed] [Google Scholar]
- 45.Wedlock, D. N., M. Denis, M. A. Skinner, J. Koach, G. W. de Lisle, H. M. Vordermeier, R. G. Hewinson, S. van Drunen Littel-van den Hurk, L. A. Babiuk, R. Hecker, and B. M. Buddle. 2005. Vaccination of cattle with a CpG ODN-formulated mycobacterial protein vaccine and BCG induces levels of protection against bovine tuberculosis superior to vaccination with BCG alone. Infect. Immun. 73:3540-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wedlock, D. N., M. A. Skinner, H. M. Vordermeier, R. G. Hewinson, R. Hecker, S. van Drunen Littel-van den Hurk, and B. M. Buddle. 2005. Vaccination of cattle with Mycobacterium bovis culture filtrate proteins and CpG oligodeoxynucleotides induces protection against bovine tuberculosis. Vet. Immunol. Immunopathol. 106:53-63. [DOI] [PubMed] [Google Scholar]
- 47.Whelan, A. O., J. C. Hope, C. J. Howard, D. Clifford, R. G. Hewinson, and H. M. Vordermeier. 2003. Modulation of the bovine delayed-type hypersensitivity responses to defined mycobacterial antigens by a synthetic bacterial lipopeptide. Infect. Immun. 71:6420-6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson, N. S., G. M. N. Behrens, R. J. Lundie, C. M. Smith, J. Waithman, L. Young, S. P. Forehan, A. Mount, R. J. Steptoe, K. D. Shortman, T. F. de Koning-Ward, G. T. Belz, F. R. Carbone, B. S. Crabb, W. R. Heath, and J. A. Villadangos. 2006. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat. Immunol. 7:165-172. [DOI] [PubMed] [Google Scholar]
- 49.Wood, P. R., L. A. Corner, J. S. Rothel, J. L. Ripper, T. Fifis, B. S. McCormick, B. Francis, L. Melville, K. Small, L. De Witte, J. Tolson, T. J. Ryan, G. W. de Lisle, J. C. Cox, and S. L. Jones. 1992. A field evaluation of serological and cellular diagnostic tests for bovine tuberculosis. Vet. Microbiol. 31:71-79. [DOI] [PubMed] [Google Scholar]
- 50.Yeremeev, V. V., I. V. Lyadova, B. V. Nikonenko, A. S. Apt, C. Abou-Zeid, J. Inwald, and D. B. Young. 2000. The 19-kD antigen and protective immunity in a murine model of tuberculosis. Clin. Exp. Immunol. 120:274-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young, L. J., N. S. Wilson, P. Schnorrer, A. Mount, R. J. Lundie, N. L. La Gruta, B. S. Crabb, G. T. Belz, W. R. Heath, and J. A. Villadangos. 2007. Dendritic cell preactivation impairs MHC class II presentation of vaccines and endogenous viral antigens. Proc. Natl. Acad. Sci. USA 104:17753-17758. [DOI] [PMC free article] [PubMed] [Google Scholar]