SUMMARY
Astrocytes constitute the most abundant cell type in the CNS, and play diverse functional roles, but the ontogenetic origins of this phenotypic diversity are poorly understood. We have investigated whether positional identity, a fundamental organizing principle governing the generation of neuronal subtype diversity, is also relevant to astrocyte diversification. We identified three positionally distinct subtypes of white matter astrocytes in the spinal cord, which can be distinguished by the combinatorial expression of Reelin and Slit1. These astrocyte subtypes derive from progenitor domains expressing the homeodomain transcription factors Pax6 and Nkx6.1, respectively. Loss- and gain-of-function experiments indicate that the positional identity of these astrocyte subtypes is controlled by Pax6 and Nkx6.1, in a combinatorial manner. Thus, positional identity is an organizing principle underlying astrocyte, as well as neuronal, subtype diversification, and is controlled by a homeodomain transcriptional code whose elements are re-utilized following the specification of neuronal identity earlier in development.
INTRODUCTION
A central problem in neural development is to elucidate the mechanisms that control the ontogenetic diversification of neuronal and glial subtypes in the central nervous system (CNS). In the last decade, positional identity has emerged as one of the fundamental organizing principles governing neuronal subtype diversification. In the ventral spinal cord, for example, molecularly distinct subtypes of motoneurons (MNs) and interneurons (INs) are generated from spatially segregated domains of progenitor cells, arranged along the dorsoventral axis of the ventricular zone (VZ) (Burrill et al., 1997; Ericson et al., 1997; Briscoe et al., 1999). These progenitor domains are generated by a combinatorial code of homeodomain (HD) transcription factors, whose expression patterns are initially established by graded morphogen signaling, and further refined by cross-repressive interactions (Briscoe et al., 2000; Goulding and Lamar, 2000; Jessell, 2000; McMahon, 2000).
While astrocytes are the most abundant cell type in the central nervous system (CNS), play varied functional roles (reviewed in (Fields and Stevens-Graham, 2002; Ullian et al., 2004)) and exhibit phenotypic heterogeneity (see below), there has been relatively little consideration of positional identity as an organizing feature of astrocyte diversity, or of positional specification as a mechanism underlying astrocyte diversification. The existence of different subtypes of astrocytes, such as fibrous and protoplasmic, has long been recognized based on morphologic (Vaughn and Pease, 1967; Mori and Leblond, 1969) and antigenic (Raff et al., 1984; Raff, 1989) criteria. However, these subtypes are thought to spatially segregate primarily according to their location in either gray or white matter (Miller and Raff, 1984). It has been speculated that spinal cord astrocytes may exhibit regional distinctions, based on studies of astrocyte phenotypes in vitro (reviewed in (Miller et al., 1994)), but whether such phenotypes are positionally distinct in vivo, or established by positional specification mechanisms, was not established. Morphologically distinct astrocyte subtypes have been identified in different layers of the olfactory bulb (Bailey and Shipley, 1993), and astrocytes with different electrophysiological properties have been described in hippocampal areas CA1 and CA3 (D’Ambrosio et al., 1998). However, with few molecular markers to differentiate these subtypes in vivo (Sharif et al., 2004), it has been difficult to study their ontogeny, phenotypic stability and function.
There is some evidence for positional heterogeneity among astrocyte precursors in the spinal cord. The bHLH transcription factor SCL is specifically expressed in the p2 progenitor domain (Briscoe et al., 2000), and is required for generic aspects of astrocyte differentiation within this domain (Muroyama et al., 2005). However these data did not provide evidence that differentiated p2-derived astrocytes are phenotypically distinct from those derived from other progenitor domains. Expression of FGFR3 is initially restricted to p2-derived astrocyte precursors (Pringle et al., 2003), but later expands to astrocytes at other positions along the dorso-ventral axis (Deneen et al., 2006). The expression of several patterning molecules controlling neuronal identity is maintained in the VZ, during the transition from the neurogenic to the gliogenic phase of development (Fu et al., 2003; Ogawa et al., 2005; Deneen et al., 2006; Sugimori et al., 2007), and it has been speculated that this may indicate the existence of positionally distinct astrocyte subtypes (Ogawa et al., 2005). However, no evidence has been presented for the existence of such subtypes in the white matter.
Here we identify three positional distinct subpopulations of white matter astrocytes (WMAs) in the ventral spinal cord, and characterize a homeodomain code that is required for their specification in the ventricular zone. Our data indicate that positional identity is an organizing feature of astrocyte, as well as neuronal, diversity in the CNS and is controlled by similar molecular mechanisms.
RESULTS
Reelin and Slit1 mark subpopulations of WMAs in ventral spinal cord
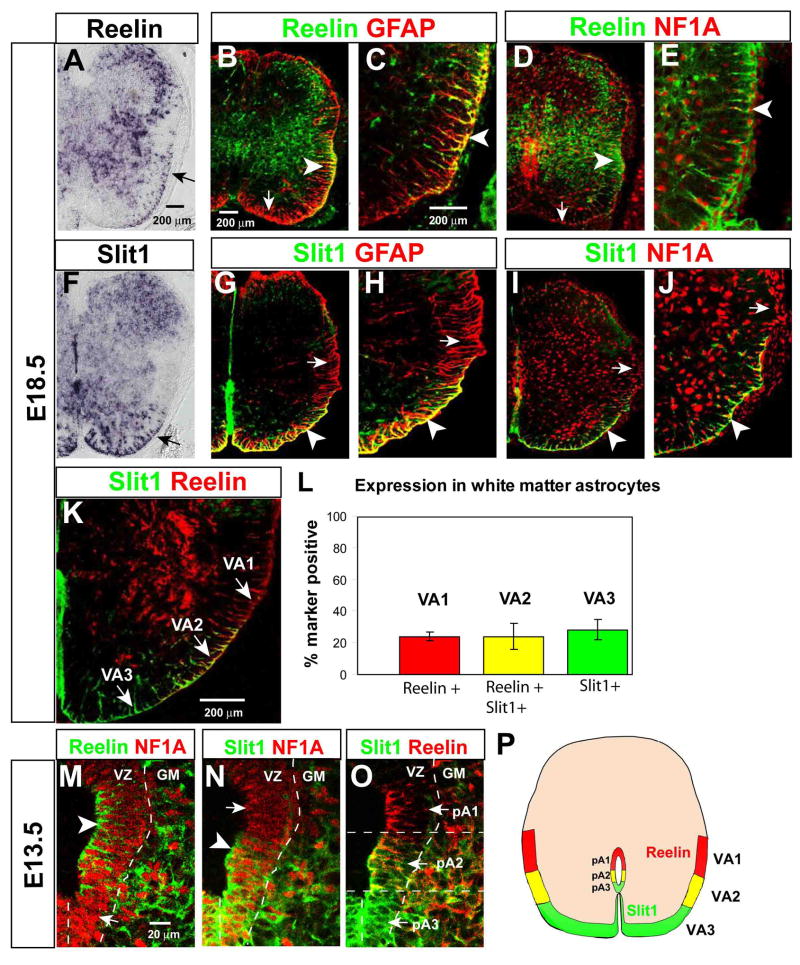
We initially identified Reelin and Slit1 as astrocyte markers in a gene expression-profiling screen for targets of Olig2. In Olig2 mutants, Olig2+ progenitors generate astrocytes instead of oligodendrocytes, at gliogenic stages (Lu et al., 2002; Zhou and Anderson, 2002). Expression profiling of Olig2-GFP-expressing glial progenitors isolated from Olig2 mutant vs. wild-type spinal cord ((Gabay et al., 2003; Mukouyama et al., 2006); see Experimental Procedures) identified Reelin and Slit1 mRNAs as up-regulated in Olig2−/− cells, suggesting that they might be expressed by astrocyte progenitors. In situ hybridization to E18.5 spinal cord sections indicated that Reelin and Slit1 mRNAs are expressed by a subset of cells at the margins of the white matter (Figure 1A, F, arrows).
Figure 1. Reelin and Slit1 define astrocyte subpopulations in the ventral spinal cord.
(A, F) In situ hybridization for mouse Reelin and Slit1 mRNAs. Arrows indicate expression in ventral white matter. (B–E) Double-label immunohistochemistry for Reelin and GFAP (B–C) or NFIA (D–E). Arrowheads indicate double-positive cells in lateral white matter, arrows Reelin− astrocytes in ventral white matter. (G–J) Double-label immunohistochemistry for Slit1-GFP and either GFAP (G–H) or NFIA (I–J), in Slit1GFP/+ embryos. Arrowheads indicate Slit1+ astrocytes in ventral white matter, arrows Slit1− astrocytes in the lateral white matter. (K) Double labeling for Reelin and Slit1-GFP reveals three subpopulations of ventral astrocytes (arrows). (L) Quantification of the percentage of NFIA+ astrocytes expressing each of the three markers. Data represent the mean±S.E.M. of 5–6 sections/embryo from 3 embryos. (M–O) Triple labeling for Reelin, Slit1-GFP and NFIA in the E13.5 ventral VZ, displayed as pairwise comparisons from the same section. Arrowheads and arrows in (M, N) indicate NFIA+ cells that are positive or negative, respectively, for Reelin (M) or Slit1 (N). (O) Overlay of Slit1 and Reelin reveals three adjacent progenitor domains in the VZ. (P) Composite schematic illustrating positions of VA1–VA3 astrocytes in the white matter at E18.5, and corresponding progenitor domains (pA1–3) in the VZ at E13.5.
To confirm that Reelin and Slit1 are expressed by WMAs, we performed double labeling studies using the generic astrocyte markers GFAP, a glial-specific intermediate filament, or NFIA, a nuclear protein expressed in differentiated astrocytes and their precursors (Deneen et al., 2006), together with antibodies to Reelin, or to GFP in a Slit1-GFP reporter mouse (Plump et al., 2002). In the white matter of embryonic day 18.5 (E18.5) spinal cord, both Reelin and Slit1 were expressed by astrocytes (Fig. 1B–E and G–J, arrowheads), but not by Olig2+ oligodendrocytes (data not shown). Both Reelin and Slit1 were also expressed by neurons in the gray matter, as previously reported (Holmes et al., 1998; Brose et al., 1999; Kubasak et al., 2004; Yip et al., 2004a). The morphology of both Reelin+ and Slit1+ white matter cells is characteristic of WMAs (Liuzzi and Miller, 1987), with NFIA+ nuclei localized at the subpial surface (Fig. 1E, J, arrowheads)) and radially oriented GFAP+ processes projecting inward (Fig. 1C, H, arrowheads; see also Supplemental Figure S1).
Unexpectedly, we found that Reelin and Slit1 were not expressed by all astrocytes, but rather by positionally distinct subsets in the ventral white matter. Reelin was expressed in the dorso-lateral and ventro-lateral white matter, but not in astrocytes close to the ventral midline (Fig. 1A, arrow; B, arrowhead). Slit1, conversely, was expressed in astrocytes in the ventro-medial and ventro-lateral white matter, but not in the dorso-lateral white matter (Fig. 1F, arrow; G, arrowhead). Double labeling for Reelin and Slit1 revealed the existence of three adjacent domains of WMAs: a dorso-lateral domain of Reelin+, Slit1− cells; a ventro-lateral domain of Reelin+, Slit1+ cells; and a ventro-medial domain of Slit1+, Reelin− cells (Fig. 1K). For convenience, we refer to these subpopulations henceforth as Ventral Astrocyte subtypes 1, 2 and 3 (VA1, VA2 and VA3, respectively; Fig. 1K, P). Quantification indicated that each of these three subpopulations is present in roughly equal numbers (Fig. 1L).
In principle, VA1–VA3 phenotypes could be established after astrocyte precursors migrate to the WM, under the influence of local environmental cues, or could be specified by positional mechanisms prior to emigration from the VZ. As a first step towards addressing this question, we asked whether Reelin and Slit1 were expressed by positionally distinct subsets of astrocyte precursors within the neuroepithelium. Examination of spinal cord sections at E13.5, a stage when most astrocyte precursors have been specified in the ventral VZ (Shibata et al., 1997; Ogawa et al., 2005; Deneen et al., 2006), revealed that Reelin and Slit1 are expressed in cells within the germinal layer. Triple-labeling for Reelin, Slit1-GFP and NFIA indicated that Reelin and Slit1 are expressed by NFIA+ glial precursors (Fig. 1M, N, arrowheads), and that the domains of their expression partially overlap (Fig. 1O). This partial overlap subdivides the ventral-most VZ into three domains: a dorsal-most Reelin+, Slit1− domain; a more ventral Reelin+, Slit1+ domain; and a ventro-medial Reelin−, Slit1+ domain (Fig. 1O). The spatial organization of these progenitor domains, which we refer to as pA1, pA2 and pA3, respectively, therefore mirrors that of the VA1, VA2 and VA3 domains in the WM (Fig. 1P).
Pax6 marks Reelin+ astrocytes
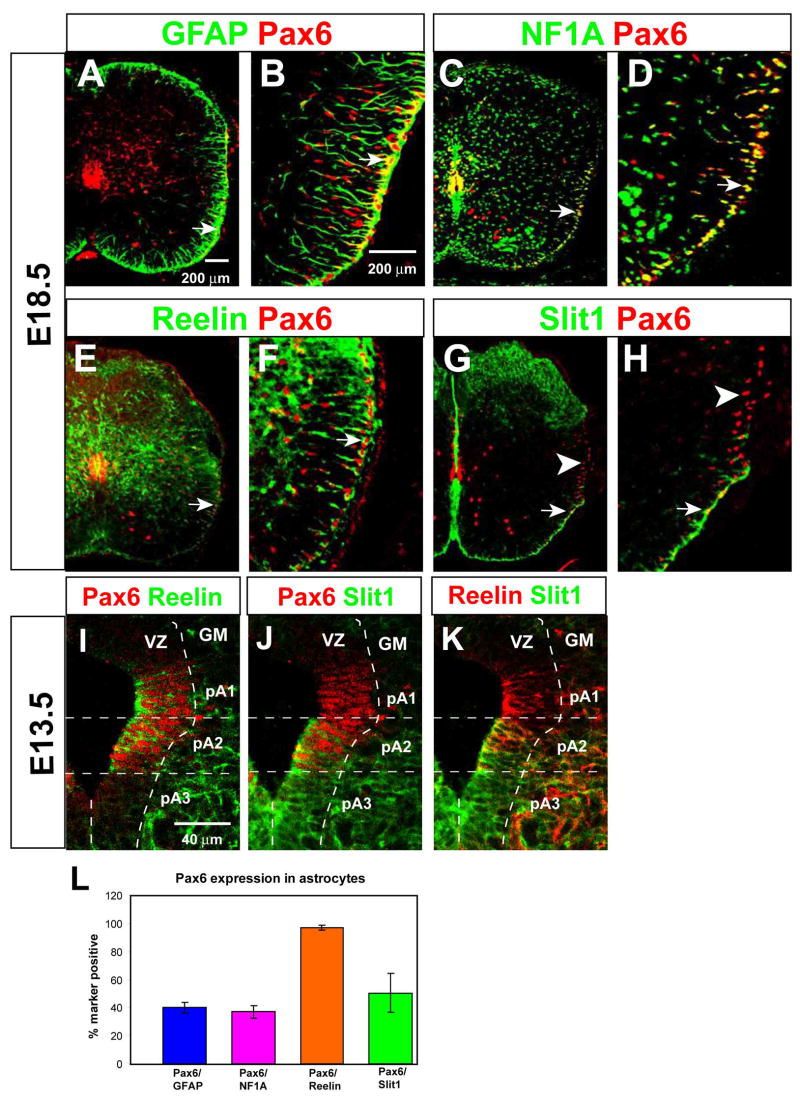
Our microarray analysis also indicated that Pax6 mRNA was up-regulated, together with Reelin and Slit1, in the Olig1,2−/− population. Double-labeling experiments indicated that Pax6 marks a subpopulation of astrocytes in the ventro-lateral white matter (Fig. 2A–D), whose distribution is similar to that of Reelin+ astrocytes (Fig. 2E–F). Pax6+ cells constitute approximately 40% of all GFAP+ and NF1A+ WMAs (Fig. 2A–D, L). Double labeling for Reelin and Pax6 confirmed that 100% of Reelin+ astrocytes co-express Pax6, suggesting that Pax6 marks the VA1 and VA2 subpopulations (Fig. 2E, F, arrows; L). Since Slit1+ astrocytes are equally distributed between the VA3 and VA2 populations, ~50% of Slit1+ astrocytes (VA2) should be Pax6+, and this was indeed the case (Fig. 2H, arrow vs. arrowhead; L). Pax6 was excluded from the ventro-medial domain of Slit1 expression (Fig. 2G), corresponding to the VA3 subset. In the VZ at E13.5, triple-labeling for Pax6, Reelin and Slit1 indicated that the spatial relationship between expression of the HD factor and the two astrocyte markers was similar to that observed in the white matter at E18.5: Pax6 overlapped with all of the Reelin-expressing progenitors (Fig. 2I), and a subset of the Slit1-expressing progenitors (Fig. 2J).
Figure 2. Expression of Pax6 in Reelin+ astrocytes and their precursors.
(A–H) Double-labeling for Pax6 (red) and the indicated markers (green) at E18.5. (B, D, F, and H) are higher magnification views of the areas indicated by arrows in (A, C, E and G), respectively. (I–K) Triple labeling for Pax6, Reelin, and Slit1-GFP in the E13.5 ventral VZ, displayed as pairwise comparisons from the same section. (L) Quantification of Pax6 expression among total (GFAP+ or NFIA+) astrocytes, and Reelin+ or Slit1+ WMAs.
To investigate whether Pax6+ cells in the VZ are precursors of Pax6+ WMAs, we pulse-labeled embryos at E13.5 with BrdU, and chased to E15.5 or E18.5. We observed a progressive shift in the distribution of both total Pax6+ cells, and of BrdU-labeled Pax6+ cells, from the VZ to the mantle zone (MZ) to the WM, during the chase period (Supplemental Figure S2J, K). The proportion of Pax6+ cells in the WM increased ~2.5-fold from E15.5 to E18.5, and this increase could be quantitatively accounted for by assuming that, during the same interval, Pax6+ cells in the VZ migrate to the MZ, and from the MZ to the WM (see Supplemental Fig. S2, legend). The most parsimonious interpretation of these data is that at least some Pax6+ cells in the E13.5 VZ are precursors of Pax6+ WMAs.
Pax6 is required for Reelin expression in astrocytes
Pax6 has been shown to control the positional identity of a subset of INs in the ventral spinal cord (Burrill et al., 1997; Ericson et al., 1997). We therefore asked whether it might also play a role in the determination of astrocyte positional identity. Pax6lacZ/lacZ homozygous mutants (St-Onge et al., 1997) exhibited a striking reduction in Reelin mRNA expression in the ventro-lateral white matter at E18.5, while expression in the gray matter appeared only modestly reduced (Fig. 3A–B, arrows vs. E–F, arrowheads). Immunostaining confirmed a loss of Reelin expression in GFAP+ and NFIA+ astrocytes (Fig. 3C–D, arrows vs. G–H, arrowheads). Quantification indicated a strong and statistically significant reduction in both the percentage (Fig. 3I) and absolute number (Fig. 3J) of Reelin+ astrocytes, using either GFAP or NFIA as counter-stains.
Figure 3. Astrocyte subtype conversion in Pax6−/− mice.
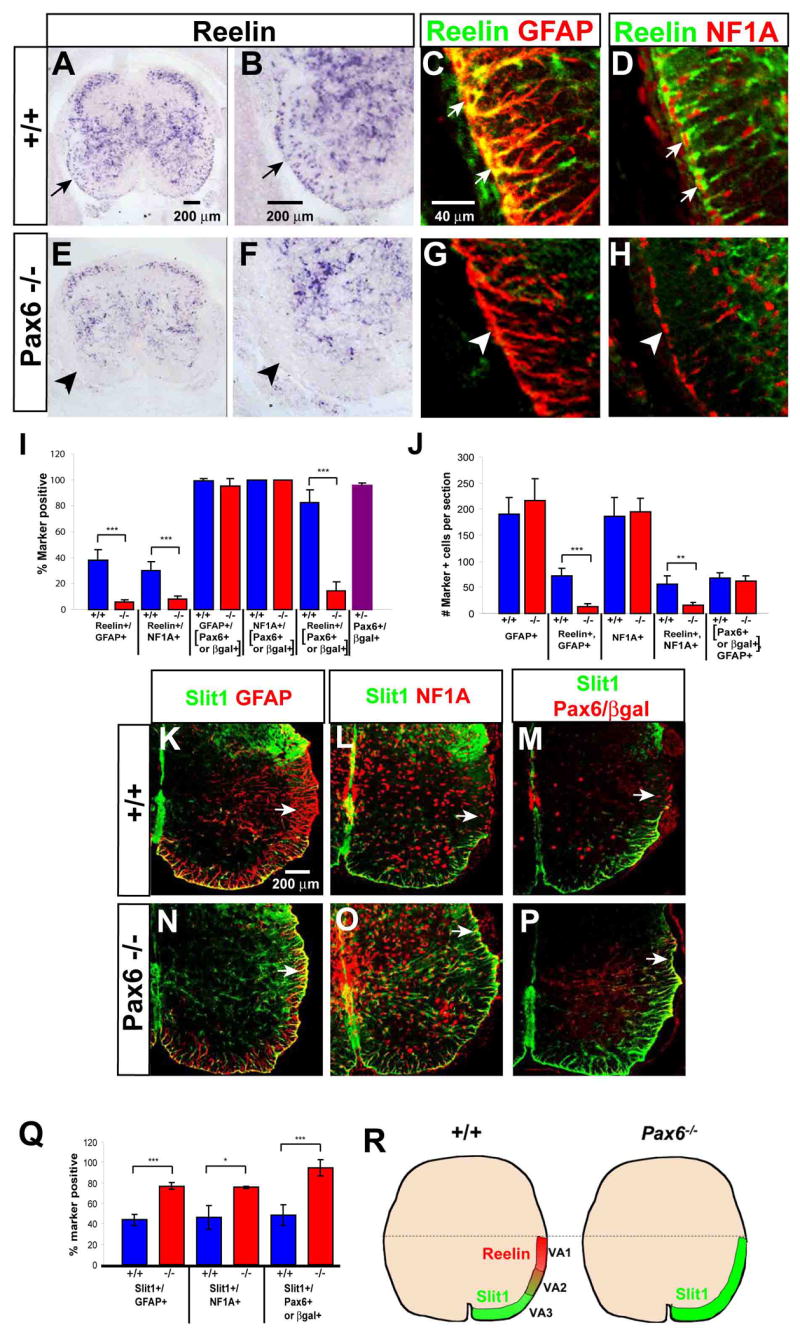
(A–B, E–F) In situ hybridization for Reelin mRNA in E18.5 spinal cord of wild-type (A–B) and Pax6 mutant (E–F) embryos. (B) and (F) are higher-magnification views of (A) and (E), respectively. Arrows in (A, B) indicate Reelin+ cells in the white matter. (C–D, G–H) Double-labeling for Reelin and GFAP (C, G) or NFIA (D, H) in wild-type (C–D) and Pax6 mutant (G–H) embryos. (I–J) Quantification of Reelin expression by WMAs in E18.5 wild type (blue bars) and Pax6−/− (red bars) spinal cord. . (I) The percentage of total GFAP+ or NFIA+ WMAs expressing Reelin is significantly reduced in the Pax6lacZ/lacZ mutant (***, p<.001). (J) The absolute number of GFAP+ and NFIA+ astrocytes in the white matter is not changed in the Pax6lacZ/lacZ spinal cord, nor is the average number of Pax6+ GFAP+ astrocytes. (*** and **, p<.001 and .002, respectively). . The data are derived from 5 wild type and 6 mutant embryos from 3 independent litters. (K–P) Spinal cord sections from Pax6+/+; Slit1GFP/+ (K–M) and Pax6lacZ/lacZ; Slit1GFP/+ (N–P) embryos double-labeled for the indicated markers. Arrows indicate VA1 astrocytes (K–M) that exhibit de-repression of Slit1 in the mutant (N–P). Red staining in (M, P) represents anti-Pax6 (M) or anti-βgal (P). (Q) Slit1 expression among total astrocytes (GFAP+ or NFIA+) is significantly increased in Pax6−/− embryos (*** and *, p = .0003, and .012, respectively). (H) Schematic illustrating the changes in Reelin and Slit1 expression by ventral astrocytes in the Pax6 mutant.
We detected no reduction in the total number of astrocytes in the spinal cord of E18.5 Pax6 mutant embryos (Fig. 3J, GFAP+ and NFIA+), consistent with an earlier analysis using GLAST as a generic astrocyte marker (Ogawa et al., 2005). Since Reelin+ cells constitute ~60% of all ventral WMAs, this argues against the idea that Reelin+ astrocytes selectively die in the absence of Pax6, otherwise there should be a measureable reduction in overall astrocyte number. However, it is possible that the mutation could cause the selective death of Pax6+ astrocytes, which is then compensated by the expansion of other, Pax6−, astrocyte subpopulations. To address this possibility, we employed the marker gene lacZ, present in the Pax6 knockout allele (St-Onge et al., 1997), to trace the fate of Pax6+ cells in the absence of Pax6 function. The percentage of Pax6+ or βgal+ cells that expressed GFAP or NFIA was identical in wild-type and mutant embryos, respectively (Fig. 3I; GFAP+/[Pax6+ or βgal+]; NFIA+/[Pax6+ or βgal+]). Thus, the loss of Pax6 did not produce a failure of generic astrocyte differentiation by Pax6+ cells. Furthermore, the absolute number of GFAP+ Pax6+ (or GFAP+ βgal+) cells was unaffected in the mutant (Fig. 3J; [Pax6+ or βgal+], GFAP+), confirming that there was no selective death of Pax6+ astrocytes. However, there was a strong reduction in the percentage of Pax6-βgal+ cells that were Reelin+ in the mutant, compared to wild-type (Fig 3I, Reelin+/[Pax6+ or βgal+]). Taken together, these data indicate that Pax6 is required for the expression of Reelin in VA1 and VA2 astrocytes, but not for their generic differentiation to astrocytes, migration to the white matter or survival.
Dorsal expansion of astrocytic Slit1 expression in the absence of Pax6
The foregoing analysis left open the question of whether the loss of Pax6 function simply caused a failure of Reelin expression by astrocytes, or rather a change in the positional identity of VA1 astrocytes. In the latter case, one might expect that presumptive VA1 astrocytes would acquire a Slit1+ phenotype. Due to a lack of adequate antibodies to Slit1, we inter-crossed the Pax6-lacZ mice with Slit1-GFP mice, and analyzed the expression of Slit1-GFP in Pax6 mutants (SlitGFP/+; Pax6lacZ/lacZ). In Pax6 mutant embryos, there was a significant increase in the percentage of GFAP+ or NFIA+ astrocytes that expressed Slit1-GFP (Fig. 3Q, red bars). Moreover, the spatial domain of Slit1-GFP expression appeared to expand dorsally in the mutant (Fig. 3K-M vs. N–P; arrows). Most importantly, the percentage of Pax6+ astrocytes that expressed Slit1 increased from ~50–60% in wild-type embryos, to virtually 100% in the mutant (Fig. 3Q, Slit1+/Pax6+ or βgal+), reflecting the acquisition of Slit1 expression by dorsal Pax6-βgal+ cells (Fig. 3M, P, arrows). Taken together, these data indicate that in the absence of Pax6 function, not only is Reelin expression lost, but Slit1 expression is de-repressed, in VA1-type astrocytes (Fig. 3R). This suggests that loss of Pax6 causes a ventralization of the VA1 domain, such that supernumerary VA3 astrocytes are now found in a more dorsal, ectopic location, analogous to the phenotypes of other spinal cord patterning mutations that change the number and distribution of interneuron subtypes (Jessell, 2000; Shirasaki and Pfaff, 2002).
Pax6 is sufficient to promote Reelin and repress Slit1 expression in astrocytes
To investigate whether Pax6 plays an instructive role in regulating the expression of markers of astrocyte positional identity, we performed in vivo gain of function experiments, in the embryonic chick neural tube. We first examined Reelin and Slit1 expression in the unmanipulated E12 chick spinal cord, to confirm that these genes labeled subsets of WMAs, as in the mouse. We observed white matter domains containing Reelin+/Slit1−, Reelin+/Slit1+ and Reelin−/Slit1+ VA1–3, astrocytes, as in the mouse (Supplemental Figure S3). We therefore examined the effect of Pax6 mis-expression on these ventral astrocyte populations. The spinal cord of E2 chick embryos was electroporated with replication competent RCAS(B) retroviruses carrying either the chick Pax6 or GFP genes. Electroporated embryos were harvested and analyzed at E12, following 10 days of development in ovo. Pax6 mis-expression significantly increased the percentage of Reelin+ cells among NFIA+ cells in the white matter, compared with control GFP mis-expressing embryos (Fig. 4F vs. H, “WM;” CC, “Reelin,” “E”). The total number of NFIA+ astrocytes was not affected by Pax6 electroporation (data not shown). These data indicate that Pax6 is sufficient, as well as necessary, for Reelin expression by ventral astrocytes in vivo.
Figure 4. Pax6 is sufficient to promote Reelin expression and repress Slit1 expression in astrocytes.
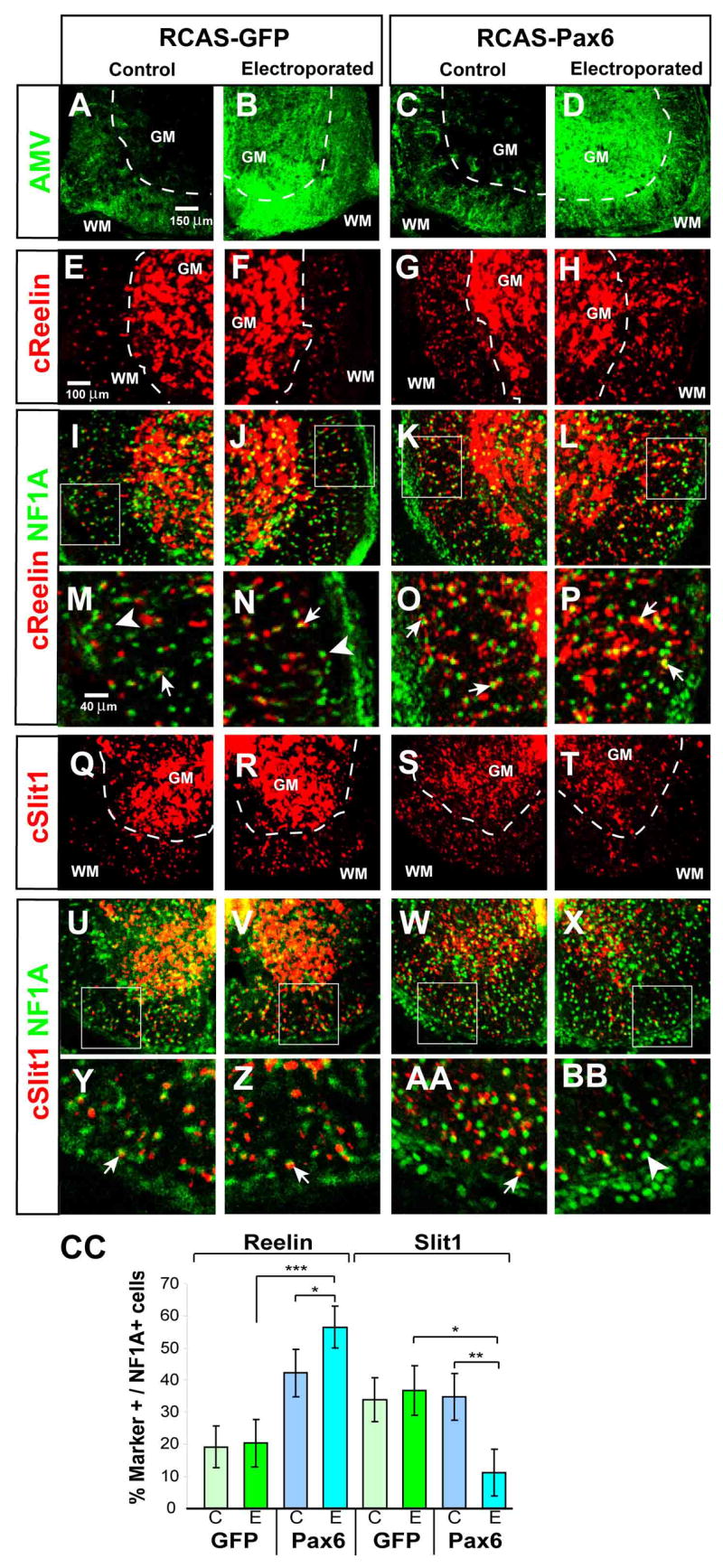
(A–D) Immunostaining for RCAS retroviral coat protein AMV in E12 spinal cord of chick embryos electroporated with either RCAS-GFP (A, B) or RCAS-Pax6 (C, D “WM,” white matter; “GM,” gray matter. (E-BB) Antibody staining for NFIA combined with fluorescent in situ hybridization for Reelin (E–P) or Slit1 (Q-BB) mRNAs. (I–L) and (U–X) are the same panels as in (E–H) and (Q–T), respectively, but include NF1A expression. (M–P) and (Y-BB) are higher power images of the boxed areas indicated in (I–L) and (U–X), respectively. Arrows indicate double-positive cells, arrowheads NFIA single-positive cells. (CC) The percentage of Reelin+ NFIA+ cells is significantly different between the electroporated (E) sides of Pax6 and GFP embryos (***, p <.001), as well as between the E and contralateral (C) sides of Pax6 embryos (*, p=.02). The reduction in Slit1 expression on the E side of Pax6 embryos is significant with respect to both the C side (**, p = .002), and the E side of GFP controls (*, p = .009). mean±S.E.M., n= 5 embryos per condition, two independent experiments.
Because we observed Pax6+, Reelin+ cells in the VZ as well as in the white matter (Fig. 2I), we also asked whether mis-expression of Pax6 could promote a ventral expansion of the domain of Reelin expression, in this germinal layer. We therefore examined embryos at E5, a stage at which ventral VZ progenitors are specified for a glial fate (Shibata et al., 1997; Deneen et al., 2006). Pax6 mis-expression indeed promoted a ventral expansion of Reelin expression within the VZ (Supplemental Figure S4C, arrow; M). Importantly, a similar result was obtained when electroporation was performed at E4.5, by which time neurogenesis has largely ceased in the ventral region of the VZ (Deneen et al., 2006) (Supplemental Figure S4I, arrow; M). This ventral expansion of Reelin expression occurred in NFIA+ cells (Fig. S4B, H, arrows), which are likely glial precursors (Deneen et al., 2006). The fact that this phenotype can be observed in embryos electroporated after ventral neurogenesis has terminated suggests a direct role for Pax6 to control Reelin expression in glial precursors, rather than an indirect role mediated by earlier effects on neuronal precursors. In support of this conclusion, we were able to observe changes in Reelin expression, caused mis-expression of Pax6, at axial levels where there was no change in the number or distribution of ventral interneuron subtypes whose development is Pax6-dependent (Burrill et al., 1997; Ericson et al., 1997; Osumi et al., 1997) (data not shown).
The de-repression of Slit1 observed in Pax6−/− embryos predicted that mis-expression of Pax6 might down-regulate Slit1 expression in WMAs. Indeed, we observed a significant reduction in the percentage of Slit1+; NFIA+ astrocytes in the ventral region of Pax6- electroporated embryos, compared to GFP-electroporated controls (Fig. 4R vs. T, “WM;” CC, “Slit1”). Pax6 mis-expression also caused a mild, but detectable, down-regulation of Slit1 mRNA in the VZ at E5 (data not shown). Thus, Pax6 is sufficient, as well as necessary, for Slit1 repression in a subset of astrocytes and their presumptive precursors. Taken together, these data suggest that Pax6 plays an instructive role in regulating astrocyte positional identity, through positive and negative regulation of Reelin and Slit1, respectively.
Expression of Nkx6.1 by Slit1+ astrocytes and their precursors
The observation that Pax6 promotes expression of Reelin, and represses that of Slit1, explained the VA1 phenotype, but raised the question of how VA2 astrocytes can express Slit1, given that they are also Pax6+. One possibility is that repression of Slit1 by Pax6 requires a cofactor, which is present in VA1 astrocytes but lacking in VA2 astrocytes. This seemed unlikely, since Pax6 was able to repress Slit1 in ventral (VA3) WMAs, in gain-of-function experiments. Alternatively, VA2 astrocytes may contain a factor absent in VA1 astrocytes, which promotes Slit1 expression and/or neutralizes the repressive activity of Pax6 towards Slit1. One candidate for the latter activity is Nkx6.1, a HD transcription factor expressed in the ventral VZ at both neurogenic and gliogenic (Fu et al., 2003) stages. Expression of Nkx6.1 partially overlaps that of Pax6, in the p2 and pMN domains, while the more dorsal p0 and p1 domains express Pax6 but not Nkx6.1 (Briscoe et al., 2000; Jessell, 2000; Briscoe and Ericson, 2001). We therefore investigated the possibility that expression of Slit1 in WMAs is under the control of Nkx6.1.
We first compared the expression of Nkx6.1 and Slit1 in E18.5 spinal cord WMAs. Triple-labeling for GFAP, Nkx6.1 and Slit1-GFP revealed that Nkx6.1 is expressed in WMAs (Fig. 5A–C, arrows), and that the dorso-ventral domain of its expression is approximately co-extensive with that of Slit1 (Fig. 5A–B, D). Quantification indicated that >90% of Slit1+ WMAs are Nkx6.1+ (Fig. 5J, green bar). We next asked whether Pax6 and Nkx6.1 are co-expressed in the region of the white matter where VA2 astrocytes (Pax6+, Slit11+) are located. Triple labeling revealed an overlap of Pax6 and Nkx6.1 expression in the ventro-lateral white matter, within Slit1+ cells (Fig. 5E–I, arrows). Quantification indicated that virtually 100% of Pax6+, Slit1+ cells were Nkx6.1+ (Fig. 5I, arrows; J, turquoise bar). These data support the idea that VA2 astrocytes are Nkx6.1+, Pax6+ and Slit1+. (It was not possible to directly compare Nkx6.1 and Reelin expression in these experiments, due to antibody incompatibility).
Figure 5. Nkx6.1 is co-expressed by Slit1+ astrocytes and their precursors.
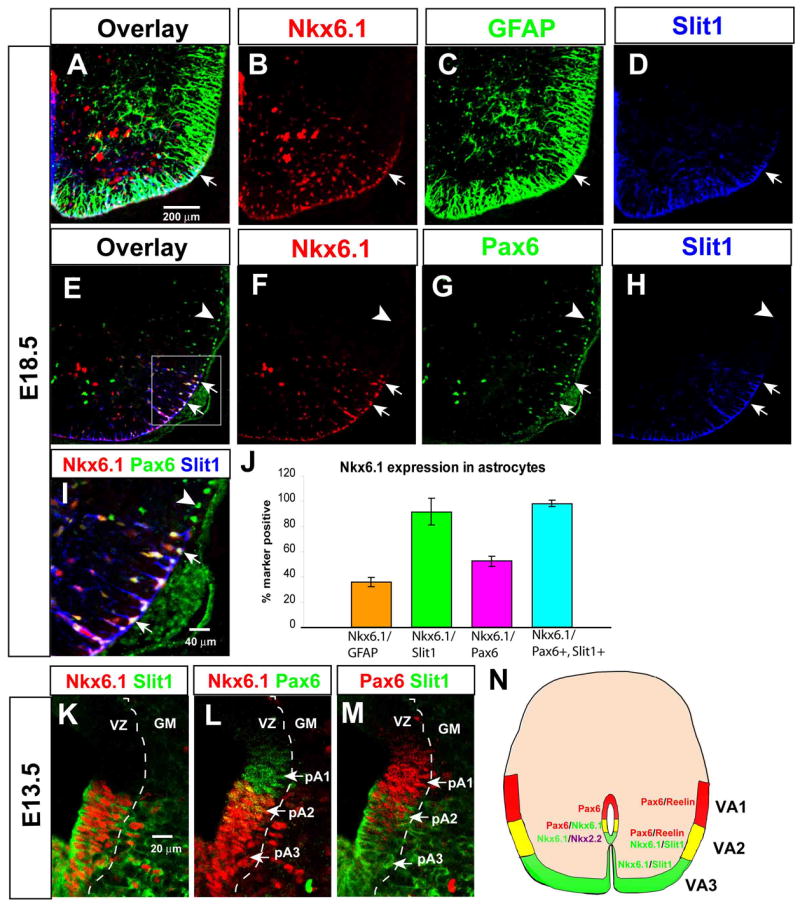
(A–I) Triple-labeling for Nkx6.1, GFAP and Slit1-GFP (A–D) or Nkx6.1, Pax6 and Slit1-GFP (E–I). (I) is a higher power image of the boxed area in (E). Note that all Slit1+ cells in this domain (boxed area, E) are Nkx6.1+ and Pax6+ (E–G, I, arrows, white nuclei), while all Pax6+ cells dorsal to the boundary of Slit1 expression are Nkx6.1− (E–G, I, arrowheads). (J) Quantification of Nkx6.1 expression in astrocyte sub-populations. Nkx6.1 is expressed in > 90% of Slit1+ astrocytes (green bar). (K–M) Triple antibody labeling for Nkx6.1, Slit1-GFP and Pax6 in the E13.5 VZ, displayed as pairwise comparisons from the same section. The three progenitor domains are indicated (L, M, arrows). “GM,” gray matter. (N) Composite schematic illustrating relationship between the domains of Reelin and Slit1 expression, and those of Pax6 and Nkx6.1 expression, in the white matter at E18.5 and in the VZ at E13.5.
In addition to their co-expression in the white matter, Nkx6.1 and Slit1 are co-expressed in the VZ, where the dorsal boundaries of their expression domains are co-extensive (Fig. 5K). Triple-labeling for Pax6, Nkx6.1 and Slit1-GFP indicated that the domain of Pax6 expression that overlaps that of Slit1 (Fig. 5M, pA2) also overlaps that of Nkx6.1 (Fig. 5L, pA2). Taken together, these data suggest that Nkx6.1 is expressed by the VA3 (Pax6−) and VA2 (Pax6+) astrocyte subpopulations, and by their presumptive precursors (pA3 and pA2, respectively), but not by the VA1 astrocytes (Fig. 5N).
Mis-expression of Nkx6.1 promotes expression of Slit1 in WMAs
We wished to examine whether the expression of Nkx6.1 in Slit1+ astrocytes reflects a functional role for this HD protein in the specification of astrocyte identity. As an initial step, we tested the prediction that mis-expression of Nkx6.1 would promote an up-regulation of Slit1 in dorso-lateral (VA1) astrocytes. Indeed, electroporation of Nkx6.1 in chick embryos using an RCAS (B) retroviral construct resulted in a clear induction of Slit1 expression in the white matter on the electroporated side (Fig. 6G–H vs. I–J, “WM,” and Fig. 6QQ, “Slit1”). Co-labeling for NFIA confirmed that this up-regulation occurred in astrocytes (Fig. 6P, V, arrow). Expression of Reelin was unaffected by mis-expression of Nkx6.1 (Fig. 6Y-BB, QQ, “Reelin”). Induction of Slit1 was particularly evident in more dorso-lateral regions of the spinal cord, where Slit1− VA1 astrocytes are normally located (Fig. 6I–J, O–P, and U–V, arrow). This phenotypic effect of Nkx6.1 mis-expression was observed not only at brachial, but also at lumbar axial levels (Supplemental Figure S7), a location where such manipulations have no effect on the differentiation of V0–V2 interneurons (Briscoe et al., 2000). Nkx6.1 mis-expression also promoted a dorsal expansion of Slit1 expression within the VZ at E5 (data not shown). Thus, this HD factor can positively regulate the positional boundaries of Slit1 expression, both in differentiated WMAs, and also in astrocytic precursors within the germinal layer of the spinal cord.
Figure 6. Nkx6.1 promotes Slit1 expression in astrocytes and overrides its repression by Pax6.
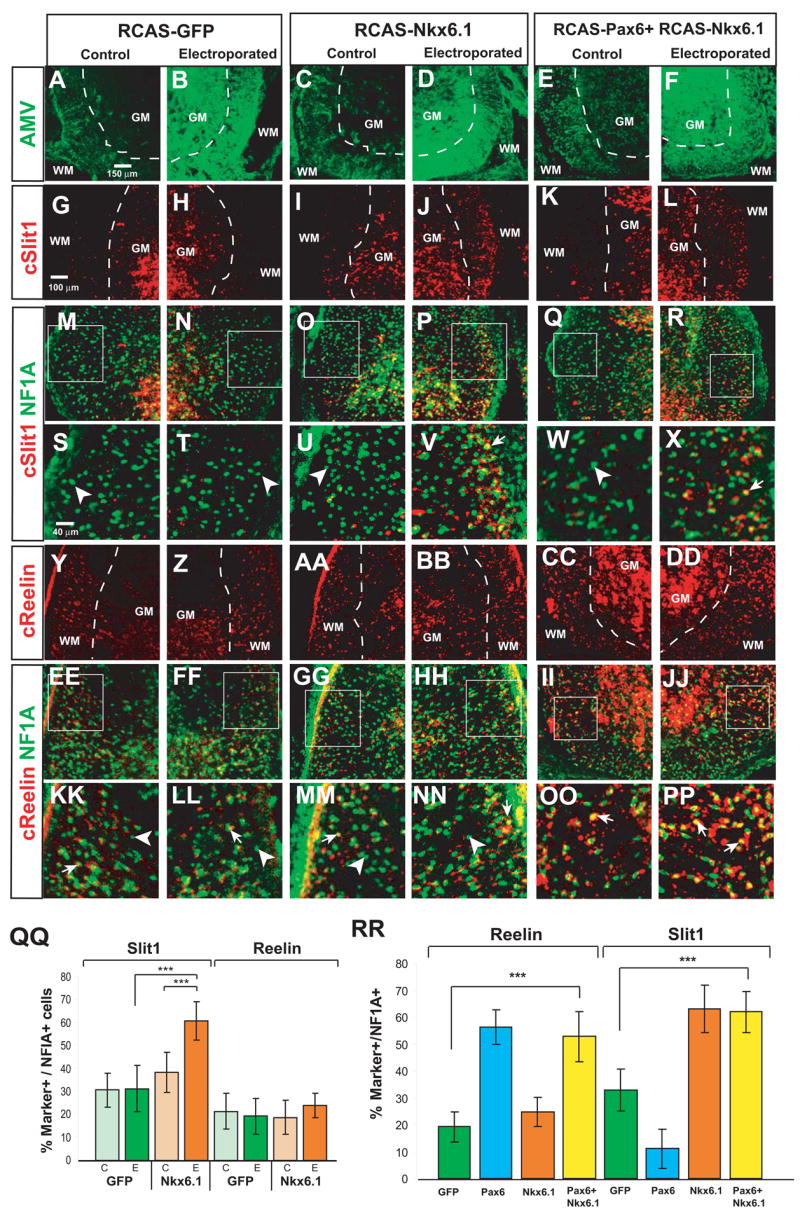
Sections through the spinal cord of E12 chick embryos electroporated with the constructs indicated above the diagram, and labeled with the antibodies (AMV, NF1A) or cRNA probes (cSlit1, cReelin) indicated to the left. Abbreviations as in Fig 4. (M–R) and (EE-JJ) are the same panels as in (G–L) and (Y-DD), respectively, but include NFIA expression. (S–X) and (KK-PP) are higher power images of the boxed areas indicated in (M–R) and (EE-JJ), respectively. Arrows in (S–X) and (KK-PP) indicate double positive cells, arrowheads NFIA single positive cells. (QQ) The percentage of Slit1+ NFIA+ cells is significantly increased on the electroporated (E) side of Nkx6.1 embryos, relative to either the control (C ) side of the same embryos (***, p=.0003), or to the E side of GFP embryos (***, p<.001). (n=4 embryos per condition, two independent experiments). (RR) Bars represent mean ± S.E.M. from the E side only. The data from embryos electroporated with Pax6 alone (blue bars) or Nkx6.1 alone (orange bars) are reproduced from Figs. 4CC and 6QQ, respectively, and included for comparative purposes. The percentage of Slit1+NFIA+ cells in embryos mis-expressing both Nkx6.1 and Pax6 is significantly higher than in GFP controls (Slit1, ***, p<.001), and is similar to that in embryos expressing Nkx6.1 alone (QQ). (n=4 embryos per condition, 2 independent experiments).
The observation that Nkx6.1 positively regulates Slit1 expression, taken together with the fact that VA2 astrocytes co-express both Nkx6.1 and Pax6, suggested that the reason Pax6 does not repress Slit1 in VA2, as it does in VA1, astrocytes is because Nkx6.1 overrides its repressive activity towards Slit1. To test this hypothesis, we asked whether co-electroporation of both Nkx6.1 and Pax6 would block the latter factor’s ability to repress Slit1 expression in WMAs. In embryos mis-expressing both Pax6 and Nkx6.1, not only did Pax6 fail to repress Slit1, but it also did not diminish the effect of Nkx6.1 to up-regulate Slit1 expression (Fig. 6H vs. L, “WM;” RR, “Slit1”). Interestingly, while Nkx6.1 overrode the ability of Pax6 to repress Slit1, it did not interfere with the activity of Pax6 to up-regulate Reelin expression (Fig. 6Z vs. DD, “WM;” RR, “Reelin”).
Finally, we investigated whether the repression of Slit1 by Pax6 in VA1 astrocytes was mediated via repression of Nkx6.1. Examination of Pax6lacZ/lacZ; Slit1eGFP/+ embryos indicated that the dorsal de-repression of Slit1-GFP (Fig. 3) was not accompanied by a de-repression of Nkx6.1 (Supplemental Figure S8B, E, arrows; G). Thus, the de-repression of Slit1 in VA1 astrocytes caused by mutation of Pax6 is Nkx6.1-independent. Examination of Nkx6.2, a related HD protein (Vallstedt et al., 2001), indicated that it is not expressed in the VZ or in WMAs at these gliogenic stages, in either wild-type or Pax6 mutant embryos (Supplemental Figure S5 I–L and data not shown). These data suggest that the Pax6-dependent repression of Slit1 in wild-type VA1 astrocytes is not mediated via repression of Nkx6.1, but by another mechanism.
DISCUSSION
We have demonstrated that the ventral spinal cord white matter contains at least three positionally distinct astrocyte subtypes in vivo, which can be identified by the combinatorial expression of the guidance molecules Reelin and Slit1. These distinct positional identities are specified by the HD transcription factors Pax6 and Nkx6.1, in a combinatorial manner. Thus, positional identity is an organizing feature of WMA diversity, and is specified by a HD code, whose elements are re-utilized following their role in the specification of neuronal identity (Fig. 7A).
Figure 7. A transcriptional code for astrocyte positional identity.
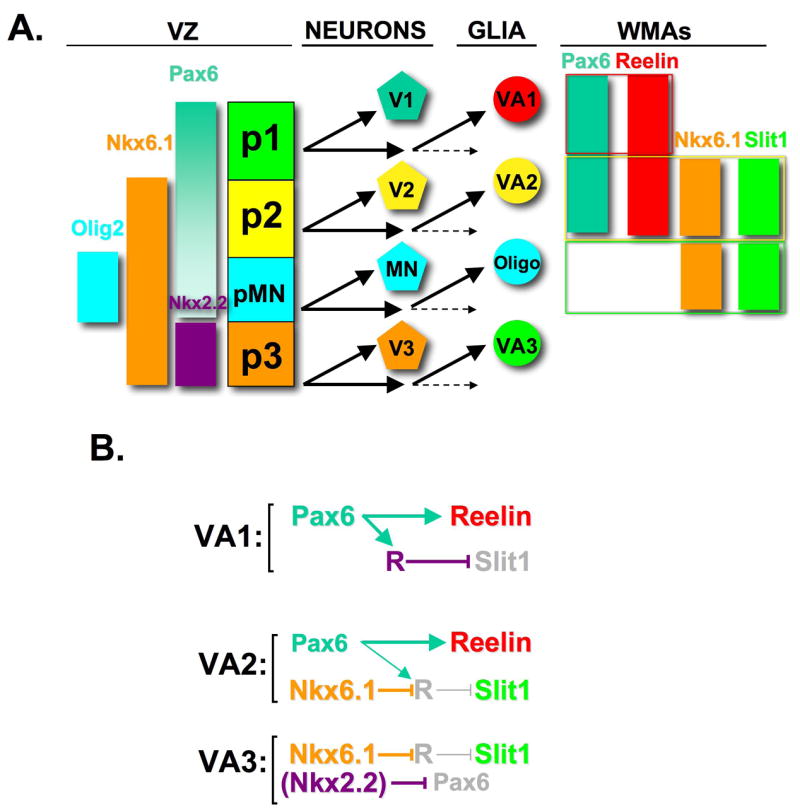
(A) Schematic illustrating sequential generation of neurons and glia from different progenitor domains in the ventral spinal cord. VZ, ventricular zone; WMAs white matter astrocytes. The molecular phenotype of each of the 3 WMA subtypes is enclosed in a colored box. The three classes of WMAs are present in roughly equal proportions. Note that the expression boundaries of some of the transcription factors in the VZ shift from the neurogenic to the gliogenic phases; these shifts are omitted for simplicity. Note also that Nkx2.2, which is co-expressed with Nkx6.1 in the ventral-most (pA3) domain of the VZ during the gliogenic phase (Supplemental Figure S6), is not co-expressed with Nkx6.1 in VA3 WMAs (Supplemental Figure S7). (B) Model for the regulation of astrocyte identity by Pax6 and Nkx6.1. ”R” represents a hypothetical repressor of Slit1, which is activated by Pax6 and repressed by Nkx6.1. See Discussion for details. Repression of Pax6 by Nkx2.2 is likely important for the initial specification of VA3 identity within the VZ (see (A)), but does not contribute to the maintenance of VA3 identity in the WM.
Astrocytes in the spinal cord white matter exhibit distinct positional identities
The recognition that positional differences underlie neuronal subtype diversity has aided both the identification and functional characterization of different neuronal subpopulations, as well as analysis of the developmental mechanisms that control their generation (Jessell, 2000; Briscoe and Ericson, 2001; Shirasaki and Pfaff, 2002). Here we provide evidence for molecular differences in the positional identities of WMA subpopulations within a specific region of the CNS. Previous studies have reported differences in the expression of markers such as ephrin-B1 and BLBP, by radial glial precursor cells at different locations along the dorso-ventral axis of the VZ (Ogawa et al., 2005). However these differences are not maintained by differentiated WMAs. The transcription factor SCL was shown to control generic aspects of astrocyte differentiation, in a position-specific manner (Muroyama et al., 2005), but no evidence was provided for any role in controlling position-specific phenotypic properties of WMAs.
The functional significance of the VA1–3 astrocyte subpopulations revealed by our studies is not yet clear. Reelin and Slit1 are secreted molecules involved in cell migration (Rice and Curran, 2001) and axon guidance (Brose et al., 1999; Plump et al., 2002), respectively. Slits in particular are expressed by astrocytic glia in the floorplate, a mid-line structure ((Holmes et al., 1998; Brose et al., 1999); reviewed in (Lemke, 2001)). The combinatorial expression of these molecules by subsets of WMAs therefore suggests a potential role for this heterogeneity in guiding axonal trajectory and/or cell migration (Powell et al., 1997). Interestingly, Reelin is expressed not only by VA1 and VA2 astrocytes, but also by V1 and V2 interneurons (Yip et al., 2004b). The dorsal restriction of Reelin expression is thought to be important in guiding the migration of sympathetic pre-ganglionic motoneurons, within the spinal cord (Yip et al., 2000; Yip et al., 2004b; Yip et al., 2007). Perhaps the expression of Reelin by VA1 and VA2 astrocytes contributes to this guidance process. In addition to their role in axon guidance, astrocytes are important for enhancing synapse formation and synaptic efficacy (Pfrieger and Barres, 1997; Ullian et al., 2001; Ullian et al., 2004). Perhaps astrocytes derived from a particular progenitor domain preferentially enhance synapse formation by neuronal subtypes derived from the same progenitor domain.
A transcriptional code for astrocyte positional identity
Our data support a combinatorial model for the specification of astrocyte positional identity, in which expression of Pax6 in the absence of Nkx6.1 specifies a VA1 phenotype (Reelin+, Slit1−), co-expression of Pax6 and Nkx6.1 specifies a VA2 phenotype (Reelin+, Slit1+), and expression of Nkx6.1 in the absence of Pax6 specifies a VA3 phenotype (Slit1+, Reelin−) (Fig. 7A). Nkx2.2 may also participate in this code, by repressing Reelin in pA3 VZ precursors (Supplemental Figure S5) via repression of Pax6 (Briscoe et al., 1999), an activity that Nkx6.1 lacks (Briscoe et al., 2000). Consistent with this, mis-expression of Nkx2.2 strongly repressed Reelin, and up-regulated Slit1, in the VZ at E5 (data not shown). However Nkx2.2, unlike Nkx6.1, is not expressed in differentiated Slit1+ WMAs (Supplemental Figure S6), but rather in oligodendrocytes (Qi et al., 2001; Zhou et al., 2001). Therefore, the maintenance of Slit1 expression in VA3 WMAs is more likely to involve Nkx6.1.
Our data suggest that the co-expression of Slit1 and Reelin in VA2 astrocytes reflects a role for Nkx6.1 to override repression of Slit1 by Pax6, without interfering with its activation of Reelin. Consistent with this view, Nkx6.1 is able to prevent repression of Slit1 by Pax6 in co-electroporation experiments, while permitting Pax6 enhancement of Reelin expression (Fig. 6RR). Since Nkx6.1 can act either as an activator or as a repressor, depending on context (Taylor et al., 2005), it could either directly activate Slit1 transcription in VA2 precursors, or do so indirectly, by repressing an as-yet unidentified repressor (Fig. 7B, “R”). The latter model seems more likely, because in Pax6 mutants, Slit1 is de-repressed in Pax6-lacZ+ VA1 astrocytes without de-repression of Nkx6.1 (Supplemental Fig. S9). Thus, Nkx6.1 is not essential for Slit1 expression in ventral WMAs under some conditions. This in turn suggests that, in normal embryos, the absence of Slit1 in VA1 astrocytes reflects either direct repression of Slit1 by Pax6, or else a Pax6-dependent Slit1 repressor. We favor the latter explanation, because in the former case, one might expect that Slit1 expression would only occur in ventral astrocytes lacking Pax6, and this is not observed (Fig. 5I, J). We suggest that in VA2 astrocytes, Nkx6.1 may repress a Pax6-dependent repressor of Slit1 (Fig. 7B, VA2). A similar layered-repression model has been proposed to explain the role of Nkx6.1 in controlling IN identity (Vallstedt et al., 2001). Interestingly, it has recently been shown that Nkx6.1 dominantly antagonizes the activation of glucagon transcription by Pax6 in pancreatic α cells, via competition for binding to common promoter elements (Gauthier et al., 2007). A similar gene-specific antagonism may allow co-expression of Pax6 and Nkx6.1 in VA2 astrocytes to permit expression of both Reelin and Slit1.
Timing and location of HD influences on astrocyte positional identity
The genetic evidence supporting a role for Pax6 and Nkx6.1 in specifying astrocyte identity leaves open the question of when and where these HD factors act. Several lines of evidence suggest that astrocyte positional identity is initially specified within the VZ. First, Pax6 and Reelin are co-expressed within the VZ in NFIA+ glial progenitor cells, as are Nkx6.1 and Slit1. With the exception of the Olig2+ domain, therefore, there is continuity in the co-expression of these HD and guidance molecules from the germinal layer to the white matter, an interpretation supported by BrdU pulse-chase experiments (Supplemental Fig. S2). Second, in embryos electroporated with Pax6 at E2, ventral expansion of Reelin expression is detected in NF1A+ glial progenitors in the VZ at E5–E6 (Supplemental Fig. S4). Importantly, a similar result is obtained when electroporation is performed at E4.5 (Supplemental Fig. S4), by which stage ventral neurogenesis has ended (Deneen et al., 2006). This latter result argues that the effect of Pax6 on Reelin is unlikely to be due to indirect effects on neuronal identity specification at earlier stages. In support of this conclusion, loss- and gain-of-function manipulations of these HD factors cause changes in astrocytic expression of Reelin and Slit1 at positions along the rostro-caudal axis where similar manipulations do not cause changes in the number or distribution of ventral IN subtypes (data not shown; (Burrill et al., 1997; Ericson et al., 1997; Osumi et al., 1997; Takahashi and Osumi, 2002; Novitch et al., 2003)). Taken together, these data support the idea that astrocyte positional identity is specified within the germinal layer, prior to emigration of astrocyte precursors into the gray matter. Importantly, however, the continued expression of Pax6 and Nkx6.1 in WMAs raises the possibility that these proteins play a role in the maintenance of astrocyte positional identity, as well as in its initial specification (Fig. 7A).
The data presented here provide evidence that positional identity is an organizing principle underlying phenotypic diversity among WMAs, as well as among neurons. They also suggest that positional mechanisms are involved in the developmental specification of this heterogeneity within the VZ. Our results argue against the view that astrocyte heterogeneity in the CNS primarily reflects the phenotypic plasticity of such glia, in response to local environmental signals, after they migrate and differentiate. Instead, the data suggest that at least some aspects of astrocyte diversity are pre-specified within the germinal zone of the CNS, by mechanisms that are not only analogous to those that specify neuronal identity at earlier stages, but which employ elements of the same transcriptional code. From this perspective, elements of this code can be thought of as coupling the specification of positionally distinct neuronal subtypes, to positionally distinct glial subtypes, a generalization of a concept first established for Olig2 and its role in specifying motoneuron and oligodendrocyte fate (Lu et al., 2002; Zhou and Anderson, 2002; Muroyama et al., 2005). Our findings open up for future study the function of the astrocyte subtypes we have identified in the spinal cord, as well as the extent to which positional specification mechanisms generate astrocyte diversity in other regions of the CNS.
EXPERIMENTAL PROCEDURES
Mouse mutants
Olig1,2−/− mice (Zhou and Anderson, 2002), Pax6-LacZ mice (St-Onge, Sosa-Pineda et al., 1997) and Slit1-GFP mice (Plump, Erskine et al. 2002) were genotyped by PCR using lacZ and GFP primers. Pax6-LacZ mice were intercrossed with Slit1-GFP mice to generate Pax6−/−, Slit1-GFP+/− and Pax6+/−, Slit1-GFP+/− embryos for analysis.
FACS and Microarray experiments
Olig2-GFP-expressing cells were FACS isolated from spinal cords of Olig1,2 +/− and −/− embryos as previously described (Mukouyama et al., 2006). RNA was extracted from the isolated cells, amplified, biotinylated and hybridized to Affymetrix mU74v2 A, B and C chips (Deneen et al., 2006). Analysis of microarray data was performed using Affymetrix Microarray Suite and Rosetta Resolver software.
In situ hybridization
Non-radioactive in situ hybridization using DIG-labelled (Zhou et al., 2000) or fluorescent probes (Choi et al., 2005) was performed on frozen sections of mouse or chick spinal cord as described. The following probes were used: mouse Reelin (gift of Gabriella D’Arcangelo), mouse Slit1 (gift of Marc Tessier-Lavigne), chick Reelin, chick Slit1 (gift of Ed Laufer). Antibodies used are listed in Supplementary Information.
Chick embryo electroporation
Chick embryos were electroporated at E2 with either RCAS(B) GFP, RCAS(B) Pax6 or RCAS(B) Nkx6.1 replication competent avian retroviruses, using established methods (Zhou, Choi et al. 2001). The electroporation conditions were 5 square wave pulses of 50 msec duration at 24 V. Embryos were incubated in a humidified 37 oC incubator until E12.
Supplementary Material
Acknowledgments
We thank A. Stoykova, P. Gruss and G. Lanuza for sharing Pax6-LacZ mice and embryos, M. Tessier-Lavigne for the Slit1-GFP mice, and K. McCarthy for pilot experiments with GFAP-CreER mice. We also thank T. Jessell for helpful discussions and antibodies, and E. Laufer and G. D’Arcangelo for in situ probes and antibody reagents. We thank S. Pease, J. Alex and the staff of the Caltech animal facility for assistance with mouse breeding and timed matings; R. Diamond for assistance with FACS; E. Zuo for Affymetrix microarray hybridization; P. Lwigale for advice and assistance with electroporation and egg husbandry techniques; M. Martinez for genotyping mouse lines; J. Chow, M. Lee, R. Ho and J. S. Chang for technical assistance; G. Mosconi for laboratory management and G. Mancuso for administrative assistance. This work was supported in part by NIH grant 1RO1-NS23476. D.J.A. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey MS, Shipley MT. Astrocyte subtypes in the rat olfactory bulb: Morphological heterogeneity and differential laminar distribution. The Journal of comparative neurology. 1993;328:501–526. doi: 10.1002/cne.903280405. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Current opinion in neurobiology. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A Homeodomain Protein Code Specifies Progenitor Cell Identity and Neuronal Fate in the Ventral Neural Tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398:622–627. doi: 10.1038/19315. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Moran L, Goulding MD, Saueressig H. PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development. 1997;124:4493–4503. doi: 10.1242/dev.124.22.4493. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong H-w, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 Delineates a Pathway Mediating Innate Reproductive Behaviors from the Amygdala to the Hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM, II, Janigro D. Functional Specialization and Topographic Segregation of Hippocampal Astrocytes. J Neurosci. 1998;18:4425–4438. doi: 10.1523/JNEUROSCI.18-12-04425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The Transcription Factor NFIA Controls the Onset of Gliogenesis in the Developing Spinal Cord. Neuron. 2006;52:953–968. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 Controls Progenitor Cell Identity and Neuronal Fate in Response to Graded Shh Signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New Insights into Neuron-Glia Communication. Science (New York, NY) 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Qi Y, Tan M, Cai J, Hu X, Liu Z, Jensen J, Qiu M. Molecular mapping of the origin of postnatal spinal cord ependymal cells: evidence that adult ependymal cells are derived from Nkx6.1+ ventral neural progenitor cells. The Journal of comparative neurology. 2003;456:237–244. doi: 10.1002/cne.10481. [DOI] [PubMed] [Google Scholar]
- Gabay L, Lowell S, Rubin LL, Anderson DJ. Deregulation of Dorsoventral Patterning by FGF Confers Trilineage Differentiation Capacity on CNS Stem Cells In Vitro. Neuron. 2003;40:485–499. doi: 10.1016/s0896-6273(03)00637-8. [DOI] [PubMed] [Google Scholar]
- Gauthier BR, Gosmain Y, Mamin A, Philippe J. The β-cell specific transcription factor Nkx6.1 inhibits glucagon gene transcription by interfering with Pax6. Biochem J. 2007;403:593–601. doi: 10.1042/BJ20070053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding M, Lamar E. Neuronal patterning: Making stripes in the spinal cord. Curr Biol. 2000;10:R565–568. doi: 10.1016/s0960-9822(00)00615-1. [DOI] [PubMed] [Google Scholar]
- Holmes GP, Negus K, Burridge L, Raman S, Algar E, Yamada T, Little MH. Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mechanisms of development. 1998;79:57–72. doi: 10.1016/s0925-4773(98)00174-9. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal Specification in the spinal cord: inductive signals and transcriptional codes. Nature Reviews Genetics. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Kubasak MD, Brooks R, Chen S, Villeda SA, Phelps PE. Developmental distribution of reelin-positive cells and their secreted product in the rodent spinal cord. The Journal of comparative neurology. 2004;468:165–178. doi: 10.1002/cne.10946. [DOI] [PubMed] [Google Scholar]
- Lemke G. Glial control of neuronal development. Annual review of neuroscience. 2001;24:87–105. doi: 10.1146/annurev.neuro.24.1.87. [DOI] [PubMed] [Google Scholar]
- Liuzzi FJ, Miller RH. Radially oriented astrocytes in the normal adult rat spinal cord. Brain Research. 1987;403:385–388. doi: 10.1016/0006-8993(87)90081-3. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common Developmental Requirement for Olig Function Indicates a Motor Neuron/Oligodendrocyte Connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- McMahon AP. Neural patterning: The role of Nkx genes in the ventral spinal cord. Genes Dev. 2000;14:2261–2264. doi: 10.1101/gad.840800. [DOI] [PubMed] [Google Scholar]
- Miller RH, Raff MC. Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J Neurosci. 1984;4:585–592. doi: 10.1523/JNEUROSCI.04-02-00585.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH, Zhang H, Fok-Seang J. Glial cell heterogeneity in the mammalian spinal cord. Perspectives on developmental neurobiology. 1994;2:225–231. [PubMed] [Google Scholar]
- Mori S, Leblond CP. Electron microscopic features and proliferation of astrocytes in the corpus callosum of the rat. The Journal of comparative neurology. 1969;137:197–225. doi: 10.1002/cne.901370206. [DOI] [PubMed] [Google Scholar]
- Mukouyama Y-s, Deneen B, Lukaszewicz A, Novitch BG, Wichterle H, Jessell TM, Anderson DJ. Olig2+ neuroepithelial motoneuron progenitors are not multipotent stem cells in vivo 10.1073/pnas.0510658103. PNAS. 2006;103:1551–1556. doi: 10.1073/pnas.0510658103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama Y, Fujiwara Y, Orkin SH, Rowitch DH. Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature. 2005;438:360–363. doi: 10.1038/nature04139. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Ogawa Y, Takebayashi H, Takahashi M, Osumi N, Iwasaki Y, Ikenaka K. Gliogenic Radial Glial Cells Show Heterogeneity in the Developing Mouse Spinal Cord. Developmental neuroscience. 2005;27:364–377. doi: 10.1159/000088452. [DOI] [PubMed] [Google Scholar]
- Osumi N, Hirota A, Ohuchi H, Nakafuku M, Iimura T, Kuratani S, Fujiwara M, Noji S, Eto K. Pax-6 is involved in the specification of hindbrain motor neuron subtype. Development (Cambridge, England) 1997;124:2961–2972. doi: 10.1242/dev.124.15.2961. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic Efficacy Enhanced by Glial Cells in Vitro. Science (New York, NY) 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Plump AS, Erskine L, Sabatier C, Brose K, Epstein CJ, Goodman CS, Mason CA, Tessier-Lavigne M. Slit1 and Slit2 Cooperate to Prevent Premature Midline Crossing of Retinal Axons in the Mouse Visual System. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Powell EM, Meiners S, DiProspero NA, Geller HM. Mechanisms of astrocyte-directed neurite guidance. Cell and tissue research. 1997;290:385–393. doi: 10.1007/s004410050945. [DOI] [PubMed] [Google Scholar]
- Pringle NP, Yu WP, Howell M, Colvin JS, Ornitz DM, Richardson WD. Fgfr3 expression by astrocytes and their precursors: evidence that astrocytes and oligodendrocytes originate in distinct neuroepithelial domains. Development (Cambridge, England) 2003;130:93–102. doi: 10.1242/dev.00184. [DOI] [PubMed] [Google Scholar]
- Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J, Qiu M. Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development. 2001;128:2723–2733. doi: 10.1242/dev.128.14.2723. [DOI] [PubMed] [Google Scholar]
- Raff MC. Glial cell diversification in the rat optic nerve. Science (New York, NY. 1989;243:1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- Raff MC, Abney ER, Miller RH. Two glial cell lineages diverge prenatally in rat optic nerve. Developmental biology. 1984;106:53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annual review of neuroscience. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- Sharif A, Renault F, Beuvon F, Castellanos R, Canton B, Barbeito L, Junier MP, Chneiweiss H. The expression of PEA-15 (phosphoprotein enriched in astrocytes of 15 kDa) defines subpopulations of astrocytes and neurons throughout the adult mouse brain. Neuroscience. 2004;126:263–275. doi: 10.1016/j.neuroscience.2004.02.039. [DOI] [PubMed] [Google Scholar]
- Shibata T, Yamada K, Watanabe M, Ikenaka K, Wada K, Tanaka K, Inoue Y. Glutamate Transporter GLAST Is Expressed in the Radial Glia-Astrocyte Lineage of Developing Mouse Spinal Cord. J Neurosci. 1997;17:9212–9219. doi: 10.1523/JNEUROSCI.17-23-09212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki R, Pfaff SL. Transcriptional codes and the control of neuronal identity. Annual review of neuroscience. 2002;25:251–281. doi: 10.1146/annurev.neuro.25.112701.142916. [DOI] [PubMed] [Google Scholar]
- St-Onge L, Sosa-Pineda B, Chowdhury K, Mansouri A, Gruss P. Pax6 is required for differentiation of glucagon-producing [alpha]-cells in mouse pancreas. Nature. 1997;387:406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Sugimori M, Nagao M, Bertrand N, Parras CM, Guillemot F, Nakafuku M. Combinatorial actions of patterning and HLH transcription factors in the spatiotemporal control of neurogenesis and gliogenesis in the developing spinal cord. Development (Cambridge, England) 2007;134:1617–1629. doi: 10.1242/dev.001255. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Osumi N. Pax6 regulates specification of ventral neurone subtypes in the hindbrain by establishing progenitor domains. Development (Cambridge, England) 2002;129:1327–1338. doi: 10.1242/dev.129.6.1327. [DOI] [PubMed] [Google Scholar]
- Taylor DG, Babu D, Mirmira RG. The C-terminal domain of the beta cell homeodomain factor Nkx6.1 enhances sequence-selective DNA binding at the insulin promoter. Biochemistry. 2005;44:11269–11278. doi: 10.1021/bi050821m. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Christopherson KS, Barres BA. Role for glia in synaptogenesis. Glia. 2004;47:209–216. doi: 10.1002/glia.20082. [DOI] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of Synapse Number by Glia. Science (New York, NY. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Vallstedt A, Muhr J, Pattyn A, Pierani A, Mendelsohn M, Sander M, Jessell TM, Ericson J. Different Levels of Repressor Activity Assign Redundant and Specific Roles to Nkx6 Genes in Motor Neuron and Interneuron Specification. Neuron. 2001;31:743–755. doi: 10.1016/s0896-6273(01)00412-3. [DOI] [PubMed] [Google Scholar]
- Vaughn JE, Pease DC. Electron microscopy of classically stained astrocytes. The Journal of comparative neurology. 1967;131:143–154. doi: 10.1002/cne.901310206. [DOI] [PubMed] [Google Scholar]
- Yip JW, Yip YP, Nakajima K, Capriotti C. Reelin controls position of autonomic neurons in the spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8612–8616. doi: 10.1073/pnas.150040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip YP, Capriotti C, Magdaleno S, Benhayon D, Curran T, Nakajima K, Yip JW. Components of the reelin signaling pathway are expressed in the spinal cord. The Journal of comparative neurology. 2004a;470:210–219. doi: 10.1002/cne.20001. [DOI] [PubMed] [Google Scholar]
- Yip YP, Kronstadt-O’Brien P, Capriotti C, Cooper JA, Yip JW. Migration of sympathetic preganglionic neurons in the spinal cord is regulated by Reelin-dependent Dab1 tyrosine phosphorylation and CrkL. The Journal of comparative neurology. 2007;502:635–643. doi: 10.1002/cne.21318. [DOI] [PubMed] [Google Scholar]
- Yip YP, Zhou G, Capriotti C, Yip JW. Location of preganglionic neurons is independent of birthdate but is correlated to reelin-producing cells in the spinal cord. The Journal of comparative neurology. 2004b;475:564–574. doi: 10.1002/cne.20212. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Anderson DJ. The bHLH Transcription Factors OLIG2 and OLIG1 Couple Neuronal and Glial Subtype Specification. Cell. 2002;109:61–73. doi: 10.1016/s0092-8674(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Choi G, Anderson DJ. The bHLH Transcription Factor Olig2 Promotes Oligodendrocyte Differentiation in Collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Wang S, Anderson DJ. Identification of a Novel Family of Oligodendrocyte Lineage-Specific Basic Helix-Loop-Helix Transcription Factors. Neuron. 2000;25:331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.