Abstract
GABA receptor systems have long been implicated in alcoholism, and GABAergic drugs have demonstrated efficacy in altering alcohol intake in some rodent models. The present study was designed to assess the effects of baclofen, muscimol, and gaboxadol (THIP) in a variation on a new mouse model of binge-like ethanol intake. Three hours into their dark cycle, male and female C57BL/6J mice were given access to a 20% unsweetened ethanol solution for 2 h each day, for four days. On day five, mice received varying doses of baclofen, muscimol or THIP and were allowed access to 20% ethanol for 60 min. Baclofen dose-dependently increased binge-like ethanol intake, while both muscimol and THIP reduced ethanol intake. Subsequent studies testing the effect of baclofen, muscimol and THIP on water intake using the same procedure revealed that whereas baclofen had no significant effect, muscimol and THIP both reduced the measure. These results add to the growing literature suggesting a role for GABA receptor systems in the modulation of ethanol intake. However, whereas the role of GABAB receptor systems seems selective in the modulation of binge-like ethanol intake, the role for GABAA receptor systems appears to also extend to general fluid intake.
Keywords: Ethanol, Mouse, GABA, Baclofen, Muscimol, THIP
1. Introduction
Alcoholism is a chronic disease that affects many individuals and their families. One potential treatment for patients with alcoholism is pharmacological intervention, and the gamma-aminobutryic acid (GABA) receptor system is a promising target for the treatment of the disease. However, a good animal model is necessary to identify potential drugs for this purpose. The gold standard for assessment of alcohol (ethanol) intake has been the two-bottle choice model in which animals are given a choice between an ethanol solution (generally in concentrations between 3 and 15%) or water over a twenty-four hour period. In this model, C57B/6J inbred mice typically exhibit a preference for ethanol, consuming most of their daily fluids from the ethanol solution. However, there is wide variability in the blood ethanol concentrations (BECs) achieved over the course of the 24 hour period, and mice rarely consume enough ethanol within a specified time period to produce pharmacologically relevant BECs (Dole and Gentry, 1984). It is difficult to determine whether the mice ever drink intoxicating amounts of ethanol (Dole and Gentry, 1984).
Recently, a new mouse model was developed by Rhodes et al. (2005, 2007) called drinking in the dark (DID). This model uses the C57BL/6J mouse, and takes advantage of the nocturnal nature of this species by allowing the mice a limited access (2 or 4 h) to an unsweetened 20% ethanol solution at their peak period of arousal, 3 h into the dark cycle. Using the DID model, mice consistently drink to pharmacologically relevant BECs that produce behavioral impairment (Rhodes et al., 2005, 2007). DID may provide a useful means by which to screen potential pharmaceuticals in the treatment of alcoholism (Kamdar et al., 2007). Naltrexone, an opioid antagonist, has been demonstrated to reduce relapse in human alcoholics (Volpicelli et al., 1992). When tested for its effectiveness at modulating DID, the drug reduced ethanol intake (Kamdar et al., 2007), an effect that appeared to be specific to ethanol because it did not reduce intake of sugar water or plain tap water.
The GABA receptor system, the primary mediator of inhibitory neurotransmission in the mammalian central nervous system, has emerged as a potential target for pharmacological intervention in drug abuse and dependence, and is clearly an important receptor system underlying the effects of alcohol. The role of GABA systems in the behavioral and pharmacological effects of ethanol has been of particular interest. Genetic linkage studies have consistently associated GABA systems with risk for alcoholism in human populations (Korpi and Sinkkonen, 2006), and clinical studies have demonstrated that GABAergic compounds can alter ethanol intake in human alcoholics (Heilig and Egli, 2006).
Two unique GABA receptor subsystems have been identified (Hill and Bowery, 1981). GABAA receptors are ionotropic receptors that regulate chloride channels. In contrast, GABAB receptors are metabotropic receptors that regulate potassium or calcium conductance. Ethanol facilitates GABAA receptor function by enhancing chloride conductance through the channel. Both GABAA and GABAB receptor systems have become targets for the treatment of alcoholism. However, pharmacological studies examining the actions of both GABAA and GABAB drugs on rodent ethanol self-administration or intake have produced mixed results.
GABAA agonists have been demonstrated to both increase and decrease ethanol intake. For example, the GABAA agonist calcium acetylhomotaurine was shown to significantly reduce voluntary ethanol intake (Boismare et al., 1984), and the GABAA agonist muscimol was shown to decrease responding for ethanol in an operant paradigm with 10% ethanol as the reinforcer (Hodge et al., 1995). However, the agonist THIP was instead shown to increase voluntary ethanol intake relative to controls in a two bottle choice paradigm (Boyle et al., 1992, 1993; Smith et al., 1992). GABAA antagonists have been shown to reverse the attenuating effects of calcium acetylhomotaurine and muscimol when administered concurrently (Boismare et al., 1984; Hodge et al., 1995). However, the opposite effect on intake is observed when the GABAA antagonist bicuculine is administered alone; it reduces responding for ethanol at high doses (Boismare et al., 1984).
The picture for GABAB receptor modulation of ethanol self-administration and intake has also been complicated. The GABAB agonist baclofen has been reported to reduce ethanol self-administration in rodents (Besheer et al., 2004; Anstrom et al., 2003; Colombo et al., 2003; Janak and Gill, 2003; Liang et al., 2006; Walker and Koob, 2007), and oral ethanol intake (Stromberg, 2004; Colombo et al., 2000) as well as withdrawal severity in alcohol preferring P rats (Colombo et al., 2000). However, others have reported that systemic administration of baclofen instead increases ethanol administration and intake (Smith et al., 1999, 1992; Petry, 1997; Czachowski et al., 2006).
The goal of the present study was to investigate the effects of several GABAergic compounds on ethanol intake in C57BL/6J mice using the DID model. Our first goal was to demonstrate that C57BL/6J mice would indeed drink sufficient amounts of ethanol to achieve pharmacologically relevant blood ethanol concentrations that produce behavioral impairment. We then used the model to assess the actions of muscimol, THIP and baclofen on binge-like ethanol intake. Based on the larger body of literature, we predicted that baclofen and muscimol would dose-dependently reduce ethanol intake, while THIP would increase the behavior.
2. Materials and methods
2.1. Animals
Seven-week old male and female C57BL/6J inbred mice were purchased from the Jackson Laboratory and shipped to the animal facility at Binghamton University. Mice were individually housed in standard shoebox mouse cages and were approximately 9 weeks old at the time of testing. Lighting was maintained on a 12 h reverse light–dark cycle with lights out at 11 AM, and the temperature of the colony room was maintained at 21±1 °C. Animals had free access to food and water at all times except during ethanol presentation when only food was freely available. All procedures were approved by the Binghamton University Institutional Animal Care and Use Committee and conformed to the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (The National Academies Press, 2003).
2.2. Drugs
Ethanol (200 proof) was obtained from Pharmco, Inc (Brookfield, CT). Ethanol solutions (20% v/v) were made with tap water. Baclofen, muscimol and THIP were obtained from Sigma Aldrich (St. Louis, MO) and were dissolved in 0.9% saline. Solutions were delivered by intraperitoneal injection in a volume of 0.1 ml per 10 g body weight.
2.3. Procedure
2.3.1. Experiment 1: Drinking in the dark
Our drinking in the dark procedure was similar to that of Rhodes et al. (2005). During the dark phase of the light–dark cycle, male C57BL/6J mice (n=23–25) were given a 2 h access to a 20% ethanol solution 3 h after lights out (2 PM). A 10 ml plastic tube affixed to a ball-bearing sipper filled with the ethanol solution was placed in the cage where the water bottle was normally located. To prevent the mice from moving the sipper tube and producing potential leakage, the animals’ water bottle was placed on top of the sipper tube to hold it steady. Sipper tube volumes were recorded both before and after the 2 h access period for five consecutive days. On the final day, retro-orbital blood samples were taken from the animals at the end of the 2 h access period to assess BECs.
A three day DID procedure was utilized to determine whether naïve male C57B/6J mice (n=25 per group) would consume enough ethanol to show motor impairment on a balance beam apparatus. On the first two days of the procedure mice were allowed access to the 20% ethanol solution for 2 h, 3 h into the dark cycle. These first two days allowed the mice to achieve stable ethanol drinking patterns. On the third day mice were trained to walk on the balance beam apparatus and were subsequently given access to either ethanol or water and assessed for motor impairment.
The balance beam apparatus consisted of a 122 cm long by 2 cm wide by 4 cm tall wood block placed on top of two ring stands measuring 48 cm high. The balance beam apparatus was situated on top of a table so that the wood block was a total of 130 cm off the floor. On the third day of the procedure mice were trained to walk across the balance beam apparatus. Training commenced approximately 2 h before lights out (9 AM) and involved encouraging the mouse to traverse the balance beam, down one way and then back again. In cases where a mouse became distracted and stopped moving it was encouraged to continue by gently nudging its hind quarters with the eraser end of a pencil. Immediately after training, mice were returned to their home cages (and the colony) until the time of fluid presentation (2 PM). Preliminary data indicated that such training was sufficient to ensure that mice would rapidly traverse the beam without the aid of the eraser in the subsequent impairment test (data not shown).
Prior to fluid presentation (and after training) on day 3 mice were randomly assigned to one of two groups: one group would be allowed access to sipper tubes containing the 20% ethanol solution, and the other would be allowed access to sipper tubes containing tap water. Starting 3 h after lights out, each group received 2 h access to its respective fluid, and was immediately tested on the balance beam. A researcher blind to the treatment groups stood behind the balance beam so that both of the mouse’s hind paws were in full view, and counted hind foot slips as the animal traversed the beam under red light. For the experiment, the mouse needed only to walk from one end of the balance beam to the other. Immediately after the animal completed the balance beam task, a retro-orbital sinus blood sample was taken for determination of BECs.
2.3.2. Experiment 2: Baclofen
A five day DID procedure was used to assess the effect of baclofen on ethanol intake. The first three days served to establish stable drinking in the procedure. On the fourth day of the ethanol access procedure, mice were injected intraperitoneally (ip) with saline vehicle prior to their ethanol access period. This served to habituate them to the baclofen injection procedure. On the fifth day, mice matched for ethanol intake on day 4 received an ip injection of baclofen (0, 5, 10, 15, and 20 mg/kg) and were immediately allowed access to the 20% ethanol solution for 1 h. Sipper tube volumes were recorded before and again at the end of the 1 h access period on day 5. Immediately upon removal of the sipper tubes retro-orbital sinus blood samples were taken for analysis of BEC. The shorter 1 h access period was chosen based on literature suggesting that baclofen’s behavioral actions wane within 30 min of systemic administration (Boehm II et al., 2002). Moreover, data from Rhodes et al. (2007) suggest that ethanol intake in the DID procedure is greatest during the first 30 min of ethanol presentation; a shorter ethanol access period is ideal to prevent significant deterioration of BECs by the time blood ethanol concentrations are determined (Middaugh et al., 2003). Finally, two passes of this experiment were conducted in order to reduce the number of animals needed to achieve sufficient statistical power; 5 animals per sex and dose were used in the first pass and then the experiment was repeated using the same animals. However, no animal received the same dose twice.
Following one week off, the same cohort of animals was used to determine whether baclofen’s actions were specific for ethanol, or also generalized to plain tap water. The effect of baclofen on water intake was measured using the same 5-day procedure described above for ethanol, with tap water replacing the ethanol in the drinking tubes. As was the case for ethanol, two separate 5-day passes of the experiment were conducted, but no animal received the same baclofen dose twice.
2.3.3. Experiment 3: Muscimol
The same 5-day procedures used to assess the effect of baclofen on ethanol and water intake were also used to determine the effect of muscimol (0, 0.5, 1.0, 1.5, and 2.0 mg/kg) on these measures. A separate group of naïve male and female mice were used. Ethanol or water solutions were again presented for just 1 h on day 5. This was done to again take advantage of the greater drinking during the first 30 min of ethanol administration. Moreover, it is consistent with the reported time course action of the drug on operant self-administration of ethanol (Hodge et al., 1995).
2.3.4. Experiment 4: THIP
A similar 5 day procedure was utilized to determine the effect of THIP (0, 2, 4, 8, 16 mg/kg) on binge-like ethanol and water intake in a naïve group of male and female mice. However, whereas saline habituation injections were administered on day 4 only in Experiments 2 and 3, saline habituation injections were administered on both days 3 and 4 in Experiment 4. This was done in an attempt to further stabilize ethanol intakes prior to drug challenge on day 5. A 1 h access period was also utilized for THIP administration. Although little is known about the time course of THIP’s action on ethanol intake, this was done to again take advantage of the greater drinking during the first 30 min of ethanol administration, and is consistent with what was done in Experiments 2 and 3.
2.4. Blood ethanol concentration
Fifty μl microcapillary tubes were used to collect the retro-orbital blood samples. Samples were centrifuged and plasma was decanted and stored at −80 °C until the time of BEC determination. Determination of blood ethanol content was achieved using an Analox Alcohol Analyzer (Analox Instruments, Lunenburg, MA).
2.5. Statistical analysis
Experiment 1 was analyzed using GraphPad Prism version 4.02 for Windows (GraphPad Software, San Diego California, USA). Ethanol intakes and corresponding BECs were compared using linear regression analysis. The balance beam data were analyzed by t-test. Data from Experiments 2, 3, and 4 were analyzed by analysis of variance (ANOVA) using Statistica release 7, (StatSoft Inc).
The baclofen, muscimol, and THIP studies (Experiments 2, 3, and 4, respectively) were each repeated using the same mice (passes 1 and 2), although a single mouse never received the same dose of drug twice. Whereas the same groups of mice were used to assess the actions of each drug on both ethanol and water intake, different groups of mice were used to assess the actions of each drug (in each experiment). Overall analyses for each experiment indicated that the patterns of responses to the second drug administration were not influenced by the first drug administration (non-significant interaction of pass), so data were collapsed on this factor prior to all subsequent analyses. Thus, the actions of two different drug doses on ethanol and water intake were assessed for each mouse, and the observed actions of each of these doses was considered independent for the purposes of subsequent statistical analyses.
Day-5 ethanol or water intakes (as well as corresponding BEC data) following administration of either baclofen, muscimol, or THIP were initially analyzed by a two-way between subjects analysis of variance with sex and dose as the between subjects factors. Sex did not participate in any important interactions. The data were therefore collapsed on this factor and re-analyzed using a one-way between subjects ANOVA with dose as the between subjects factor. Tukey post hoc tests were performed where appropriate. Differences were considered significant at p<0.05.
3. Results
3.1. Experiment 1: Drinking in the dark
Our adapted drinking in the dark procedure yielded similar results as initially reported by Rhodes et al. (2005, 2007). Male C57BL/6J mice consumed 5.9±0.3 mg/kg ethanol, achieving average BECs of 65±7.1 mg/dL, when given access to the 20% ethanol solution for 2 h, 3 h into the dark cycle (Fig. 1A). Regression analysis indicated that ethanol intake predicted BEC (r2=0.68, p<0.0001; Fig. 1B). Two-hour access to DID also produced behavioral impairment on the balance beam; mice that had been allowed access to ethanol exhibited a significant increase in the number of foot slips compared to mice that had been allowed access to water [t(48)=3.2, p<0.01; Fig. 1C]. Mice with access to ethanol prior to being tested on the balance beam also displayed physiologically relevant blood ethanol concentrations (mean BEC of 81.3±9.4 mg/dL).
Fig. 1.
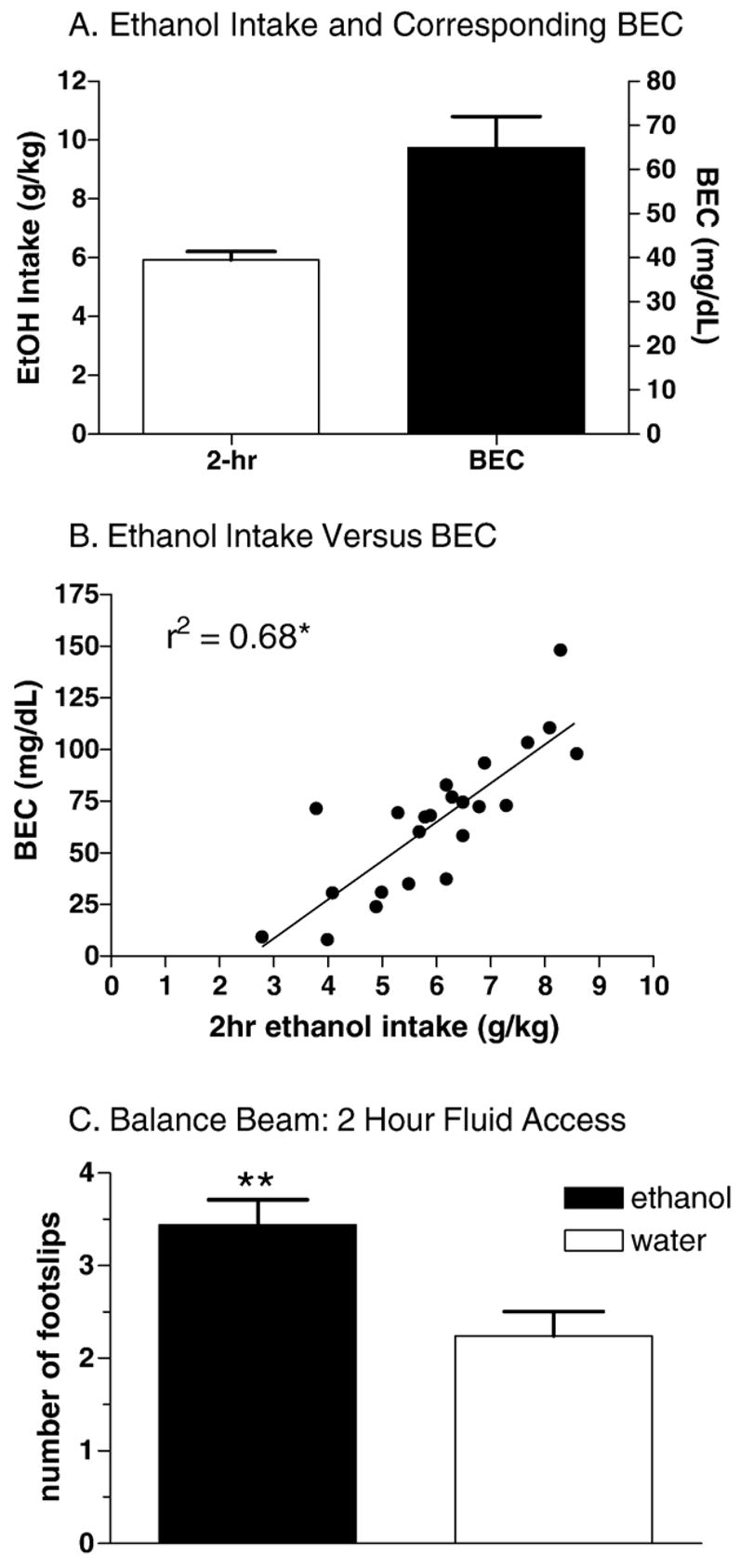
Binge-like ethanol intake in male C57BL/6J mice allowed access to a 20% solution for 2 h, 3 h into the dark cycle (n=23–25). A. Average ethanol intakes and corresponding blood ethanol concentrations. Mean ethanol intake was 5.9±0.3 g/kg, whereas the corresponding mean BEC was 65±7.1 mg/dL. B. Ethanol intake positively predicted blood ethanol concentration (p<0.0001). C. C57BL/6J mice displayed impaired motor performance on a balance beam task after being allowed access to a 20% ethanol solution for 2 h, 3 h into the dark cycle (n=25 per group). One group of mice was allowed a 2 hour access to tap water while the other was allowed a 2 h access to the ethanol solution. Mice allowed access to the ethanol drank an average of 5.0±0.3 g/kg, achieving average blood ethanol concentrations of 81.3±9.4 mg/dL. This level of intake produced a significant increase in the number of hind foot slips on a balance beam apparatus (p<0.01).
3.2. Experiment 2: Baclofen
To limit the number of mice necessary to determine whether baclofen altered ethanol and water intake, each mouse received two different doses of drug (over two different passes) of Experiment 2. The effect of each of these doses on intake was considered an independent observation for purposes of statistical analysis.
Mean ethanol intakes prior to baclofen administration were fairly stable. Intakes on days 1–4 were 4.6±0.1, 5.0±0.1, 4.6±0.1, and 4.2±0.2 g/kg, respectively. The effect of baclofen on binge-like ethanol intake and resulting blood ethanol concentration are shown in Fig. 2. Analysis of the ethanol intakes revealed a significant main effect of baclofen dose [F(4,95)=4.8, p=0.001]. Tukey post hoc tests revealed that the 10.0 mg/kg baclofen dose significantly enhanced ethanol intake (p<0.01; Fig. 2A). Analysis of the blood ethanol concentrations following the 1 h ethanol access period were consistent with the ethanol intake findings, with a significant main effect of dose [F(4,90)=3.9, p<0.01]. Consistent with the intake data, post hoc tests revealed a significant enhancement of BEC following administration of the 10.0 mg/kg baclofen dose (p<0.01; Fig. 2B).
Fig. 2.
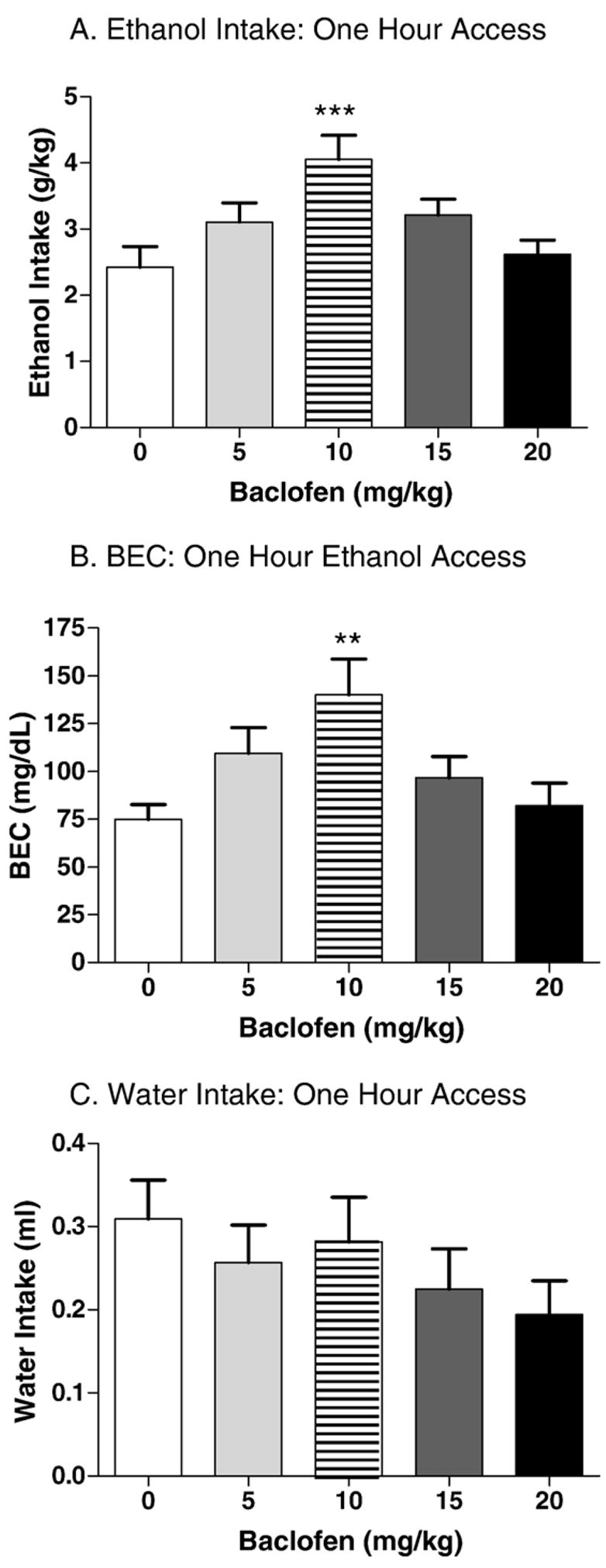
Effect of baclofen on 1 h DID in C57BL/6J mice (n=18–20 per baclofen dose). Each animal received two different doses of baclofen, and the corresponding effects of each dose on ethanol and water intake were considered independent observations for the purposes of statistical analysis. A. Baclofen dose-dependently increased binge-like ethanol intake during the 1 h ethanol access period. This effect reached significance at the 10 mg/kg baclofen dose (p<0.001). B. Baclofen dose-dependently increased blood ethanol concentration during the 1 h ethanol access period. The peak effect of baclofen occurred at the 10 mg/kg dose (p<0.01). C. Baclofen did not significantly alter water intake.
Water intake was assessed to determine whether baclofen’s actions were specific for ethanol intake, or also generalized to intake of other fluids. Intake of tap water over days 1–4 was 0.8±0.0, 0.9±0.0, 0.9±0.0, 0.6±0.0 ml, respectively. The effect of baclofen on water intake on day 5 was analyzed using a one-way between subjects ANOVA. No significant main effect of baclofen dose was found (Fig. 2C). These results suggest that baclofen dose-dependently enhances binge-like ethanol intake in C57BL/6J mice at doses that do not significantly alter water intake.
3.3. Experiment 3: Muscimol
Each mouse received two different doses of muscimol (over two passes) immediately prior to determination of ethanol and water intake in Experiment 3. As was the case for baclofen above, the actions of each of these muscimol doses on intake were considered an independent observation for purposes of statistical analysis.
Ethanol intake over days 1–4 were also stable prior to day 5 administration of muscimol. Mean ethanol intakes were 5.0±0.1, 5.6±0.1, 5.1±0.1, and 4.4±0.1 g/kg on days 1–4, respectively. Analysis of day-5 ethanol intakes revealed a significant effect of dose [F(4,91)=10.7, p<0.001]. Post-hoc tests indicated that muscimol reduced binge-like ethanol intake at all the doses tested (p’s<0.05; Fig. 3A). Analysis of the blood ethanol concentrations following the 1 h ethanol access period corresponded with the ethanol intake findings, with a main effect of dose [F(4,85)=22.9, p<0.001]. Post hoc tests revealed that all the muscimol doses tested resulted in significantly lower BECs compared saline controls (p’s<0.001; Fig. 3B).
Fig. 3.
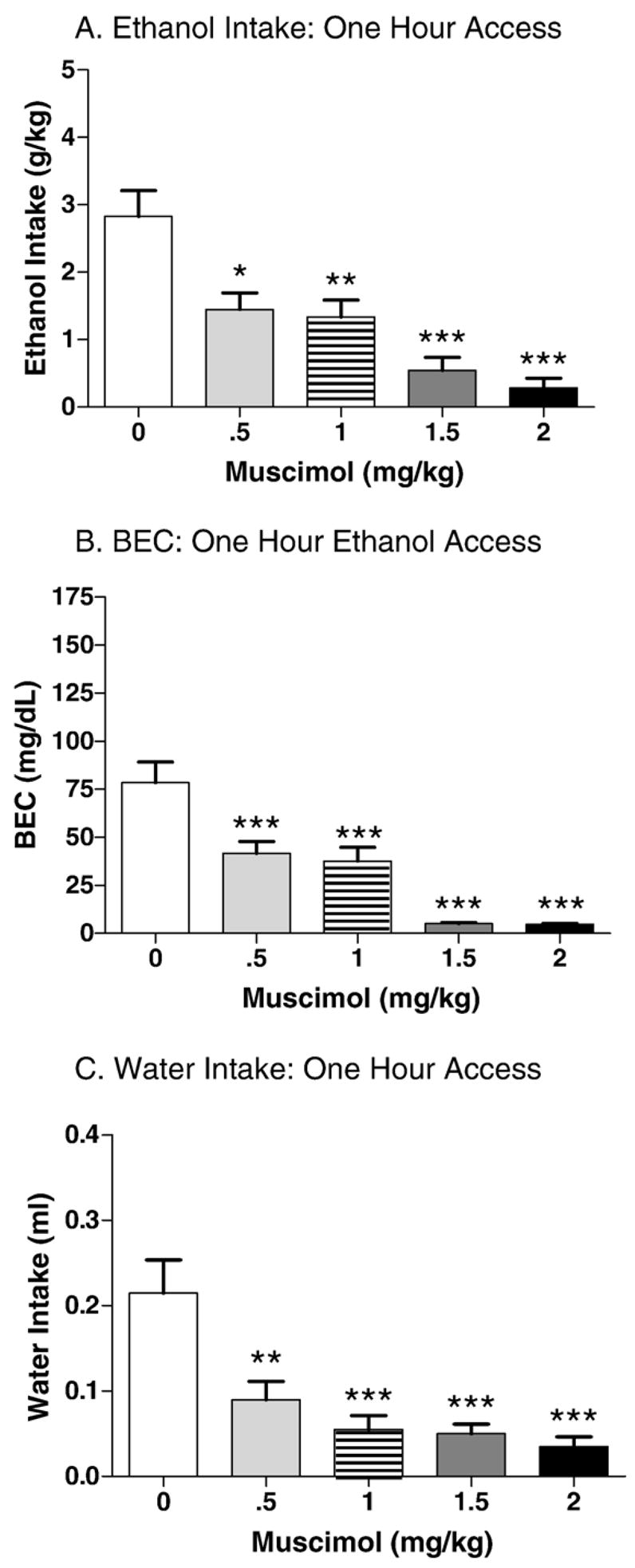
Effect of muscimol on 1 h DID in C57BL/6J mice (n=16–20 per muscimol dose). Each animal received two different doses of muscimol, and the corresponding effects of each dose on ethanol and water intake were considered independent observations for the purposes of statistical analysis. A. Muscimol dose-dependently reduced binge-like ethanol intake during the 1 h ethanol access period. This effect reached significance at all the doses tested (p’s<0.05). B. Muscimol dose-dependently decreased blood ethanol concentration during the 1 h ethanol access period following a similar pattern as that of ethanol intake (p’s<0.001). C. Muscimol significantly reduced water intake at all the doses tested (p’s<0.01).
Water intakes prior to muscimol administration on days 1–4 were 0.8±0.0, 0.7±0.0, 0.8±0.0, and 0.5±0.0 ml, respectively. The effect of muscimol on water intake on day 5 is shown in Fig. 3C. The analysis of water intakes revealed significant main effect of dose [F(4,90)=10.1, p<0.001]. Post hoc tests revealed a significant reduction of water intake at all the muscimol doses tested (p’s<0.01). Muscimol’s attenuating actions on binge-like ethanol intake also extended to water intake.
3.4. Experiment 4: THIP
Similar to the procedures described for Experiments 2 and 3 above, each mouse received two different doses of THIP prior to assessment of ethanol and water intake over two passes of Experiment 4. However, for purposes of statistical analysis, each of these observations was considered independent.
Ethanol intakes prior to THIP administration on days 1–4 were 4.2±0.1, 3.9±0.1, 3.9±0.1, and 3.7±0.1 mg/kg, respectively. The effect of THIP on binge-like ethanol intake and resulting blood ethanol concentrations are shown in Fig. 4A and B. Analysis of ethanol intakes revealed a significant main effect of dose [F(4,95)=7.5, p<0.001]. Post hoc tests revealed that, compared to saline-treated controls, THIP administration attenuated DID at the 8.0 and 16.0 mg/kg doses (p’s<0.05). The analysis of the blood ethanol concentrations following the 1-hour ethanol access period was similar to the ethanol intake findings; a significant main effect of dose was revealed [F(4,91)=7.1, p<0.001]. Post hoc tests revealed a significant reduction in BECs at the 8.0 and 16.0 mg/kg doses (p’s=0.05).
Fig. 4.
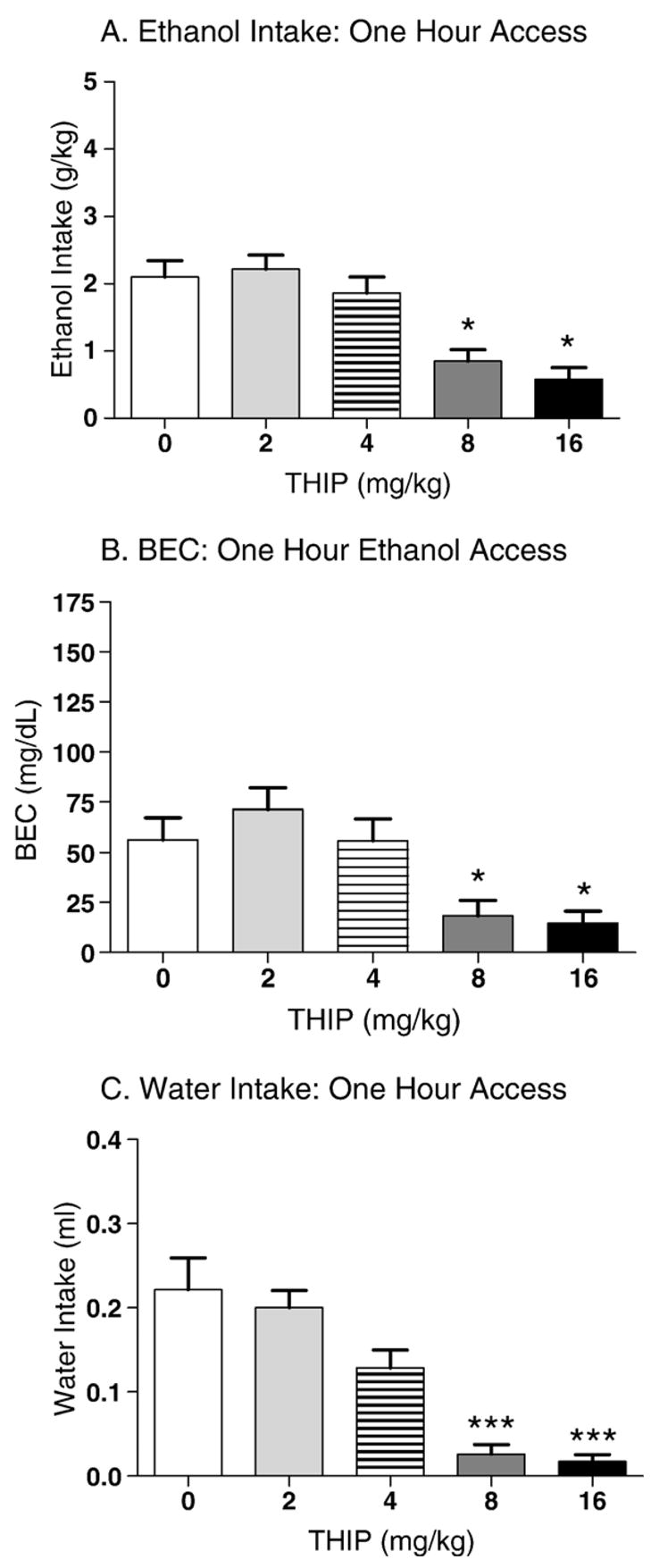
Effect of THIP on 1 h DID in C57BL/6J mice (n=18–20 per THIP dose). Each animal received two different doses of THIP, and the corresponding effects of each dose on ethanol and water intake were considered independent observations for the purposes of statistical analysis. A. THIP dose-dependently reduced binge-like ethanol intake during the 1 h ethanol access period. This effect reached significance at the two highest doses tested in male and female mice (p’s<0.05). B. THIP dose-dependently decreased blood ethanol concentration during the 1hr ethanol access period following the same pattern as the ethanol intake data (p’s<0.05). C. THIP significantly reduced water intake at the 8 and 16 mg/kg doses (p’s<0.001).
Water intakes were 0.6±0.0, 0.7±0.0, 0.5±0.0, and 0.5±0.0 ml on days 1–4, respectively. Analysis of water intakes revealed a significant main effect of dose [F(4,93)=16.1, p<0.001]; Tukey post hoc tests indicated that THIP reduced general fluid intake at the 8.0 and 16.0 mg/kg dose (p’s<0.001; Fig. 4C). Thus, as was the case for muscimol, THIP’s actions on ethanol intake generalized to water intake.
4. Discussion
The goal of the current work was to examine the actions of the GABAergic compounds baclofen, muscimol, and THIP on binge-like ethanol intake using a new mouse model developed by Rhodes et al. (2005), called Drinking in the Dark (DID). The DID model was chosen for its simplicity, as well as its unique ability to rapidly screen pharmaceuticals for modulation of ethanol intake (Kamdar et al., 2007). The duration of the ethanol access period is short, not more than 2 h. In our hands, mice given a daily 2 h access to the 20% unsweetened ethanol solution (3 h into the dark cycle) over several consecutive days drank nearly 6 g/kg ethanol resulting in blood ethanol concentrations approaching 70 mg/dL, a level of ethanol intake sufficient to produce significant motor impairment on a balance beam apparatus. Other models yielding high ethanol intakes and corresponding BECs often require lengthy acclimation and/or training periods, or food and/or water restriction (Lê et al., 1994; Becker and Lopez, 2004; Finn et al., 2005; Sharpe et al., 2005). In the classic 2-bottle choice model in which mice are given a choice between a less concentrated ethanol solution (generally between 3 and 15%) and water over a 24 h period, C57B/6J mice typically exhibit a preference for ethanol, consuming most of their daily fluids from the ethanol solution. However, there is wide variability in the BECs achieved over the course of the day, and mice rarely consume enough ethanol within a specified time period to produce pharmacologically relevant BECs (Dole and Gentry, 1984).
In the DID model, the GABAB receptor agonist baclofen dose-dependently enhanced DID, whereas the GABAA receptor agonists muscimol and THIP dose-dependently reduced this measure in C57BL/6J mice. However, baclofen was the only drug which altered ethanol intake in the absence of any observable actions on water intake; suggesting that the actions of muscimol and THIP on binge-like ethanol intake may also generalize to other fluids. Nevertheless, the present work demonstrates that DID can be utilized to assess the actions of GABAergic compounds on ethanol intake and adds to literature suggesting its utility in assessing the action of various drugs on ethanol intake in mice (Kamdar et al., 2007).
However, it is worth noting that our procedure differs slightly from the one used by Kamdar et al. (2007) to test the effects of naltrexone. Whereas we used a five day procedure with 2 h access on days 1–4 and a 1 hour access on day 5 to test the effects of baclofen, muscimol and THIP; Kamdar et al. (2007) used a shorter 2 day procedure with naltrexone administration on day 2. The difference in the duration of the procedures raises a few questions. First, it is possible that with our slightly longer procedure that our animals may have developed greater tolerance and/or dependence to ethanol. Tolerance and/or dependence to ethanol and the underlying neural changes associated with them may contribute to the results we obtained. Second, in our procedure on the testing day our animals had access to ethanol for a shorter period of time (1 h) compared to their access time on the first 4 days of ethanol presentation (2 h). A different pattern of results might have been obtained if the ethanol solution was made available for a longer period of time. However, our preliminary data indicated that, at least for baclofen, the effects of the drug seemed to diminish after an hour (data not shown). Therefore, it did not seem necessary or appropriate for us to give a longer access time to the animals on the testing day (day 5). Future work will need to address whether DID can produce tolerance and/or dependence to ethanol, as well as the time necessary to produce such effects.
Another important difference between our data and that of Rhodes et al. (2005, 2007) and Kamdar et al. (2007) is that whereas their intake data were fairly consistent across experiments, in our hands the ethanol intakes varied considerably across different cohorts of animals. One possible explanation is that different degrees of leakage occurred across the different experiments. Leakage would not only explain the variability in ethanol intakes across experiments, but also the discrepancy in Experiment 1 between the high ethanol intake and the relatively low BEC. However this explanation seems unlikely considering we controlled for leakage by placing ethanol (or water) sipper tubes into empty “control cages” and monitored the amount of leakage in these cages over the 2 h fluid access period. We then took this “control leakage” and subtracted it from the fluid volume that the animals consumed. Although there was variability in ethanol intakes across the experiments, the ethanol intakes were consistently high; and these high intakes are consistent with those reported by Rhodes et al. (2005, 2007). As far as the low BECs in Experiment 1 are concerned, it is more likely that animals consumed most of their ethanol intake during the first 30 min of the experiment; this large binge by C57BL/6J mice in the first 30 min has also been reported by Rhodes et al. (2005). If the animals consumed most of their ethanol in the first 30 min of the experiment, it would follow that the BECs would be lower when measured at the conclusion of the ethanol access period (2 h in the case of Experiment 1). Indeed, Middaugh et al. (2003) reported that for C57BL/6 mice the longer the duration of ethanol exposure, the weaker the relation between ethanol intake and BEC.
Our results for muscimol are consistent with literature suggesting that the drug decreases ethanol intake (Petry, 1997). However, our THIP results are opposite of those found in the literature, and the baclofen data are only consistent with the findings of a few studies. Although Smith et al. (1999, 1992) and Petry (1997) both found that baclofen enhances ethanol intake, the majority of the published data support the notion that baclofen reduces ethanol intake (Besheer et al., 2004; Anstrom et al., 2003; Colombo et al., 2000, 2003; Janak and Gill, 2003; Stromberg, 2004) and that THIP enhances ethanol intake (Boyle et al., 1992, 1993; Smith et al., 1992) and ethanol self administration. The discrepant results may be due to a number of issues, including differences in the procedures used, the species and age of animals, the concentration of ethanol solution, and dose ranges of the drugs. For example, Colombo et al. (2003) reported that baclofen suppresses motivation to consume ethanol (10% ethanol solution) in an operant procedure using baclofen doses ranging from 0 to 3 mg/kg. In contrast, Anstrom et al. (2003) described baclofen’s (0, 1.8, 3.2, and 5.6 mg/kg doses) actions as reducing ethanol intake using a 10% ethanol solution in an operant procedure. However, Anstrom et al. (2003) reported that this reduction by baclofen extended to sucrose solutions as well, suggesting that the reduction was not specific to ethanol. Boyle et al. (1992, 1993) reported increases in ethanol intake after THIP administration when using a free choice procedure in which ethanol was introduced beginning with a 2% concentration and increasing this concentration after every two ethanol presentations until the desired concentration was reached (between 9 and 10%). The reported enhancement was seen at the same doses in which we observed a significant reduction in both ethanol intake and corresponding BEC. Thus, it is possible the action of THIP and/or baclofen on ethanol intake may depend on the concentration of the ethanol solution presented, or even the level of intoxication achieved.
Another possible explanation for the enhancement of ethanol intake observed with baclofen administration and the dose-dependent reduction observed with THIP could be the timing of drug treatment with respect to the onset of the dark cycle. On day 5 of ethanol exposure in the current study, animals were treated with drug 3 h after lights out. Goldstein and Kakihana (1977) showed that the rhythm of food and fluid consumption in mice resembles a sinusoidal curve, with the most consumption around the first few hours after lights out. In the DID model, ethanol is presented (and drug is administered) 3 h into the dark cycle; this particular time happens to coincide with the time that animals consume most of their daily fluids. Smith et al. (1999) examined the effect of baclofen administration on ethanol intake in rats using an operant procedure in which 10 mg/kg baclofen or saline (1 h prior to dark cycle) was administered to animals for five days while the animals had access to a 10% ethanol solution. During the first hour of ethanol access (prior to dark phase), a reduction in ethanol intake was observed after baclofen administration. However, after the first hour a substantial increase in ethanol intake was observed (during the dark phase of the light cycle), and the increase continued on into the next light phase. These results may suggest that baclofen produces biphasic effects on ethanol intake. The timing of the baclofen (and possibly THIP) injection and subsequent access to ethanol may be critical in determining how the drug will ultimately influence ethanol intake.
A critical question in studies demonstrating a change in ethanol intake following administration of a drug concerns the mechanism of drug action. Indeed, the drug may produce competing behaviors that interfere with the motivation to drink the solution presented. For example, baclofen has been widely prescribed as a muscle relaxant for patients with back spasms (Chou et al., 2004), and GABAA receptor agonists are known to produce sedative-hypnotic actions. This raises an important problem for studies in which baclofen, muscimol, or THIP are shown to reduce ethanol intake or self-administration; these drugs may reduce ethanol intake because they are producing a general sedative effect. This issue was clearly a concern for our muscimol and THIP work. In both cases, the doses that produced the reduction in ethanol intake also produced decreases in water intake. This issue might have been of greater concern for the current baclofen work if not for the fact that baclofen enhanced DID without altering water intake. If the drug had been producing a general sedative effect, we would have expected to see a reduction in both ethanol and water intake.
Another question in studies demonstrating a change in ethanol intake following administration of a drug concerns the generalizability of the drug effect to intake of other potential reinforcers, such as saccharine or sugar water. Such generalizability may indicate that the drug alters general motivation to drink, and that this action is not specific to ethanol intake per se. We did not assess intake of any alternative reinforcer in the present work. Although it is possible (and probably likely) that muscimol and THIP would each reduce intake of a saccharine or sugar solution, the question of generalizability to other potential reinforcers is less interesting given the attenuating effects of the drugs on water intake and the resulting conclusion that competing behaviors are reducing overall fluid intake. However, the question is a potentially relevant one for baclofen given its apparently selective action on ethanol (and not water) intake, and worth considering in any future work examining the actions of the drug in the DID procedure.
A possible limitation or at least methodological consideration of the present work is that we chose to limit the number of animals we used by testing the same animals’ response to a drug over two experimental passes. Therefore, each animal received two different doses of a drug, yet the corresponding observations were considered independent for purposes of statistical analysis. This raises the possibility that order effects could have influenced the data; the effects of the initial drug exposure may have influenced the effects of the second. However, visual inspection of our data did not indicate any differences in the animals’ response to the drugs between the passes. Moreover, we subjected the data to an initial overall ANOVA that included pass as a factor. This analysis did not reveal any interactions of pass with any other factor. We therefore felt justified in our decision to collapse across the passes for purposes of statistical analyses.
In conclusion, the current work adds to the literature suggesting that the DID model is a useful tool for determining whether various neuromodulatory compounds alter ethanol intake in mice. It also provides additional support to an extensive literature linking GABAA and GABAB receptor systems with this ethanol-related behavior. However, the current results do not support the literature suggesting that THIP enhances ethanol intake, and adds to the growing literature indicating that baclofen may not always reduce ethanol self-administration and/or intake as the majority of the literature suggests.
Acknowledgments
This work was supported in part by NIAAA grant AA015434.
Footnotes
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author’s institution, sharing with colleagues and providing to institution administration. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- Anstrom KK, Cromwell HC, Markowski T, Woodward DJ. Effect of baclofen on alcohol and sucrose self-administration in rats. Alcohol Clin Exp Res. 2003;27:900–8. doi: 10.1097/01.ALC.0000071744.78580.78. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28(12):1829–38. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Hodge CW. GABAB receptor agonists reduce operant ethanol self-administration and enhance ethanol sedation in C57BL/6J mice. Psychopharmacology (Berl) 2004;74:358–66. doi: 10.1007/s00213-003-1769-3. [DOI] [PubMed] [Google Scholar]
- Boehm SL, II, Piercy MM, Bergstrom HC, Phillips TJ. Ventral tegmental area region governs GABAB receptor modulation of ethanol-stimulated activity in mice. Neuroscience. 2002;115:185–200. doi: 10.1016/s0306-4522(02)00378-0. [DOI] [PubMed] [Google Scholar]
- Boismare F, Daoust M, Moore N, Saligaut C, Lhuintre JP, Chretien P, et al. A homotaurine derivative reduces the voluntary intake of ethanol by rats: are cerebral GABA receptors involved? Pharmacol Biochem Behav. 1984;21(5):787–9. doi: 10.1016/s0091-3057(84)80020-9. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Smith BR, Amit Z. Microstructural analysis of the effects of THIP, a GABAA agonist, on voluntary ethanol intake in laboratory rats. Pharmacol Biochem Behav. 1992;43(4):1121–7. doi: 10.1016/0091-3057(92)90491-w. [DOI] [PubMed] [Google Scholar]
- Boyle AE, Segal R, Smith BR, Amit Z. Bidirectional effects of GABAergic agonists and antagonists on maintenance of voluntary ethanol intake in rats. Pharmacol Biochem Behav. 1993;46(1):179–82. doi: 10.1016/0091-3057(93)90338-t. [DOI] [PubMed] [Google Scholar]
- Chou R, Peterson K, Hefland M. Comparitive efficacy and safety of skeletal muscle relaxants for spasticity and musculoskeletal conditions: a systemic review. J Pain Symptom Manage. 2004;28(2):140–75. doi: 10.1016/j.jpainsymman.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Carai MAM, Lobina C, Pani M, Reali R, et al. Ability of baclofen in reducing alcohol intake and withdrawal severity: I-preclinical evidence. Alcohol Clin Exp Res. 2000;24:58–66. [PubMed] [Google Scholar]
- Colombo G, Vacca G, Serra S, Brunetti G, Carai MAM, Gessa GL. Baclofen suppresses motivation to consume alcohol in rats. Psychopharmaclogy (Berl) 2003;167:221–4. doi: 10.1007/s00213-003-1397-y. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Legg BH, Stansfield KH. Ethanol and sucrose seeking and consumption following repeated administration of the GABAB agonist baclofen in rats. Alcohol Clin Exp Res. 2006;30(5):812–8. doi: 10.1111/j.1530-0277.2006.00094.x. [DOI] [PubMed] [Google Scholar]
- Dole VP, Gentry TR. Toward an analogue of alcoholism in mice: scale factors in the model. Proc Natl Acad Sci U S A. 1984;81:3543–6. doi: 10.1073/pnas.81.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Belknap JK, Cronise K, Yoneyama N, Murillo A, Crabbe JC. A procedure to produce high alcohol intake in mice. Psychopharmacology (Berl) 2005;178:471–80. doi: 10.1007/s00213-004-2039-8. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Kakihana R. Circadian rhythms of ethanol consumption by mice: a single computer analysis for chronopharmacology. Psychopharmacology (Berl) 1977;52(1):41–5. doi: 10.1007/BF00426598. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–76. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hill DR, Bowery NG. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981;290(5802):149–52. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19(6):1486–93. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- Janak PH, Gill TM. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Kamdar MK, Miller SA, Syed YM, Bahyana R, Gupta T, Rhodes JS. Acute effects of Naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 2007;192:207–17. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Sinkkonen ST. GABAA receptor subtype as targets for neuropsy-chiatric drug development. Pharmacol Ther. 2006;109:12–32. doi: 10.1016/j.pharmthera.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Lê AD, Ko J, Chow S, Quan B. Alcohol consumption by C57BL/6, BALB/c and DBA/2 mice in a limited access paradigm. Pharmacol Biochem Behav. 1994;47(2):375–8. doi: 10.1016/0091-3057(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Liang JH, Chen F, Krstew E, Cowen MS, Carroll FY, Crawford D, et al. The GABAB receptor allosteric modulator CGP7930, like baclofen, reduces operant self-administration of ethanol in alcohol-preferring rats. Neuropharmacology. 2006;50:632–9. doi: 10.1016/j.neuropharm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Szumlinski KK, Van Patten Y, Marlowe AL, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res. 2003;27(12):1892–900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- National Research Council of the National Academies. Guidelines for the care and use of mammals in neuroscience and behavioral research. The National Academies Press; 2003. [PubMed] [Google Scholar]
- Petry NM. Benzodiazepine-GABA modulation of concurrent ethanol and sucrose reinforcement in the rat. Exp Clin Psychopharmacol. 1997;5(3):183–94. [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Jr, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Tsvkovskaia NO, Ryabinin AE. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol Clin Exp Res. 2005;29(8):1419–26. doi: 10.1097/01.alc.0000174746.64499.83. [DOI] [PubMed] [Google Scholar]
- Smith BR, Robidoux J, Amit Z. GABAergic involvement in the acquisition of voluntary ethanol intake in laboratory rats. Alcohol Alcohol. 1992;27:227–31. [PubMed] [Google Scholar]
- Smith BR, Boyle AEL, Amit Z. The effects of the GABAB agonist baclofen on the temporal and structural characteristics of ethanol intake. Alcohol. 1999;17:231–40. doi: 10.1016/s0741-8329(98)00053-6. [DOI] [PubMed] [Google Scholar]
- Stromberg MF. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacol Biochem Behav. 2004;78:743–50. doi: 10.1016/j.pbb.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. The c-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol Clin Exp Res. 2007;31(1):11–8. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


