Abstract
We have previously shown that Campylobacter jejuni strains do not produce a functional cholera toxin-like toxin (CTLT) detectable in a Chinese hamster ovary cell assay. Instead, the 53-kDa major outer membrane protein (OMP) of C. jejuni, PorA, reacts with cholera toxin (CT) antibody on immunoblots. Here, we have extended this observation to other species of Campylobacter, including C. coli, C. lari, C. fetus, C. hyointestinalis, and C. upsaliensis, the common 53-kDa OMP of which reacted with CT antibody in immunoblotting assays. There were additional reactive bands for C. fetus. As with C. jejuni, this finding may lead to the erroneous conclusion that these additional species produce a functional CTLT. However, this common cross-reactive OMP can be explored as a vaccine candidate to prevent campylobacteriosis.
The genus Campylobacter consists of several species, including Campylobacter jejuni, C. coli, C. fetus, C. lari (previously C. laridis), C. upsaliensis, and C. hyointestinalis (22). All of these species are food-borne pathogens and cause diarrheal diseases worldwide. They also cause extraintestinal infections and sequelae (2). Since C. jejuni is the predominant species, most studies have been directed at this pathogen. C. jejuni causes predominantly inflammatory diarrhea in individuals in developed countries and watery diarrhea in individuals in developing countries (2, 25). The putative virulence factors of C. jejuni include the ability to adhere to and invade epithelial cells, iron acquisition systems (13), cytotoxins, cytolethal distending toxin, and an enterotoxin that resembles cholera toxin (CT) and the heat-labile enterotoxin (LT) of Escherichia coli (29). We refer to this enterotoxin as CT-like toxin (CTLT). It is believed that CTLT may contribute to watery diarrhea (29). However, there has been controversy on the existence of CTLT.
Many groups have reported the production of CTLT by C. jejuni strains (6, 8, 9, 16, 19, 20, 27), while others have failed to do so (17, 25, 26, 28). Attempts to demonstrate genetic sequences homologous to the genes encoding CT and LT have also failed (24, 26). In an attempt to clarify whether or not C. jejuni produces CTLT, we carried out a study using well-characterized strains from different laboratories in different media reported to promote CTLT production. We found that C. jejuni does not produce a functional CTLT that is capable of causing a cytotonic alteration of Chinese hamster ovary (CHO) cells, but the major outer membrane protein (OMP) of C. jejuni, PorA, reacted with CT antibody in enzyme-linked immunosorbent and immunoblotting assays. We concluded that this reaction of PorA with CT antibody has led to the erroneous conclusion that C. jejuni produces CTLT (1).
Previously, we found that the primers amplifying the gene encoding PorA in C. jejuni also amplify the corresponding genes from other species of Campylobacter (14). This finding suggested that porA genes from different species of Campylobacter are homologous.
There are also reports of the production of enterotoxin by C. coli (11), C. lari (11), and C. hyointestinalis (12) strains. However, there are no reports of the production of enterotoxin by C. upsaliensis and C. fetus strains. In this study, we examined C. coli, C. fetus, C. lari, C. hyointestinalis, and C. upsaliensis for functional CTLT production and investigated whether, as PorA from C. jejuni, the PorA proteins from these species cross-react with CT. The latter finding would have implications for the interpretation of results regarding the possible production of CTLT by these species, as with C. jejuni. Moreover, the identification of a common immunogenic protein in many species of Campylobacter would present opportunities to explore the antigen as a potential candidate vaccine to combat campylobacteriosis.
MATERIALS AND METHODS
Bacteria.
Two strains each of the following species were provided by G. Hogg, Microbiological Diagnostic Unit, University of Melbourne, Parkville, Victoria, Australia: C. jejuni, C. coli, C. fetus, C. lari, C. hyointestinalis, C. upsaliensis, C. fetus, C. lari, and C. hyointestinalis. The other isolates were from our culture collection. The species of all isolates were confirmed by standard bacteriological tests and PCR assays (7, 10, 15, 22).
The Campylobacter organisms were stored in brucella broth (BBL; Becton Dickinson, Sparks, MD) with 15% glycerol at −70°C. For the study, the organisms were cultured on 7% sheep blood agar in a microaerophilic atmosphere generated with a BBL Campy GasPak (Becton Dickinson) in a jar with a catalyst at 42°C for 48 h. An enterotoxigenic Escherichia coli strain, H10407, producing LT served as a positive control for enterotoxin production in a CHO cell assay (see below).
Production of CTLT.
Isolates were tested for CTLT production in Casamino Acids-yeast extract broth supplemented with ferric chloride in a shaker incubator as described previously (1). Serial doubling dilutions of bacterium-free filtrate were tested for enterotoxin on CHO cell monolayers in a microtiter plate. The elongation of ≥50% of cells at a dilution of ≥1:4 was considered to indicate positivity for CTLT (1).
Preparation of crude OMPs and purified major OMPs.
The PorA major OMPs from different Campylobacter species were prepared by the Sarkosyl method of Blaser et al. (4). Briefly, for each preparation, bacterial cells were disrupted by sonication and the preparation was centrifuged at 5,000 × g to remove whole cells. The supernatant was centrifuged for 1 h at 100,000 × g at 4°C in an L8-70 ultracentrifuge (Beckman Instruments, Fullerton, CA), and the pellet was suspended in sterile distilled water and used as the crude membrane fraction. The crude membrane preparation was further treated with sodium lauryl sarcosinate. The Sarkosyl-insoluble portion was used as the purified outer membrane fraction.
SDS-PAGE and immunoblotting.
Proteins were separated by discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 5.0% stacking gel and a 12.0% separating gel by the method of Laemmli (18) and stained with Coomassie blue. For immunoblotting, the separated proteins were transferred electrophoretically onto nitrocellulose (Bio-Rad, Hercules, CA) and then blocked with 5% skim milk in phosphate-buffered saline (pH 7.2). The membrane was allowed to react with rabbit CT antibody (Sigma, St. Louis, MO) or normal rabbit serum, as appropriate, both diluted 1:1,000. The secondary antibody (peroxidase-conjugated, affinity-purified goat anti-rabbit immunoglobulin G [Fc fragment specific], at a 1:1,000 dilution [Jackson Immunoresearch Laboratories, West Grove, PA]) was added, after which the results were developed with enhanced chemiluminescence Western blotting detection reagents according to the instructions of the manufacturer (Amersham Pharmacia Biotech, Piscataway, NJ).
Amplification of porA genes.
The porA genes from Campylobacter species strains were amplified using boiled cells as templates with primers and amplification parameters as described previously (14). The amplified products were separated by electrophoresis in 1.0% agarose gels in Tris-borate-EDTA buffer at 90 V for 90 min. The bands were visualized under UV light after staining with a 1-μg/ml ethidium bromide solution.
RESULTS AND DISCUSSION
All Campylobacter species strains were negative for CTLT production in the CHO cell assay, while the positive control enterotoxigenic E. coli strain was positive.
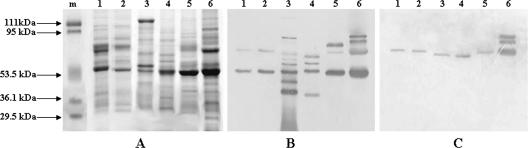
Crude OMPs from all Campylobacter species produced several bands that were seen on Coomassie blue-stained gels. However, 53-kDa bands from all species were prominent (Fig. 1A). From two bands for C. jejuni and C. coli up to seven bands for C. fetus reacted with CT antibody in Western blotting assays, with a common prominent band of approximately 53 kDa for all species (Fig. 1B).
FIG. 1.
Analysis of crude outer membrane fractions. (A) SDS-PAGE-separated proteins were stained with Coomassie blue. (B and C) The separated proteins were transferred onto nitrocellulose membranes and probed with CT antibody (B) or normal rabbit serum (C). Lane m in panel A contains molecular mass markers. Other lanes in all three panels are loaded with 5 μg each of OMPs from the same strains. Lanes 1, C. jejuni; lanes 2, C. coli; lanes 3, C. fetus; lanes 4, C. hyointestinalis; lanes 5, C. lari; and lanes 6, C. upsaliensis. The positions of molecular mass markers are shown on the left of panel A.
To rule out nonspecific activity, the crude OMPs were subjected to immunoblotting with normal rabbit serum. The higher band corresponding to the approximate molecular mass of 79 kDa from all species reacted with normal rabbit serum, as observed in a previous study with C. jejuni strains (1). There was a reaction with two additional higher-molecular-mass bands from C. upsaliensis (Fig. 1C).
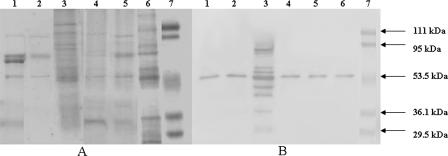
In the Sarkosyl-purified OMP preparations, Coomassie blue-stained bands were less numerous and less prominent than those in the crude OMP preparations (Fig. 2A). However, when these Sarkosyl-purified OMPs were allowed to react with CT antibody in immunoblotting assays, all species except C. fetus produced the unique single band of 53 kDa. The purified OMP preparation from C. fetus produced several bands in a ladder-like pattern, as did the crude membrane preparation. However, the nonspecific band of 79 kDa that reacted with normal rabbit serum was absent (Fig. 2B).
FIG. 2.
Analysis of Sarkosyl-purified outer membrane fractions. (A) SDS-PAGE-separated proteins were stained with Coomassie blue. (B) The separated proteins were transferred onto a nitrocellulose membrane and probed with CT antibody. The first six lanes in both panels are loaded with 1.4 μg each of OMPs from the same strains. Lanes 7 in both panels contain molecular mass markers. Lanes 1, C. jejuni; lanes 2, C. coli; lanes 3, C. fetus; lanes 4, C. hyointestinalis; lanes 5, C. lari; and lanes 6, C. upsaliensis. The positions of molecular mass markers are shown on the right of panel B.
When purified outer membranes were allowed to react with normal rabbit serum in immunoblotting assays, no band from any species was visible (data not shown).
Both isolates of all the six species of Campylobacter produced identical banding patterns on Coomassie blue-stained SDS-PAGE gels as well as on immunoblots (data not shown).
Like the strains we described previously (14), all Campylobacter species strains in the present study generated an amplicon of 1,275 bp corresponding to the porA gene (data not shown). In a previous study (1) using protein sequencing and recombinant PorA protein, we identified the 53-kDa C. jejuni protein reacting with CT antibody as PorA. In the present study also, we demonstrated the presence of a 53-kDa protein reactive with CT antibody in the outer membrane preparations from both C. jejuni strains. Interestingly, purified OMPs from other species of Campylobacter exhibited reactive bands corresponding to similar molecular masses. Therefore, it is reasonable to assume that the PorA major OMPs from all the tested species of Campylobacter cross-react with CT. The only exception was C. fetus, which had several additional bands that reacted specifically with CT antibody and appeared as a ladder-like structure. C. fetus strains are reported to possess a unique S-layer OMP, which separates into a ladder-like pattern upon gel electrophoresis (5). This observation suggested that the ladder-like structure from C. fetus was the CT-cross-reactive major OMP PorA.
Previously, we failed to show functional CTLT production in a CHO cell assay using many well-characterized strains of C. jejuni. Instead, we concluded that the cross-reactivity of PorA of C. jejuni with CT would have misled investigators to the erroneous conclusion that C. jejuni strains produce CTLT (1). Similarly, there are some reports of CTLT production by other species of Campylobacter (11, 12). However, as with C. jejuni, we did not find evidence for functional CTLT production by these isolates in CHO cell assays. On the other hand, outer membranes from all these species of Campylobacter, like those from C. jejuni, reacted with CT antibody. Therefore, indications of CTLT production by non-C. jejuni species of Campylobacter should be interpreted with caution. It appears that the PorA major OMPs from all Campylobacter species share a common antigenic determinant(s) that cross-reacts with CT. This observation could be exploited for protection against disease caused by Campylobacter species strains. Patients as well as volunteers recovering from C. jejuni infection mount a strong antibody response to this major OMP (3, 21, 23).
Acknowledgments
This study was supported by a Kuwait University research grant (number MI02/01) and by Australian Research Council grant (CE)562063.
We thank G. Hogg, Microbiological Diagnostic Unit, University of Melbourne, Parkville, Victoria, Australia, for the provision of some Campylobacter strains.
Footnotes
Published ahead of print on 19 March 2008.
REFERENCES
- 1.Albert, M. J., S. Haridas, D. Steer, G. S. Dhaunsi, A. I. Smith, and B. Adler. 2007. Identification of a Campylobacter jejuni protein that cross-reacts with cholera toxin. Infect. Immun. 75:3070-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allos, B. M., and M. J. Blaser. 1995. Campylobacter jejuni and expanding spectrum of related infections. Clin. Infect. Dis. 20:1092-1099. [DOI] [PubMed] [Google Scholar]
- 3.Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. [DOI] [PubMed] [Google Scholar]
- 4.Blaser, M. J., J. A. Hopkins, R. M. Berka, M. L. Vasil, and W.-L. L. Wang. 1983. Identification and characterization of Campylobacter jejuni outer membrane proteins. Infect. Immun. 42:276-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser, M. J., P. F. Smith, J. A. Hopkins, I. Heinzer, J. H. Bryner, and W. L. Wang. 1987. Pathogenesis of Campylobacter fetus infections: serum resistance associated with high-molecular-weight surface proteins. J. Infect. Dis. 155:696-706. [DOI] [PubMed] [Google Scholar]
- 6.Bok, H. E., A. S. Greeff, and H. H. Crewe-Brown. 1991. Incidence of toxigenic Campylobacter strains in South Africa. J. Clin. Microbiol. 29:1262-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, P. E., O. F. Christensen, H. E. Clough, P. J. Diggle, C. A. Hart, S. Hazel, R. Kemp, A. J. Leatherbarrow, A. Moore, J. Sutherst, J. Turner, N. J. Williams, E. J. Wright, and N. P. French. 2004. Frequency and spatial distribution of environmental Campylobacter spp. Appl. Environ. Microbiol. 70:6501-6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everest, P. H., H. Goossens, J.-P. Butzler, D. Lloyd, S. Knutton, J. M. Ketley, and P. H. Williams. 1992. Differentiated Caco-2 cells as a model for enteric invasion by Campylobacter jejuni and C. coli. J. Med. Microbiol. 37:319-325. [DOI] [PubMed] [Google Scholar]
- 9.Goossens, H., J.-P. Butzler, and Y. Takeda. 1985. Demonstration of cholera-like enterotoxin production by Campylobacter jejuni. FEMS Microbiol. Lett. 29:73-76. [Google Scholar]
- 10.Hum, S., K. Quinn, J. Brunner, and S. L. W. On. 1997. Evaluation of a PCR assay for identification and differentiation of Campylobacter fetus subspecies. Aust. Vet. J. 75:827-831. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, W. M., and H. Lior. 1986. Cytotoxic and cytotonic factors produced by Campylobacter jejuni, Campylobacter coli, and Campylobacter laridis. J. Clin. Microbiol. 24:275-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, W. M., and H. Lior. 1988. A new heat-labile cytolethal distending toxin (CLDT) produced by Campylobacter spp. Microb. Pathog. 4:115-126. [DOI] [PubMed] [Google Scholar]
- 13.Ketley, J. M. 1997. Pathogenesis of enteric infection by Campylobacter. Microbiology 143:5-21. [DOI] [PubMed] [Google Scholar]
- 14.Khan, I., B. Adler, S. Haridas, and M. J. Albert. 2005. PorA protein of Campylobacter jejuni is not a cytotoxin mediating inflammatory diarrhoea. Microb. Infect. 7:853-859. [DOI] [PubMed] [Google Scholar]
- 15.Klena, J. D., C. T. Parker, K. Knibb, J. C. Ibbitt, P. M. L. Devane, S. T. Horn, W. G. Miller, and M. E. Konkel. 2004. Differentiation of Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis by a multiplex PCR developed from the nucleotide sequence of the lipid gene lpxA. J. Clin. Microbiol. 42:5549-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klipstein, F. A., and R. F. Engert. 1985. Immunological relationship of the B subunits of Campylobacter jejuni and Escherichia coli heat-labile enterotoxins. Infect. Immun. 48:629-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konkel, M. E., Y. Lobet, and W. Cieplak. 1992. Examination of multiple isolates of Campylobacter jejuni for evidence of cholera toxin-like activity, p. 193-198. In I. Nachamkin, M. J. Blaser, and L. S. Tomkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, DC.
- 18.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 19.Lindblom, G.-B., M. Johny, K. Khalil, K. Mazhar, G. M. Ruiz-Palacios, and B. Kaijser. 1990. Enterotoxigenicity and frequency of Campylobacter jejuni, Campylobacter coli and Campylobacter laridis in human and animal stool isolates from different countries. FEMS Microbiol. Lett. 54:163-168. [DOI] [PubMed] [Google Scholar]
- 20.McCardell, B. A., J. M. Madden, and J. T. Stanfield. 1986. Effect of iron concentration on toxin production in Campylobacter jejuni and C. coli. Can. J. Microbiol. 32:395-401. [DOI] [PubMed] [Google Scholar]
- 21.Mills, S. D., and W. C. Bradbury. 1984. Human antibody response to outer membrane proteins of Campylobacter jejuni during infection. Infect. Immun. 43:739-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nachamkin, I. 1999. Campylobacter and Arcobacter, p. 716-726. In P. R. Murray, E. J. Barron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, DC.
- 23.Nachamkin, I., and A. M. Hart. 1985. Western blot analysis of the human antibody response to Campylobacter jejuni cellular antigens during gastrointestinal infection. J. Clin. Microbiol. 21:33-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsvik, Ø., K. Wachsmuth, G. Morris, and J. C. Feeley. 1984. Genetic probing of Campylobacter jejuni for cholera toxin and E. coli heat-labile enterotoxin. Lancet i:449. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Perez, G. I., D. L. Cohn, R. L. Guerrant, C. M. Patton, L. B. Reller, and M. J. Blaser. 1989. Clinical and immunological significance of cholera-like toxin and cytotoxin production by Campylobacter species in patients with acute inflammatory diarrhea in the USA. J. Infect. Dis. 160:460-468. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Perez, G. I., D. N. Taylor, P. D. Echeverria, and M. J. Blaser. 1992. Lack of evidence of enterotoxin involvement in pathogenesis of Campylobacter diarrhea, p. 184-192. In I. Nachamkin, M. J. Blaser, and L. S. Tomkins (ed.), Campylobacter jejuni: current status and future trends. American Society for Microbiology, Washington, DC.
- 27.Ruiz-Palacios, G. M., N. I. Torres, B. R. Ruiz-Palacios, J. Torres, E. Escamilla, and J. Tamayo. 1983. Cholera-like enterotoxin produced by Campylobacter jejuni: characterisation and clinical significance. Lancet ii:250-253. [DOI] [PubMed] [Google Scholar]
- 28.Wadstrom, T. S., S. B. Baloda, K. Krovacek, A. Faris, S. Bengtson, and M. Walder. 1983. Swedish isolates of Campylobacter jejuni/coli do not produce cytotonic or cytotoxic enterotoxins. Lancet ii:911. [DOI] [PubMed] [Google Scholar]
- 29.Wassenaar, T. M. 1997. Toxin production by Campylobacter spp. Clin. Microbiol. Rev. 10:466-476. [DOI] [PMC free article] [PubMed] [Google Scholar]