Abstract
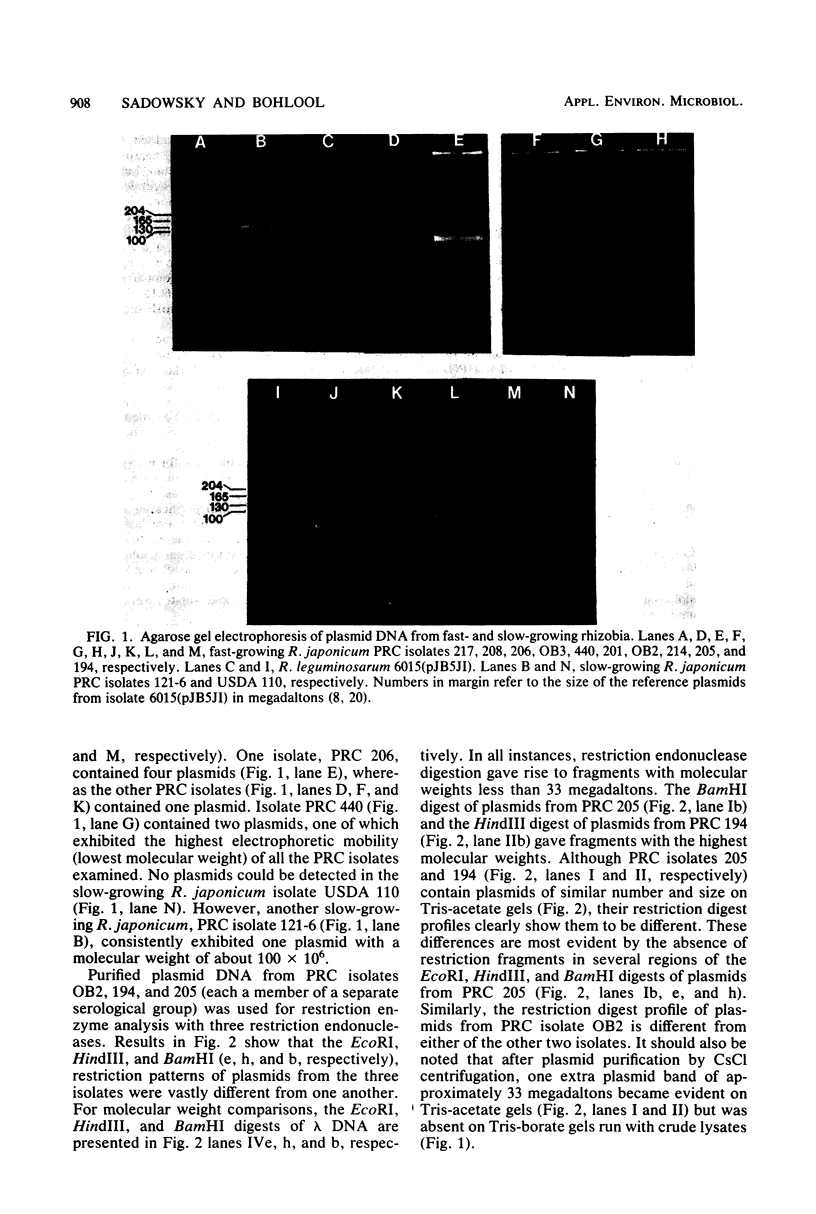
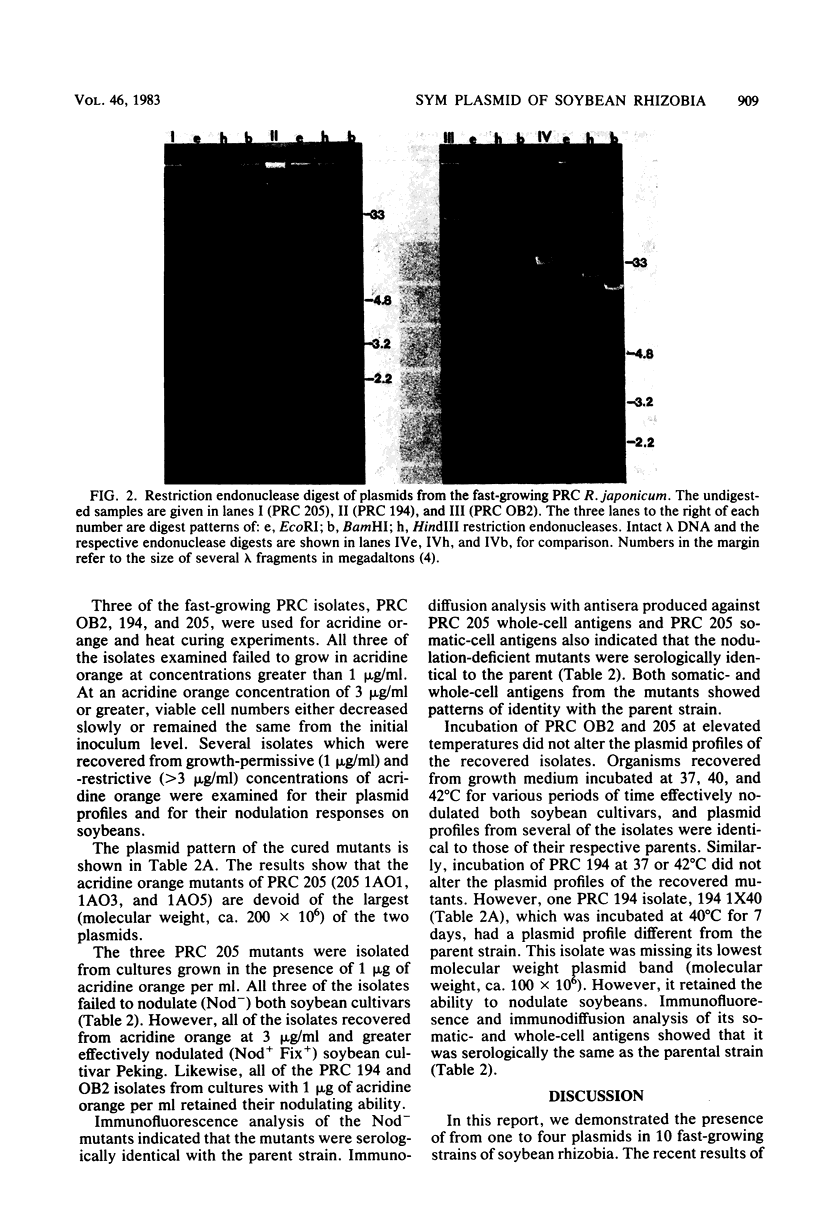
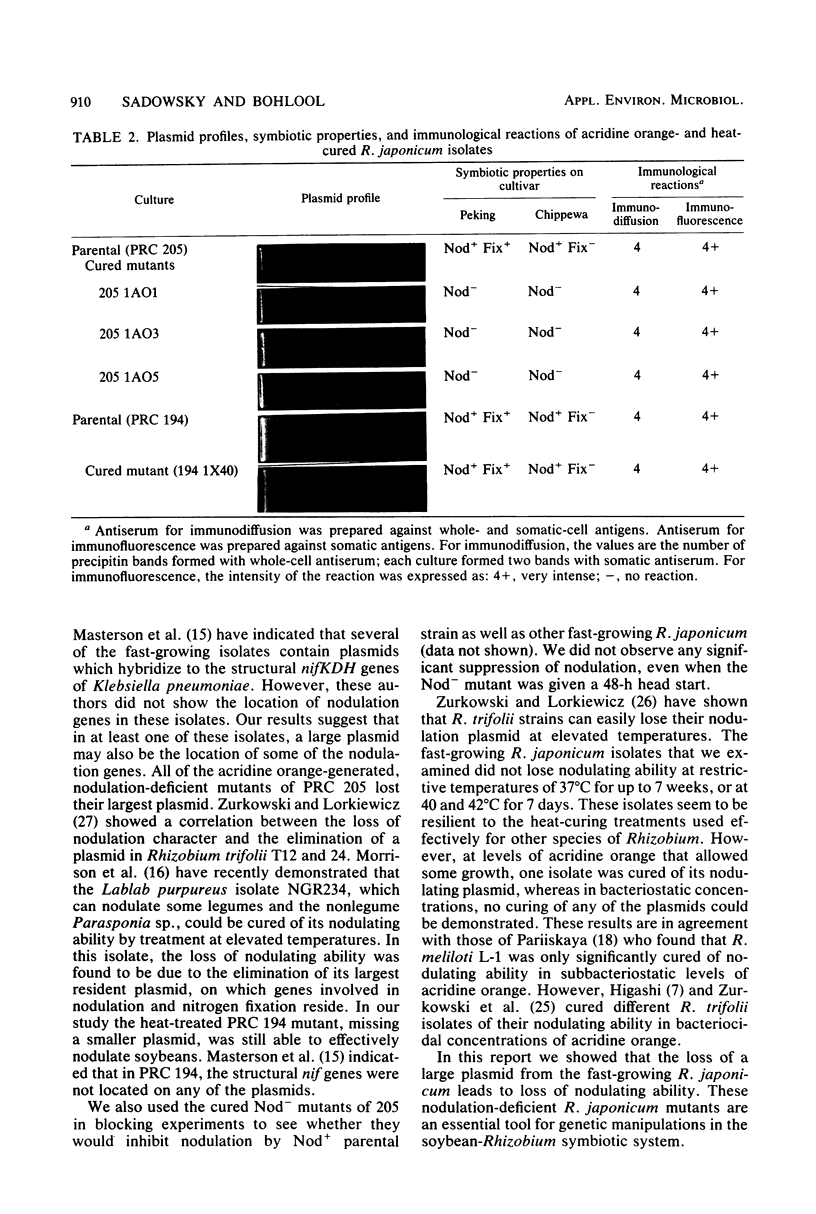
Several isolates from a newly described group of fast-growing acid-producing soybean rhizobia, Rhizobium japonicum, were analyzed for plasmid content. All contained from one to four plasmids with molecular weights of 100 × 106 or larger. Although most of the isolates shared plasmids of similar size, the restriction endonuclease (BamHI, EcoRI, and HindIII) patterns of the plasmids from three of the isolates were vastly different. Growth in the presence of acridine orange was effective in producing mutants cured of the largest plasmid in one of the strains. These mutants also lost the ability to form nodules on soybeans. High-temperature curing of a smaller plasmid in another strain did not lead to loss of nodulating ability or alteration of symbiotic effectiveness on soybean cultivars. The identities of all of the isolates and mutants were ascertained by immunofluoresence and immunodiffusion. The new fast-growing strains of R. japonicum may provide a better genetic system for the study of the soybean symbiosis than the slow-growing R. japonicum, not all of which can be shown to contain plasmids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. Fast-growing rhizobia isolated from root nodules of soybean. Science. 1982 Mar 26;215(4540):1631–1632. doi: 10.1126/science.215.4540.1631. [DOI] [PubMed] [Google Scholar]
- Leonard L. T. A Simple Assembly for Use in the Testing of Cultures of Rhizobia. J Bacteriol. 1943 Jun;45(6):523–527. doi: 10.1128/jb.45.6.523-527.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masterson R. V., Russell P. R., Atherly A. G. Nitrogen fixation (nif) genes and large plasmids of Rhizobium japonicum. J Bacteriol. 1982 Nov;152(2):928–931. doi: 10.1128/jb.152.2.928-931.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison N. A., Hau C. Y., Trinick M. J., Shine J., Rolfe B. G. Heat curing of a sym plasmid in a fast-growing Rhizobium sp. that is able to nodulate legumes and the nonlegume Parasponia sp. J Bacteriol. 1983 Jan;153(1):527–531. doi: 10.1128/jb.153.1.527-531.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilacinski W. P., Schmidt E. L. Plasmid transfer within and between serologically distinct strains of Rhizobium japonicum, using antibiotic resistance mutants and auxotrophs. J Bacteriol. 1981 Feb;145(2):1025–1030. doi: 10.1128/jb.145.2.1025-1030.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Bakole R. O., Bohlool B. B. Fluorescent-antibody approach to study of rhizobia in soil. J Bacteriol. 1968 Jun;95(6):1987–1992. doi: 10.1128/jb.95.6.1987-1992.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurkowski W., Hoffman M., Lorkiewicz Z. Effect of acriflavine and sodium dodecyl sulphate on infectiveness of Rhizobium trifolii. Acta Microbiol Pol A. 1973;5(2):55–60. [PubMed] [Google Scholar]