Abstract
Dissecting the specificities of human antibody responses following disease caused by serogroup A meningococci may be important for the development of improved vaccines. We performed a study of Ethiopian patients during outbreaks in 2002 and 2003. Sera were obtained from 71 patients with meningitis caused by bacteria of sequence type 7, as confirmed by PCR or culture, and from 113 Ethiopian controls. Antibody specificities were analyzed by immunoblotting (IB) against outer membrane antigen extracts of a reference strain and of the patients' own isolates and by enzyme-linked immunosorbent assay for immunoglobulin G (IgG) levels against lipooligosaccharide (LOS) L11 and the proteins NadA and NspA. IB revealed that the main antigens targeted were the proteins PorA, PorB, RmpM, and Opa/OpcA, as well as LOS. MenA disease induced significant increases in IgG against LOS L11 and NadA. The IgG levels against LOS remained elevated following disease, whereas the IgG anti-NadA levels returned to acute-phase levels in the late convalescent phase. Among adults, the anti-LOS IgG levels were similar in acute-phase patient sera as in control sera, whereas anti-NadA IgG levels were significantly higher in acute-phase sera than in controls. The IgG antibody levels against LOS and NadA correlated moderately but significantly with serum bactericidal activity against MenA strains. Future studies on immune response during MenA disease should take into account the high levels of anti-MenA polysaccharide IgG commonly found in the population and seek to clarify the role of antibodies against subcapsular antigens in protection against MenA disease.
In the meningitis belt of sub-Saharan Africa (30), the incidence rate of meningococcal disease is higher than in any other region of the world. In this area, epidemics of meningococcal disease can sweep through several countries during the same year, and large epidemics occur roughly every 8 to 12 years (21). Historically, most of the cases are caused by Neisseria meningitidis bacteria harboring the capsular polysaccharide of serogroup A. In the last two decades, most serogroup A meningococci have belonged to the genetic lineage subgroup III, as determined by multilocus enzyme electrophoresis (1, 10). Most subgroup III strains belonged to sequence type 5 (ST-5) or ST-7, as determined by multilocus sequence typing (38). While ST-5 strains dominated from 1989 to the mid-1990s, ST-7 strains have since then replaced them in the area (38). In Ethiopia, which lies in the easternmost part of the meningitis belt, the replacement of ST-5 by ST-7 strains occurred between 1995 and 2000 (42). Both of these STs express the same PorA and PorB porins (P1.20,9 and P3.4/21, respectively). However, we recently showed that Ethiopian ST-5 and ST-7 strains differed from each other at several loci associated with outer membrane antigens, changes that could be relevant for clonal replacement (42). Since such replacements may be related to immunological selection pressure, it is of interest to determine which antigens induce an antibody response following disease.
The antibody response against meningococcal antigens following disease caused by serogroup A meningococci has been the subject of several studies (2, 6, 7, 28, 40, 49). The advent of whole-genome sequencing and improved protein characterization techniques have resulted in the identification of numerous novel meningococcal proteins (3, 32), but the naturally acquired human immunity against these antigens is less explored. Outer membrane vesicle (OMV)-based vaccines provide protection against serogroup B meningococcal (MenB) disease in humans (4, 48) and are able to induce bactericidal and opsonophagocytic antibodies against serogroup A meningococcal (MenA) strains in mice (39, 41). Therefore, generation of antibodies against outer membrane proteins or lipooligosaccharide (LOS) might be an alternative option to provide protection against meningococcal disease in the meningitis belt. Exploring the specificity of the non-polysaccharide antibody responses following MenA disease may thus contribute to vaccine development.
A study of meningococcal meningitis in Ethiopian patients was performed in 2002 and 2003 (40, 42). Besides characterization of the etiology, another major objective was to characterize the kinetics, functional activity, and specificity of the capsular and subcapsular antibody responses following MenA disease caused by subgroup III ST-7 meningococci in Ethiopia. We have previously reported the serum bactericidal activities and the immunoglobulin G (IgG) responses against MenA polysaccharide (APS) and OMVs in Ethiopian patient and control sera (40). We then confirmed that there was a significant anti-MenA background immunity in the Ethiopian population, as shown by a high proportion of both acute patient sera and controls with a putatively protective titer in the serum bactericidal activity (SBA) assay. We also found that MenA meningitis could induce bactericidal IgGs in nearly all of the patients. The IgG responses were directed both against APS and against subcapsular antigens. Besides showing a strong association between anti-APS IgG concentration and SBA titers, the results also indicated that the IgGs against subcapsular antigens could have a role in providing protection against MenA disease.
To characterize the specificity of the subcapsular antibody response associated with the putative protection induced by MenA meningitis, the Ethiopian patient and control sera were therefore in the present study analyzed by immunoblotting (IB) and by enzyme-linked immunosorbent assay (ELISA) against LOS and the proteins NadA and NspA. The latter three antigens were selected for analyses for their ability to induce bactericidal antibodies and their potential as future vaccine components.
(Part of this study was presented at the 15th International Pathogenic Neisseria Conference in Cairns, Australia, September 2006.)
MATERIALS AND METHODS
Patients.
Ninety-five suspected cases of meningitis residing in Ethiopia were included in the present study. The study was conducted from April 2002 to June 2003, after ethical clearance was obtained from the relevant authorities in Ethiopia and Norway, as described previously (42). The study area, population, layout, patient demographic characteristics, clinical data, and hospital laboratory investigation findings have been described previously (42). The inclusion criteria were clinical diagnosis of meningitis (58), presentation of a turbid cerebrospinal fluid, and age of ≥6 months (40). Of the 95 suspected cases, 71 were confirmed, either by culture or PCR, to have serogroup A N. meningitidis in the cerebrospinal fluid (42).
Sera and bacterial isolates.
Patient and control sera analyzed in the present study were obtained from the Ethiopian individuals as described previously (40). These sera had been analyzed in ELISA for IgG against APS and against deoxycholate-extracted OMVs (dOMVs) of the Mk 686/02 isolate. In addition, the sera had been analyzed in an SBA assay against two serogroup A isolates (Mk 686/02 and F8238) using rabbit or human serum as the complement source. The patient sera analyzed by IB were only from patients that had been laboratory confirmed for MenA meningitis (42) and for whom the serum from at least one phase had been collected. Of the 113 previously described Ethiopian control sera (40), 111 were available for analysis. All 111 sera were analyzed by LOS ELISA, and smaller samples of these (n = 30) were selected for the NadA ELISA analysis, and another smaller sample of these (n = 22) was selected for the IB analysis. The sera analyzed by IB against the dOMVs of the Mk 686/02 isolate were from 2 patients in the infant and toddler age group, 7 in the young children's age group, 21 in the older children and teenager age group, and 18 in the adult group. The corresponding numbers for control sera in the IB against dOMVs were five, five, four, and eight individuals, respectively. The corresponding numbers for sera analyzed by IB against the OMVs of the patients' own isolates were 1, 3, 15, and 11 patients, respectively. The six patients selected for NspA ELISA were of 3, 4.5, 7, 15, 16, and 38 years of age, respectively. In addition, sera from healthy Norwegian teenagers (median age = 13.5 years [n = 37]) (40) were used as controls in antibody analyses with LOS and NadA. The meningococcal isolates used to prepare LOS ELISA and IB antigens have been described previously (42); they were all ST-7 organisms with the characteristics A:4/21:P1.20,9, possessing the FetA VR epitope 3-1 and the NadA3 variant.
Preparation of OMVs.
Native meningococcal OMVs (nOMVs) were prepared by extraction with lithium chloride/lithium acetate (LiCl/LiAc), as described previously (16, 39), from MenA isolates of patients from whom sera also were available. The dOMVs, derived from the Ethiopian ST-7 strain Mk 686/02, as well as the nOMVs, derived from the patients' own isolates and used in IB, were previously characterized (42). The nOMV preparations showed higher amounts of Opa proteins and LOS than the dOMV preparations (39).
IB.
IB was carried out as described previously (41, 52), using both dOMVs from strain Mk 686/02 and nOMVs from the patients' own isolates as antigenic targets. Positive control strips from the blot, which were incubated with murine monoclonal antibodies (MAbs) against NadA (1079-B6) (19), PorA P1.9 (W316) (2), PorB P3.21 (14-1-P21) (54), RmpM (185, H-8) (46), OpcA (279 5c) (45), NspA (236, B-2) (41), LOS L11 (4C4) (29), LOS L10 (14-1-L10) (47), or polyclonal rabbit antisera against Omp85 (80K-1) and FetA (70K-1) (17), identified the positions of the major antigens. Binding of antibodies to each antigen was scored visually on a scale from 0 to 5 by two observers independently as follows: 0, no reaction; 1, very weak reaction; 2 to 4, progressively stronger reaction; and 5, very strong reaction.
Preparation of LOS.
The LOS of immunotype L11 was prepared from strain Mk 686/02 as described by Wu et al. (59), with some modifications. Briefly, bacteria were grown in 2.8-liter Fernbach flasks in chemically defined medium (Modified Catlin 6 [MC.6*]) (18, 39). The cell mass was harvested by centrifugation, suspended in 70% ethanol and centrifuged again. After evaporation of ethanol from the pellet and redissolution in 40 mM NaPO4 buffer with 5 mM sodium EDTA and 0.05% NaN3 (pH 7.0), it was treated with lysozyme (Sigma-Aldrich, St. Louis, MO) (25) and RNase (BDH Chemicals, Ltd., Poole, United Kingdom) (14). The LOS was then extracted by the hot-phenol method (56), and the pellet was washed in ethanol and dissolved in 0.05 M Tris-HCl with 2 mM sodium EDTA, 2.5% deoxycholate and 0.01% merthiolate (pH 8.95). LOS was eluted in a Sephacryl S-400 column (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) with 20 mM Tris-HCl with 2 mM sodium EDTA, 1% sodium deoxycholate (wt/vol), and 0.02% NaN3 (pH 8.5) as the elution buffer. Fractions with a high LOS content were identified by 16.5% Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis and silver staining (31). These fractions were pooled, and the LOS was precipitated overnight at −20°C with ethanol and NaCl. After centrifugation, the pellet was dissolved in distilled water. Purification of the LOS of the L11 immunotype from culture biomass of strain Mk 686/02 yielded a product with undetectable protein contamination, as evaluated by Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis with silver and Coomassie brilliant blue staining (data not shown). A single LOS band was detected by silver staining, and this band reacted strongly with the anti-L11 MAb.
LOS ELISA.
IgG antibodies against L11 LOS were analyzed as described previously (44), except that microtiter plates were incubated with dilutions of sera overnight at 37°C. Concentrations of test antibody were calculated from a four-parameter curve derived from the twofold dilutions of the convalescent-phase serum standard pool. This was made by pooling early-convalescent-phase sera from five confirmed N. meningitidis patients determined to be positive by screening and was defined as 1,000 arbitrary units (AU)/ml for anti-LOS L11 IgG response. Positive and negative or weak sera were included as internal references on all plates.
NadA ELISA.
The purified recombinant NadA (rNadA) protein used in ELISA was of allele variant 3—the same variant harbored by the Ethiopian ST-5 and ST-7 strains (42). Maxisorp plates (Nunc, Roskilde, Denmark) were coated with 100 μl/well of 2 μg protein/ml of rNadA (NadA3 as in strain 2996, 961c; Chiron S.p.A., Siena, Italy) (9) in phosphate-buffered saline (PBS) buffer (pH 7.2). Plates were then allowed to stand overnight at 4°C in PBS and were blocked with 200 μl of serum dilution buffer (Dulbecco PBS without Ca or Mg but with 0.5% bovine serum albumin, 0.02% [wt/vol] NaN3, and 0.05% [wt/vol] Tween 20; pH 7.4) for 30 min at room temperature. Plates were then washed three times in PBS with 0.5% (vol/vol) Tween 20, and 100 μl of serum diluted 1:100 in serum dilution buffer was added. The plates were incubated for 2 h at 37°C and washed as described above. Bound antibodies were detected by using alkaline-phosphatase anti-human IgG conjugate and p-nitrophenyl phosphate substrate (both from Sigma-Aldrich), as described previously (40). The optical densities (ODs) at 405 nm were read after 35 min.
rNspA ELISA.
Specific antibodies to recombinant NspA protein (rNspA) were determined by ELISA as described previously (39). Microtiter plates were coated with Escherichia coli nOMVs, genetically modified with (+) and without (−) the expression of rNspA (36). Bound antibodies were detected as described above. The difference in OD (ΔOD) between rNspA(+) and rNspA(−) nOMVs at the serum dilution 1:800 was assumed to represent the binding of antibodies to rNspA.
Statistical methods.
The statistical analyses were performed by using SPSS version 13.0.1 for Windows (SPSS, Inc., Chicago, IL). Geometric mean fold rises from acute phase to early convalescent or to late convalescent phase were calculated by dividing individual paired-serum data from early- or late-convalescent-phase serum results by the acute-phase serum result. For nonpaired data, group-to-group comparisons using Mann-Whitney t tests were performed if an analysis of variance test (Kruskal-Wallis) had verified a significant overall difference between all groups. Comparison of paired data between groups of sera collected at different points of time from the same patients was performed by using a Wilcoxon signed-rank sum test. Differences in the proportions of responders of the above predefined threshold values were compared by using a chi-square test. After log transformation of data, the relation between the results from various assays were analyzed by using a Pearson correlation test and univariate linear regression. The relationship between IB results and results of other assays was analyzed by the Spearman correlation (rs) test.
Sera with an undetectable SBA were assigned to have a titer of 2 (40). With rabbit complement and target strain F8238, an SBA titer of ≥128 was defined as putatively protective against MenA disease (40). With human complement and target strain Mk 686/02, an SBA titer of ≥4 was defined as putatively protective (16). A titer of a serum analyzed with the rabbit complement SBA (rSBA) assay was referred to as an rSBA titer, whereas a titer of a serum analyzed with the human complement SBA (hSBA) assay was referred to as an hSBA titer. A seroresponder was defined as an individual demonstrating a ≥4-fold rise in SBA titer.
RESULTS
IgG antibody binding analyzed by IB.
The specificity of the subcapsular antibody responses in sera from Ethiopian MenA disease patients was analyzed by using either IB with dOMVs from isolate Mk 686/02 or IB with nOMVs from the patient's own isolate. Sera from Ethiopian controls were analyzed against dOMVs from Mk 686/02. The percentages of sera with medium to strong binding in the IB (band intensity score of ≥2) are shown in Table 1.
TABLE 1.
Proportions of Ethiopian MenA patient and control sera with immunoblot band reaction scores of ≥2 and proportions of patient sera with an increase or decrease in score of ≥1 from acute to early convalescent phasea
| Antigen band identity | Reaction against dOMVs of strain Mk 686/02 in:
|
Reaction against nOMVs of patients' own isolates in Ethiopian patients
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethiopian patients
|
% of Ethiopian controls (n = 22) | ||||||||||
| % Acute (n = 48) | % Early (n = 35) | % Late (n = 31) | No. (%) increased (n = 26) | No. (%) decreased (n = 26) | % Acute (n = 30) | % Early (n = 26) | % Late (n = 19) | No. (%) increased (n = 26) | No. (%) decreased (n = 26) | ||
| NadA | 0 | 3 | 3 | 3 (9) | 1 (3) | 0 | 17 | 17 | 16 | 4 (15) | 3 (12) |
| Omp85 | 4 | 20 | 6 | 11 (31) | 2 (6) | 5 | 43 | 38 | 42 | 8 (31) | 7 (27) |
| FetA | 0 | 6 | 0 | 1 (11) | 2 (6) | 5 | 7 | 8 | 0 | 2 (8) | 2 (8) |
| PorA | 40 | 43 | 48 | 8 (23) | 7 (20) | 23 | 70 | 73 | 74 | 8 (31) | 4 (15) |
| PorB | 44 | 54 | 35 | 7 (20) | 4 (11) | 23 | 70 | 77 | 74 | 5 (19) | 4 (15) |
| RmpM | 69 | 74 | 48 | 9 (26) | 6 (17) | 64 | 73 | 77 | 84 | 7 (27) | 6 (23) |
| Opa/OpcA | 69 | 63 | 52 | 9 (26) | 5 (14) | 27 | 23 | 46 | 21 | 6 (23) | 4 (15) |
| NspA | 0 | 0 | 0 | 0 (0) | 1 (3) | 0 | 20 | 38 | 37 | 8 (31) | 4 (15) |
| LOS | 35 | 51 | 32 | 14 (40) | 4 (11) | 9 | 50 | 62 | 53 | 11 (44) | 4 (16) |
As graded by a visual score scale of 0 to 5 against OMV antigens. n, number of sera analyzed.
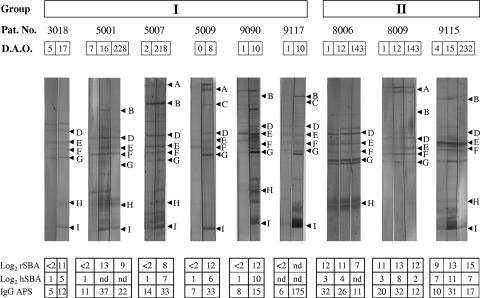
The IgG binding in the acute-phase sera from MenA patients ranged from absent to very strong. The results from IB analysis using dOMVs of the strain Mk 686/02 as the antigen showed that early-convalescent-phase sera most commonly reacted with an IB band intensity score of ≥2 against the antigens RmpM (74%), Opa/OpcA (63%), and PorB (54%). To demonstrate the binding strength changes over time in individual patients, we subtracted the IB score value in acute-phase patient serum from the score value in early-convalescent-phase patient serum (Table 1). An increase in intensity score of ≥1 in IB between the acute and early convalescent phases was observed only in a minority of the patients, but was most frequent against LOS (40%), Omp85 (31%), and RmpM and Opa/OpcA (26% each). The only antigen for which a significant mean score increase was seen from acute to early convalescent phase was the Omp85 antigen (P = 0.009).
In IB using nOMVs of the patients' own isolates, early-convalescent-phase sera from MenA meningitis patients most commonly reacted with an IB band intensity score of ≥2 against PorB and RmpM (77% each), PorA (73%), and LOS (62%). Also, an increase in intensity score of ≥ 1 in IB between acute and early convalescent phase was observed only in a minority of patients, but was most frequent for LOS (44%) and PorA, NspA, and Omp85 (31% each) in IB with nOMVs (Table 1). Despite the fact that LOS was the most frequent antigen patients responded to, some individuals did not respond to LOS (e.g., patients 8006 and 8009 in Fig. 1), and for all sera the increase in anti-LOS IgG antibodies as detected by IB was not statistically significant (P = 0.076). A significant decrease from early to late convalescent phase was observed for antibodies against NadA (P = 0.039) and Opa/OpcA (P = 0.046).
FIG. 1.
Examples of immunoblotting results from selected Ethiopian MenA patients. Patients in group I (patients 3018, 5001, 5007, 5009, 9090, and 9117) were observed to have no detectable rSBA titer in their acute-phase serum, whereas those in group II (patients 8006, 8009, and 9115) showed high rSBA titers already in their acute-phase serum. Letters indicate the positions of individual antigen bands, as determined by the guidestrips incubated with MAbs (PorA, PorB, RmpM, OpcA, NspA, and L11) or polyclonal sera (Omp85 and FetA) as follows: A, NadA; B, Omp85; C, FetA; D, PorA; E, PorB; F, RmpM; G, OpcA; H, NspA; I, LOS L11. Pat. No., patient number; D.A.O., days after reported onset of disease; rSBA, SBA against MenA strain F8238 with rabbit complement; hSBA, SBA against MenA strain Mk 686/02 with human complement; IgG APS, approximate IgG concentration (μg/ml) against MenA polysaccharide as measured by ELISA (40); nd, not done due to limited amount of serum available.
Sera from nine MenA disease patients were selected for illustration of reaction patterns commonly observed in the IB analyses (Fig. 1). Selection was based on differences in IB pattern and on previous results from SBA analyses (40). Two typical response patterns are shown: (i) group I representing patients without SBA activity in acute-phase sera but with an increase in intensity score to several antigens between acute- and convalescent-phase sera and (ii) group II representing patients with SBA activity in acute-phase sera but with only minor differences observed in IB between the sera collected at different time points.
Anti-LOS IgG by ELISA.
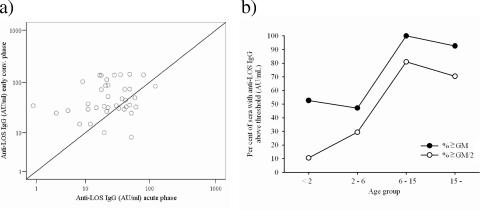
The geometric mean concentration (GMC) of IgG against immunotype L11 LOS increased significantly from acute to early convalescent phase (n = 38, P = 0.002), when we analyzed paired sera from individual patients only (Table 2). The IgG levels remained elevated in the late convalescent phase, as demonstrated by a higher IgG GMC than for the acute phase (n = 32, P = 0.033). However, in some MenA patients there was no significant increase or even a decline in IgG levels against LOS. The proportion of patients with a ≥4-fold increase in IgG from acute to early convalescent phase was 26%, while the similar proportion with a ≥2-fold increase was 45% (Fig. 2a). We detected a higher level of IgG in early-convalescent-phase sera than in controls (P = 0.004) (Table 2), but no statistically significant differences were found when we compared Ethiopian control sera with either acute-phase or late-convalescent-phase patient sera.
TABLE 2.
GMCs of anti-LOS L11 ELISA IgG levels and anti-NadA IgG levels in Ethiopian MenA patient and control seraa
| Parameter and age group (in yr) | Ethiopian patients
|
Ethiopian controls
|
Norwegian controls
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acute phase
|
Early convalescent phase
|
Late convalescent phase
|
||||||||
| GMC (range) | n | GMC (range) | n | GMC (range) | n | GMC (range) | n | GMC (range) | n | |
| LOS IgG (AU/ml) | ||||||||||
| All ages | 24.6 (0.8-135) | 63 | 46.7 (8-143) | 41 | 36.1 (11-281) | 33 | 28.8 (4-232) | 111 | 1.5 (1-5) | 37 |
| 0.5 to ≤2 | 9.8 (8-12) | 2 | 25 | 1 | 51.2 (25-105) | 2 | 14.0 (5-51) | 19 | 0 | |
| >2 to ≤6 | 18.8 (2-53) | 10 | 38.5 (15-136) | 8 | 34.6 (17-74) | 4 | 21.9 (8-232) | 17 | 0 | |
| >6 to ≤15 | 31.0 (3-135) | 26 | 40.8 (8-143) | 17 | 31 (13-68) | 15 | 41.3 (16-81) | 21 | 1.5 (1-5) | 37 |
| >15 | 23.2 (1-52) | 25 | 62.8 (31-140) | 15 | 41.8 (11-281) | 12 | 35.1 (4-153) | 54 | 0 | |
| NadA-IgG (OD U/ml)b | ||||||||||
| All ages | 0.26 (0.11-0.86) | 62 | 0.37 (0.13-1.20) | 42 | 0.28 (0.05-1.23) | 34 | 0.16 (0.05-0.34) | 30 | 0.09 (0.05-0.15) | 10 |
| 0.5 to ≤2 | 0.11 (0.11-0.12) | 2 | 0.13 | 1 | 0.18 (0.12-0.26) | 2 | 0.17 (0.12-0.34) | 10 | 0 | |
| >2 to ≤6 | 0.24 (0.13-0.86) | 10 | 0.31 (0.15-0.62) | 8 | 0.26 (0.16-0.54) | 4 | 0 | 0 | ||
| >6 to ≤15 | 0.25 (0.14-0.70) | 26 | 0.33 (0.14-0.69) | 17 | 0.28 (0.15-1.23) | 15 | 0.18 (0.14-0.23) | 10 | 0.09 (0.05-0.15) | 10 |
| >15 | 0.31 (0.13-0.73) | 23 | 0.49 (0.20-1.2) | 16 | 0.31 (0.05-0.70) | 13 | 0.13 (0.05-0.34) | 10 | 0 | |
n, number of sera analyzed.
Anti-LOS IgG antibody concentrations were calculated using a four-parametric fitting model for standard curve dilutions of a positive serum assigned to 1,000 AU/ml, which is assumed to correlate well with actual antibody concentrations. In contrast, the direct OD values of anti-NadA IgG binding in 1:100 dilution of sera do not correlate with antibody concentrations in a proportional manner.
FIG. 2.
Development of IgG against LOS L11 in sera from MenA disease patients as measured by ELISA. (a) Response in paired sera from acute versus early convalescent phase. The 45° line indicates the location of values that show no difference in IgG binding between acute- and early-convalescent-phase sera. (b) Age-related differences in anti-LOS IgG levels among Ethiopian control sera, expressed as percentages of sera above or equal to thresholds (the thresholds used are either the GMCs of these control sera or 50% of the GMC).
Significant differences in IgG levels were observed between age groups of Ethiopian controls (P < 0.001), with the IgG levels increasing with age, as demonstrated by increasing proportions of sera with IgG levels above two different thresholds up to the age of 15 years (Fig. 2b). For the acute-phase sera from the patients, the same trend was observed, but the differences were not statistically significant (P = 0.062). The IgG levels were significantly higher in Ethiopian control sera (age, >6 to ≤15 years; GMC = 41.3 AU/ml; n = 21) than in Norwegian teenager sera (Table 2) (P < 0.001).
Anti-rNadA IgG by ELISA.
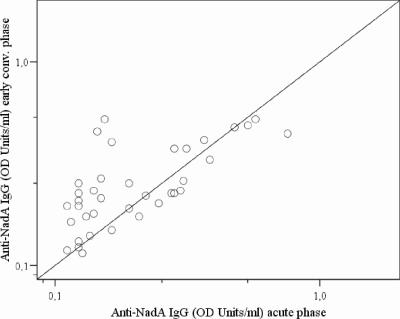
Many of the patients showed a weak IgG response against rNadA in ELISA from acute to early convalescent phase, as demonstrated by only 19% mounting a ≥2-fold increase in OD (Table 2 and Fig. 3). On overall, the increase was, however, statistically significant (n = 37, P = 0.012). In late convalescent phase, the IgG levels were comparable to those of the acute phase (n = 33, P = 0.491) (Table 2). The levels of IgG were higher in acute-phase sera (P = 0.009) and early-convalescent-phase sera (P = 0.001) from patients infected with an isolate reacting strongly with an anti-NadA MAb in dot blot (42) than in those with a weak or no reaction in the dot blot.
FIG. 3.
Development of IgG against NadA in sera from MenA disease patients as measured by ELISA indicated by the response in acute-phase versus early-convalescent-phase paired sera. The 45° line indicates the location of values that show no difference in IgG binding between acute-and early-convalescent-phase sera.
Among adults (age ≥ 15 years), a higher level of anti-rNadA IgG was found both in acute-phase and in late-convalescent-phase patient sera than in control sera (P < 0.001 and P = 0.002, respectively). No significant differences in IgG levels were revealed between age groups among the controls (P > 0.05). The anti-rNadA IgG levels were higher in Ethiopian teenager control sera (GMC = 0.16 OD units, n = 30) than in Norwegian teenager sera (Table 2) (P < 0.001).
Anti-rNspA IgG by ELISA.
Acute- and convalescent-phase sera from six MenA cases, who had demonstrated significant increase in IgG against OMVs, were tested by ELISA against rNspA. No significant increase in the GMC of anti-rNspA IgG from acute to early convalescent phase was observed. However, one patient demonstrated a strong increase in IgG of 0.52 OD units from day 6 to day 16 after onset of disease (data not shown).
Correlation between immune responses analyzed by IB, ELISA, and SBA.
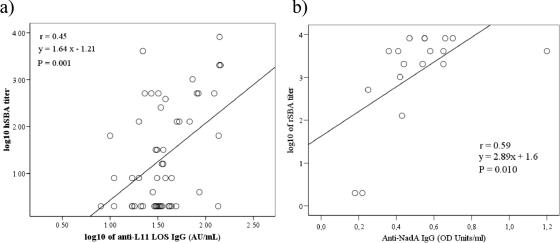
Among sera from patients who had an isolate with positive reaction with an anti-L11 MAb in dot blot analyses, there was a correlation between having an IB score of ≥2 against LOS in dOMVs from Mk 686/02 and a protective hSBA level (titer ≥ 4) (P = 0.006). We further analyzed whether there were any significant correlations between anti-LOS IgG levels and previously obtained hSBA and rSBA titers with the same sera (40). Using pooled results from all patient sera, we observed overall weak correlations between log-transformed rSBA titers and ELISA anti-LOS IgG (r = 0.18, P = 0.041, n = 133). Parallel analyses for data on hSBA titers showed similarly weak significant correlations with anti-LOS IgG (r = 0.30, P = 0.001, n = 109). In the patients infected by LOS L11 strains, we found a moderate correlation between log-transformed anti-LOS IgG levels and hSBA titers (r = 0.45, P = 0.001, n = 52 sera) (Fig. 4a) and between anti-LOS L11 IgG and rSBA titers (r = 0.28, P = 0.028, n = 61 sera). With all patient sera, the anti-LOS L11 IgG levels also correlated with the IB LOS band reaction scores, both against LOS in nOMVs from own isolates (rs = 0.37, P = 0.001, n = 72) and against the Mk 686/02 dOMVs (rs = 0.25, P = 0.008, n = 112). In contrast, among sera from patients infected with an L10 isolate (n = 12) there was no correlation between having a strong binding to LOS in IB and the bactericidal titer.
FIG. 4.
(a) Scatter plot showing the correlation between results in the hSBA assay and in the anti-L11 LOS IgG ELISA in all sera (acute, early convalescent, and late convalescent phases) from Ethiopian MenA disease patients infected with L11-positive strains (n = 52). (b) Similarly, the correlation between log-transformed results in the rSBA assay and in the anti-NadA IgG ELISA in all sera from patients infected with NadA-positive strains (n = 18) is shown. The Pearson correlation coefficient r and the least-squares linear regression line equation are given.
We further analyzed correlations between anti-rNadA levels and previously obtained hSBA and rSBA titers (40). With pooled results from all patient sera, we observed overall weak correlations between log-transformed rSBA titers and ELISA anti-rNadA IgG (r = 0.29, P = 0.001, n = 132), as well as between hSBA titers and anti-rNadA IgG (r = 0.23, P = 0.017, n = 109). When only results from sera from patients who had an isolate with positive reaction with an anti-NadA MAb in dot blotting were considered, we observed a correlation between log-transformed rSBA titers (40) and anti-rNadA IgG OD values in ELISA of r = 0.59 (n = 18) (Fig. 4b).
When analyzing all patient sera, we found significant correlation between having an IB reaction (score ≥ 2) against conserved antigens as the Omp85 band of Mk 686/02 dOMVs or NspA and RmpM bands of nOMVs and having a protective SBA titer (data not shown). However, no statistically significant correlations were observed between SBA titers and IgG binding in IB for PorA, PorB, or Opa/OpcA.
Use of LOS ELISA for serological evaluation of probable MenA cases.
A total of 71 of 95 patients were confirmed MenA cases by culture or PCR (42). Among those 10 nonconfirmed patients for whom serum pairs were available, 6 were previously shown to have a significant increase in anti-APS IgG or anti-OMV IgG and thus termed “probable MenA cases” (40). In the present study, 4 of the 10 nonconfirmed patients also showed a ≥2-fold increase in anti-LOS IgG, all among those 6 already assigned as probable MenA cases. Of the remaining patients with paired sera available and not showing significant anti-APS IgG or anti-OMV IgG increases (40), two showed a remarkable fall from acute to early-convalescent-phase serum in IgG against LOS.
DISCUSSION
IB results and disease associated changes.
We generally found high frequencies of sera with strong antibody reaction in IB against the major outer membrane antigens PorA, PorB, RmpM, Opa/OpcA, and LOS (Table 1). However, large individual differences between patients were evident (Fig. 1). This is comparable to results from previous IB studies of sera from patients with MenA (15, 49) and MenB (55, 57) disease, although the identity of the protein antigens was not always clearly defined in the previous MenA studies.
The proportions of sera with binding scores of ≥2 in early-convalescent-phase sera in our study were higher for PorA and PorB than those reported among MenB cases in Norway (55), while the proportions of patient sera binding to Omp85, FetA, Opa/OpcA, and LOS were comparable. In late-convalescent-phase sera, higher proportions of the MenA cases were responders against PorA than reported for the MenB cases (55); against Opa/OpcA and LOS the proportions were similar and were lower against Omp85 and FetA. The differences may be explained by the fact that the IB analyses in the present study were performed with OMVs from the patients' infecting MenA strain, whereas the previous MenB IB study also included patients infected with strains different from the one used in IB. Alternatively, it could be due to a difference in immunogenicity between the PorA porins expressed by MenA and MenB strains, or differences in background immunity in Ethiopia compared to in Norway (Table 1). This background activity was probably stimulated by previous contact with neisseriae or with other bacteria with cross-reacting proteins (26, 27, 44, 46).
In general, only a few of the patients demonstrated IB band reaction scores that differed significantly between serum phases. However, the relatively low proportion of Ethiopian MenA patient sera showing increased binding to PorA (23% with dOMV) from the acute to the convalescent phase is comparable to that observed with MenB patients (55). This is partly due to the high proportion of acute-phase sera showing an anti-PorA binding score of ≥2, possibly explained by a delayed collection of acute-phase sera, or by a stimulus (e.g., carriage) prior to the onset of disease, as described previously (39). Other explanations could be saturation of the antigens with antibodies on the nitrocellulose membrane, or that our visual scoring method was not sensitive enough. However, the latter explanation is less likely, since a previous study of sera from MenB cases reported good correlation between visual scoring and the more accurate method of digital scanning (55).
Our results may also have been affected by the OMV extraction method and the isolate-specific differences in proteins and LOS. Although dOMVs and nOMVs from the same MenA isolate are known to contain approximately similar amounts of PorA, PorB, and RmpM (39), the nOMVs contain more LOS and Opa/OpcA proteins, as well as a much wider array of minor proteins (39). We also observed that the sera presented with higher band intensities when analyzed in IB against nOMVs, than when analyzed against dOMVs from the same isolate (data not shown). This may be due to conformational changes of the antigens caused by the different extraction procedures. The ST-7 isolates used in the present study were previously shown to express relatively stable amounts of PorA, widely varying amounts of NadA, and varying types and amounts of Opa/OpcA (42). In addition, different LOS immunotypes were observed among these isolates, although LOS L11 was dominant.
IB results and associations with SBA.
The previous study of the same Ethiopian patient and control sera demonstrated that there was a significant association between IgG against dOMVs of Mk 686/02 and SBA (r = 0.69 and r = 0.30 with human or rabbit complement, respectively) (40). In order to single out antigens that may have contributed to the observed SBA, we correlated IB results from the current study with the previously obtained SBA results (40). Significant relationships between IB scores and SBA were observed for LOS and the conserved proteins Omp85, RmpM, and NspA. Such covariations have been described previously in some studies (53, 55), whereas other studies did not confirm that antibodies to RmpM and NspA could exert bactericidal activity (23, 46).
Previous studies of MenB patients and MenB OMV vaccinees have shown a strong association between antibodies against PorA and SBA titers (34, 55) and that PorA is critical for mounting a protective immune response (44). In the Ethiopian MenA cases, 40 to 74% of the patient sera had antibodies strongly binding to PorA (scores ≥ 2) in all phases (Table 1), but no statistically significant association with SBA titers was found. The association between anti-APS antibodies and SBA was previously shown to be very strong (r ≈ 0.6 for both human and rabbit complement) (40). The lack of association between PorA and SBA may thus, in part, be due to the high concentration of anti-APS IgG in the Ethiopian sera.
Unlike the PorA of MenB meningococci, the PorA of MenA bacteria shows low antigenic variation (50). This could be explained by a lack of immunological selection pressure on the P1.20,9 PorA variable region epitopes (50). In a study of MenA OMV vaccine response in mice, however, we found that the typical PorA of ST-5 complex strains (P1.20,9) was highly immunogenic and induced bactericidal antibodies, as demonstrated by a significant correlation between Mk 686/02 hSBA titers and anti-PorA IB reactions (39). Immunization with OMV vaccines may, however, be a more efficient way than disease to induce anti-PorA antibodies. In order to evaluate the possibility of using PorA as part of future vaccines against MenA disease, further studies should determine naturally acquired immunity to PorA and its role in protection against MenA disease.
ELISA results and associations with SBAs.
Using the LOS L11-specific ELISA, we observed that anti-LOS IgG levels increased significantly in patients during early convalescent phase (Table 2), in line with a previous study in Egyptian MenA patients (49) and in patients with MenB and MenC disease (13, 22, 33, 43, 57). Although anti-LOS L11 IgG increased significantly during disease and remained elevated in the late convalescent phase, the GMCs in acute-phase or late-convalescent-phase sera were not statistically different from that found in Ethiopian control sera.
Among sera from patients infected with an L11 positive isolate, we found that anti-L11 LOS IgG levels correlated both with hSBA (Fig. 4a) and rSBA titers. In addition, having a significant IB reaction against LOS L11 was correlated with having a protective hSBA titer. Thus, L11 LOS seemed to induce a protective immune response against L11 isolates, whereas infection with L10 isolates did not seem to be effective in inducing SBA against L11 isolates. This is in line with the results from a previous study demonstrating an immunotype-specific effect of bactericidal antibodies directed against LOS (60).
Using the NadA-specific ELISA, we found that acute-phase patient sera had higher levels of anti-rNadA IgG than Ethiopian controls and that developing MenA disease induced a significant increase in such antibodies (Table 2). This finding is in agreement with the results from a study of MenB and MenC patients in the United Kingdom (32). The higher levels of anti-rNadA IgG in acute-phase sera than in controls could indicate that an immune response already was initiated at the time of sampling serum in the acute phase. As discussed elsewhere (40), the IgG levels against APS and OMVs in the same sera showed the same tendency. Anti-rNadA IgG ELISA levels correlated with rSBA titers (Fig. 4b). This is in agreement with previous studies in animals (5, 11, 12, 20, 39) and indicates NadA to be a favorable component of vaccines against MenA disease.
We found no indication that a significant anti-NspA IgG response was mounted following MenA meningitis disease. Whereas the ability of anti-NspA IgG in conferring a protective immune response seems promising in mice (8, 35, 37), immunization of humans with an rNspA vaccine did not induce bactericidal antibodies (23), and immunization of humans with the NspA-containing OMV vaccine “MeNZB” did not usually elicit any anti-NspA IgG response (53). Conformation or presentation of the NspA protein may, however, be critical for the induction of functional anti-NspA immune response in humans (23, 24, 51).
Based on findings here and in previous studies (39, 41), further work may focus on the potential of IgG antibodies specific for LOS, NadA, and PorA to provide a protective immune response against MenA disease. In addition, the specificity of naturally acquired antibodies that may show opsonophagocytic activity against MenA bacteria should be investigated.
Acknowledgments
We thank Tsegaye Alebel from the North Gondar Zonal Health Bureau for participating in the field study and Ulla Heggelund, Kirsten Konsmo, and Berit Nyland at the Norwegian Institute of Public Health for excellent technical assistance. We thank Maurizio Comanducci and Novartis Vaccines (Siena, Italy) for the generous gift of rNadA protein and Gregory Moe at the Children's Hospital Oakland Research Institute (Oakland, CA) for the generous gift of plasmid pGMS 1.0 containing the nspA gene. We thank M. Achtman, C. E. Frasch, J. T. Poolman, and W. D. Zollinger for the generous gifts of MAbs.
This study was in part supported by grant 146185/730 from the Research Council of Norway.
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Achtman, M. 1997. Microevolution and epidemic spread of serogroup A Neisseria meningitidis: a review. Gene 192:135-140. [DOI] [PubMed] [Google Scholar]
- 2.Achtman, M., B. Kusecek, G. Morelli, K. Eickmann, J. F. Wang, B. Crowe, R. A. Wall, M. Hassan-King, P. S. Moore, and W. Zollinger. 1992. A comparison of the variable antigens expressed by clone IV-1 and subgroup III of Neisseria meningitidis serogroup A. J. Infect. Dis. 165:53-68. [DOI] [PubMed] [Google Scholar]
- 3.Bernardini, G., G. Renzone, M. Comanducci, R. Mini, S. Arena, C. D'Ambrosio, S. Bambini, L. Trabalzini, G. Grandi, P. Martelli, M. Achtman, A. Scaloni, G. Ratti, and A. Santucci. 2004. Proteome analysis of Neisseria meningitidis serogroup A. Proteomics 4:2893-2926. [DOI] [PubMed] [Google Scholar]
- 4.Bjune, G., E. A. Høiby, J. K. Grønnesby, O. Arnesen, J. H. Fredriksen, A. Halstensen, E. Holten, A. K. Lindbak, H. Nøkleby, and E. Rosenqvist. 1991. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338:1093-1096. [DOI] [PubMed] [Google Scholar]
- 5.Bowe, F., E. C. Lavelle, E. A. McNeela, C. Hale, S. Clare, B. Arico, M. M. Giuliani, A. Rae, A. Huett, R. Rappuoli, G. Dougan, and K. H. Mills. 2004. Mucosal vaccination against serogroup B meningococci: induction of bactericidal antibodies and cellular immunity following intranasal immunization with NadA of Neisseria meningitidis and mutants of Escherichia coli heat-labile enterotoxin. Infect. Immun. 72:4052-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brieske, N., M. Schenker, T. Schnibbe, M. J. Quentin-Millet, and M. Achtman. 1999. Human antibody responses to A and C capsular polysaccharides, IgA1 protease and transferrin-binding protein complex stimulated by infection with Neisseria meningitidis of subgroup IV-1 or ET-37 complex. Vaccine 17:731-744. [DOI] [PubMed] [Google Scholar]
- 7.Brooks, G. F., C. J. Lammel, M. S. Blake, B. Kusecek, and M. Achtman. 1992. Antibodies against IgA1 protease are stimulated both by clinical disease and asymptomatic carriage of serogroup A Neisseria meningitidis. J. Infect. Dis. 166:1316-1321. [DOI] [PubMed] [Google Scholar]
- 8.Cadieux, N., M. Plante, C. R. Rioux, J. Hamel, B. R. Brodeur, and D. Martin. 1999. Bactericidal and cross-protective activities of a monoclonal antibody directed against Neisseria meningitidis NspA outer membrane protein. Infect. Immun. 67:4955-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capecchi, B., J. Adu-Bobie, F. Di Marcello, L. Ciucchi, V. Masignani, A. Taddei, R. Rappuoli, M. Pizza, and B. Arico. 2005. Neisseria meningitidis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 55:687-698. [DOI] [PubMed] [Google Scholar]
- 10.Caugant, D. A. 1998. Population genetics and molecular epidemiology of Neisseria meningitidis. APMIS 106:505-525. [PubMed] [Google Scholar]
- 11.Comanducci, M., S. Bambini, B. Brunelli, J. Adu-Bobie, B. Arico, B. Capecchi, M. M. Giuliani, V. Masignani, L. Santini, S. Savino, D. M. Granoff, D. A. Caugant, M. Pizza, R. Rappuoli, and M. Mora. 2002. NadA, a novel vaccine candidate of Neisseria meningitidis. J. Exp. Med. 195:1445-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comanducci, M., S. Bambini, D. A. Caugant, M. Mora, B. Brunelli, B. Capecchi, L. Ciucchi, R. Rappuoli, and M. Pizza. 2004. NadA diversity and carriage in Neisseria meningitidis. Infect. Immun. 72:4217-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estabrook, M. M., R. E. Mandrell, M. A. Apicella, and J. M. Griffiss. 1990. Measurement of the human immune response to meningococcal lipooligosaccharide antigens by using serum to inhibit monoclonal antibody binding to purified lipooligosaccharide. Infect. Immun. 58:2204-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, J. S., and M. C. Maiden. 1996. Purification of meningococcal lipo-oligosaccharide by FPLC techniques. Microbiology 142(Pt. 1):57-62. [DOI] [PubMed] [Google Scholar]
- 15.Filatova, T. N., and L. N. Gamzulina. 2000. Antibodies to meningococcal antibodies in the sera of patients and donors. Zh. Mikrobiol. Epidemiol. Immunobiol. May-June:58-62. (In Russian.) [PubMed] [Google Scholar]
- 16.Frasch, C. E., and L. F. Mocca. 1978. Heat-modifiable outer membrane proteins of Neisseria meningitidis and their organization within the membrane. J. Bacteriol. 136:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredriksen, J. H., E. Griffiths, E. M. Grotterød, E. A. Høiby, E. Rosenqvist, P. Stevenson, and E. Wedege. 1992. Characterization of high molecular weight components in MenB-vaccine “Folkehelsa”: an outer membrane vesicle vaccine against group B meningococcal disease, p. 818-824. In C. J. Conde-Glez, S. Morse, P. Rice, F. Sparling, and E. Calderon (ed.), Proceedings of the 8th International Pathogenic Neisseria Conference. Instituto Nacional de Salud Publica, Cuernavaca, Mexico.
- 18.Fu, J., F. J. Bailey, J. J. King, C. B. Parker, R. S. Robinett, D. G. Kolodin, H. A. George, and W. K. Herber. 1995. Recent advances in the large scale fermentation of Neisseria meningitidis group B for the production of an outer membrane protein complex. Biotechnology 13:170-174. [DOI] [PubMed] [Google Scholar]
- 19.Fukasawa, L. O., M. C. Gorla, A. P. Lemos, R. P. Schenkman, M. C. Brandileone, J. W. Fox, I. Raw, C. E. Frasch, and M. M. Tanizaki. 2003. Immune response to native NadA from Neisseria meningitidis and its expression in clinical isolates in Brazil. J. Med. Microbiol. 52:121-125. [DOI] [PubMed] [Google Scholar]
- 20.Giuliani, M. M., J. Adu-Bobie, M. Comanducci, B. Arico, S. Savino, L. Santini, B. Brunelli, S. Bambini, A. Biolchi, B. Capecchi, E. Cartocci, L. Ciucchi, F. Di Marcello, F. Ferlicca, B. Galli, E. Luzzi, V. Masignani, D. Serruto, D. Veggi, M. Contorni, M. Morandi, A. Bartalesi, V. Cinotti, D. Mannucci, F. Titta, E. Ovidi, J. A. Welsch, D. Granoff, R. Rappuoli, and M. Pizza. 2006. A universal vaccine for serogroup B meningococcus. Proc. Natl. Acad. Sci. USA 103:10834-10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenwood, B. 1999. Manson Lecture. Meningococcal meningitis in Africa. Trans. R. Soc. Trop. Med. Hyg. 93:341-353. [DOI] [PubMed] [Google Scholar]
- 22.Griffiss, J. M., B. L. Brandt, D. D. Broud, D. K. Goroff, and C. J. Baker. 1984. Immune response of infants and children to disseminated infections with Neisseria meningitidis. J. Infect. Dis. 150:71-79. [DOI] [PubMed] [Google Scholar]
- 23.Halperin, S. A., J. M. Langley, B. Smith, P. Wunderli, L. Kaufman, A. Kimura, and D. Martin. 2006. Phase 1 first-in-human studies of the reactogenicity and immunogenicity of a recombinant meningococcal NspA vaccine in healthy adults. Vaccine 25:450-457. [DOI] [PubMed] [Google Scholar]
- 24.Hou, V. C., G. R. Moe, Z. Raad, T. Wuorimaa, and D. M. Granoff. 2003. Conformational epitopes recognized by protective anti-neisserial surface protein A antibodies. Infect. Immun. 71:6844-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson, K. G., and M. B. Perry. 1976. Improved techniques for the preparation of bacterial lipopolysaccharides. Can. J. Microbiol. 22:29-34. [DOI] [PubMed] [Google Scholar]
- 26.Jones, G. R., M. Christodoulides, J. L. Brooks, A. R. Miller, K. A. Cartwright, and J. E. Heckels. 1998. Dynamics of carriage of Neisseria meningitidis in a group of military recruits: subtype stability and specificity of the immune response following colonization. J. Infect. Dis. 178:451-459. [DOI] [PubMed] [Google Scholar]
- 27.Jordens, J. Z., J. N. Williams, G. R. Jones, M. Christodoulides, and J. E. Heckels. 2004. Development of immunity to serogroup B meningococci during carriage of Neisseria meningitidis in a cohort of university students. Infect. Immun. 72:6503-6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kayhty, H., H. Jousimies-Somer, H. Peltola, and P. H. Maketa. 1981. Antibody response to capsular polysaccharides of groups A and C Neisseria meningitidis and Haemophilus influenzae type b during bacteremic disease. J. Infect. Dis. 143:32-41. [DOI] [PubMed] [Google Scholar]
- 29.Kim, J. J., N. J. Phillips, B. W. Gibson, J. M. Griffiss, and R. Yamasaki. 1994. Meningococcal group A lipooligosaccharides (LOS): preliminary structural studies and characterization of serotype-associated and conserved LOS epitopes. Infect. Immun. 62:1566-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapeyssonnie, L. 1963. Cerebrospinal meningitis in Africa. Bull. W. H. O. 28:3-114. [PMC free article] [PubMed] [Google Scholar]
- 31.Lesse, A. J., A. A. Campagnari, W. E. Bittner, and M. A. Apicella. 1990. Increased resolution of lipopolysaccharides and lipooligosaccharides utilizing Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Immunol. Methods 126:109-117. [DOI] [PubMed] [Google Scholar]
- 32.Litt, D. J., S. Savino, A. Beddek, M. Comanducci, C. Sandiford, J. Stevens, M. Levin, C. Ison, M. Pizza, R. Rappuoli, and J. S. Kroll. 2004. Putative vaccine antigens from Neisseria meningitidis recognized by serum antibodies of young children convalescing after meningococcal disease. J. Infect. Dis. 190:1488-1497. [DOI] [PubMed] [Google Scholar]
- 33.Maeland, J. A., and E. Wedege. 1989. Serum antibodies to cross-reactive Neisseria outer membrane antigens in healthy persons and patients with meningococcal disease. APMIS 97:774-780. [DOI] [PubMed] [Google Scholar]
- 34.Mandrell, R. E., and W. D. Zollinger. 1989. Human immune response to meningococcal outer membrane protein epitopes after natural infection or vaccination. Infect. Immun. 57:1590-1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin, D., N. Cadieux, J. Hamel, and B. R. Brodeur. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 185:1173-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moe, G. R., S. Tan, and D. M. Granoff. 1999. Differences in surface expression of NspA among Neisseria meningitidis group B strains. Infect. Immun. 67:5664-5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moe, G. R., P. Zuno-Mitchell, S. S. Lee, A. H. Lucas, and D. M. Granoff. 2001. Functional activity of anti-neisserial surface protein A monoclonal antibodies against strains of Neisseria meningitidis serogroup B. Infect. Immun. 69:3762-3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nicolas, P., G. Norheim, E. Garnotel, S. Djibo, and D. A. Caugant. 2005. Molecular epidemiology of Neisseria meningitidis isolated in the African meningitis belt between 1988 and 2003 shows dominance of sequence type 5 (ST-5) and ST-11 complexes. J. Clin. Microbiol. 43:5129-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norheim, G., A. Aase, D. A. Caugant, E. A. Høiby, E. Fritzsønn, T. Tangen, P. Kristiansen, U. Heggelund, and E. Rosenqvist. 2005. Development and characterisation of outer membrane vesicle vaccines against serogroup A Neisseria meningitidis. Vaccine 23:3762-3774. [DOI] [PubMed] [Google Scholar]
- 40.Norheim, G., A. Aseffa, M. A. Yassin, G. Mengistu, A. Kassu, D. Fikremariam, W. Tamire, Y. Merid, E. A. Høiby, D. A. Caugant, E. Fritzsønn, T. Tangen, T. Alebel, D. Berhanu, M. Harboe, and E. Rosenqvist. 2007. Serum antibody responses in Ethiopian meningitis patients infected with serogroup A sequence type 7 Neisseria meningitidis. Clin. Vaccine Immunol. 14:451-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norheim, G., E. A. Høiby, D. A. Caugant, E. Namork, T. Tangen, E. Fritzsønn, and E. Rosenqvist. 2004. Immunogenicity and bactericidal activity in mice of an outer membrane protein vesicle vaccine against Neisseria meningitidis serogroup A disease. Vaccine 22:2171-2180. [DOI] [PubMed] [Google Scholar]
- 42.Norheim, G., E. Rosenqvist, A. Aseffa, M. A. Yassin, G. Mengistu, A. Kassu, D. Fikremariam, W. Tamire, E. A. Hoiby, T. Alebel, D. Berhanu, Y. Merid, M. Harboe, and D. A. Caugant. 2006. Characterization of Neisseria meningitidis isolates from recent outbreaks in Ethiopia and comparison with those recovered during the epidemic of 1988 to 1989. J. Clin. Microbiol. 44:861-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poolman, J. T., C. T. Hopman, and H. C. Zanen. 1983. Immunogenicity of meningococcal antigens as detected in patient sera. Infect. Immun. 40:398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenqvist, E., E. A. Høiby, E. Wedege, K. Bryn, J. Kolberg, A. Klem, E. Rønnild, G. Bjune, and H. Nøkleby. 1995. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect. Immun. 63:4642-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenqvist, E., E. A. Høiby, E. Wedege, B. Kusecek, and M. Achtman. 1993. The 5C protein of Neisseria meningitidis is highly immunogenic in humans and induces bactericidal antibodies. J. Infect. Dis. 167:1065-1073. [DOI] [PubMed] [Google Scholar]
- 46.Rosenqvist, E., A. Musacchio, A. Aase, E. A. Høiby, E. Namork, J. Kolberg, E. Wedege, A. Delvig, R. Dalseg, T. E. Michaelsen, and J. Tommassen. 1999. Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis. Infect. Immun. 67:1267-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scholten, R. J., B. Kuipers, H. A. Valkenburg, J. Dankert, W. D. Zollinger, and J. T. Poolman. 1994. Lipo-oligosaccharide immunotyping of Neisseria meningitidis by a whole-cell ELISA with monoclonal antibodies. J. Med. Microbiol. 41:236-243. [DOI] [PubMed] [Google Scholar]
- 48.Sierra, G. V., H. C. Campa, N. M. Varcacel, I. L. Garcia, P. L. Izquierdo, P. F. Sotolongo, G. V. Casanueva, C. O. Rico, C. R. Rodriguez, and M. H. Terry. 1991. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 14:195-207. [PubMed] [Google Scholar]
- 49.Sugasawara, R. J. 1985. Recognition of serogroup A Neisseria meningitidis serotype antigens by human antisera. Infect. Immun. 48:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suker, J., I. M. Feavers, M. Achtman, G. Morelli, J. F. Wang, and M. C. Maiden. 1994. The porA gene in serogroup A meningococci: evolutionary stability and mechanism of genetic variation. Mol. Microbiol. 12:253-265. [DOI] [PubMed] [Google Scholar]
- 51.Vandeputte-Rutten, L., M. P. Bos, J. Tommassen, and P. Gros. 2003. Crystal structure of neisserial surface protein A (NspA), a conserved outer membrane protein with vaccine potential. J. Biol. Chem. 278:24825-24830. [DOI] [PubMed] [Google Scholar]
- 52.Wedege, E. 2001. Immunoblot analysis of sera from patients and vaccinees, p. 275-288. In A. J. Pollard and M. C. Maiden (ed.), Methods in molecular medicine: meningococcal vaccines. Humana Press, Totowa, NJ. [DOI] [PubMed]
- 53.Wedege, E., K. Bolstad, A. Aase, T. K. Herstad, L. McCallum, E. Rosenqvist, P. Oster, and D. Martin. 2007. Functional and specific antibody responses in adult volunteers vaccinated with two different meningococcal serogroup B outer membrane vesicle vaccines in New Zealand. Clin. Vaccine Immunol. 7:830-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wedege, E., D. A. Caugant, L. O. Frøholm, and W. D. Zollinger. 1991. Characterization of serogroup A and B strains of Neisseria meningitidis with serotype 4 and 21 monoclonal antibodies and by multilocus enzyme electrophoresis. J. Clin. Microbiol. 29:1486-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wedege, E., E. A. Høiby, E. Rosenqvist, and G. Bjune. 1998. Immune responses against major outer membrane antigens of Neisseria meningitidis in vaccinees and controls who contracted meningococcal disease during the Norwegian serogroup B protection trial. Infect. Immun. 66:3223-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westphal, O., and K. Jann. 1965. Bacterial lipopolysaccharide extraction with phenol-water and further application of the procedure. Methods Carbohydr. Chem. 5:91. [Google Scholar]
- 57.Williams, J. N., G. R. Jones, M. Christodoulides, and J. E. Heckels. 2003. Serological correlates of protection against meningococci in a cohort of university students, before and during an outbreak of serogroup C infection. J. Infect. Dis. 187:1433-1441. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. 1998. Control of epidemic meningococcal disease. WHO practical guidelines. Document no. WHO/EMC/BAC/98.3. World Health Organization, Geneva, Switzerland.
- 59.Wu, L. H., C. M. Tsai, and C. E. Frasch. 1987. A method for purification of bacterial R-type lipopolysaccharides (lipooligosaccharides). Anal. Biochem. 160:281-289. [DOI] [PubMed] [Google Scholar]
- 60.Zollinger, W., E. Moran, D. Schmiel, and B. Brandt. 2006. Specificity of cross reactive bactericidal antibodies in normal and convalescent human serum, p. 124. In J. Sofronidis, J. Davies, and M. Jennings (ed.), IPNC 2006 Australia program and abstract book. 15th International Pathogenic Neisseria Conference. Cambridge Publishing, West Leederville, Australia.