Abstract
Hepatic steatosis has been reported in human immunodeficiency virus (HIV)/hepatitis C virus (HCV) coinfection. However, the features of steatohepatitis, including cytologic ballooning and pericellular fibrosis, its risk factors, and the impact on disease severity in such patients are unknown. To assess this, we prospectively reviewed liver histology in consecutive coinfected patients to define the prevalence and severity of the features of steatohepatitis, its risk factors, and its impact on the severity of liver disease. A total of 222 subjects (74% male, mean age 45, 78% African American, 90% genotype 1) were studied. The mean body mass index (BMI) was 26, and 18% had a BMI >30. The prevalence of risk factors for steatosis were: diabetes (31%), hypertension (15%), dyslipidemia (8%), metabolic syndrome (9%), and alcohol abuse (21%). Steatosis was present in 23% and steatohepatitis was present in 17%. The steatosis was mild (5%–33%) in 19%, and moderate to severe (>33%) in 4%. Cytologic ballooning and pericellular fibrosis were present in 30% and 13%, respectively. The mean Ishak score was 6.9, and 33% had bridging fibrosis or cirrhosis. Both steatosis and cytologic ballooning were associated with BMI, metabolic syndrome, and insulin resistance, and presence of either was strongly associated with advanced fibrosis (P < 0.0001). By multiple logistic regressions, the following associations were identified: increased BMI, diabetes, and genotype 3 with steatosis; diabetes with cytologic ballooning; and longer duration of infection with steatohepatitis.
Conclusion
Steatosis and steatohepatitis are present in 23% and 30%, respectively, of patients with HIV/HCV coinfection, and both are associated with an increased risk of having advanced fibrosis. Although we did identify genotype 3, increased BMI, and diabetes as risk factors, we found no independent association with antiretroviral therapy.
Human immunodeficiency virus (HIV) infection is a global health concern with an estimated 1,200,000 infected in the United States and more than 39 million worldwide (data from www.unaids.org, accessed October 7, 2007). With the advent of highly active antiretroviral therapy, which combines various nucleoside/nucleotide analogue reverse transcriptase inhibitors (NRTIs), nonnucleoside reverse transcriptase inhibitors (NNRTIs), and protease inhibitors (PIs), the morbidity and mortality related to HIV have decreased significantly.1,2 As a result, patients are now living longer with HIV infection and other comorbidities, and hepatic events have emerged as a key issue in the management of HIV-infected patients.3
Much of the focus on liver disease in patients with HIV has been on coinfection with hepatitis C virus (HCV) and hepatitis B virus (HBV). Consequently, there has been little attention to other liver diseases such as nonalcoholic fatty liver disease (NAFLD). NAFLD represents a spectrum of liver diseases characterized mainly by macrovesicular steatosis in the absence of significant alcohol consumption and is now recognized as a major cause of abnormal liver enzymes.4,5 Hepatic histology can vary from isolated hepatic steatosis alone to steatohepatitis which can progress to cirrhosis and liver failure.6,7 In addition to steatosis, nonalcoholic steatohepatitis (NASH) requires the additional presence of varying combinations of findings, including cytologic ballooning, Mallory’s hyaline, scattered inflammation, and pericellular fibrosis.5 It is now recognized that the majority of patients with NAFLD have insulin resistance (IR) and dyslipidemia, both of which are often associated with other features of the metabolic syndrome such as central obesity, diabetes mellitus, and hypertension.8,9 Although the exact mechanism in the development of NASH has not been fully elucidated, it appears to be largely dependent on mitochondrial dysfunction10 and shares similar pathogenic mechanisms as those described with the metabolic abnormalities associated with NRTIs and PIs as part of highly active antiretroviral therapy.11–17 As such, the prevalence and impact of steatohepatitis in the HIV population needs to be studied.
There have been several reports on hepatic steatosis in patients with HCV18–20 and HIV/HCV coinfection with a prevalence of 40%–75%.21–31 Because hepatic steatosis is associated with lower response rates to interferon and increased fibrosis progression,21,32 its presence is clinically significant. There are, however, little data on the other features of steatohepatitis, such as cytologic ballooning, Mallory bodies, and pericellular fibrosis in patients with HIV/HCV coinfection.24 Therefore, the presence of features of steatohepatitis including cytologic ballooning, its risk factors, and impact on disease severity in such patients are largely unknown. To assess this, we prospectively reviewed liver histology in consecutive HIV/HCV coinfected patients to (1) define the prevalence, severity, and risk factors for hepatic steatosis and its impact on HCV disease severity; (2) define the prevalence and risk factors of cytologic ballooning in the presence and absence of steatosis and its impact on HCV disease severity; and (3) determine the prevalence of steatohepatitis, its risk factors, and its impact on the severity of liver disease in HIV/HCV coinfection.
Patients and Methods
Study Population
The present study included consecutive patients with HIV/HCV coinfection prospectively evaluated for chronic HCV at Virginia Commonwealth University Medical Center between 1998 and 2006. All patients were ≥ 18 years of age, positive for anti–HIV antibody, and positive for HCV RNA by commercial assay. Quantitative HCV RNA testing was performed via polymerase chain reaction assay (Amplicor; Roche Molecular Systems, Branchburg, NJ) and HCV genotype using Inno-Lipa (InnoGenetics, Tarrytown, NY). Patients were excluded from analysis if they had a prolonged prothrombin time of >2 seconds from control; had presence of ascites; had thrombocytopenia (platelet count <70,000); had active or recent (within 3 months) opportunistic infection related to HIV; had advanced HIV disease with life expectancy less than 1 year; had renal failure defined as a creatinine level >2.5; were HBV surface antigen–positive; had any other form of chronic liver disease such as autoimmune hepatitis, hemochromatosis, α1-antitrypsin deficiency, or Wilson’s disease; or were unable to give informed consent.
Liver Histology
Percutaneous liver biopsy was performed in the standard fashion. Formalin-fixed, paraffin-embedded liver tissue was stained with hematoxylineosin and Masson’s trichrome. The Ishak Histologic Activity Index was used to assess for inflammation and fibrosis33 and Ishak 3–6 fibrosis represented advanced fibrosis. Findings of fatty liver disease were graded by a modification of the Brunt scoring system as described.34,35 Specifically, grade of steatosis (<5%, 5%–33%, 34%–66%, and >66%), lobular inflammation at 20× magnification (0, 1–2, 2–4, and >4), cytologic ballooning (none, few, many), presence of Mallory’s hyaline and megamitochondria, and presence of perisinusoidal fibrosis away from septa was assessed and used in a semiquantitative manner as described recently for the NAFLD Activity Score (NAS) which ranges from 0 to 7.36 A diagnosis of steatohepatitis was made based on the pattern of steatosis and the presence of hepatocyte ballooning and lobular/portal inflammation.34,36 To minimize interobserver bias, all slides were reviewed simultaneously by 2 investigators (M.J.C./R.K.S. or A.J.S./R.K.S.) trained in liver pathology blinded to clinical information.
Variables Examined
At the time of biopsy, a comprehensive physical examination was performed and the following information was collected: age, sex, race, HIV risk factor, estimated duration of infection, pathologic alcohol use, and presence of diabetes, hypertension, lipodystrophy, and dyslipidemia. For those undergoing more than 1 biopsy, data were recorded separately at each biopsy. Both past and current antiretroviral use at the time of biopsy was recorded. Because the use of stavudine and didanosine have been reported to be associated with steatosis,22,30 they were analyzed separately. Weight, body mass index (BMI), and anthropomorphic assessment for percent body fat by skin fold thickness and bioelectrical impedance were assessed by a registered dietician. Prior to biopsy, biochemical tests for fasting glucose, insulin and lipid panel for total cholesterol, triglycerides, high-density lipoprotein (HDL) and low-density lipoprotein, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), bilirubin, albumin, complete blood count, CD4 and CD8 lymphocyte counts, HIV RNA, HCV RNA, and HCV genotype were performed by commercial assays in our hospital laboratory. Because quantitative testing for HCV RNA changed over time during the study period, HCV RNA levels are not reported.
Definitions
Data on current and prior antiretroviral medication were extracted from the chart at the time of liver biopsy. A normal ALT was defined as ≤60 U/L (the upper limit of normal in our laboratory) at the time of biopsy. IR was measured by the homeostasis model assessment of insulin resistance (HOMA-IR) as a continuous variable. The metabolic syndrome was defined by the Adult Treatment Panel (ATP III) criteria and included 3 or more of the following factors: a waist circumference >40 inches in men and >35 inches in women, triglyceride level ≥150 mg/dL, HDL <40 mg/dL in men and <50 mg/dL in women, blood pressure >130/85 mm Hg, and fasting plasma glucose ≥110 mg/dL.15 Obesity was defined as a BMI ≥30 and diabetes was defined as a presence of fasting glucose >126 mg/dL or by a history of hyperglycemia managed by antidiabetic medications. Hypertension and dyslipidemia were defined clinically in a similar manner, whereas lipodystrophy was assessed via chart review, physical examination, or patient self report. Pathologic alcohol consumption was defined as self reporting of >20 g/day in females and >30g/day in males.7 Hepatic steatosis was categorized as <5%, 5%–33%, and >33%; cytologic ballooning was dichotomized as absent or present (few or many); and steatohepatitis was defined as the presence of steatosis with cytologic ballooning and lobular inflammation.34–36 Advanced fibrosis was defined as the presence of bridging fibrosis or cirrhosis. This study was approved by the Office of Research Subjects Protection at the Virginia Commonwealth University Health System.
Statistical Analysis
Demographic, clinical, laboratory, and histologic data are presented as mean and standard deviation for normally distributed data, median and interquartile range [IQR] for non-normal distributed data, and proportions for categorical data as indicated. The 3 primary outcomes were the histologic presence of hepatic steatosis (≥5% versus <5%), cytologic ballooning (few or many versus none), and steatohepatitis (present versus absent). For each of these, demographic, clinical, and laboratory variables were compared. Differences in continuous variables were compared via 2-tailed Student t test and analysis of variance and categorical variables were assessed by chi-square or Fisher’s exact test as appropriate. Associations between variables were assessed via Pearson correlation. Stepwise multivariable logistic regression of factors identified on univariate analysis with a P value ≤0.25 to enter and ≤0.10 to remain in the model were used to identify predictors for each outcome. A P value <0.05 was considered significant. All analyses were performed using JMP IN 7.0 (SAS Institute, Cary, NC).
Results
Study Population
During the study period, 270 patients with HIV and liver disease were evaluated. Forty-eight patients were excluded from analysis for the following reasons: HBV (28), end-stage renal disease (9), hepatic decompensation (2), refused biopsy (3), and significant comorbid disease that precluded liver biopsy (6). A total of 277 biopsies in 222 subjects were available for review. The mean time between biopsies in the 50 patients with more than 1 biopsy was 50 months. Because demographic, laboratory, and clinical characteristics were similar between those undergoing 1 and more than 1 biopsy, the cohort was analyzed collectively. When patients with more than 1 biopsy were excluded, the results did not change (data not shown). The characteristics of the subjects meeting inclusion and exclusion criteria are shown in Table 1. All patients were HCV RNA–positive at the time of biopsy, and 90% were genotype 1 and only 4% were genotype 3. Antiretroviral therapy was used in 83% at the time of biopsy and included an NRTI in 93%, an NNRTI in 30%, and a PI in 47%. Of those on an NRTI, the most commonly used were zidovudine (34%), tenofovir (16%), lamivudine (11%), stavudine (16%), abacavir (6%), and didanosine (5%). Of those on an NNRTI, 57% were on efavirenz and 42% were talking nevirapine. Of those on a PI, the most commonly used were nelfinavir (30%), lopinavir/ritonavir (27%), indinavir (13%), atazanavir (17%), and saquinavir (5%). Fasting insulin was available in 68 subjects with a mean HOMA-IR of 2.08 (range, 0.05–11.68). There were no significant differences in demographic, clinical, or histologic characteristics between those with and without HOMA-IR available (data not shown). The mean (standard deviation) Ishak score in the cohort was 6.9 (4.6), and 33% had advanced fibrosis. The presence of advanced fibrosis was associated with longer duration of infection (P = 0.007); increased AST, ALT, and ALP (all P < 0.0001); HOMA-IR (P = 0.01); presence of diabetes (P = 0.002); lipodystrophy (P = 0.005); and use of stavudine at time of biopsy (P = 0.02). When all clinical factors with a P value <0.25 were entered into a stepwise multivariable logistic regression model with advanced fibrosis as the outcome variable, HOMA-IR (P = 0.009), BMI (P = 0.007), and duration of infection (P = 0.006) were independently associated with advanced fibrosis.
Table 1.
Patient Characteristics
| Factor | Values |
|---|---|
| Demographics | |
| Number of patients | 222 |
| Age* | 45.4 (8) |
| Sex (% male) | 74 |
| Race (%) (white/African American) | 22/78 |
| CDC class (% A1/A2/A3/B1/B2/B3/C1/C2/C3)† | 27/22/11/6/4/1/2/26 |
| Duration of infection (years)* | 18.7 (10.5) |
| Weight (kg)* | 74 (2.2) |
| Body mass index* | 26 (4.9) |
| Body mass index ≥30 (%) | 18 |
| Waist/hip ratio*‡ | 0.9 (0.09) |
| Body fat* (%) (skin fold/bioimpedence)‡ | 29 (8.8)/26(11.3) |
| Clinical factors | |
| On antiretroviral therapy (%) | 83 |
| NRTI use (%) | 82 |
| Didanosine/stavudine use (%) | 10/25 |
| NNRTI use (%) | 30 |
| PI use (%) | 47 |
| Diabetes mellitus (%) | 11 |
| Hypertension (%) | 15 |
| Dyslipidemia (%) | 8 |
| Metabolic syndrome (%) | 9 |
| Alcohol use (%) | 21 |
| Laboratory | |
| CD4 (cells/mL)* | 535 (329) |
| CD4%* | 24.9 (10.7) |
| CD8 (cells/mL)* | 957 (540) |
| CD8% | 43.9 (12.8) |
| CD4 3200, 201–500, 3500 cells/mL | 10%,42%,48% |
| HIV RNA 3400 copies/mL (%) | 45 |
| HCV genotype 1 (%) | 90 |
| AST (U/L)§ | 62 (43–102) |
| ALT (U/L)§ | 68 (43–101) |
| ALP (U/L)§ | 112 (85–112) |
| Normal ALT (%) | 38 |
| Bilirubin (mg/dL)* | .84 (0.84) |
| Albumin (g/L)* | 3.97 (0.44) |
| Platelet ×1000* | 204 (72) |
| Triglyceride (mg/dL)*| | 169 (101) |
| Cholesterol (mg/dL)* | | 169 (36) |
| High-density lipoprotein (mg/dL)* | | 44.4 (15) |
| Low-density lipoprotein (mg/dL)* | | 89 (31) |
| HOMA-IR* ¶ | 2.08 (1.9) |
| Histology | |
| Ishak score (total)* | 6.94 (4.58) |
| Ishak inflammation* | 5.17 (3.43) |
| Ishak fibrosis* | 1.76 (1.61) |
| Advanced fibrosis (%) | 33 |
| Steatosis (>5%; yes/no) | 23 |
| Cytologic ballooning (yes/no) | 30 |
| Pericellular fibrosis (yes/no) | 13 |
| Mallory’s hyaline (%) | 9 |
| NASH activity score* | 2.18 (1.42) |
| Steatohepatitis (yes/no) | 17 |
Abbreviation: CDC, Centers for Disease Control and Prevention.
Mean (standard deviation).
Data available in 153 patients.
Data available in 70 patients.
Median (interquartile range).
Data available in 160 patients.
Data available in 68 patients.
Prevalence, Severity, and Risk Factors for Hepatic Steatosis and Its Impact on HCV Disease Severity
Two hundred twenty-four biopsies were available for review for steatosis; the distribution is shown in Fig. 1. Steatosis was predominantly macrovesicular and present in 23%, mild (5%–33%) in 19%, and moderate to severe (>33%) in 4%. Table 2 compares those with (n = 53) to those without (n = 171) ≥5% steatosis. On univariate analysis, coinfected patients with steatosis had a higher ALT (107 versus 81 U/L; P = 0.022), a higher BMI (28 versus 25; P =.001), a BMI ≥30 (28% versus 16%; P = .04), longer duration of infection (22 versus 18 years; P = .036), had diabetes (19 versus 9%; P = .04), had a higher HOMA-IR (3.6 versus 1.85), and were more likely to have the metabolic syndrome (17% versus 6%; P = .03). Conversely, those with ≥5% steatosis were less likely to have a positive HIV RNA (>400 copies/mL) 24 versus 42%; P = .026), less likely to be on antiretroviral therapy (77 versus 88%; P = .048) overall, and specifically less likely to be on a PI (33 versus 51%; P = 0.03) compared with those with <5% steatosis. Although there were only 9 patients (4%) in the cohort with genotype 3, it was more common in those with steatosis (14% versus 2%; P = .035). Those with steatosis also had higher Ishak scores for portal, periportal, and lobular inflammation (all P< ;.0001), had higher fibrosis scores (2.64 versus 1.91; P < .003), and were more likely to have advanced fibrosis (62% versus 28%; P < 0.0001), cytologic ballooning (51% versus 10%; P < .0001), Mallory’s hyaline (25% versus 4%; P < 0.0001), and pericellular fibrosis (25% versus 10%). We observed no differences in other clinical variables. Stepwise multivariable logistic regression of clinical factors identified increased BMI [odds ratio (OR) 1.17; 95% confidence interval (CI) 1.07–1.29; P = 0.0004], diabetes (OR 2.05, 95% CI 1.16–3.59; P = 0.014) and genotype 3 (OR 4.06; 95% CI 1.83–9.89; P = 0.0007) as an independent clinical factors associated with the presence of hepatic steatosis.
Fig. 1.
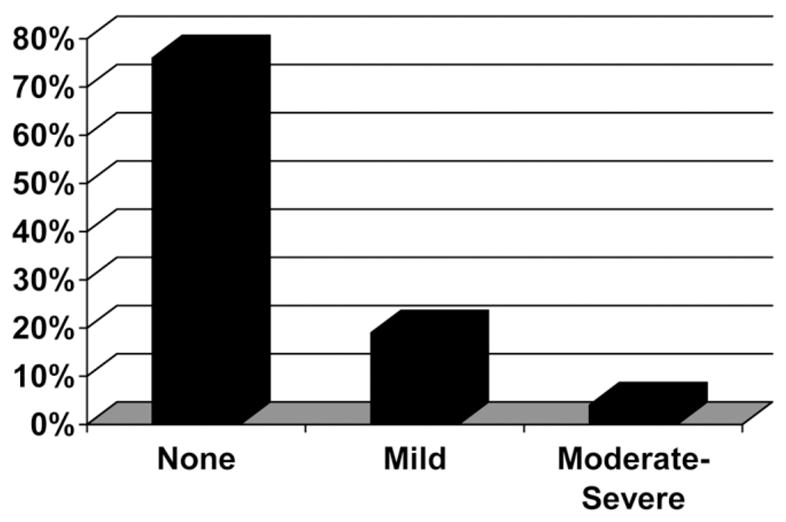
Distribution of steatosis in 224 patients with HIV/HCV coinfection. None = <5%. Mild = 5%–33%. Moderate-Severe = >33%. Data represent the percentage of patients.
Table 2.
Comparison of Patients with and without Steatosis (>5%)
| Steatosis
|
P Value§§ | ||
|---|---|---|---|
| Factor | <5% | >5% | |
| Demographics | |||
| Number of patients | 171 | 53 | |
| Age* | 45 (0.61) | 45 (1.1) | NS |
| Sex (% male) | 77 | 70 | NS |
| Duration of infection (years) | 18.5 (0.95) | 22.63 (0.75) | 0.036 |
| Weight (kg) | 73 (1.8) | 76 (3.2) | 0.35 |
| Body mass index | 25.66 (0.41) | 28.51 (0.75) | 0.001 |
| Body mass index ≥30 (%) | 16 | 28 | 0.03 |
| Waist/hip ratio† | 0.89 (0.01) | 0.91 (0.02) | NS |
| Body fat (%; skin fold/bioimpedence)† | 27/31 | 25/29 | NS |
| Clinical factors | |||
| On antiretroviral therapy (%) | 88 | 77 | 0.048 |
| NRTI use (%) | 87 | 77 | 0.08 |
| Didanosine/stavudine use (%) | 10/25 | 15/30 | NS |
| NNRTI use (%) | 30 | 37 | NS |
| PI use (%) | 51 | 33 | 0.034 |
| Diabetes mellitus (%) | 9 | 19 | 0.04 |
| Hypertension (%) | 11 | 20 | 0.09 |
| Dyslipidemia (%) | 8 | 9 | NS |
| Metabolic syndrome (%) | 6 | 17 | 0.01 |
| Alcohol use (%) | 18 | 24 | 0.36 |
| Laboratory | |||
| CD4 (cells/mL) | 514 (26) | 598 (47) | 0.12 |
| CD4% | 24 (0.89) | 25 (1.6) | NS |
| CD8 (cells/mL) | 940 (45) | 994 (81) | NS |
| CD8% | 44 (1.1) | 41 (1.9) | 0.24 |
| CD4 <200 (%) | 12 | 15 | 0.22 |
| HIV RNA <400 copies/mL (%) | 43 | 47 | 0.024 |
| HCV genotype 3 (%) | 2 | 14 | 0.0035 |
| AST (U/L) | 86 (6) | 106 (11) | 0.13 |
| ALT (U/L) | 82 (5) | 107 (9) | 0.025 |
| ALP (U/L) | 130 (8) | 141 (10) | 0.39 |
| Normal ALT (%) | 41 | 26 | 0.13 |
| Platelet ×1000 | 208 (6) | 193 (10) | 0.19 |
| Triglyceride (mg/dL)‡ | 165 (10) | 179 (18) | NS |
| Cholesterol (mg/dL)‡ | 169 (3) | 164 (6) | 0.44 |
| High-density lipoprotein (mg/dL)‡ | 45 (1.6) | 42 (2.8) | 0.29 |
| Low-density lipoprotein (mg/dL)‡ | 90 (3.4) | 86 (5.7) | NS |
| HOMA-IR§ | 1.85 (0.39) | 3.6 (0.65) | 0.024 |
| Histology | |||
| Ishak score (total) | 8.11 (0.28) | 9.0 (.52) | 0.13 |
| Ishak inflammation | 6.2 (0.21) | 6.3 (0.38) | NS |
| Ishak fibrosis | 1.91 (.11) | 2.64 (0.21) | 0.0034 |
| Advanced fibrosis (%) | 28 | 62 | <0.0001 |
| Cytologic ballooning (yes/no) | 18 | 68 | <0.0001 |
| Pericellular fibrosis (yes/no) | 10 | 25 | <0.0059 |
| Mallory’s hyaline (%) | 4 | 25 | <0.0001 |
| Steatohepatitis (yes/no) | 6 | 54 | <0.0001 |
Abbreviation: NS, not significant.
Mean (standard error of the mean).
Data available in 70 patients.
Data available in 160 patients.
Data available in 68 patients.
For univariate analysis.
Prevalence and Risk factors of Cytologic Ballooning in the Presence and Absence of Steatosis and Its Impact on HCV Disease Severity
Two hundred twenty-four biopsies were available for review for cytologic ballooning; the distribution is shown in Fig. 2. Cytologic ballooning was present in 67 [30% (few in 21% and many in 9%)] patients. When comparing patients with (n = 66) to patients without (n = 154) cytologic ballooning, patients with ballooning had higher AST levels (130 versus 78 U/L; P < 0.0001), had higher ALP levels (163 versus 121 U/L; P = 0.0002), had lipodystrophy (18% versus 9%; p = 0.05), and were less likely taking either stavudine (20% versus 29%; P = 0.04) and more likely taking didanosine (17% versus 9%; p = 0.059) compared to those without ballooning. Similar to those with steatosis, those with cytologic ballooning had higher ALT (107 versus 78 U/L; P = 0.006), longer duration of disease (23 versus 17 years; P = 0.0009), diabetes (19% versus 8%; P = 0.01), a higher HOMA-IR (3.35 versus 1.86; P = 0.006), higher Ishak scores for portal, periportal and lobular inflammation (all P < 0.0001), fibrosis (both P < 0.0001), and Mallory bodies (30% versus none; P < 0.0001). Unlike those with steatosis, we observed no significant differences in BMI, percent with BMI ≥30, proportion with the metabolic syndrome, undetectable HIV RNA, or protease inhibitor use. The proportion of patients with steatosis (5%–33% and >33%) was higher in those with (38% and 13%) than those without (10% and 0%) cytologic ballooning respectively (P < 0.0001). Stepwise multivariable logistic regression of clinical factors identified only a history of elevated fasting glucose or diabetes as an independent clinical factor associated with the presence of cytologic ballooning (OR 3.89; 95% CI 1.19–19.78; P = 0.023).
Fig. 2.
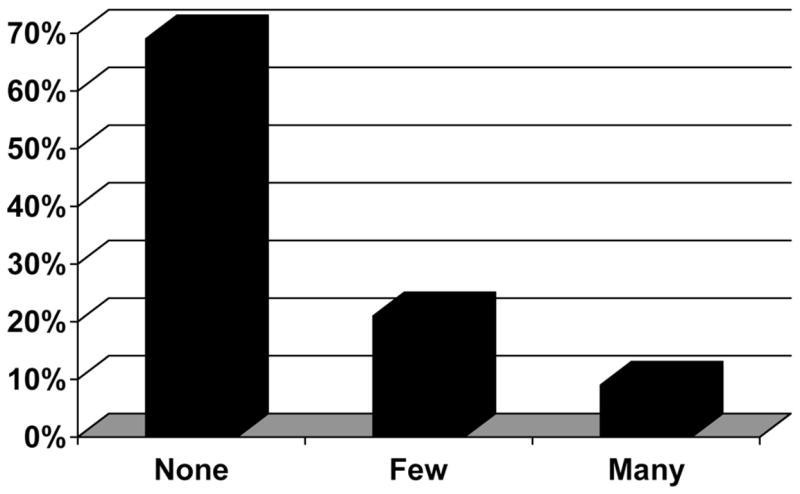
Distribution of cytologic ballooning in 224 patients with HIV/HCV coinfection. Data represent the percentage of patients.
Although steatosis was highly associated with cytologic ballooning (P < 0.0001), approximately 10% of patients with no ballooning had ≥5% steatosis, whereas 33% of patients with cytologic ballooning had <5% steatosis. Therefore, we also stratified the analysis of factors associated with cytologic ballooning in the absence (32/171: 19%) and presence (34/50: 68%) of steatosis. In the absence of steatosis, the presence of cytologic ballooning was associated with increased AST, ALT, and ALP (all P < 0.001); hepatic inflammation (P < 0.0001) and fibrosis (P < 0.0001); and the presence of advanced fibrosis (65% versus 19%; P < 0.0001). On univariate analysis, only duration of disease (23.5 versus 17 years; P = 0.005) and increased HOMA-IR (1.55 versus 2.89; P = 0.036) were associated with cytologic ballooning. On multivariable logistic regression, only increasing HOMA-IR was independently associated with cytologic ballooning in the absence of steatosis (P = 0.047). Conversely, when we compared patients with (n = 34) and without (n = 16) cytologic ballooning in only those patients with steatosis, the only variables associated with ballooning were lower weight (71 versus 89 kg; P = 0.04), lower cholesterol (156 versus 190 mg/dL; P = 0.04), and higher fibrosis scores (3.11 versus 2.12; P = 0.032) with no differences in other laboratory studies or liver inflammation.
Prevalence and Risk Factors of Steatohepatitis and Its Impact on HCV Disease Severity
Two hundred twenty-four biopsies were available for review for steatohepatitis; the distribution of NAFLD Activity Score is shown in Fig. 3. A histologic diagnosis of steatohepatitis defined as steatosis in the presence of cytologic ballooning and lobular inflammation was present in 38 (17%) patients. Mallory’s hyaline was seen in 8% of patients, and pericellular fibrosis was seen in 13%. Table 3 compares patients with (n = 37) and without (n = 179) a diagnosis of steatohepatitis. Univariate analysis identified that patients with steatohepatitis were more likely to be female (26% versus 13%; P = 0.02); to be shorter (66 versus 68 inches; P = 0.01); to have longer duration of disease (24 versus 18 years; P = 0.0053); to have higher CD8 (1165 versus 918 cells/mL; P = 0.017), AST (124 versus 85 U/L; P = 0.0054) and ALT levels (108 versus 83 U/L; P = 0.047); and to have lower HDL (38 versus 46 mg/dL; P = 0.035) and total cholesterol (155 versus 171 mg/dL; P = 0048). Although NRTI use as a class was lower in patients with steatohepatitis (14% versus 34%; P = 0.0048), specific use of didanosine was much higher in patients with steatohepatitis (33% versus 12%; P = 0.0086). Similar to steatosis and cytologic ballooning, the composite diagnosis of steatohepatitis was associated with higher Ishak portal, periportal, and lobular inflammation (all P < 0.001) and higher fibrosis (P < 0.0001). We did not observe any significant differences in diabetes, BMI, alcohol use, hypertension, percent body fat, triglyceride levels, HOMA-IR, or PI use between patients with and without steatohepatitis. Stepwise multivariable logistic regression of clinical factors identified only longer duration of disease (OR 1.06; 95% CI 1.01–1.11; P = 0.0125) as an independent clinical factor associated with the presence of steatohepatitis.
Fig. 3.
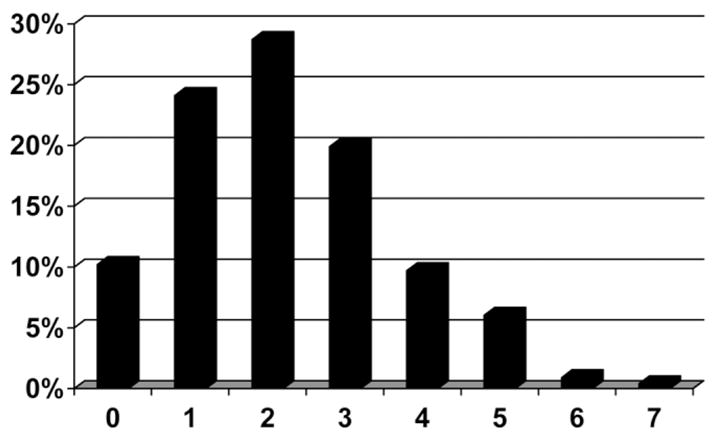
Distribution of NASH Activity Score in 224 patients with HIV/HCV coinfection. Data represent the percentage of patients.
Table 3.
Comparison of Patients with and without Steatohepatitis
| Steatohepatitis
|
P Value§§ | ||
|---|---|---|---|
| Factor | No | Yes | |
| Demographics | |||
| Number of patients | 179 | 37 | |
| Age* | 44(0.59) | 45(1.3) | NS |
| Sex (% male) | 87 | 74 | 0.025 |
| Duration of infection (years) | 18(0.93) | 24(1.8) | 0.0053 |
| Weight (kg) | 75(1.7) | 67(3.9) | 0.07 |
| Body mass index | 26.01(0.41) | 27.6(0.97) | 0.12 |
| Body mass index ≥30 (%) | 16 | 22 | 0.31 |
| Waist/hip ratio† | 0.90(0.01) | 0.91 (0.02) | NS |
| Body fat (%) (skin fold/bioimpedence)† | 28/25 | 33/32 | 0.12 |
| Clinical factors | |||
| On antiretroviral therapy (%) | 88 | 72 | 0.01 |
| NRTI use (%) | 88 | 70 | 0.005 |
| Didanosine/stavudine use (%) | 12/15 | 33/14 | 0.008/NS |
| NNRTI use (%) | 34 | 14 | 0.0048 |
| PI use (%) | 20 | 12 | 0.12 |
| Diabetes mellitus (%) | 15 | 29 | 0.09 |
| Hypertension (%) | 17 | 17 | NS |
| Dyslipidemia (%) | 17 | 16 | NS |
| Metabolic syndrome (%) | 16 | 25 | 0.32 |
| Alcohol use (%) | 16 | 19 | 0.65 |
| Laboratory | |||
| CD4 (cells/mL) | 529 (28) | 572 (56) | 0.48 |
| CD4% | 24.6 (0.85) | 24.5 (1.8) | NS |
| CD8 (cells/mL) | 917 (44) | 1165 (93) | 0.018 |
| CD8% | 43.7 (1.03) | 44.4 (2.2) | NS |
| CD4 <200 (%) | 14 | 5 | 0.16 |
| HIV RNA <400 copies/mL (%) | 14 | 18 | NS |
| HCV genotype 3 (%) | 2 | 10 | 0.05 |
| AST (U/L) | 85 (6) | 124 (13) | 0.0054 |
| ALT (U/L) | 83 (5) | 108 (11) | 0.047 |
| ALP (U/L) | 129 (0.83) | 154 (.08) | 0.08 |
| Normal ALT (%) | 41 | 27 | 0.19 |
| Bilirubin (mg/dL) | 0.79 (0.06) | 1.2 (.015) | 0.012 |
| Albumin (g/L) | 3.9 (0.03) | 3.8 (0.07) | 0.12 |
| Platelet ×1000 | 207 (5) | 192 (12) | 0.28 |
| Triglyceride (mg/dL)‡ | 169 (10) | 179 (23) | NS |
| Cholesterol (mg/dL)‡ | 171 (3.2 | 155 (7.3) | 0.048 |
| High-density lipoprotein (mg/dL)‡ | 46 (1.6) | 38 (3.5) | 0.035 |
| Low-density lipoprotein (mg/dL)‡ | 91 (3) | 81 (7) | 0.21 |
| HOMA-IR§ | 2.04 (0.42) | 3.28 (0.69) | 0.13 |
| Histology | |||
| Ishak score (total) | 7.9 (0.24) | 11.44 (.055) | <0.0001 |
| Ishak inflammation | 6.04 (0.18) | 8.22 (0.41) | <0.0001 |
| Ishak fibrosis | 1.87 (0.11) | 3.16 (0.24) | <0.0001 |
| Advanced fibrosis (%) | 15 | 40 | 0.0003 |
| Steatosis (>5%; yes/no) | 6 | 64 | <0.0001 |
| Cytologic ballooning (yes/no) | 1 | 53 | <0.0001 |
| Pericellular fibrosis (yes/no) | 10 | 30 | 0.0001 |
| Mallory’s hyaline (%) | 4 | 33 | <0.0001 |
Abbreviation: NS, not significant.
Mean (standard error of the mean).
Data available in 70 patients.
Data available in 160 patients.
Data available in 68 patients.
For univariate analysis.
Discussion
Liver disease is now recognized as an important cause of morbidity and mortality in patients living with HIV.37–39 Although the focus has been on chronic HCV and HBV, hepatic steatosis has emerged as an important comorbidity.3,40 Several reports have found that steatosis is common in patients with both chronic HCV13,18,19,27 and HIV/HCV coinfection21–31 and greater than expected from the general population.41 Because steatosis is associated with fibrosis progression and reduced response to anti-HCV therapy,21,22,32 its identification has an important clinical implication. Possible causes of hepatic steatosis in patients with HIV may be due to HIV itself, concurrent viral hepatitis, pathologic alcohol use, diabetes mellitus, obesity, or antiretroviral medications.3,40
The current study in a cohort of consecutive patients with HIV/HCV coinfection with and without elevated liver enzymes identified steatosis in 23%: mild (5%–33%) in 19%, and moderate to severe (>33%) in 4%. This is somewhat lower than in other reports that found varying degrees of steatosis in 40%–72%.22–25,27–31 In these studies, any steatosis was associated with several factors, including white race,22 age,23 increased weight or BMI,23–25,31 hyperglycemia,22 low HDL,24 lipodystrophy,24 hypertriglyceridemia,28 HCV RNA level,25 HCV genotype 3,24,25,30,31 and exposure to stavudine22,30 or di-danosine.30 Of these studies, 2 compared coinfected patients with patients with HCV alone with dichotomous results. Monto et al.23 found a low (2%) prevalence of significant steatosis (>33%) in 92 coinfected patients compared with 9% in 372 HCV controls (P = 0.02) which was unrelated to HIV therapy; however, Gaslight-wala and Bini28 found a higher proportion with any steatosis (72 versus 52%, P < 0.001) and grade 2/3 steatosis (48 versus 20%; P < 0.001) in 154 coinfected patients compared with 554 HCV controls. One study that compared steatosis-coinfected patients with elevated and persistently normal levels of ALT found no difference in the proportion with steatosis (57% versus 54%; P = 0.07).27 Similar to these previous studies, we identified increasing BMI, diabetes mellitus, and genotype 3 as independent predictors of steatosis. Similar to studies in HCV monoinfection, steatosis was also associated with increased insulin resistance and the presence of the metabolic syndrome.18–20,42 Differences between our findings and those of prior studies may be due to the high proportion of African Americans in our patient population, because this group may have a lower prevalence of steatosis43 and the differences in defining steatosis.
Unlike other studies, this is the first study that specifically analyzed for the presence of other features of steatohepatitis such as cytologic ballooning, Mallory body formation, and pericellular fibrosis.35 Interestingly, 33% of patients with cytologic ballooning had < 5% steatosis. In this subset, cytologic ballooning in the absence of steatosis was associated with more hepatic inflammation and a higher likelihood of having advanced fibrosis. Although diabetes was not more common in this group, insulin resistance was. This suggests that factors that might lead to insulin resistance—such as certain PIs and NRTIs11–17,44,45—may increase cytologic ballooning and result in increased liver disease progression in some coinfected patients.
In addition to steatosis and cytologic ballooning, we also examined our cohort for the composite score of steatohepatitis34,35 and found similar associations with disease duration, insulin resistance, and liver enzymes. However, unlike when steatosis or cytologic ballooning were assessed separately, patients with steatohepatitis were also more likely to be female, to be shorter, to have a higher CD8 count, and to have lower HDL and total cholesterol compared with coinfected patients without steatohepatitis. Although NRTI use as a class was lower in patients with steatohepatitis, similar to cytologic ballooning, specific use of didanosine was much higher in patients with steatohepatitis. Similar to steatosis and cytologic ballooning, the composite diagnosis of steatohepatitis was associated with higher portal, periportal, and lobular inflammation and higher fibrosis scores. Unlike associations with steatosis or cytologic ballooning, we did not observe any significant differences in diabetes or BMI, nor did we observe significant differences in other factors associated with the metabolic syndrome, such as hypertension, percent body fat, or triglyceride levels or PI use between those with and without steatohepatitis. Importantly, there was no association with self-reported pathologic alcohol use. However, multivariate logistic regression identified only longer duration of disease as an independent clinical factor associated with the presence of steatohepatitis.
There were several limitations to our analysis. All patients seen in our HIV clinic are tested for HCV. However, referral for further evaluation is dependent on the HIV provider. We recognize that our patient population, which is comprised of mostly black males, may not be representative of all patients with HIV. This could have resulted in selection bias of patients included in this cohort analysis. We also recognize that although the presence of steatosis may be lower in blacks than in whites,43 these data were not based on liver histology and were not obtained in HIV-positive patients. Therefore, the impact of race on the presence of steatosis in HIV-positive patients in the absence of HCV is not known. Although liver biopsy has always been the gold standard in the evaluation of liver disease, it provides a relatively small core (2–3 cm) and may be subject to intraobserver variation and sample error similar to what is now appreciated in HCV.46 Similar to other studies, we did not account for prior antiretroviral use and compliance with these medications. Due to the dynamic process of HIV disease and therapies, patients may change their antiretroviral regimen due to drug toxicities, compliance issues, or development of multidrug resistance. In addition, not all medications within each class have similar metabolic effects. Only prospective, longitudinal studies in antiretroviral-naïve patients that include paired liver biopsies that take into account these factors mentioned would be specifically able to adequately determine the impact of medication class on liver disease. That type of study may never be possible due to the ethical problems of obtaining liver histology in those without HCV as well as the numbers needed to detect statistical differences.
Concomitant alcohol use contributes to HCV disease severity.47,48 Although all patients included in this analysis abstained from alcohol for at least 6 months prior to biopsy, because we relied on self-reported historical information and not random alcohol testing, unreported alcohol use could have affected our analysis. In addition, we were not able to adequately assess for duration of disease in all patients as not all patients were able to give an accurate time of infection. However, because these factors were true for both patients with and without steatohepatitis, it should have minimized any differences between groups. Given the dynamic processes of HIV and HCV infections, the single point in time laboratory values and missing data in some patients may have limited our analysis. Because we did not measure historical glucose values, we were not able to differentiate those with uncontrolled glucose levels from those with a diagnosis of diabetes regardless of level of control. Lastly, we did not compare our population to patients with HCV alone. However, the lack of association of unique HIV factors, such as HIV RNA, CD4 counts, or antiretroviral therapy should minimize this limitation.
In conclusion, steatosis, cytologic ballooning and steatohepatitis are relatively common in HIV/HCV coinfection and are all associated with an increased risk of having advanced fibrosis, underscoring the importance of these histologic findings and their contribution to the pathogenesis of liver disease progression in coinfected individuals. As such, all future studies should include these histologic findings. Importantly, we identified that risk factors for these conditions, including increased BMI, diabetes, genotype 3 and disease duration, are similar to those in HCV-infected patients without HIV. We found no independent association with antiretroviral therapy. Finally, given the adverse impact of steatosis, cytologic ballooning, and steatohepatitis on fibrosis progression, additional studies on efforts to reduce steatosis by addressing modifiable factors, such as weight reduction and treatment of HCV— especially in those with genotype 3 that might reduce steatosis46,50 in HIV-HCV coinfected patients—are needed.
Acknowledgments
We thank the staff and providers of the HIV clinic and the General Clinical Research Center at VCUHS for their support, referral, and care of coinfected patients.
Supported by a grant from the National Institutes of Health (K23-DK064578) to Richard Sterling and by a grant to the General Clinical Research Center at Virginia Commonwealth University (M01RR00065). This work was presented in part at the American Association for the Study of Liver Diseases Annual Meeting, Boston, MA, November 2–6, 2007.
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HDL
high-density lipoprotein
- HIV
human immunodeficiency virus
- HOMA-IR
homeostasis model assessment of insulin resistance
- IR
insulin resistance
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NRTI
nucleoside/nucleotide analog reverse transcriptase inhibitor
- NNRTI
nonnucleoside/nucleotide analogue reverse transcriptase inhibitor
- PI
protease inhibitor
- U/L
upper limit of normal
Footnotes
Potential conflict of interest: Dr. Sterling is a consultant for, advises, is on the speakers’ bureau of, and received grants from Roche. He is on the speakers’ bureau of Schering-Plough. He is also a consultant for and received grants from Wako.
References
- 1.Lee LM, Karon JM, Selik R, Neal JJ, Fleming PL. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984–1997. JAMA. 2001;285:1308–1315. doi: 10.1001/jama.285.10.1308. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Pol S, Lebray P, Vallet-Pichard A. HIV infection and hepatic enzyme abnormalities: intricacies of the pathogenic mechanisms. Clin Infect Dis. 2004;38(Suppl 2):S65–S72. doi: 10.1086/381499. [DOI] [PubMed] [Google Scholar]
- 4.Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol. 2005;42(Suppl 1):S2–S12. doi: 10.1016/j.jhep.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 5.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Banas C, Sargearnt C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 7.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical pathologic severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 9.Loria P, Lonardo A, Carulli L, Verrone AM, Ricchi M, Lombardini S, et al. Review article: the metabolic syndrome and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2005;22(Suppl 2):31–36. doi: 10.1111/j.1365-2036.2005.02592.x. [DOI] [PubMed] [Google Scholar]
- 10.Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42:928–940. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Leow MK, Addy CL, Mantzoros CS. Clinical review 159: human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. J Clin Endocrinol Metab. 2003;88:1961–1976. doi: 10.1210/jc.2002-021704. [DOI] [PubMed] [Google Scholar]
- 12.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS. 1998;12:F51–F58. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Walli R, Herfort O, Michl GM, Demant T, Jager H, Dieterle C, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS. 1998;12:F167–F173. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Carr A. HIV protease inhibitor-related lipodystrophy syndrome. Clin Infect Dis. 2000;30(Suppl 2):S135–S142. doi: 10.1086/313854. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 16.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 17.Grunfeld C, Tien P. Difficulties in understanding the metabolic complications of acquired immune deficiency syndrome. Clin Infect Dis. 2003;37(Suppl 2):S43–S46. doi: 10.1086/375886. [DOI] [PubMed] [Google Scholar]
- 18.Matos CA, Perez RM, Pacheco MS, Figueiredo-Mendes CG, Lopes-Neto E, Oliveira EB, Jr, et al. Steatosis in chronic hepatitis C: relationship to the virus and host risk factors. J Gastroenterol Hepatol. 2006;21:1236–1239. doi: 10.1111/j.1440-1746.2006.04308.x. [DOI] [PubMed] [Google Scholar]
- 19.Leandro G, Mangia A, Hui J, Fabris P, Rubbia-Brandt L, Colloredo G, et al. Relationship between steatosis, inflammation, and fibrosis in chronic hepatitis C: a meta-analysis of individual patient data. Gastroenterology. 2006;130:1636–1642. doi: 10.1053/j.gastro.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Sanyal AJ. Review article: non-alcoholic fatty liver disease and hepatitis C—risk factors and clinical implications. Aliment Pharmacol Ther. 2005;22(Suppl 2):48–51. doi: 10.1111/j.1365-2036.2005.02596.x. [DOI] [PubMed] [Google Scholar]
- 21.Urial A, Moorehead L, Agarwal K, et al. Insulin resistance associated with poorer HCV virologic response in HCV/HIV coinfected patients. Program and Abstracts of the 12th Conference on Retroviruses and Opportunistic Infections; Boston, MA. February 22–25, 2005; [Abstract 925] [Google Scholar]
- 22.Sulkowski MS, Mehta SH, Torbenson M, Afdhal NH, Mirel L, Moore RD, et al. Hepatic steatosis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS. 2005;19:585–592. doi: 10.1097/01.aids.0000163935.99401.25. [DOI] [PubMed] [Google Scholar]
- 23.Monto A, Dove LM, Bostrom A, Kakar S, Tien PC, Wright TL. Hepatic steatosis in HIV/hepatitis C coinfection: prevalence and significance compared with hepatitis C monoinfection. Hepatology. 2005;42:310–316. doi: 10.1002/hep.20805. [DOI] [PubMed] [Google Scholar]
- 24.Marks KM, Petrovic LM, Talal AH, Murray MP, Gulick RM, Glesby MJ. Histologic findings and clinical characteristics associated with hepatic steatosis in patients coinfected with HIV and hepatitis C virus. J Infect Dis. 2005;192:1943–1949. doi: 10.1086/497608. [DOI] [PubMed] [Google Scholar]
- 25.Bani-Sadr F, Carrat F, Bedossa P, Piroth L, Cacoub P, Perronne C, et al. Hepatic steatosis in HIV-HCV coinfected patients: analysis of risk factors. AIDS. 2006;20:525–531. doi: 10.1097/01.aids.0000210606.63138.f5. [DOI] [PubMed] [Google Scholar]
- 26.Lapoile E, Vona G, Canioni D, Chaix M-L, Nalpas B, Fontaine C, et al. Factors participating in severe HCV-related liver disease in HIV/HCV coinfection [Abstract] J Hepatol. 2002;36(Suppl 1):172. [Google Scholar]
- 27.Sanchez-Conde M, Berenguer J, Miralles P, Alvarez F, Lopez JC, Cosin J, et al. Liver biopsy findings for HIV-infected patients with chronic hepatitis C and persistently normal levels of alanine aminotransferase. Clinical Infectious diseases. 2006;43:640–644. doi: 10.1086/506440. [DOI] [PubMed] [Google Scholar]
- 28.Gaslightwala I, Bini EJ. Impact of human immunodeficiency virus infection on the prevalence and severity of steatosis in patients with chronic hepatitis C virus infection. J Hepatol. 2006;44:1026–1032. doi: 10.1016/j.jhep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Merchante N, Macias J, Ramayo E, Vergara S, Garcia-Garcia A, Mira JA, et al. Insulin resistance is not associated with liver fibrosis progression in HIV/hepatitis C virus-coinfected patients. J Viral Hep. 2006;13:449–456. doi: 10.1111/j.1365-2893.2005.00708.x. [DOI] [PubMed] [Google Scholar]
- 30.McGovern BH, Ditelberg JS, Taylor LE, Gandhi RT, Christopoulos KA, Chapman S, et al. Hepatic steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin Infect Dis. 2006;43:365–372. doi: 10.1086/505495. [DOI] [PubMed] [Google Scholar]
- 31.Neau D, Winnock M, Castéra L, Le Bail B, Loko MA, Géraut L, et al. the Groupe d’Epidemiologie Clinique du SIDA en Aquitaine. J Acquir Immune Defic Syndr. 2007;45:168–173. doi: 10.1097/QAI.0b013e318042e1db. [DOI] [PubMed] [Google Scholar]
- 32.Adinolfi LE, Durante-Mangoni E, Zampino R, Ruggiero G. Review article: hepatitis C virus-associated steatosis—pathologic mechanisms and clinical implications. Aliment Pharmacol Ther. 2005;22(Suppl 2):52–55. doi: 10.1111/j.1365-2036.2005.02597.x. [DOI] [PubMed] [Google Scholar]
- 33.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 34.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 35.Contos MJ, Sanyal AJ. The clinicopathologic spectrum and management of nonalcoholic fatty liver disease. Adv Anat Pathol. 2002;9:37–51. doi: 10.1097/00125480-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Kleiner DE, Brunt EM, Van NM, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 37.Sherman KE, Peters M, Koziel MJ. HIV and liver disease forum: conference proceedings. Hepatology. 2007;45:1566–1577. doi: 10.1002/hep.21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 39.Salmon-Ceron D, Lewden C, Morlat P, Bévilacqua S, Jougla E, Bonnet F, et al. The Mortality 2000 Study Group. J Hepatol. 2005;42:799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 40.Tien PC, Grunfeld C. The fatty liver in AIDS. Semin Gastrointest Dis. 2002;13:47–54. [PubMed] [Google Scholar]
- 41.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 42.Grassi A, Ballardini G, Susca M, Bianchini F, Bonoli S, Bianchi FB, et al. HCV liver infection and liver steatosis: evidence for indirect mechanisms in genotype 3? Aliment Pharmacol Ther. 2005;22(Suppl 2):79–82. doi: 10.1111/j.1365-2036.2005.02603.x. [DOI] [PubMed] [Google Scholar]
- 43.Browning JD, Szczepaniak LS, Dobbins R, Nurenberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 44.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999;354:1112–1115. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 45.Nolan D, John M, Mallal S. Antiretoviral therapy and the lipodystrophy syndrome, part 2: concepts in aetiopathogenesis. Antivir Ther. 2001;6:145–160. [PubMed] [Google Scholar]
- 46.Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 47.Allory Y, Charlotte F, Benhamou Y, Opolon P, Le Charpentier Y, Poynard T. Impact of human immunodeficiency virus infection on the histological features of chronic hepatitis C: a case-control study. The Multivirc group. Hum Pathol. 2000;31:69–74. doi: 10.1016/s0046-8177(00)80201-4. [DOI] [PubMed] [Google Scholar]
- 48.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 49.Hickman IJ, Clouston AD, Macdonald GA, Purdie DM, Prins JB, Ash S, et al. Effect of weight reduction on liver histology and biochemistry in patients with chronic hepatitis C. Gut. 2002;51:89–94. doi: 10.1136/gut.51.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar D, Farrell GC, Fung C, George J. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002;36:1266–1276. doi: 10.1053/jhep.2002.36370. [DOI] [PubMed] [Google Scholar]


