Abstract
This was a phase I study to assess the safety, tolerability, and immunogenicity of escalating doses of AG-702, a noncovalent complex of an HLA A*0201-restricted epitope in the glycoprotein B protein of herpes simplex virus type 2 (gB2) and truncated human constitutive heat shock protein 70. Similar vaccines have been immunogenic in animals. Three injections of 10 to 250 μg were administered intradermally to HLA A*0201-bearing subjects who were either herpes simplex virus type 2 (HSV-2)-infected or HSV uninfected. Sixty-two participants received the vaccine, 60 completed the protocol, and T-cell data were accrued for 56 subjects. The vaccine was safe and well tolerated. New or boosted responses to the HSV-2 CD8 epitope were not detected. Baseline responses to an epitope in virion proteins 13/14 were higher than responses to the gB2 epitope. A heat shock protein vaccine with an HSV-2 peptide appears to be safe at the doses studied in healthy adults with or without HSV infection. Modifications of the dose, adjuvant, route, schedule, or HSV antigen may be required to improve responses.
Herpes simplex virus type 2 (HSV-2) is a major cause of genital ulcer disease, causing severe infections in neonates and immunocompromised persons, and it is a cofactor in human immunodeficiency virus type 1 (HIV-1) transmission (5). Existing HSV control strategies, including oral nucleoside therapy (6) and regular use of condoms (50), have logistic, biological, or behavioral limitations. It has been proven that limiting HSV-2 shedding can limit HSV-2 transmission (6). Intensive research has focused on therapeutic and prophylactic vaccines to limit HSV-2 shedding and disease. Immunotherapy with HSV-2 glycoproteins has demonstrated short-term efficacy but substantial reactogenicity with some adjuvants (8, 43, 44). This vaccine format is known to elicit or boost antibody and CD4-cell responses but is unlikely to boost CD8 responses (23, 42).
Several lines of evidence support critical roles for CD8 T cells in the control of HSV infections. Local infiltration of HSV-specific cytotoxic T lymphocytes (CTL) correlates with the clearance of infectious HSV-2 in human recurrent genital herpes (26). HSV-2-specific CD8 T cells persistently infiltrate healed genital herpes lesions and localize near sensory nerve endings (53). HSV-specific CD8 T cells with cytotoxic and gamma interferon (IFN-γ) functional activity also localize to latently infected human trigeminal ganglia (48). The precursor frequency of HSV-2-specific CD8 CTL is correlated with HSV-2 severity in HIV-1/HSV-2-coinfected men (37), and cross-sectional studies show human leukocyte antigen (HLA) class I associations with HSV-2 severity (29). In mice, HSV-2-specific CD8 T cells infiltrate acutely and latently infected ganglia and mediate control over viral reactivation in an IFN-γ-dependent manner (20). Vaccines containing a CD8 epitope as the sole HSV component can protect mice from HSV challenge (4). Candidate replication-competent and -incompetent HSV strains have entered clinical trials as therapeutic or preventative vaccines, but CD8 responses have not been demonstrated in humans. Virus-mediated immune cell inactivation may limit priming or boosting with attenuated HSV (38). Safety concerns also suggest that other formats be investigated for boosting CD8 responses (23).
Recently, heat shock proteins (HSP) have been shown to have adjuvant activity for priming antigen-specific CD8 T-cell responses to proteins and peptides. Relevant dendritic cell receptors and uptake mechanisms have been proposed (3). Preclinical studies with mice, using major histocompatibility complex-appropriate CD8 T-cell epitopes and murine HSP, show priming of CD8 T cells in day 7 splenocytes and partial protection from HSV challenge (27, 35). Typically, peptide-only vaccines have not been immunogenic in humans, but combination of peptides with suitable adjuvants has yielded detectable antigen-specific responses (11, 14).
Given the importance of CD8 responses in the control of HSV-2 infection and the potential for a novel adjuvant system to enhance or elicit CD8 responses, we performed a phase I, dose-escalation, nonblinded, two-site trial of safety and immunogenicity of an HSP-based vaccine with both HSV-2-infected and HSV-uninfected subjects. For proof of the concept, the HSV-2 component of the vaccine was an HSV-2 envelope glycoprotein B (gB2) peptide epitope known to be present in HLA A*0201 (R. L. Burke, personal communications, 2001; 46). This HLA allele is present in 30 to 40% of people (31). The HLA A*0201-positive subjects were thus theoretically capable of CD8 T-cell responses to the vaccine. The doses used were relatively low, as this was a first-in-humans trial. Assays did not detect priming or boosting of peptide-specific CD8 responses to the vaccine immunogen. However, the vaccine appeared safe over a 25-fold dose range, and novel observations concerning baseline HSV-2-specific CD8 T cells were accrued with a large, defined cohort.
MATERIALS AND METHODS
Clinical protocol and specimens.
Participants 18 to 65 years of age participated in a protocol approved by local institution review boards and conducted according to International Conference on Harmonization Good Clinical Practice (ICH GCP) guidelines. All subjects gave written informed consent, were HLA A*02 positive by DNA testing (Puget Sound Blood Center, Seattle, WA) (16), and, if subjects were female, were nonpregnant and practicing contraception. Inclusion criteria included the lack of either HSV-1 or HSV-2 infection, as determined by type-specific serology (2) (comprising group 1), or HSV-2 seropositivity with a clinical history of genital herpes (comprising group 2). There were no criteria regarding the duration or severity of recurrent genital herpes for group 2. Group 2 subjects took either 1,000 mg valacyclovir daily or 400 mg acyclovir twice daily to suppress HSV replication for 2 weeks before and throughout the 24-week study period. Exclusion criteria included chronic or acute medical conditions requiring medical therapy, a history of ocular HSV infection or HSV-associated erythema multiforme, immunodeficiency, receipt of nontopical immunosuppressive medication, serum antibodies to HIV-1 or myeloperoxidase, allergy to acyclovir or valacyclovir, previous HSV vaccinations, or the presence of antinuclear antibodies at 1:80 or greater.
The doses studied were 10, 100, and 250 μg of AG-702 per dose. As a control, some subjects received 4 μg of the gB2 amino acids 442 to 451 (gB2 442-451) dissolved in 200 μl phosphate-buffered saline. This corresponded to the amount of peptide present in the highest dose of AG-702. AG-702 was given first, and escalation was allowed after five group 1 and five group 2 subjects were observed 2 weeks after receiving the second vaccine to be without serious side effects or laboratory evidence of toxicity. The targeted enrollment was 5 subjects in each group who were to receive a 10-μg dose and peptide control and 5 and 15 group 1 and group 2 subjects, respectively, who were to receive both 100- and 250-μg dose levels.
Single-use vials were thawed on ice, and vaccine was given at 0, 2, and 4 weeks by intradermal injection with a 25-gauge needle. Volumes were 0.2 ml of 0.05 or 0.5 mg/ml vaccine for 10- and 100-μg doses, respectively, or two injections, 2 cm apart, of 0.25 ml of the 0.5 mg/ml vaccine for 250-μg doses. For immunogenicity testing, blood was obtained at 1 week and just prior to the first vaccination for baselines and at 4, 6, 12, and 24 weeks. Acid citrate dextrose-anticoagulated blood was centrifuged with Ficoll-Hypaque (45), and peripheral blood mononuclear cells (PBMC) were frozen in 20% human serum (Gemini, West Sacramento, CA), 10% dimethyl sulfoxide (DMSO; Sigma, St. Louis, MO), and 70% RPMI 1640 medium (Invitrogen, Carlsbad, CA) in liquid nitrogen. Blood test samples for safety monitoring were obtained at baseline before each vaccination and at weeks 6, 12, and 24, and tests included erythrocyte sedimentation rate; C-reactive protein level; electrolytes; glucose, renal, and hepatic function; muscle enzyme levels; antinuclear antibodies; and antimyeloperoxidase. Vital signs and local or systemic reactions were checked after vaccinations were given and on the next day. Laboratory abnormalities and signs and symptoms were graded by the National Cancer Institute Common Toxicity Criteria (CTC), version 2.0 (47).
Vaccine.
The full-length cDNA encoding the human constitutive HSP70 gene HSPA8 (GenBank accession no. Y00371) was expressed in Escherichia coli strain BL21(DE3), using a pET-24a-based vector (Novagen, San Diego, CA) without the initial methionine. The synthetic gB2 442-451 peptide (GFLIAYQPLL) was synthesized using tert-butoxycarbonyl (t-Boc) chemistry and obtained at high purity (CS Bio Inc., San Carlos, CA). The vaccine compound AG-702 is a protein-peptide complex consisting of recombinant human recombinant heat shock protein HSC70 (rHSC70) complexed with the synthetic gB 442-451 peptide at a 1:1 molar ratio. Complexes were sterile filtered, put into vials at 50 or 500 μg/ml, and stored at −80°C until used. The vaccine contained AG-702 in phosphate-buffered saline and no additional excipients, stabilizers, or adjuvants. Characterizations included testing the rHSC70 identity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis and immunoblot detection and the peptide identity by amino acid analysis, as well as Edman sequencing, electrospray mass spectrometry and reversed-phase high-performance liquid chromatography, and sterility and purity. Stability tests of the rHSC70 monomer, the gB2 peptide, and the rHSC70-peptide complex were performed for between 18 and 54 months, spanning the storage period of the vaccine at −80°C relevant to this trial, using size exclusion chromatography. The presence of a physical association between the rHSC70 and the gB2 peptide was also confirmed by column chromatography using size exclusion media. Preclinical animal toxicology testing was conducted with Sprague-Dawley rats, in compliance with the Food and Drug Administration Good Laboratory Practice Regulations (GLP) as set forth in Title 21 of the U.S. Code of Federal Regulations, part 58, issued December 22, 1978. The test article was well tolerated with no evidence of systemic toxicity. Immunogenicity studies with the murine construct have been reported previously (35).
Immunogenicity testing.
IFN-γ enzyme-linked immunospot (ELISPOT) assays followed protocols and used the reagents and supplies described previously (16). Briefly, each well contained antigen-presenting cells, responder CD8+ cells, and a test stimulus in a final volume of 200 μl T-cell medium (RPMI 1640 medium, 1% penicillin-streptomycin, 2 mM l-glutamine, and 10% of a single heat-inactivated human serum batch [Serologics, Norcross, VA]). Antigen-presenting cells were dendritic cells (DC) made from plastic-adherent cells cultured with granulocyte-macrophage colony-stimulating factor and interleukin-4 (IL-4) for 6 days (16). DC from week 24 were used with CD8 responders from each time point to run all samples from each subject in parallel. DC were irradiated (3,300 rad, γ-irradiation) and used at 2.5 × 104/well. Positively selected CD8+ cells (Miltenyi, Auburn, CA) were used at 3 × 105/well. These were typically >90% CD8+ CD4− cells, by flow cytometry, performed as described previously (26). Peptide gB2 442-451 was stored at 10 mg/ml in DMSO at −20°C and was tested at 1 μM. Preliminary studies had shown that responses to HSV-2-infected persons were maximal at this peptide concentration. Phytohemagglutinin (PHA-P; Remel, Kansas City, KA) at 1.6 μg/ml, HSV-2 strain 333 (21), UV irradiated (24) from a preirradiation titer of 108 PFU/ml and diluted 1:100, and DMSO (0.1%) were used as controls. Typically, DMSO and gB2 442-451 samples were run in quadruplicate, and other stimuli were run in duplicate. Replicates were reduced if required by responder cell availability. Plates were read on a Zeiss ELISPOT reader. Data are reported as the mean spot-forming units (sfu)/106 CD8 PBMC. For the gB2 442-451 peptide, the mean value for DMSO was subtracted from the mean value for the peptide. For whole UV-irradiated HSV-2 antigen and PHA, the mean value was used. If one of the duplicate PHA or HSV-2 wells had too many sfu to count, the sfu from the countable well was used. If both wells contained sfu too numerous to count, results were recorded as positive. PHA was used as a quality control for PBMC preservation and assay procedures. If less than 30 sfu/106 CD8 responders were detected for PHA, all data from that specimen were discarded.
To use phycoerythrin-labeled HLA-peptide tetramers, we restimulated antigen-specific precursors as described previously (22, 28). In brief, PBMC in T-cell medium were pulsed with 100 μM gB2 442-451 or HSV-2 UL47 (the gene encoding virion proteins 13/14 [VP13/14]) amino acids 550 to 559 for 1 h, followed by 10 days of incubation with 5 μM or less of the peptide. Cytokines (recombinant human IL-2 and IL-7) were added (22, 28) to support T-cell survival and expansion, and cells split as needed. Tetramers containing HLA *0201 and either gB2 443-451 (FLIAYQPLL) or HSV-2 VP13/14 amino acids 551 to 559 (VP13/14 551-559; GLADTVVAC) were purchased from Immunomics (San Diego, CA). Day 10 cultured cells were stained with 1 μl tetramer for 60 min at room temperature in 50 μl of T-cell medium, followed by anti-CD8-phycoerythrin-cyanine 5 (Caltag, Burlingame, CA), washes, and fixation. At least 200,000 events were accrued (FACScan; Becton Dickenson, San Jose, CA), and data were analyzed with WinMDI 2.8 software (http://facs.scripps.edu/software.html). The presence or absence of cells staining brightly for both anti-CD8 and tetramer binding was recorded.
Statistical methods.
To examine a possible vaccine effect, linear mixed-effect models were used to test whether the average peptide response, measured in IFN-γ sfu/106 CD8 PBMC, to prevaccination was similar to that postvaccination. Models included a term identifying the visits occurring postvaccination, as well as interaction terms between the postvaccination effect and the dose groups (using the peptide as the reference). Prevaccination average responses were computed to compare baseline responses by HSV serostatus. Dichotomous prevaccination measures were combined to reflect being “ever positive” at baseline. The Mann-Whitney test and Fisher's exact test, two-tailed, were used to compare baseline continuous and dichotomous responses, respectively, between groups 1 and 2 (Instat version 3.05; GraphPad, San Diego, CA). Spearman's correlation coefficient was used to test whether continuous measures were associated at baseline.
RESULTS
Cohort and enrollment.
HSV seronegative (group 1) or HSV-2 seropositive (group 2) individuals were enrolled to receive a total of three vaccinations of either 10 μg, 100 μg, or 250 μg of AG-702 or 4 μg of peptide alone as a control. Overall, 60 subjects completed the vaccination program (Table 1). Overall, 45% of the subjects were female and 90% were Caucasian, and the median age was 36.0 years, with a range of 20.5 to 57.0 years. There were no significant differences in gender, age, or ethnicity between the HSV-seronegative and HSV-2-seropositive subjects. All subjects receiving the 10-μg and 100-μg doses for AG-702 and those for the 4-μg peptide control completed three immunizations. At the 250-μg dose level, 4 subjects in group 1 and 16 in group 2 completed three immunizations. One additional subject in each group at this dose level withdrew after receiving one dose of vaccine because the subjects moved away from the geographic area.
TABLE 1.
Number of study participants completing the three-dose vaccination protocol
| Vaccine or peptide | Dose (μg) | Subjects completing the protocol in:
|
|||||
|---|---|---|---|---|---|---|---|
| Group 1 (HSV seronegative)
|
Group 2 (HSV-2 seropositive)
|
||||||
| No. of subjects | % Female subjects | Median age (yr) | No. of subjects | % Female subjects | Median age (yr) | ||
| AG-70 | 210 | 5 | 20 | 38 | 5 | 60 | 38 |
| AG-702 | 100 | 5 | 40 | 30 | 16 | 44 | 44 |
| AG-702 | 250a | 4 | 50 | 35 | 16 | 44 | 39 |
| Peptide | 4 | 5 | 40 | 35 | 4 | 50 | 35 |
| Total | 19 | 41 | |||||
One subject in group 1 and one subject in group 2 withdrew after receiving one 250-μg dose because they moved from the geographic area.
Safety and tolerability.
The vaccine was well tolerated. A total of 62 persons received at least one dose of vaccine (Table 2). The adverse experiences most frequently reported were injection site induration (reported by 100% of patients), injection site erythema (96.8%), injection site tenderness (56.5%), headache (41.9%), injection site warmth (38.7%), injection site pain (29.0%), and injection site pruritus (17.7%). One subject reported grade-2 injection site pain, and one subject reported grade-2 fatigue. The local vaccine site reactions were generally brief in duration (less than 2 days). One subject developed a documented iron deficiency anemia; specimens for week 12 and 24 T-cell immunogenicity studies were not obtained. A separate group 2 subject had normal serum phosphorus levels and then received three doses of 250 μg of AG-702. The phosphorus value was 1.8 mg/dl (grade 3 per CTC) at week 6, 2 weeks after the third immunization was received. The subject was asymptomatic. Phosphorus levels returned to normal, without specific therapy 2 days later and remained normal.
TABLE 2.
Adverse effects reported by AG-702 recipients or noted by clinicians
| System | Sign or symptom | No. of subjects (% of total) receiving peptide or vaccine at the dose level shown (total subjects/group)
|
||||
|---|---|---|---|---|---|---|
| 4 μg peptide (n = 9) | 10 μg AG-702 (n = 10) | 100 μg AG-702 (n = 21) | 250 μg AG-702 (n = 22) | Total (n = 62) | ||
| Blood and lymphatic | Lymphadenopathy | 1 (11.1) | 0 (0.0) | 1 (4.8) | 3 (13.6) | 5 (8.1) |
| Gastrointestinal | Nausea | 0 (0.0) | 0 (0.0) | 3 (14.3) | 1 (4.5) | 4 (6.5) |
| General and administration site | Injection site induration | 9 (100.0) | 10 (100.0) | 21 (100.0) | 22 (100.0) | 62 (100) |
| Injection site erythema | 8 (88.9) | 9 (90.0) | 21 (100.0) | 22 (100.0) | 60 (96.8) | |
| Injection site tenderness | 0 (0.0) | 3 (30.0) | 19 (90.5) | 13 (59.1) | 35 (56.5) | |
| Injection site warmth | 0 (0.0) | 3 (30.0) | 13 (61.9) | 8 (36.4) | 24 (38.7) | |
| Injection site pain | 0 (0.0) | 2 (20.0) | 5 (23.8) | 11 (50.0) | 18 (29.0) | |
| Injection site pruritus | 0 (0.0) | 1 (10.0) | 8 (38.1) | 2 (9.1) | 11 (17.7) | |
| Fatigue | 3 (33.3) | 3 (30.0) | 3 (14.3) | 0 (0.0) | 9 (14.5) | |
| Feeling hot | 0 (0.0) | 1 (10.0) | 1 (4.8) | 2 (9.1) | 4 (6.5) | |
| Malaise | 0 (0.0) | 1 (10.0) | 1 (4.8) | 2 (9.1) | 4 (6.5) | |
| Immune | Seasonal allergy | 0 (0.0) | 1 (10.0) | 3 (14.3) | 0 (0.0) | 4 (6.5) |
| Infections and infestations | Herpes simplex symptomatic recurrence | 1 (11.1) | 2 (20.0) | 4 (19.0) | 2 (9.1) | 9 (14.5) |
| Upper respiratory tract infection | 0 (0.0) | 1 (10.0) | 5 (23.8) | 2 (9.1) | 8 (12.9) | |
| Nasopharyngitis | 1 (11.1) | 0 (0.0) | 0 (0.0) | 4 (18.2) | 5 (8.1) | |
| Sinusitis | 0 (0.0) | 1 (10.0) | 2 (9.5) | 2 (9.1) | 5 (8.1) | |
| Musculoskeletal and | Myalgia | 1 (11.1) | 3 (30.0) | 3 (14.3) | 4 (18.2) | 11 (17.7) |
| connective tissue | Back pain | 2 (22.2) | 1 (10.0) | 2 (9.5) | 3 (13.6) | 8 (12.9) |
| Nervous system | Headache | 5 (55.6) | 6 (60.0) | 9 (42.9) | 6 (27.3) | 26 (41.9) |
| Dizziness | 1 (11.1) | 0 (0.0) | 3 (14.3) | 0 (0.0) | 4 (6.5) | |
| Tension headache | 0 (0.0) | 1 (10.0) | 3 (14.3) | 0 (0.0) | 4 (6.5) | |
| Respiratory, thoracic, and | Nasal congestion | 0 (0.0) | 3 (30.0) | 4 (19.0) | 2 (9.1) | 9 (14.5) |
| mediastinal | Cough | 0 (0.0) | 3 (30.0) | 2 (9.5) | 2 (9.1) | 7 (11.3) |
| Pharyngolaryngeal pain | 1 (11.1) | 1 (10.0) | 3 (14.3) | 1 (4.5) | 6 (9.7) | |
Overall, the elevation of blood creatine phosphokinase (CK) to grade 1 or higher, per CTC, occurred in 12 (19.4%) of the subjects. Five subjects had a grade 3 (5 to 10 times the upper limit of normal) elevation. Four of the subjects had participated in physical activity that was vigorous or uncharacteristic or both. Two subjects reported muscle pain and soreness. One subject had concomitant elevations of serum transaminases associated with serologically documented primary Epstein-Barr virus infection and had an uneventful recovery, while another subject had streptococcal pharyngitis. No dose-related trend was observed. There were no elevations of CK for subjects receiving 4 μg peptide control; two subjects had grade 1 elevations and two had grade 3 elevations at the 10-μg AG-702 dose; two subjects had a grade 1 elevation and one had a grade 3 elevation at the 100-μg AG-702 dose; and three subjects had a grade 1 elevation and two had grade 3 elevations at the 250 μg AG-702 dose. There was no association with baseline HSV serostatus. Serum CK values were closely monitored and found to return to normal within several days, without specific intervention. There were low elevations in CK-MB (CK myocardial band isoenzyme) fractions in two subjects, consistent with the total elevations. The CK changes were judged to be unrelated to vaccine administration, because of the clinical histories of exertion or the relationship to intercurrent illness.
Vaccine immunogenicity.
Blood samples were obtained for immunogenicity testing at two prevaccine time points and at weeks 4, 6, 12, and 24. Immunogenicity tests for each subject were run side by side in single assays, using responder cells from each available blood sample and DC from the previously available blood sample. A total of 56 participants underwent immunogenicity testing. (Table 3). All subjects at the 10- and 100-μg dose levels had samples from each of six time points evaluated. At the 250-μg dose level, 17 subjects had complete tests at all time points, three subjects had no immunogenicity tests, and two subjects had partial testing at early time points only. The three who had no tests performed included the two early dropouts, mentioned above, who received only one dose of vaccine, and a group 2 subject whose vaccine sequence was interrupted as discussed above. The two who had partial testing were the group 2 subject who developed anemia and the group 1 subject who received all vaccines and was lost to follow-up at week 6. Among the nine subjects receiving 4-μg of peptide control, complete specimen sets were available and run on six subjects. We excluded data from specimens which yielded <30 sfu/106 CD8 responder cells after PHA stimulation (11% of specimens obtained after vaccination started).
TABLE 3.
Subjects receiving AG-702 or control vaccine from whom immunogenicity data were obtained
| AG-702 or peptide dose (μg) | No. of subjects whose HSV serostatus was:
|
|
|---|---|---|
| Negative | HSV-2 positive and HSV-1 negative or positive | |
| 10 | 5 | 5 |
| 100 | 5 | 16 |
| 250 | 3 | 16 |
| Peptide (4) | 3 | 3 |
| Total | 16 | 40 |
Among the HSV-seronegative persons, we predicted that responses to peptide gB2 442-451 would increase from undetectable to detectable if we primed CD8 T cells in vivo. Both ELISPOT and tetramer assays were considered suitable for observing de novo responses. Responses to a whole UV-treated HSV-2 antigen increase might be detected if gB2 in viral lysates were cross-presented in vitro, while responses to PHA, a control measure for PBMC cryopreservation and recovery, should not change. Among HSV-2-infected persons, we tried to detect any increase in baseline responses to gB2 peptide, or whole HSV-2, while similarly monitoring PHA responses to assess PBMC quality. The tetramer assay was not quantitative, so temporal trends in the proportion of tetramer-positive cells were not analyzed in HSV-2 seropositive subjects.
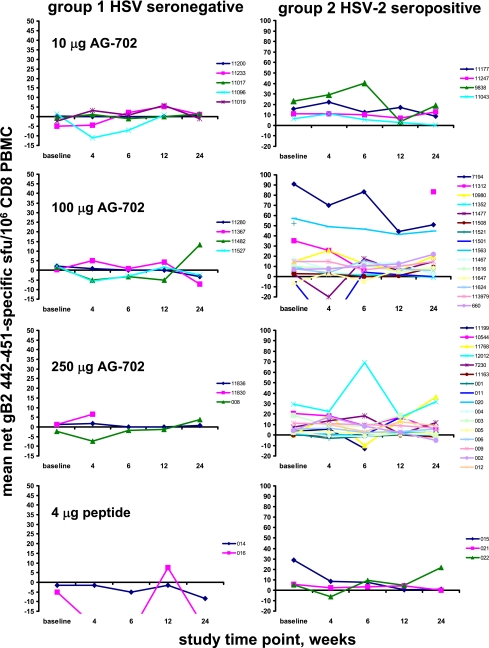
There was no statistically significant de novo induction of gB2 442-451-specific cells in the HSV-seronegative subjects following vaccination, as tested by using linear mixed-effect models for an average within-person change (P = 0.73; individual curves are shown in Fig. 1). Interaction terms were also nonsignificant, indicating that the lack of effect was consistent over doses. There was no increase in preexisting responses to gB2 442-451 in the HSV-2 seropositive subjects (Fig. 1, right).
FIG. 1.
CD8 cells in PBMC reactive in IFN-γ ELISPOT with peptide gB2 442-451 prior to and 24 weeks after vaccination with AG-702 or control peptide. Each panel represents one dose level and subject group as indicated. Individual subjects each have a colored line. Data are mean peptide-specific sfu per 106 CD8 PBMC. Note the association of the y-axis scale with subjects’ HSV serostatus.
Baseline immune responses to HSV.
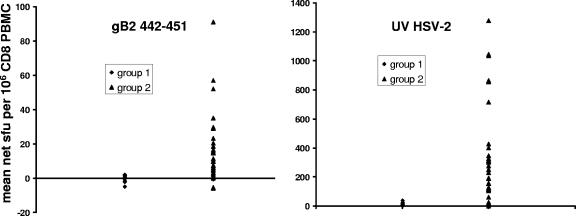
We expected that group 2, the HSV-2 seropositive subjects, would have higher baseline CD8 IFN-γ responses to the gB2 442-451 peptide and to whole HSV-2 antigen than the HSV-seronegative subjects (group 1). We observed that HSV-2-infected persons had higher mean net baseline responses to gB2 442-451 than the HSV-seronegative persons (P < 0.0001, Mann-Whitney test, two tail-ed) (Fig. 2). For group 1 subjects (n = 14), the mean ± standard deviation baseline responses were −0.4 ± 2.4 sfu/106 CD8 PBMC, with a range of −5 to 2.1 sfu/106 CD8 PBMC. The mean net baseline response ± standard deviation among HSV-2-infected persons (n = 35) was 14.3 ± 19.3 sfu/106 CD8 PBMC, with a range of −6 to 91 sfu/106 CD8 PBMC. Similarly, HSV-2-infected persons had much higher baseline CD8 responses to whole UV-treated HSV-2 (P < 0.0001, Mann-Whitney test, two tailed) (Fig. 3). Group 1 subjects (n = 14) had 11.1 ± 10.7 sfu/106 CD8 PBMC, with a range of 0 to 39 sfu/106 CD8 PBMC. In contrast, group 2 subjects (n = 33) had 348 ± 346 sfu/106 CD8 PBMC, with a range of 0 to 1,278 sfu/106 CD8 PBMC. PHA stimulates lymphocytes in a nonspecific fashion (34). Therefore, we predicted that the responses to PHA should not segregate with HSV infection status. There were no differences in the mean PHA responses at baseline between the subjects in group 1 and those in 2 (P = 0.102).
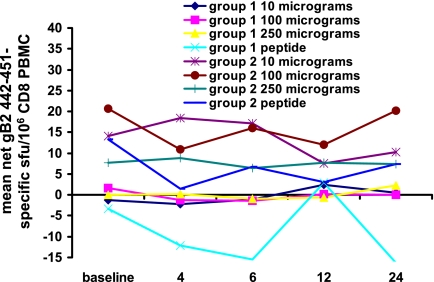
FIG. 2.
CD8 cells in PBMC reactive in IFN-γ ELISPOT with peptide gB2 442-451 prior to and 24 weeks after vaccination with AG-702 or control peptide. Data are mean peptide-specific sfu per 106 CD8 PBMC, with dose levels arranged by rows.
FIG. 3.
Mean baseline IFN-γ ELISPOT response among CD8 T cells to (left panel) HSV-2 gB2 442-451 peptide and (right panel) whole UV-inactivated HSV-2. Note differences in y-axis scales.
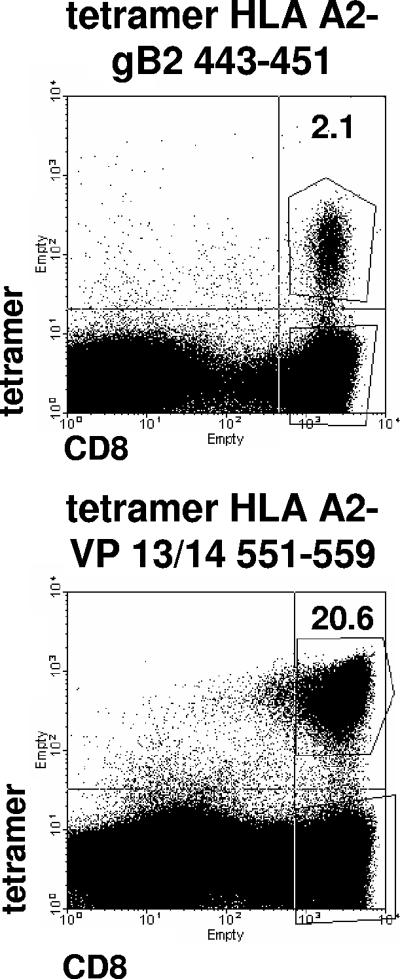
The prevalence of responses to an HSV epitope in the community of HLA-appropriate HSV-2 seropositive persons could correlate with vaccine immunogenicity. An epitope that is immunogenic in most persons in the context of infection would be preferred. We therefore compared baseline responses to HSV-2 gB2 442-451 with baseline responses to a second HLA A*0201-restricted epitope, HSV-2 VP 13/14 551-559 (22). Fluorescent HLA-peptide tetramers and flow cytometry were used in a peptide-restimulated assay to detect epitope-specific cells. We restimulated PBMC to increase sensitivity and analyzed the tetramer results in a dichotomous (yes/no) fashion. Among the HSV-2 seropositive group 2 subjects, tetramer responses were typically robust (Fig. 4, top). There was a higher population prevalence of responses to VP13/14 551-559 than to gB2 442-451 (P = 0.0005, McNemar's test). For gB2 442-451, 20 of 40 HSV-2-infected persons (50%) had positive responses at baseline time points compared to 27 of 33 (82%) for VP13/14 551-559. Among the 33 persons who had tetramer tests for both HSV-2 CD8 epitopes, baseline responses to gB2 were more common among those who also had responses to VP13/14 (P = 0.0213, Fisher's exact test, two sided) (Table 4). One explanation for a higher prevalence of baseline responses to VP13/14 could be amino acid sequence variations in circulating HSV-2 strains in regions in or flanking the gB2 epitope. Persons infected with a variant could have either strain-specific responses that would not be picked up with our test, based on the consensus peptide (10), or no response at all. We therefore sequenced >50 circulating HSV-2 strains at the relevant gB2 (and also VP13/14) loci. We have not encountered any coding polymorphisms leading to amino acid changes in or flanking these epitopes (D. M. Koelle, C. Liu, B. Byrd, A. Sette, J. Sidney, and A. Wald, presented at the 30th International Herpesvirus Workshop, Turku, Finland, 2005). A second explanation is that the gB2 tetramer test was insensitive to low numbers of gB2-specific CD8 T cells in PBMC. To investigate this, we compared tetramer and ELISPOT assay results from among the 33 HSV-2-infected persons who had both tests for the gB2 peptide. The 15 HSV-2-infected persons with negative tetramer test results had a mean net ± standard deviation of 9.4 ± 11.5 gB2 peptide-specific sfu/106 CD8 PBMC compared to 17.2 ± 23.7 gB2 peptide-specific sfu/106 CD8 PBMC for the 18 persons with positive tetramer test results. This difference was not statistically significance (P = 0.35, Mann-Whitney test, two-tail).
FIG. 4.
Detection of epitope-specific CD8 T-cell responses to HSV-2 in humans, using peptide-restimulated tetramer assays. PBMC from HSV-2-infected subjects were restimulated with peptide for 10 days and then stained with the indicated HLA A*0201-containing tetramers and anti-CD8. Numbers are the percentages of CD8high cells (right upper and lower quadrants) that are tetramerhigh (boxed in right upper quadrant).
TABLE 4.
Detection of CD8+ responses to HLA A*0201-restricted HSV-2 T-cell epitopes in gB2 or VP13/14a
| Response to gB2 442-451 | No. of subject responses to VP13/14 551-559
|
||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 15 | 0 | 15 |
| Negative | 12 | 6 | 18 |
| Total | 27 | 6 | 33 |
Values show detection of CD8+ responses to HLA A*0201-restricted HSV-2 T-cell epitopes in gB2 or VP13/14, using tetramers, at baseline time points in HSV-2-infected, HLA A*02-positive subjects who had both tests.
The tetramer assay was specific. The 16 HSV-seronegative group 1 subjects were tested at two baseline time points using tetramers containing either gB2 443-451 or VP13/14 551-559. Among the 64 baseline tests, 63 were negative (<0.01% of CD8 T cells were tetramer positive). One prevaccine specimen yielded a trace positive (0.08% of CD8 T cells) cell line for the gB2 442-451 tetramer. No tetramer-positive cells were detected in samples from this subject after the vaccination was received.
DISCUSSION
This is the first human trial of a compound containing the recombinant human HSP of ∼70 kDa molecular mass, encoded by the HSPA8 gene. The HSP70 was noncovalently associated with a synthetic peptide encoded by both HSV-1 and HSV-2 and was administered intradermally in a dose-escalation, nonblinded format to healthy adults both with and without prior infection with HSV. The safety and tolerability end points did not reveal any unexpected toxicity when three doses of up to 250 μg/dose were administered over 4 weeks. Neither de novo responses primed by the vaccine nor anamnestic responses by preexisting memory cells were severe enough to limit dosing. Within the limits of the CD8+ T-cell assay technologies used, we were unable to document either priming or boosting of peptide-specific responses. However, the lack of toxicity in this initial clinical trial and the biological activity of this approach in animals (27, 35, 41) suggest that further investigations of this approach in humans are rational.
This proof-of-concept trial was designed using available information about the CD8 response to HSV. The epitope picked resides with gB2, whose open reading frame is generally invariant between circulating HSV-2 strains. The epitope is also type common between HSV-2 and HSV-1, relevant to the prevention or treatment of genital herpes, because up to 50% of clinical first-episode genital herpes cases are due to HSV-1 infection (18). We chose to study an A*02-restricted epitope because the associated allele is present in about 30% to 40% of persons, facilitating enrollment. For unknown reasons, among HSV-2-infected persons, the prevalence of responses to an A*02-restricted epitope in the tegument protein VP13/14 is higher than that of responses to the gB2 epitope in AG-702. The quantitative response to VP13/14 551-559, as measured by direct PBMC tetramer assays (25) as well as direct PBMC IFN-γ ELISPOT (D. M. Koelle et al., unpublished data), is also greater than the responses to gB2 442-451. It may be that the VP13/14 epitope is inherently more immunogenic and thus a better candidate for HSV-2 vaccines.
Several alternative HSV-2 vaccine formats have been investigated that have achieved immunogenicity without unacceptable toxicity. One of these, recombinant truncated gD2 in alum/monophosphoryl lipid A adjuvant, has clinical activity as a preventative vaccine in HSV-1- and HSV-2-uninfected women (42). No evidence has been presented that this vaccine elicits CD8 responses, and it is not clear that the vaccine formulation would be likely to do so. The vaccine candidate studied in this report was designed to elicit CD8 rather than CD4 and antibody responses, with the eventual hope that similar polyclonal vaccines could boost existing CD8 responses in HSV-2-infected persons and positively influence recurrent disease. It is recognized that CD4 responses are also important for several phases of immunity to HSV, such that a final vaccine formulation would optimally elicit or expand both types of T cells. CD8 T cells primed in the absence of CD4 help are short-lived in some systems (33, 39). While the results of the current study were negative, boosting CD8 responses in the setting of chronic infection has been extremely challenging, and continuing research is needed.
The HSP-peptide vaccine was also tried with HSV-uninfected persons. It has generally been difficult to prime T-cell responses to isolated antigenic peptides. Tolerance or deletion may be elicited if synthetic peptides alone are administered by some routes (9). Dosing is an important issue, with some trials administering several milligrams of peptide together with adjuvant (11, 14). Further studies administering larger doses of viral peptide will be required to determine if heat shock protein adjuvants can prime CD8 responses without dose-limiting toxicity. HSP are abundant in unstressed and stressed cells and appear to have a role in chaperoning proteins and peptides. As such, they have an inherent and relatively non-sequence-specific peptide-binding function (36). AG-702, as well as similar compounds used in preclinical toxicology and animal efficacy studies, contains recombinant HSP70 that is noncovalently complexed to an HSV peptide.
This phase I dose-escalation trial was designed primarily to monitor safety and tolerability. No dose-limiting toxicity was noted. In previous studies of HSV vaccines, local reactogenicity was noted with HSV-seropositive subjects (23). The activation of innate immunity and/or elicitation of recall responses by an adjuvant has the potential to lead to toxicity in immune individuals. Thus far, this does not seem to be a problem with the noncovalently associated HSP70-peptide approach and HSV infection. We did note elevations of CK, but in no cases were there associated elevations of markers of cardiac inflammation. Each elevation of CK was associated with an unusual or marked physical activity or intercurrent illness and returned to normal. Elevations of CK detected in a recent clinical trial of flavivirus vaccines were also ascribed to physical exertion, leading to an adjustment in study design in which contact sports or new exercise regimens were discouraged among study participants (17).
It is not known why we failed to observe vaccine immunogenicity. Biological reasons include inadequate dosing, suboptimal coupling to the HSP70 protein leading to the administration of a free peptide that could lead to tolerance, poor inherent immunogenicity, or the need for a better route or schedule of vaccine administration. HSP70 is known to prefer hydrophobic peptides, and the HSV-2 peptide used is quite hydrophobic (30). Future studies could include a physical assessment of peptide-HSP interactions in vaccine compounds. The vaccine was stored at −80°C prior to use, reducing but not eliminating the possibility of degradation prior to administration. It is possible that transitory, small immune responses could have been detected at time points earlier than 2 weeks. The intradermal route, as some data suggest, is more immunogenic than the intramuscular route (19, 32). It is possible that the use of an alternative administration method could increase immunogenicity. Technical and assay factors are considered below.
Our two-site study design dictated that PBMC were typically processed the day after phlebotomy. It has recently been reported that Ficoll processing of blood after overnight storage, in comparison to same-day PBMC enrichment, can significantly reduce the number of IFN-γ-positive, virus-specific T cells detected with ELISPOT tests performed after thawing the responder cells (40). In addition, our assays were directed at a limited spectrum of T-cell effector functions. The immunogenicity studies in this trial were designed to detect HSV-2-specific CD8 T cells that could either secrete IFN-γ or bind an HLA-peptide tetramer. In some situations, antigen-specific CD8 T cells may not secrete IFN-γ but may still have biologically significant responses such as secretion of other cytokines (13). The binding of HLA-peptide tetramers is dependent on the avidity of T-cell receptor for peptide/HLA (52), so it is possible we missed low-avidity T cells. Our culture-boosted tetramer assay is also dependent on the ability of peptide-specific T cells to proliferate in response to peptide, IL-2, and IL-7 in vitro. Peptide-specific CD8 T cells with a defect in proliferation have been documented in human HIV infection (1). Thus, it is possible that the end points used in this trial were inadequate to detect vaccine-induced CD8 T cells. The monitoring of future HSP-based vaccine trials will optimally use multiple parameters to measure the possible induction or boosting of antigen-specific immunity.
The study design included suppressive antiviral medication for HSV-2-infected persons. Some immune responses in PBMC have been noted to fluctuate temporally in association with HSV infection recurrences (7), although studies are absent with regard to epitope-specific CD8 responses. We administered antiviral drugs to reduce the detection of possible fluctuations in the level of antigen-specific CD8 T cells in response to symptomatic or asymptomatic episodes of HSV reactivation. In HIV-1 infection, epitope-specific CD8 responses may wane over the course of years with profound suppression of viral replication (15). We felt there was less risk of provoking a similar decline in this study, as our observation period was limited to 24 weeks, the degree of suppression of viral shedding obtained with the antiviral mediations and doses we used are generally only in the 90 to 95% range (49, 51), and previous studies of HSV-specific CD4 responses have generally not observed changes during suppressive antiviral therapy (12). It is unknown whether endogenous antigen will assist or hinder either the efficacy or detection of HSV-specific T-cell responses in an immunotherapeutic setting.
In summary, we tested a novel compound, representative of a new class of candidate vaccines, with human volunteers in a phase I trial and found that it was safe and well tolerated. AG-702 is a noncovalent complex of the human constitutive HSP70 protein, produced in E. coli, and a synthetic peptide corresponding to a type-common CD8 epitope in HSV glycoprotein B. AG-702 was administered intradermally, in doses ranging from 10 to 250 μg per injection, to healthy adult volunteers. No sign of untoward vaccine toxicity was noted in either HSV-naïve or HSV-2-infected subjects who were receiving antiviral suppressive therapy. Peptide-specific tests of PBMC did not reveal elicitation or boosting of IFN-γ responses or in vivo priming of cells capable of proliferating to peptide in vitro and then binding to an appropriate tetramer. The comparison of baseline responses to the vaccine epitope and a separate HLA A*02-restricted epitope in VP13/14 showed that responses to the latter are more prevalent in the population. Continuing research with human constitutive HSP70-based vaccines could include variations in the microbial antigen(s) chosen, the conjugation and formulation technology including the provision of additional adjuvants, the dose, and the route and schedule of administration.
Acknowledgments
Funding was provided by Antigenics to Terri Warren and David Koelle to conduct the clinical trial and immunogenicity testing. Florentina Teofilovici is an employee of Antigenics. Resources from NIH grants AI50132, AI30731, and AI42528 contributed to the performance of this trial.
We thank the study participants and Mary Shaughnessy and Ellen Lairson for clinical assistance with study visits, Rhoda Ashley Morrow for HSV serology, Roselie Montero for HLA typing, Jonathan Lewis, Axel Hoos, and Renu Gupta for assistance with the clinical protocol and monitoring and the staff of the Virology Research Clinic and the Combined Program in Infectious Disease and Virology at the University of Washington and Fred Hutchinson Cancer Research Center for the clinical and administrative tasks work without which this study would not have been possible.
Footnotes
Published ahead of print on 19 March 2008.
REFERENCES
- 1.Arrode, G., J. S. Finke, H. Zebroski, F. P. Siegal, and R. M. Steinman. 2005. CD8+ T cells from most HIV-1-infected patients, even when challenged with mature dendritic cells, lack functional recall memory to HIV gag but not other viruses. Eur. J. Immunol. 35:159-170. [DOI] [PubMed] [Google Scholar]
- 2.Ashley, R. A., J. Militoni, F. Lee, A. Nahmias, and L. Corey. 1988. Comparison of Western blot (immunoblot)and glycoprotein G-specific immunoblot for detecting antibodies to herpes simplex types 1 and 2 in human sera. J. Clin. Microbiol. 26:662-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binder, R. J., and P. K. Srivastava. 2005. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat. Immunol. 6:593-599. [DOI] [PubMed] [Google Scholar]
- 4.Blaney, J. E., E. Nobusawa, M. A. Brehm, R. H. Bonneau, L. M. Mylin, T. M. Fu, Y. Kawoaka, and S. S. Tevethia. 1998. Immunization with a single major histocompatibility class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 72:9567-9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corey, L., A. Wald, C. L. Celum, and T. C. Quinn. 2004. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J. Acquir. Immune Defic. Syndr. 35:435-445. [DOI] [PubMed] [Google Scholar]
- 6.Corey, L., A. Wald, R. Patel, S. L. Sacks, S. K. Tyring, T. Warren, J. M. Douglas, Jr., J. Paavonen, R. A. Morrow, K. R. Beutner, L. S. Stratchounsky, G. Mertz, O. N. Keene, H. A. Watson, D. Tait, and M. Vargas-Cortes. 2004. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N. Engl. J. Med. 350:11-20. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham, A. L., and T. C. Merigan. 1983. Gamma interferon production appears to predict time of recurrence of herpes labialis. J. Immunol. 130:2397-2400. [PubMed] [Google Scholar]
- 8.Dekker, C. L., S. F. Adair, R. Sekulovich, N. Niland, and R. L. Burke. 1992. A phase I study of recombinantly produced herpes simplex virus (HSV) glycoproteins gD2 and gB2 combined with a novel adjuvant MF59/MTP-PE. Abstr. 32nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1431, p. 349.
- 9.Diehl, L., A. T. den Boer, S. P. Schoenberger, E. I. van der Voort, T. N. Schumacher, C. J. Melief, R. Offringa, and R. E. Toes. 1999. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat. Med. 5:774-779. [DOI] [PubMed] [Google Scholar]
- 10.Dolan, A., F. E. Jamieson, C. Cunningham, B. C. Barnett, and D. J. McGeoch. 1998. The genome sequence of herpes simplex virus type 2. J. Virol. 72:2010-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engler, O. B., W. J. Dai, A. Sette, I. P. Hunziker, J. Reichen, W. J. Pichler, and A. Cerny. 2001. Peptide vaccines against hepatitis B virus: from animal model to human studies. Mol. Immunol. 38:457-465. [DOI] [PubMed] [Google Scholar]
- 12.Frenkel, L., E. Pineda, H. Hall, M. Dillon, and Y. Bryson. 1989. A prospective study of the effects of acyclovir treatment on the HSV-2 lymphoproliferative response of persons with frequently recurring HSV-2 genital infections. J. Infect. Dis. 159:845-850. [DOI] [PubMed] [Google Scholar]
- 13.Fu, C. L., Y. L. Ye, Y. L. Lee, and B. L. Chiang. 2003. Both allergen-specific CD4 and CD8 type 2 T cells decreased in asthmatic children with immunotherapy. Pediatr. Allergy Immunol. 14:284-291. [DOI] [PubMed] [Google Scholar]
- 14.Gahery, H., N. Daniel, B. Charmeteau, L. Ourth, A. Jackson, M. Andrieu, J. Choppin, D. Salmon, G. Pialoux, and J. G. Guillet. 2006. New CD4+ and CD8+ T cell responses induced in chronically HIV type-1-infected patients after immunizations with an HIV type 1 lipopeptide vaccine. AIDS Res. Hum. Retroviruses 22:684-694. [DOI] [PubMed] [Google Scholar]
- 15.Gray, C. M., J. Lawrence, E. A. Ranheim, M. Vierra, M. Zupancic, M. Winters, J. Altman, J. Montoya, A. Zolopa, J. Schapiro, A. T. Haase, and T. C. Merigan. 2000. Highly active antiretroviral therapy results in HIV type 1 suppression in lymph nodes, increased pools of naive T cells, decreased pools of activated T cells, and diminished frequencies of peripheral activated HIV type 1-specific CD8+ T cells. AIDS Res. Hum. Retroviruses 16:1357-1369. [DOI] [PubMed] [Google Scholar]
- 16.Hosken, N., P. McGowan, A. Meier, D. M. Koelle, P. Sleath, F. Wegener, M. Elliott, L. Grabstein, C. Posavad, and L. Corey. 2006. Diversity of the CD8+ T-cell response to herpes simplex virus type 2 proteins among persons with genital herpes. J. Virol. 80:5509-5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, C., T. P. Monath, N. Kanesa-Thasan, D. Mathis, C. Miller, S. Shapiro, R. Nichols, K. McCarthy, A. Deary, and P. Bedford. 2005. Exercise-induced serum enzyme elevations confounding the evaluation of investigational drug toxicity. Report of two cases in a vaccine trial. Hum. Vaccin. 1:24-29. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson, M. K., and B. Wahren. 2004. Sexually transmitted herpes simplex viruses. Scand. J. Infect. Dis. 36:93-101. [DOI] [PubMed] [Google Scholar]
- 19.Karahocagil, M. K., T. Buzgan, H. Irmak, H. Karsen, H. Akdeniz, and N. Akman. 2006. Comparison of intramuscular and intradermal applications of hepatitis B vaccine in hemodialysis patients. Ren. Fail. 28:561-565. [DOI] [PubMed] [Google Scholar]
- 20.Khanna, K. M., A. J. Lepisto, and R. L. Hendricks. 2004. Immunity to latent viral infection: many skirmishes but few fatalities. Trends Immunol. 25:230-234. [DOI] [PubMed] [Google Scholar]
- 21.Kit, S., M. Kit, H. Qavi, D. Trkula, and H. Otsuka. 1983. Nucleotide sequence of the herpes simplex virus type 2 (HSV-2) thymidine kinase gene and predicted amino acid sequence of thymidine kinase polypeptide and its comparison with the HSV-1 thymidine kinase gene. Biochim. Biophys. Acta 741:158-170. [DOI] [PubMed] [Google Scholar]
- 22.Koelle, D. M., H. Chen, M. A. Gavin, A. Wald, W. W. Kwok, and L. Corey. 2001. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J. Immunol. 166:4049-4058. [DOI] [PubMed] [Google Scholar]
- 23.Koelle, D. M., and L. Corey. 2003. Recent progress in herpes simplex virus immunobiology and vaccine research. Clin. Microbiol. Rev. 16:96-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koelle, D. M., L. Corey, R. L. Burke, R. J. Eisenberg, G. H. Cohen, R. Pichyangkura, and S. J. Triezenberg. 1994. Antigenic specificity of human CD4+ T-cell clones recovered from recurrent genital herpes simplex virus type 2 lesions. J. Virol. 68:2803-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koelle, D. M., Z. Liu, C. M. McClurkan, M. Topp, S. R. Riddell, E. G. Pamer, A. S. Johnson, A. Wald, and L. Corey. 2002. Expression of cutaneous lymphocyte-associated antigen by CD8+ T-cells specific for a skin-tropic virus. J. Clin. Investig. 110:537-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumaraguru, U., M. Gierynska, S. Norman, B. D. Bruce, and B. T. Rouse. 2002. Immunization with chaperone-peptide complex includes low-avidity cytotoxic T lymphocytes providing transient protection against herpes simplex virus infection. J. Virol. 76:136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalvani, A., T. Dong, G. Ogg, A. A. Patham, H. Newell, A. V. Hill, A. J. McMichael, and S. Rowland-Jones. 1997. Optimization of a peptide-based protocol employing IL-7 for in vitro restimulation of human cytotoxic T lymphocyte precursors. J. Immunol. Methods 15:65-77. [DOI] [PubMed] [Google Scholar]
- 29.Lekstrom-Himes, J. A., P. Hohman, T. Warren, A. Wald, J.-M. Nam, L. Corey, and S. E. Straus. 1999. Association of major histocompatibility complex determinants with the development of symptomatic and asymptomatic genital herpes simplex virus type 2 infections. J. Infect. Dis. 179:1077-1085. [DOI] [PubMed] [Google Scholar]
- 30.Maeda, H., H. Sahara, Y. Mori, T. Torigo, K. Kamiguchi, Y. Tamura, K. Hirata, and N. Sato. 2007. Biological heterogeneity of the peptide-binding motif of the 70-kDa heat shock protein by surface plasmon resonance analysis. J. Biol. Chem. 282:26956-26962. [DOI] [PubMed] [Google Scholar]
- 31.Marsh, S. G. E., P. Parham, and L. D. Barber. 2000. The HLA factsbook. Academic Press, San Diego, CA.
- 32.Mikszta, J. A., V. J. Sullivan, C. Dean, A. M. Waterston, J. B. Alarcon, J. P. Dekker III, J. M. Brittingham, J. Huang, C. R. Hwang, M. Ferriter, G. Jiang, K. Mar, K. U. Saikh, B. G. Stiles, C. J. Roy, R. G. Ulrich, and N. G. Harvey. 2005. Protective immunization against inhalational anthrax: a comparison of minimally invasive delivery platforms. J. Infect. Dis. 191:278-288. [DOI] [PubMed] [Google Scholar]
- 33.Novy, P., M. Quigley, X. Huang, and Y. Yang. 2007. CD4 T cells are required for CD8 T cell survival during both primary and memory recall responses. J. Immunol. 179:8243-8251. [DOI] [PubMed] [Google Scholar]
- 34.O'Flynn, K., A. M. Krensky, P. C. Beverley, S. J. Burakoff, and D. C. Linch. 1985. Phytohaemagglutinin activation of T cells through the sheep red blood cell receptor. Nature 313:686-687. [DOI] [PubMed] [Google Scholar]
- 35.Pack, C. D., U. Kumaraguru, S. Suvas, and B. T. Rouse. 2005. Heat-shock protein 70 acts as an effective adjuvant in neonatal mice and confers protection against challenge with herpes simplex virus. Vaccine 23:3526-3534. [DOI] [PubMed] [Google Scholar]
- 36.Pockley, A. G. 2003. Heat shock proteins as regulators of the immune response. Lancet 362:469-476. [DOI] [PubMed] [Google Scholar]
- 37.Posavad, C. M., D. M. Koelle, M. F. Shaughnessy, and L. Corey. 1997. Severe genital herpes infections in HIV-infected individuals with impaired HSV-specific CD8+ cytotoxic T lymphocyte responses. Proc. Natl. Acad. Sci. USA 94:10289-10294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sloan, D. D., J. Y. Han, T. K. Sandifer, M. Stewart, A. J. Hinz, M. Yoon, D. C. Johnson, P. G. Spear, and K. R. Jerome. 2006. Inhibition of TCR signaling by herpes simplex virus. J. Immunol. 176:1825-1833. [DOI] [PubMed] [Google Scholar]
- 39.Smith, C. M., N. S. Wilson, J. Waithman, J. A. Villadangos, F. R. Carbone, W. R. Heath, and G. T. Belz. 2004. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat. Immunol. 5:1143-1148. [DOI] [PubMed] [Google Scholar]
- 40.Smith, J. G., X. Liu, R. M. Kaufhold, J. Clair, and M. J. Caulfield. 2001. Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicella-zoster virus. Clin. Diagn. Lab. Immunol. 8:871-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava, P. K. 2000. Immunotherapy of human cancer: lessons from mice. Nat. Immunol. 1:363-366. [DOI] [PubMed] [Google Scholar]
- 42.Stanberry, L. R., S. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denes, P. Vandepapeliere, and G. Dubin. 2002. Prophylactic vaccination against genital herpes with adjuvanted recombinant glycoprotein D vaccine: two randomized controlled trials. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 43.Straus, S. E., L. Corey, R. L. Burke, B. Savarese, G. Barnum, P. R. Krause, R. G. Kost, J. L. Meier, R. Sekulovich, S. F. Adair, and C. L. Dekker. 1994. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 343:1460-1463. [DOI] [PubMed] [Google Scholar]
- 44.Straus, S. E., A. Wald, R. G. Kost, R. McKenzie, A. G. M. Langenberg, P. Hohman, J. Lekstrom, E. Cox, M. Nakamura, R. Sekulovich, A. Izu, C. Dekker, and L. Corey. 1997. Immunotherapy of recurrent genital herpes with recombinant herpes simplex virua type 2 glycoproteins B and D: results of a placebo-controlled vaccine trial. J. Infect. Dis. 176:1129-1134. [DOI] [PubMed] [Google Scholar]
- 45.Tigges, M. A., D. M. Koelle, K. Hartog, R. E. Sekulovich, L. Corey, and R. L. Burke. 1992. Human CD8+ herpes simplex virus-specific cytotoxic T lymphocyte clones recognize diverse virion protein antigens. J. Virol. 66:1622-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tigges, M. A., S. Leng, D. C. Johnson, and R. L. Burke. 1996. Human herpes simplex (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J. Immunol. 156:3901-3910. [PubMed] [Google Scholar]
- 47.Trotti, A., R. Byhardt, J. Stetz, C. Gwede, B. Corn, K. Fu, L. Gunderson, B. McCormick, M. Morrisintegral, T. Rich, W. Shipley, and W. Curran. 2000. Common toxicity criteria: version 2.0, an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 47:13-47. [DOI] [PubMed] [Google Scholar]
- 48.Verjans, G. M. G. M., R. Q. Hintzen, J. M. van Dun, A. Poot, J. C. Milikan, J. D. Laman, A. W. Langerak, P. R. Kinchington, and A. D. M. E. Osterhaus. 2007. Selective retention of herpes simplex virus specific T cells in latently infected human trigeminal ganglia. Proc. Natl. Acad. Sci. USA 104:3496-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wald, A., L. Corey, R. Cone, A. Hobson, G. Davis, and J. Zeh. 1997. Frequent genital herpes simplex virus 2 shedding in immunocompetent women: effect of acyclovir treatment. J. Clin. Investig. 99:1092-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wald, A., A. G. Langenberg, E. Krantz, J. M. Douglas, Jr., H. H. Handsfield, R. P. DiCarlo, A. A. Adimora, A. E. Izu, R. A. Morrow, and L. Corey. 2005. The relationship between condom use and herpes simplex virus acquisition. Ann. Intern. Med. 143:707-713. [DOI] [PubMed] [Google Scholar]
- 51.Wald, A., J. Zeh, G. Barnum, L. G. Davis, and L. Corey. 1996. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann. Intern. Med. 124:8-15. [DOI] [PubMed] [Google Scholar]
- 52.Yang, S., K. Y. Tsang, and J. Schlom. 2005. Induction of higher-avidity human CTLs by vector-mediated enhanced costimulation of antigen-presenting cells. Clin. Cancer Res. 11:5603-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, J., D. M. Koelle, J. Cao, J. Vezquez, M. L. Huang, F. Hladik, A. Wald, and L. Corey. 2007. Peripheral virus-specific CD8+ T cells contiguous to sensory nerve endings limit HSV-2 reactivation in human genital skin. J. Exp. Med. 204:595-603. [DOI] [PMC free article] [PubMed] [Google Scholar]