Abstract
Chelonid fibropapillomatosis-associated herpesvirus (CFPHV) is an alphaherpesvirus believed to cause marine turtle fibropapillomatosis (FP). A serodiagnostic assay was developed for monitoring sea turtle populations for CFPHV exposure. CFPHV glycoprotein H (gH) expressed in recombinant baculovirus was used in an enzyme-linked immunosorbent assay (ELISA) to detect virus-specific 7S turtle antibodies. Using captive-reared green turtles (Chelonia mydas) with no history of virus exposure as “known negatives” and others with experimentally induced FP as “known positives,” the assay had 100% specificity but low sensitivity, as seroconversion was detected in only half of the turtles bearing experimentally induced tumors. Antibodies were detected only in samples collected after cutaneous fibropapillomas appeared, consistent with observations that tumors are significant sites of virion production and antigen expression and the possibility that prolonged/repeated virus shedding may be required for adequate stimulation of 7S antibody responses to gH. Natural routes of infection, however, may produce higher seroconversion rates. High gH antibody seroprevalences (∼80%) were found among wild green turtles in three Florida localities with different FP prevalences, including one site with no history of FP. In addition, all eight loggerhead turtles (Caretta caretta) tested were seropositive despite FP being uncommon in this species. The possibility that CFPHV infection may be common relative to disease suggests roles for environmental and host factors as modulators of disease expression. Alternatively, the possibility of other antigenically similar herpesviruses present in wild populations cannot be excluded, although antibody cross-reactivity with the lung/eye/trachea disease-associated herpesvirus was ruled out in this study.
Marine turtles with fibropapillomatosis (FP) are infected with a novel alphaherpesvirus known as chelonid fibropapillomatosis-associated herpesvirus (CFPHV) (17). Detection of viral genomic DNA, primarily within fibropapillomas of all turtles with spontaneous FP, virus shedding from tumor epidermal keratinocytes, and cotransmission of virus with disease in experimentally induced FP makes CFPHV infection the major risk factor and strongly implicates it as the etiologic agent of this disease (17, 20, 32, 33, 36, 37). In recent decades, the prevalence of FP has increased dramatically, and the disease has spread to new localities around the world (16, 45). A phylogenetic study of CFPHV variants found in tumors showed, however, that this group of viruses has been present in turtle populations far longer than the FP panzootic (17). Thus, the current panzootic is not due to a newly emergent pathogenic virus variant. This raises questions about the relative importance of ecological factors affecting the rate of virus transmission within and between populations compared to factors affecting disease expression (the development, progression, and regression of tumors) in influencing the prevalence and spread of epizootic FP (10, 17).
Epidemiologic studies designed to answer these questions and to evaluate populations for susceptibility to FP outbreaks require methods to detect the major risk factor, CFPHV exposure, independently of clinically apparent disease. To date, the spread of CFPHV infections in sea turtle populations has been documented through surveillance for turtles with clinically apparent tumors and detection of virus DNA within these tissues. To address the need to detect viral exposure independently of disease, a primary goal of our research program has been to produce sensitive and specific diagnostic assays to detect antibodies to this virus.
CFPHV has not been propagated successfully in culture as a source of viral antigens. An immunohistochemical assay using formalin-fixed tumor tissue sections as targets demonstrated that turtles with either experimental or spontaneous FP had antibodies to antigens present in epidermal foci of productive virus replication (18). The utility of this assay has been limited, however, by the availability of tissue containing these foci. Furthermore, the antigens in such foci are uncharacterized and likely to vary among tissue preparations. Further complicating matters, antibody cross-reactivity with lung/eye/trachea disease-associated herpesvirus (LETHV), another alphaherpesvirus that is associated with pneumonia, conjunctivitis, and tracheitis in green turtles (28), has been demonstrated with this assay (5, 7). Specifically, turtles that had been immunized with inactivated LETHV culture lysates showed antibody reactivity to CFPHV inclusions in FP tissue sections (5). Some wild green turtles with FP also had antibody reactivity to LETHV-infected cells and culture lysates (7). One cross-reacting antigen, UL26 protease, has been identified (6).
In vitro expression of recombinant viral proteins provides a source of target antigens that can be evaluated separately for their utility in diagnostic assay development. Since large portions of the unique long (UL) region of the CFPHV genome have been cloned and sequenced, open reading frames for a number of viral proteins are available for expression and evaluation (13, 17). Glycoproteins were selected for recombinant expression because their location on the surface of viral particles made them likely candidates for humoral immune responses (35, 44). In addition, glycoproteins may be relatively virus specific. For example, glycoprotein G has been found to distinguish antibody reactivity to the closely related human herpesviruses 1 and 2 (HSV-1 and HSV-2) (15, 34).
This paper reports baculovirus expression of recombinant CFPHV glycoprotein H with and without glycoprotein L coexpression and the development and application of an enzyme-linked immunosorbent assay (ELISA) to detect turtle antibodies to recombinant CFPHV glycoprotein H. This assay was used to evaluate plasma immune responses of turtles that were experimentally infected with CFPHV and to describe the seroprevalence of CFPHV glycoprotein H antibodies in free-ranging turtles from three sites with very different FP disease prevalences.
MATERIALS AND METHODS
CFPHV glycoprotein peptides.
The open reading frame sequences for CFPHV gH (UL22) and gL (UL1) were retrieved from the partially sequenced UL region of the CFPHV Florida variant A genome (GenBank accession number AY644454) (17). Candidate immunogenic peptides were designed and synthesized, and purity was confirmed by mass spectrometry (SigmaGenosys, The Woodlands, TX). Rabbits were immunized with keyhole limpet hemocyanin-conjugated peptides to produce peptide-specific antisera for detecting protein expression. Unconjugated peptides were also used as antigens to screen plasma samples from turtles with spontaneous FP for immunoreactivity to identify samples that could serve as positive controls in the recombinant glycoprotein H protein ELISA.
Expression of recombinant gH (UL22) and gL (UL1).
Full-length open reading frames for viral glycoproteins were PCR amplified from a reference DNA sample containing CFPHV Florida variant A (17) using primers in the flanking sequences upstream and downstream of each open reading frame. UL22 was amplified using primers 5′-AACCTGTTGAGCGACTTCCA-3′ and 5′-TCTAGCCCCTTTTTCCACCT-3′, and UL1 was amplified using primers 5′-TCCGACAACCGTCTTCGA-3′and 5′-CACCTCGCTGCTCTTAGTTA-3′. Each product was cloned into a plasmid vector (Invitrogen, Carlsbad, CA), and clones were fully sequenced to confirm the absence of PCR-induced mutations. Viral open reading frames were fused in frame to myc and six-histidine epitopes in the plasmid vector pcDNA4myc-His (Invitrogen), such that the epitope tags were located at the carboxyl termini of the fusion proteins. The myc-His tag was positioned at the carboxyl terminus of gL, while the tag was fused slightly upstream of the hydrophobic membrane-spanning domain near the carboxyl terminus of gH. The myc-His-tagged gL and gH were then inserted into a baculovirus transfer vector. The vector used was pCPE20. This is a derivative of the baculovirus transfer vector pUL1393 into which the amino terminus of human carboxypeptidase E (CPE) (42) has been cloned. The CPE segment provided a proven signal peptide for effective expression of type I membrane proteins in the baculovirus system followed by a furin cleavage site. Immediately downstream of the furin cleavage site was a unique XbaI cloning site and further downstream was a unique XhoI site. The myc-His-tagged gL and gH were PCR amplified from immediately downstream of the predicted N-terminal signal peptides through the stop codon immediately following the epitope tags. XbaI sites were included in the 5′ PCR primers and XhoI sites in the 3′ PCR primers. The epitope-tagged gL and gH were fused in frame downstream of the CPE signal sequence. Thus, the final plasmid contained from N to C termini, the CPE signal peptide, the CPE furin cleavage site, the viral protein, Myc epitope, six-His tag, and the stop codon. Sequences of all plasmids were confirmed again to rule out PCR-induced errors. The CPE-fusion vectors were then used to generate infectious baculoviruses, which were then cloned, and the proteins were expressed in baculovirus-infected SF9 cells (Kinnakeet Biotechnology, Richmond, VA). Pellets from approximately 1 liter of infected cells were solubilized in 20 mM HEPES pH 7.4 plus protease inhibitors (Sigma Chemical Co., St. Louis, MO), sonicated, and then centrifuged at 15,000 rpm for 30 min in a Sorvall SS-34 rotor. The pellet (membrane-enriched fraction) was resuspended in 1% Nonidet P-40 (NP-40) in phosphate-buffered saline on ice and centrifuged under the same conditions, yielding NP-40-soluble (supernatant) and insoluble (pellet) fractions. Proteins were enriched from the various fractions using the six-His tag by Ni chelate-Sepharose chromatography. Total culture lysates and the proteins from various fractions were tested by Western blotting using anti-six-His antibodies (Kinnakeet Biotechnology).
ELISA detection of antibodies to recombinant gH.
The antigen used in the ELISA was protein enriched from NP-40-soluble fractions of the initial lysate pellet of gH-expressing cells. A series of ELISAs were conducted to optimize the antigen-coating concentration and concentrations of reagents. The following ELISA conditions were used in this study. Recombinant glycoprotein H (affinity-enriched NP-40-soluble fraction) was immobilized at a concentration of 2 μg ml−1 in Tris-buffered saline (TBS), pH 7.4, in Maxisorp plates (NUNC, Kamstrup, Denmark). Half of the wells on each ELISA plate were coated with antigen, and the remaining half were coated with 1% bovine serum albumin (BSA) in TBS as controls for nonspecific binding. Following overnight incubation at 4°C, the plates were blocked with 1% BSA in TBS. After washing with TBS containing 0.1% Tween 20 (TBST), sea turtle plasma was tested in duplicate on both antigen-coated and BSA-coated wells at a dilution of 1:5 in TBST adjusted to 0.5 M NaCl containing 1% BSA (hi-salt TBST-BSA). Each assay plate included blank (no plasma) wells and wells containing negative and positive assay control plasma samples. Following incubation for 1 h and washing (three cycles) with TBST, biotinylated mouse monoclonal antibody HL858, specific for green turtle 7S immunoglobulin heavy chain (23), was added at a concentration of 1 μg ml−1 in hi-salt TBST-BSA. Detection used streptavidin-conjugated alkaline phosphatase (Sigma) diluted 1:1,000 in hi-salt TBST-BSA followed by 100 μl per well of substrate (p-nitrophenyl phosphate disodium [Zymed Laboratories, San Francisco, CA] in diethanolamine buffer, pH 9.8). The average of three optical density readings taken at 405 nm (OD405) per plate was used for subsequent calculations.
Antigen-specific optical densities were calculated as the difference between the average OD405 measured in recombinant glycoprotein H-coated wells and that measured in BSA-coated wells for each sample. The antigen-specific immunoreactivity of each sample was scored relative to negative pool and positive assay control samples as follows: ELISA score = (sample OD - negative control OD)/(positive control OD).
The negative assay control included on each plate was pooled plasma from a set of 10 “known negative” captive-reared green turtles (below). The positive assay control was plasma from a wild green turtle with FP that had strong immunoreactivity to the glycoprotein H-derived synthetic peptide (CKALKSGKIEGEDRK). This turtle was selected from a group of 10 green turtles with FP that were tested for immunoreactivity to a set of CFPHV glycoprotein-derived synthetic peptides. The peptide ELISA was run as described above, except that each unconjugated peptide at 100 ng ml−1 in TBS served as antigen.
Sample ELISA scores of >3 standard deviations above the mean antigen-specific ELISA scores of a set of 10 “known negative” samples (below) were interpreted as positive. Selected plasma samples that tested positive at a 1:5 dilution were tested at serial dilutions ranging from 1:5 to 1:500. Titers were defined as the highest dilution at which the sample OD remained greater than 10% above the negative pool OD. Western blot assays were performed on selected ELISA-positive samples to confirm antigen-specific immunoreactivity.
Plasma samples from FP transmission experiments.
A series of green turtles that had been raised from eggs in captivity for use in transmission experiments provided a source of plasma samples with well-controlled exposure to CFPHV and limited exposure to other pathogens (21, 24). Samples that were collected from these turtles prior to any experimental exposure to CFPHV (preinoculation samples and controls) were used as our population of “known negatives.” Pooled plasma from 10 of these animals was used as the negative pool sample in all ELISA plates (above). Plasma samples from 20 additional “known negative” turtles were tested independently to evaluate assay specificity.
Captive-reared green turtles that were experimentally exposed to CFPHV were used to characterize the 7S antibody response (4, 23) to recombinant CFPHV glycoprotein H. These transmission experiments are described in reference 21. Briefly, four turtles were assigned to one of four groups, and each group was inoculated by intradermal injection and skin scarification with FP tumor homogenate from a single donor, a free-ranging green turtle with spontaneous disease. An additional turtle was assigned to each group as an uninoculated contact control. Blood samples were collected from all animals prior to inoculation and at several time points (at 3- to 6-month intervals) over the following 20 months. Turtles were checked weekly for tumor development. Tumors developed at sites of inoculation in all the treated turtles from three of the groups. Uninoculated contact controls and all turtles from the fourth group, inoculated with tumor homogenate from donor 4, failed to develop FP (21). Since infection could only be verified by PCR in turtles that developed tumors, only those experimentally exposed turtles that developed FP were classified as “known positives.”
In another transmission experiment, two captive-reared turtles were immunized by repeated injections with inactivated LETHV until they had developed strong anti-LETHV antibody responses (up to 1:3,200) as detected by ELISA (5, 7). Both immunized turtles and an unimmunized control were subsequently challenged with FP tumor homogenates containing CFPHV. Plasma samples collected prior to LETHV immunization and at various time points throughout the experiment were tested by ELISA at a 1:5 dilution.
Retrospectively, all donor tumor homogenates used in these transmission studies were tested by PCR using consensus primers (17, 41), CFPHV-specific primers (10), and LETHV-specific primers to confirm the presence of CFPHV and check for the presence of herpesviruses other than CFPHV.
Plasma samples from free-ranging turtle populations.
Archived plasma samples were tested from free-ranging green turtles (Chelonia mydas) and loggerheads (Caretta caretta) that were netted at three study sites on the Atlantic coast of Florida in 1999. Turtle populations at these sites have been monitored for FP since 1982, 1988, and 1993, respectively, by one of the authors (L. M. Ehrhart). These sites represent three distinct near-shore feeding habitats for juvenile green turtles, have markedly different disease prevalences, defined as the percentage of captured animals with detectable cutaneous tumors, and are in close geographic proximity. The Indian River Lagoon site (Indian River County, FL; 27Ε49′N, 80Ε26′W) is located about 3 km south of Sebastian Inlet and consists of shallow water (1 to 3 m) and a muddy bottom with drift algae and seagrass beds. The prevalence of FP among green turtles at this site has averaged 50% (ranging from 28 to 72%) since monitoring began in 1982 (26). The Wabasso Beach Reef site is approximately 1 km due east of the Indian River Lagoon site in open water east of the barrier island and is a Sabellariid worm reef located in about 2 to 3 m of water. The prevalence of FP at this site since monitoring began in 1988 until 1997 was 0%. Since 1997, however, the FP prevalence has ranged from 8 to 21% (26). The Trident Submarine Basin is a human-made embayment located near the mouth of the ship channel of Port Canaveral, Brevard County, FL, approximately 60 km north of the other two sites. The basin is approximately 600 m wide by 1,200 m long with a water depth of 13 m and a marginal shelf up to 3 m in depth. Prevalence of FP at this locality has been 0% since monitoring began in 1993.
Blood samples (3 to 10 ml) were collected in heparinized syringes from the dorsal cervical sinus. Plasma was separated by centrifugation and frozen in aliquots at −20°C and then stored at −70°C.
RESULTS
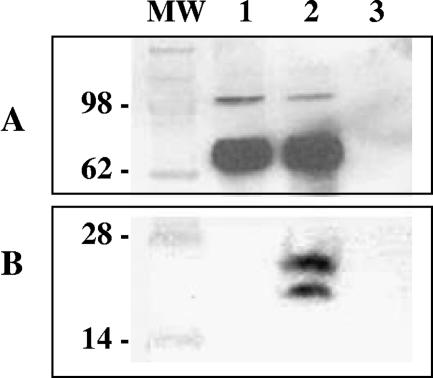
Baculovirus vectors were engineered to express full-length CFPHV gH and gL with six-His tags at their carboxyl termini. Western blot analysis of lysates from gH-encoding baculovirus-infected cells revealed expression proteins with apparent molecular masses of approximately 75 and 105 kDa that were detected with anti-six-His monoclonal antibodies (not shown). Rabbit antiserum generated against gH-specific peptide confirmed the identity of these proteins, which may represent differentially processed forms of gH (Fig. 1). Identically sized proteins were identified in cultures coexpressing gH with gL. In both cultures, gH proteins were recovered predominantly from the detergent (NP-40)-soluble and insoluble fractions, although some (105-kDa) gH protein was also found in the lysate supernatant from cells coinfected with gH and gL. Immunoreactive proteins of 20 and 24 kDa were detected in the gH-plus-gL culture lysate by Western blotting with CFPHV gL peptide-specific rabbit antiserum (Fig. 1), as well as anti six-His antibodies (not shown), confirming gL expression.
FIG. 1.
Expression of CFPHV glycoprotein H alone or in combination with glycoprotein L in recombinant baculovirus-infected insect cells. Lane 1, culture lysate expressing glycoprotein H alone; lane 2, culture lysate coexpressing glycoprotein H and glycoprotein L; lane 3, control baculovirus lysate; MW, molecular size markers. Proteins with apparent molecular masses of 75 and 105 kDa were detected with rabbit antisera to glycoprotein H peptide (CKALKSGKIEGEDRK) at a dilution of 1:50,000. Proteins with apparent molecular masses of 20 and 24 kDa were detected with rabbit antisera to glycoprotein L peptide (CGKPESILTEGLKSE) at a 1:50,000 dilution.
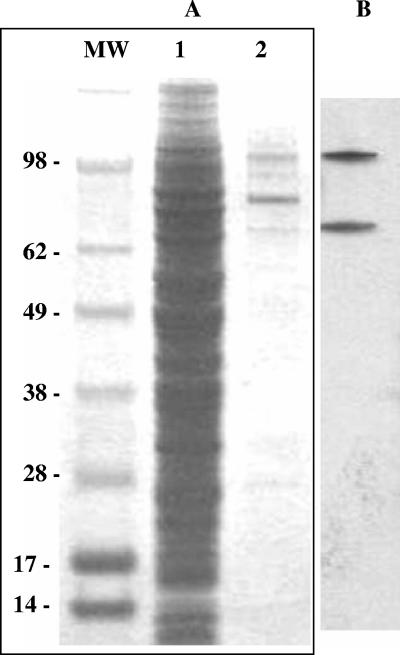
Glycoprotein H for use as the ELISA antigen was enriched from the NP-40-soluble fraction of culture lysate infected with recombinant baculovirus expressing gH alone using a nickel-Sepharose affinity chromatography column (Fig. 2A). Both 75- and 105-kDa immunoreactive proteins were recovered from this fraction (Fig. 2B).
FIG. 2.
Enrichment of recombinant glycoprotein H from NP-40 detergent-soluble culture lysate using Ni-Sepharose. (A) Coomassie blue-stained proteins from total culture lysate expressing glycoprotein H alone (lane 1) and Ni-Sepharose-bound proteins eluted with imidazole (lane 2). (B) Immunoblot of eluted Ni-Sepharose-bound proteins using rabbit antisera to glycoprotein H peptide (CKALKSGKIEGEDRK) at a dilution of 1:100,000.
ELISA development.
Preliminary ELISA screening of 10 plasma samples from wild turtles with spontaneous FP against a set of synthetic peptides derived from CFPHV glycoproteins found two samples that reacted strongly with the glycoprotein H-derived synthetic peptide (CKALKSGKIEGEDRK). The sample with the highest immunoreactivity was included as the positive assay control in every ELISA plate. The ELISA score for this sample averaged 0.90 and had a coefficient of variation of 6% in 25 independent assays. Plasma samples from 10 uninfected captive reared green turtles were used to establish expected optical density values and ELISA scores for negative samples. Pooled plasma from these 10 uninfected turtles was used as the negative assay control, with an ELISA score defined as 0. When individual samples were tested relative to this negative pool assay control, they had ELISA scores ranging from <0 to 0.06. An ELISA score of 0.12, equivalent to 3 standard deviations above 0, was established as the cutoff value for a negative score.
Serology of turtles used in FP transmission experiments.
Plasma collected from 20 additional uninfected captive-reared turtles, sampled prior to experimental inoculation with tumor homogenates containing CFPHV, served as the “known negative” population to evaluate assay specificity. The ELISA scores of these samples all fell below the cutoff value and within the same range (<0 to 0.06) as those used in the negative pool control. Consequently, this ELISA had a specificity of 100%. Furthermore, additional samples collected from uninfected contact controls at various time points throughout the course of transmission experiments also fell below an ELISA score of 0.06.
Specific antibody responses to CFPHV glycoprotein H were detected only among green turtles that developed detectable fibropapillomas after they were experimentally infected via intradermal inoculation with tumor homogenates. Four turtles that never developed FP following inoculation with tumor homogenate from a single donor were excluded from the population of “known positives” because CFPHV infection could not be confirmed. They did not have detectable 7S antibody responses to gH in any of the samples tested (ELISA scores of <0.06).
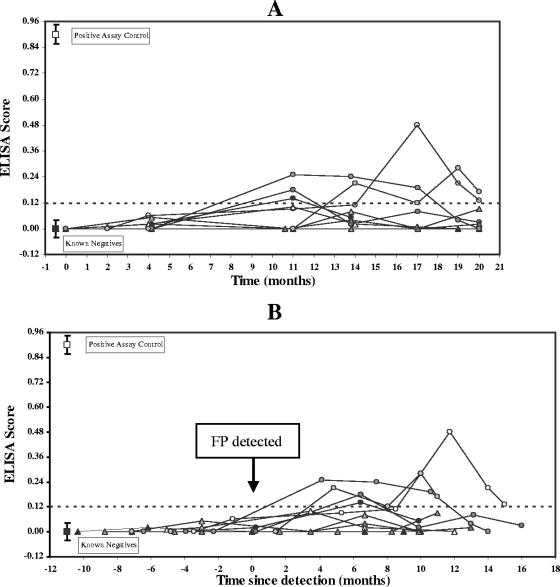
Ten turtles that did develop experimentally induced tumors were defined as the “known positive” population. Figure 3A presents the ELISA scores of samples from these turtles relative to time since the start of the transmission experiment. Figure 3B presents the same ELISA data relative to the time when tumors were first detected (time zero). Plasma samples collected 2 to 4 months after inoculation but prior to development of visible fibropapillomas had ELISA scores of <0.06 (Fig. 3A). Several of these ELISA-negative samples were collected on the days that individual cases of FP were first detected (Fig. 3B). The earliest samples that were interpreted as ELISA positive (ELISA scores of >0.12) were collected from 9 to 11 months after experimental infection (Fig. 3A). This corresponded to seroconversion being detected initially in samples collected between 4 and 11 months after tumors were first observed (Fig. 3B).
FIG. 3.
Plasma 7S antibody reactivity to Ni-Sepharose affinity-enriched recombinant CFPHV glycoprotein H among 10 “known positive” green turtles over time. “Known positives” were experimentally infected turtles that developed FP at inoculation sites. CFPHV infection was confirmed by PCR of tumor DNA. (A) ELISA scores plotted against time relative to the start of the experiment. (B) ELISA scores plotted against time relative to the first detection of FP in each animal (time zero). Time points sampled prior to time zero were from turtles that did not have tumors, while those sampled after time zero were from turtles with detectable tumors. Reference ELISA scores (means ± standard deviations) for 10 “known negative” controls (▪) and the positive assay control sample (□) are included for comparison. The cutoff value (0.12) corresponds to 3 standard deviations above the mean score of “known negative” pool sample.
Ultimately, half of the “known positive” turtles had at least one plasma sample that was interpreted as ELISA positive during the course of experiment, while the other half did not. Thus, the sensitivity of this ELISA was 50% across all time samples. The 7S antibody response appeared to be sustained for several months in three turtles, with positive scores found in two or more consecutive plasma samples over a 3- to 6-month period. In two other turtles the response was more transient, with only a single time point testing positive (Fig. 3A and B). Thus, on any particular sampling date in this experiment, no more than three turtles with tumors tested positive, yielding a sensitivity estimate of 30% for a single time point population sample. Seropositive samples had anti-gH titers ranging from 1:50 to 1:100.
Lack of cross-reactivity of plasma from LETHV-immunized turtles.
Additional experimental turtles were studied to address concerns about potential assay cross-reactivity with a distantly related turtle herpesvirus, LETHV (6, 7). Plasma samples from two turtles that had been serially immunized with inactivated LETHV and subsequently challenged via inoculation with FP tumor homogenates were tested for antibodies to CFPHV recombinant glycoprotein H. All samples collected prior to CFPHV inoculation were seronegative (titers < 1:5), including samples collected 2 and 3 months post-LETHV immunization that had robust anti-LETHV antibody titers (up to 1:3,200) detected in an ELISA using LETHV-infected culture lysate (5, 7). Thus, despite developing robust antibody responses against LETHV, in neither turtle were these antibodies cross-reactive with CFPHV glycoprotein H. A third unimmunized (control) turtle was ELISA negative to both LETHV and CFPHV glycoprotein H at these time points.
All three turtles were subsequently challenged by intradermal injection with FP homogenates containing CFPHV and within 6 months developed FP at the inoculation sites. Thus, the antibody response against inactivated LETHV that occurred in two turtles was unable to protect against FP tumorigenesis (5). Plasma samples collected approximately 17 months after tumor development (23 months after CFPHV infection) were available for testing. One previously LETHV-immunized turtle tested positive for anti-CFPHV glycoprotein H antibodies and had a titer of >1:500. This turtle eventually also developed tumors at anatomic sites other than those inoculated. The samples from the other previously LETHV-immunized and the unimmunized control turtles were seronegative for CFPHV. Thus, seroprevalence was 33% among these known CFPHV-exposed turtles in plasma sampled at approximately 2 years postinfection.
Donor FP tissues used to experimentally infect turtles and transmit FP were tested retrospectively by PCR using consensus primers and LETHV-specific primer sets and found to be LETHV negative. The only products that were amplified using consensus primers were CFPHV sequences.
Serology of wild turtles.
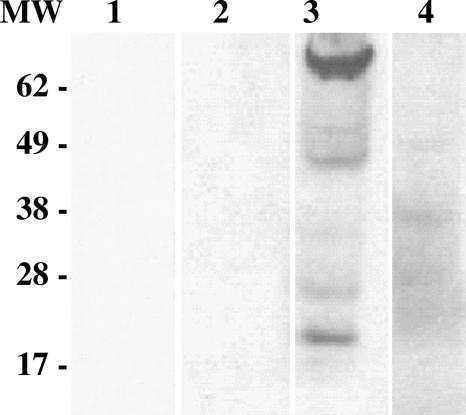
Plasma was collected from 171 free-ranging green turtles at three study sites on the east central Atlantic coast of Florida. These sites historically differed in their FP prevalences. The population samples collected for analysis had FP tumor prevalences in the ranges reported for these sites in long-term monitoring studies (26), with Indian River Lagoon having the highest FP prevalence (61.8%), followed by Wabasso Beach Reef (18.2%), and then Trident Submarine Basin (0%). Table 1 presents the FP disease prevalences and the 7S anti-glycoprotein H seroprevalences for turtles from these three localities. ELISA scores for wild green turtle samples at the three sites ranged from 0 to 2.12. There were no statistically significant differences in ELISA scores either between these three localities or between turtles with tumors and those without, based on Kruskal-Wallis tests (46). High-scoring samples from each locality had anti-glycoprotein H titers of up to 1:500. Immunoblot assays confirmed the antigen-binding specificity of selected plasma samples with positive ELISA scores (Fig. 4). Seroprevalence (percentage of turtles with ELISA scores of >0.12) ranged from 80% to 87.3% among the three localities. There was no association between seroprevalence and locality (chi-square P = 0.59) and no correlation between seroprevalence and FP prevalence across sites. Positive ELISA scores were not associated with current disease status in either of the two sites with FP (Indian River Lagoon and Wabasso Beach Reef; chi-square P = 0.79 and P = 0.08, respectively). That is, no more individuals with FP had positive serology than those without disease.
TABLE 1.
Seroprevalence versus FP tumor prevalence among free-ranging green turtles at three Florida localities
| Locality (no. of turtles) | FP tumor prevalence (%) | Anti-CFPHV gH seroprevalence (%)
|
||
|---|---|---|---|---|
| All turtles | Turtles with no apparent FP | Turtles with FP | ||
| Indian River Lagoon (55) | 61.8 | 87.3 | 85.7 | 88.2 |
| Wabasso Beach Reef (55) | 18.2 | 80 | 75.6 | 100 |
| Trident Basin (61) | 0 | 83.6 | 83.6 | NAa |
NA, not applicable (no turtles with apparent FP).
FIG. 4.
Immunoblot demonstrating the anti-gH antibody-binding specificity of ELISA-positive turtle plasma. The antigen used was recombinant baculovirus-infected insect cell culture lysate coexpressing glycoprotein H and glycoprotein L (lanes 1 to 3) and control baculovirus culture lysate (lane 4). Plasma samples were diluted 1:20 in blocking buffer. Lane 1, blocking buffer-only control; lane 2, ELISA negative control; lanes 3 and 4, ELISA-positive field sample. Turtle 7S antibodies were detected with monoclonal antibody HL858 (1 μg ml−1) followed by peroxidase-conjugated goat anti-mouse IgG (diluted 1:5,000) in blocking buffer. The ELISA-positive sample specifically reacted with proteins consistent with gH (∼75 kDa) and gL (∼20 kDa).
The sample population of green turtles from the Trident Basin had an anti-CFPHV glycoprotein H seroprevalence of 83.6% despite there being no history of FP in that population for over 15 years of monitoring (26). Changing the ELISA score cutoff value had no effect on these overall relationships.
Several of the field samples used in this study (n = 29) had been tested previously by ELISA against LETHV (8). This sample population included 4 LETHV negatives that tested CFPHV negative, 20 LETHV negatives that tested CFPHV positive, 4 that were double positive, and 1 that was LETHV positive but CFPHV negative. Thus, there was no association between 7S immunoreactivity to LETHV and CFPHV in this sample (chi-square P = 0.86).
Plasma samples from eight juvenile loggerhead turtles (Caretta caretta) from Indian River Lagoon were also tested by ELISA. ELISA scores ranged from 0.21 to 2.13 (average of 1.02 ± 0.78). Thus, all were seropositive despite having a low FP prevalence in this sample (12.5%) and in long-term monitoring of this study site (L. Ehrhart, unpublished).
DISCUSSION
Specificity of gH as an assay target.
Selection of gH was guided by concern about assay cross-reactivity between CFPHV and LETHV (6, 7). Among the glycoproteins for which published nucleotide sequences were available for comparison (UL22 and UL27), gH (UL22) was chosen for antigen development because it had lower sequence identity (30%) with its cognate sequence from LETHV (GenBank accession number EU194838). In contrast, gB (UL27) had about 40% amino acid sequence identity with the LETHV gB (GenBank accession number AY124577). Glycoprotein B is relatively conserved among herpesviruses, and cross-reactions have been reported among the gB homologues of several human herpesviruses as well as among other mammalian herpesviruses (2, 9, 30, 31).
Baculovirus-expressed glycoprotein H provided a well-characterized and readily produced antigen for assay development. Studies with other herpesviruses have shown that gL helps transport gH to the cell surface (43). While coexpression of gL with gH might facilitate gH antigen recovery, there was concern that the presence of gL in the ELISA would increase cross-reactivity. Fortunately, the overall amount of gH antigen recovered by expression of gH alone was similar to that with gL coexpression.
High specificity and limited sensitivity for 7S antibody responses of captive-reared green turtles.
Captive-reared green turtles used in FP transmission experiments provided a population with known exposure history to herpesviruses (known negatives and known positives) essential for ELISA development. They also provided initial insights on the development of virus-specific antibody responses under relatively controlled conditions that are useful in interpreting serologic results from wild turtle populations (unknowns). The ELISA developed in this study was highly specific (100%), as only infected turtles developed seroreactivity. Consequently, the predictive value of a positive test result is high, and positive results should reliably indicate exposure to CFPHV. On the other hand, assay sensitivity was low, as seroconversion was detected only in some turtles that developed FP, even though PCR confirmed that all were infected. As a consequence of low sensitivity, the predictive value of negative assay results is low (19), and negative serology cannot rule out infection.
A number of factors may explain why some turtles with FP did not seroconvert while others evidenced only transient seroconversion in this experiment. Glycoprotein H is expressed late (gamma phase) during productive virus infection, which to date has been observed sporadically and focally only in the epidermis of cutaneous tumors (20, 25). During early stages of tumor development virus may be in an eclipse phase with limited exposure of structural antigens such as gH to the host immune system. Also, it is well known in some herpesvirus-induced neoplasias for infection to remain latent with only a fraction of viral genes being expressed in proliferating transformed cells while virion production involving expression of the full set of viral structural proteins occurs in other tissues (1, 29). FP appears to be a proliferative disorder of dermal fibroblasts with variable involvement of tumor epidermis, and there is no evidence that these tumor fibroblasts themselves produce viral particles (20, 25). Thus, even in clinically apparent FP, some tumors may not produce viral particles, or express enough gH to stimulate seroconversion. Furthermore, plasma 7S immunoglobulin Y (IgY) immune responses, like those of mammalian IgG, result from heavy chain class switching following repeated antigenic stimulation (4, 23). Thus, the probability of eliciting detectable 7S IgY immunoreactivity to gH may vary as a function of how much virus is produced and shed over time and possibly whether there is subcutaneous exposure and/or systemic spread (viremia). All tumors that occurred in this study were surgically resected approximately 11 months after the experiment was begun, and recurrent tumors were periodically resected thereafter, thus reducing antigen exposure from primary skin tumors. Nevertheless, plasma samples collected from these turtles when tumors were first removed all (100%) showed immunoreactivity to viral antigens present in epidermal foci of virus production in tumor sections by an immunohistochemical assay (18). Thus, experimentally infected turtles produce antibodies targeted to additional viral antigens than just gH. These other viral antigens could lead to more sensitive assays; however, they must be evaluated to understand their effects on specificity.
High prevalences of anti-CFPHV gH antibodies among wild turtles.
Wild green turtle populations at three localities in Florida all had high seroprevalences of antibody reactivity to CFPHV gH despite large differences in FP prevalences. The lack of association between antibody immunoreactivity to gH and current clinical disease in the two localities with ongoing FP outbreaks, Indian River Lagoon and Wabasso Beach Reef, can be explained as follows. Based on the experimental findings that seroconversion occurred late relative to disease onset, if at all, some free-ranging individuals with FP were expected to test negative. Furthermore, the apparently unaffected (tumor-free) portion of the population may include turtles that had FP but have fully recovered. Hirama (26) documented that in the Indian River Lagoon population approximately 87% of turtles with FP that were recaptured at intervals greater than 10 months showed evidence of tumor regression. Assuming that turtles that are recovering from FP seroconvert, it is possible that a high percentage of apparently unaffected turtles in the sites with ongoing FP outbreaks may have anti-CFPHV antibodies. It was surprising, however, that seroprevalences were similar despite a nearly threefold difference in FP prevalence between these two sites, especially since the outbreak at the Wabasso Beach Reef site was more recent and had been going on for only about 2 years at the time samples were collected.
The fact that turtles from the Trident Basin also had a high seroprevalence (80%) despite no history of FP in the population (0% prevalence) since monitoring began in 1993 (26) cannot be readily explained by a model that requires tumor development for seroconversion. Given the long monitoring history at this site, it is highly improbable that this is a population that has fully recovered from a past FP outbreak. Extrapolating from transmission studies, the ELISA was not expected to detect antibodies to gH in individuals that had not developed FP (subclinical infection). It is conceivable, however, that the intradermal inoculations used in experimental infections may not fully reflect the routes of transmission in nature and that robust antibody responses to natural infection might develop independently of the appearance of cutaneous tumors. This hypothesis postulates that nontumor tissues, which have yet to be identified, support CFPHV replication and gH expression in cryptically infected turtles. To date, however, CFPHV has only reliably been detected in turtles with FP (20, 27).
The possibility that virus infection and seroconversion may occur in the absence of clinically apparent FP suggests that CFPHV infection alone is not sufficient for disease expression. Although studies showing a tight association of CFPHV with tumors are consistent with the hypothesis that this virus is involved in FP tumorigenesis (10, 13, 17, 32, 36), a variety of biological and physical factors may modulate whether or not CFPHV infection leads to FP disease (22). In this regard, CFPHV infection may be understood in light of other proliferative skin diseases caused by alphaherpesviruses and may even serve as a model for these diseases. In humans, for example, HSV, which typically causes focal vesicular and ulcerative skin lesions, may cause hyperplastic lesions in the context of chronic host immunodeficiency (3). Many factors may modulate host immune function. One possibility is that another virus may interact with CFPHV in FP pathogenesis, perhaps by causing host immunodeficiency (20). Such interactions are recognized in human herpesvirus-associated proliferative diseases such as Kaposi's sarcoma, involving human immunodeficiency virus and human herpesvirus-8, and cutaneous verrucaeform lesions, involving human immunodeficiency virus and human herpesvirus-3 (11, 12, 14, 39).
Cross-reactivity with related chelonid herpesviruses.
Another possible explanation for the high prevalence of CFPHV gH-seropositive turtles in the wild Florida populations is that the ELISA also detected antibodies produced in response to infection with antigenically similar nontumorigenic herpesviruses. Cross-reactivity with LETHV was a particular concern in this study because this had been demonstrated in other assays and because there was serologic evidence of exposure to LETHV in the Florida population (7, 8). However, there was no association between immunoreactivity to recombinant CFPHV glycoprotein H and immunoreactivity to LETHV culture lysate among wild turtles. Furthermore, the reported seroprevalence of LETHV antibodies in the Florida population of approximately 21.6% across the same three sites during the same time period (8) is too low to account for the ∼80% seroprevalence to CFPHV in this study. In addition, both captive-reared turtles that were immunized with inactivated LETHV developed high anti-LETHV titers but remained seronegative to CFPHV glycoprotein H.
The presence of cross-reacting antibodies to other chelonid herpesviruses that may infect the wild population cannot be ruled out until antigens from these viruses become available for study. Possible candidates include two herpesviruses recently reported in Florida loggerheads (40). Although one of these appears to be highly similar to LETHV, the other is novel. Another candidate is chelonid herpesvirus-1, Greypatch disease herpesvirus, which has been observed in posthatchling green turtles raised in mariculture in the Caribbean (38). Nevertheless, serological assays that use well-characterized recombinant antigens, such as those described here, will be the best way to differentiate among antibody responses to antigenically similar viruses.
Implications for FP epizootiology.
The ELISA to detect antibody exposure to CFPHV glycoprotein H described in this report allowed an important hypothesis about the relationship between viral infection and FP disease in free-ranging sea turtle populations to be tested for the first time (10, 17). The finding that CFPHV infection (as measured by antibody exposure to gH) is highly prevalent along the east coast of Florida, even in a population historically free of FP, suggests that FP outbreaks in this region are less limited by environmental factors affecting virus transmission than they are by environmental or host factors affecting disease expression and whether or not turtles develop FP. It will be important to survey turtle populations in other regions to determine if similar seroprevalences exist elsewhere and to begin to correlate CFPHV infection with other environmental and host cofactors that may play a role in FP outbreaks around the world. In addition, the spatial distribution and association of specific closely related CFPHV variants found in turtles with FP with particular nearshore localities (such as the Indian River Lagoon) suggests that turtles become infected with CFPHV after they migrate as juveniles to these localities (10). The epizootic transmission cycle is perpetuated in areas with high FP prevalences, as tumors are a known source of transmissible virus (21, 24). The high prevalence of CFPHV gH antibodies in the Trident Basin population suggests, however, that there may be alternate virus transmission cycles involving virus replication and shedding from tissues other than cutaneous tumors. How CFPHV or antigenically similar chelonid herpesviruses are transmitted in apparently healthy FP-free populations is a major question.
Acknowledgments
This work was supported by grant D05ZO-049 from the Morris Animal Foundation.
We thank Susan Buhl from the AECOM Hybridoma Core Laboratory, Ronald Magnusson from Kinnakeet Biotechnology, and Richie Moretti, Susan Schaf, Corinne Rose, and Douglas Mader from the Turtle Hospital, Marathon, FL, for their help in this study.
Footnotes
Published ahead of print on 26 March 2008.
REFERENCES
- 1.Baigent, S. J., and F. Davidson. 2004. Marek's disease virus: biology and life cycle, p. 62-77. In F. Davidson and V. Nair (ed.), Marek's disease: an evolving problem. Elsevier Academic Press, Amsterdam, The Netherlands.
- 2.Balachandran, N., D. E. Oba, and L. M. Hutt-Fletcher. 1987. Antigenic cross-reactions among herpes simplex virus types 1 and 2, Epstein-Barr virus, and cytomegalovirus. J. Virol. 61:1125-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley, K. L., G. E. Cooley, G. F. Kao, M. H. Lowitt, J. W. Burnett, and L. Aurelian. 1997. Herpes simplex vegetans: atypical genital herpes infection in a patient with common variable immunodeficiency. J. Am. Acad. Dermatol. 37:860-863. [DOI] [PubMed] [Google Scholar]
- 4.Benedict, A. A., and L. W. Pollard. 1972. Three classes of immunoglobulins found in the sea turtle, Chelonia mydas. Folia Microbiol. (Prague) 17:75-78. [DOI] [PubMed] [Google Scholar]
- 5.Coberley, S. S. 2002. The role of herpesviruses in diseases of marine turtles. Ph.D. dissertation. University of Florida, Gainesville.
- 6.Coberley, S. S., R. C. Condit, L. H. Herbst, and P. A. Klein. 2002. Identification and expression of immunogenic proteins of a disease-associated marine turtle herpesvirus. J. Virol. 76:10553-10558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coberley, S. S., L. H. Herbst, D. R. Brown, L. M. Ehrhart, D. A. Bagley, S. A. Schaf, R. H. Moretti, E. R. Jacobson, and P. A. Klein. 2001. Detection of antibodies to a disease-associated herpesvirus of the green turtle, Chelonia mydas. J. Clin. Microbiol. 39:3572-3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coberley, S. S., L. H. Herbst, L. M. Ehrhart, D. A. Bagley, S. Hirama, E. R. Jacobson, and P. A. Klein. 2001. Survey of Florida green turtles for exposure to a disease-associated herpesvirus. Dis. Aquat. Organ. 47:159-167. [DOI] [PubMed] [Google Scholar]
- 9.Emini, E. A., J. Luka, M. E. Armstrong, P. M. Keller, R. W. Ellis, and G. R. Pearson. 1987. Identification of an Epstein-Barr virus glycoprotein which is antigenically homologous to the varicella-zoster virus glycoprotein II and the herpes simplex virus glycoprotein B. Virology 157:552-555. [DOI] [PubMed] [Google Scholar]
- 10.Ene, A., M. Su, S. Lemaire, C. Rose, S. Schaff, R. Moretti, J. Lenz, and L. H. Herbst. 2005. Distribution of chelonid fibropapillomatosis-associated herpesvirus variants in Florida: molecular genetic evidence for infection of turtles following recruitment to neritic developmental habitats. J. Wildl. Dis. 41:489-497. [DOI] [PubMed] [Google Scholar]
- 11.Fagan, W. A., P. C. Collins, and D. R. Pulitzer. 1996. Verrucous herpes virus infection in human immunodeficiency virus patients. Arch. Pathol. Lab. Med. 120:956-958. [PubMed] [Google Scholar]
- 12.Feller, L., N. H. Wood, and J. Lemmer. 2007. HIV-associated Kaposi sarcoma: pathogenic mechanisms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 104:521-529. [DOI] [PubMed] [Google Scholar]
- 13.Greenblatt, R. J., S. L. Quackenbush, R. N. Casey, J. Rovnak, G. H. Balazs, T. M. Work, J. W. Casey, and C. A. Sutton. 2005. Genomic variation of the fibropapilloma-associated marine turtle herpesvirus across seven geographic areas and three host species. J. Virol. 79:1125-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greene, W., K. Kuhne, F. Ye, J. Chen, F. Zhou, X. Lei, and S. J. Gao. 2007. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat. Res. 133:69-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashido, M., F. K. Lee, S. Inouye, and T. Kawana. 1997. Detection of herpes simplex virus type-specific antibodies by an enzyme-linked immunosorbent assay based on glycoprotein G. J. Med. Virol. 53:319-323. [DOI] [PubMed] [Google Scholar]
- 16.Herbst, L. H. 1994. Fibropapillomatosis of marine turtles. Annu. Rev. Fish Dis. 4:389-425. [Google Scholar]
- 17.Herbst, L., A. Ene, M. Su, R. Desalle, and J. Lenz. 2004. Tumor outbreaks in marine turtles are not due to recent herpesvirus mutations. Curr. Biol. 14:R697-R699. [DOI] [PubMed] [Google Scholar]
- 18.Herbst, L. H., E. C. Greiner, L. M. Ehrhart, D. A. Bagley, and P. A. Klein. 1998. Serological association between spirorchidiasis, herpesvirus infection, and fibropapillomatosis in green turtles from Florida. J. Wildl. Dis. 34:496-507. [DOI] [PubMed] [Google Scholar]
- 19.Herbst, L. H., and E. R. Jacobson. 2003. Practical approaches for studying sea turtle health and disease, p. 385-410. In P. L. Lutz, J. A. Musick, and J. Wyneken (ed.), The biology of sea turtles, vol. 2. CRC Press, Boca Raton, FL. [Google Scholar]
- 20.Herbst, L. H., E. R. Jacobson, P. A. Klein, G. H. Balazs, R. Moretti, T. Brown, and J. P. Sundberg. 1999. Comparative pathology and pathogenesis of spontaneous and experimentally induced fibropapillomas of green turtles (Chelonia mydas). Vet. Pathol. 36:551-564. [DOI] [PubMed] [Google Scholar]
- 21.Herbst, L. H., E. R. Jacobson, R. Moretti, T. Brown, J. P. Sundberg, and P. A. Klein. 1995. Experimental transmission of green turtle fibropapillomatosis using cell-free tumor extracts. Dis. Aquat. Organ. 22:1-12. [Google Scholar]
- 22.Herbst, L. H., and P. A. Klein. 1995. Green turtle fibropapillomatosis: challenges to assessing the role of environmental cofactors. Environ. Health Perspect. 103(Suppl. 4):27-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst, L. H., and P. A. Klein. 1995. Monoclonal antibodies for the measurement of class-specific antibody responses in the green turtle, Chelonia mydas. Vet. Immunol. Immunopathol. 46:317-335. [DOI] [PubMed] [Google Scholar]
- 24.Herbst, L. H., R. Moretti, T. Brown, and P. A. Klein. 1996. Sensitivity of the transmissible green turtle fibropapillomatosis agent to chloroform and ultracentrifugation conditions. Dis. Aquat. Organ. 25:225-228. [Google Scholar]
- 25.Herbst, L. H., J. P. Sundberg, L. D. Shultz, B. A. Gray, and P. A. Klein. 1998. Tumorigenicity of green turtle fibropapilloma-derived fibroblast lines in immunodeficient mice. Lab. Anim. Sci. 48:162-167. [PubMed] [Google Scholar]
- 26.Hirama, S., and L. M. Ehrhart. 2007. Description, prevalence and severity of green turtle fibropapillomatosis in three developmental habitats on the east coast of Florida. Fla. Sci. 70:435-448. [Google Scholar]
- 27.Jacobson, E. R., C. Buergelt, B. Williams, and R. K. Harris. 1991. Herpesvirus in cutaneous fibropapillomas of the green turtle Chelonia mydas. Dis. Aquat. Organ. 12:1-6. [Google Scholar]
- 28.Jacobson, E. R., J. M. Gaskin, M. Roelke, E. C. Greiner, and J. Allen. 1986. Conjunctivitis, tracheitis, and pneumonia associated with herpesvirus infection in green sea turtles. J. Am. Vet. Med. Assoc. 189:1020-1023. [PubMed] [Google Scholar]
- 29.Kieff, E., and D. Liebowitz. 1990. Epstein-Barr virus and its replication, p. 1889-1920. In B. N. Fields, D. M. Knipe, R. M. Chanock, M. S. Hirsch, J. L. Melnick, T. P. Monath, and B. Roizman (ed.), Virology, 2nd ed., vol. 2. Raven Press, New York, NY. [Google Scholar]
- 30.Kitamura, K., J. Namazue, H. Campo-Vera, T. Ogino, and K. Yamanishi. 1986. Induction of neutralizing antibody against varicella-zoster virus (VZV) by VZV gp3 and cross-reactivity between VZV gp3 and herpes simplex viruses gB. Virology 149:74-82. [DOI] [PubMed] [Google Scholar]
- 31.Kuhn, J. E., K. Klaffke, K. Munk, and R. W. Braun. 1990. HSV-1 gB and VZV gp-II crossreactive antibodies in human sera. Arch. Virol. 112:203-213. [DOI] [PubMed] [Google Scholar]
- 32.Lackovich, J. K., D. R. Brown, B. L. Homer, R. L. Garber, D. R. Mader, R. H. Moretti, A. D. Patterson, L. H. Herbst, J. Oros, E. R. Jacobson, S. S. Curry, and P. A. Klein. 1999. Association of herpesvirus with fibropapillomatosis of the green turtle Chelonia mydas and the loggerhead turtle Caretta caretta in Florida. Dis. Aquat. Organ. 37:89-97. [DOI] [PubMed] [Google Scholar]
- 33.Lu, Y., Y. Wang, Q. Yu, A. A. Aguirre, G. H. Balazs, V. R. Nerurkar, and R. Yanagihara. 2000. Detection of herpesviral sequences in tissues of green turtles with fibropapilloma by polymerase chain reaction. Arch. Virol. 145:1885-1893. [DOI] [PubMed] [Google Scholar]
- 34.Marsden, H. S., K. MacAulay, J. Murray, and I. W. Smith. 1998. Identification of an immunodominant sequential epitope in glycoprotein G of herpes simplex virus type 2 that is useful for serotype-specific diagnosis. J. Med. Virol. 56:79-84. [DOI] [PubMed] [Google Scholar]
- 35.Peng, T., M. Ponce-de-Leon, H. Jiang, G. Dubin, J. M. Lubinski, R. J. Eisenberg, and G. H. Cohen. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 72:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quackenbush, S. L., R. N. Casey, R. J. Murcek, T. A. Paul, T. M. Work, C. J. Limpus, A. Chaves, L. duToit, J. V. Perez, A. A. Aguirre, T. R. Spraker, J. A. Horrocks, L. A. Vermeer, G. H. Balazs, and J. W. Casey. 2001. Quantitative analysis of herpesvirus sequences from normal tissue and fibropapillomas of marine turtles with real-time PCR. Virology 287:105-111. [DOI] [PubMed] [Google Scholar]
- 37.Quackenbush, S. L., T. M. Work, G. H. Balazs, R. N. Casey, J. Rovnak, A. Chaves, L. duToit, J. D. Baines, C. R. Parrish, P. R. Bowser, and J. W. Casey. 1998. Three closely related herpesviruses are associated with fibropapillomatosis in marine turtles. Virology 246:392-399. [DOI] [PubMed] [Google Scholar]
- 38.Rebell, G., A. Rywlin, and H. Haines. 1975. A herpesvirus-type agent associated with skin lesions of green turtles in aquaculture. Am. J. Vet. Res. 36:1221-1224. [PubMed] [Google Scholar]
- 39.Smith, K. J., H. G. Skelton III, D. M. Frissman, P. Angritt, et al. 1992. Verrucous lesions secondary to DNA viruses in patients infected with the human immunodeficiency virus in association with increased factor XIIIa-positive dermal dendritic cells. J. Am. Acad. Dermatol. 27:943-950. [DOI] [PubMed] [Google Scholar]
- 40.Stacy, B. A., J. F. Wellehan, A. M. Foley, S. S. Coberley, L. H. Herbst, C. A. Manire, M. M. Garner, M. D. Brookins, A. L. Childress, and E. R. Jacobson. 2008. Two herpesviruses associated with disease in wild Atlantic loggerhead sea turtles (Caretta caretta). Vet. Microbiol. 126:63-73. [DOI] [PubMed] [Google Scholar]
- 41.VanDevanter, D. R., P. Warrener, L. Bennett, E. R. Schultz, S. Coulter, R. L. Garber, and T. M. Rose. 1996. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 34:1666-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varlamov, O., E. H. Leiter, and L. Fricker. 1996. Induced and spontaneous mutations at Ser202 of carboxypeptidase E. Effect on enzyme expression, activity, and intracellular routing. J. Biol. Chem. 271:13981-13986. [DOI] [PubMed] [Google Scholar]
- 43.Westra, D. F., K. L. Glazenburg, M. C. Harmsen, A. Tiran, A. Jan Scheffer, G. W. Welling, T. H. The, and S. Welling-Wester. 1997. Glycoprotein H of herpes simplex virus type 1 requires glycoprotein L for transport to the surfaces of insect cells. J. Virol. 71:2285-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westra, D. F., G. M. Verjans, A. D. Osterhaus, A. van Kooij, G. W. Welling, A. J. Scheffer, T. H. The, and S. Welling-Wester. 2000. Natural infection with herpes simplex virus type 1 (HSV-1) induces humoral and T cell responses to the HSV-1 glycoprotein H:L complex. J. Gen. Virol. 81:2011-2015. [DOI] [PubMed] [Google Scholar]
- 45.Williams, E. H. J., L. Bunkley-Williams, E. C. Peters, B. Pinto-Rodriguez, R. Matos-Morales, A. A. Mignucci-Giannoni, K. V. Hall, J. V. Rueda-Almonacid, J. Sybesma, I. Bonnelly de Calventi, and R. Boulon. 1994. An epizootic of cutaneous fibropapillomas in green turtles Chelonia mydas of the Caribbean: part of a panzootic? J. Aquat. Animal Health 6:70-78. [Google Scholar]
- 46.Zar, J. H. 1974. Biostatistical analysis. Prentice-Hall, Englewood Cliffs, NJ.