Abstract
The objective of this study was to compare PLC/PRF/5 and BGM cell lines for use in a total culturable viral assay (TCVA) of treated sewage effluents. Samples were collected before and after chlorination from an activated sludge wastewater treatment plant and from the effluent of a high-rate enhanced flocculation system, followed by UV light disinfection. Cell monolayers were observed for cytopathic effect (CPE) after two passages of 14 days each. Monolayers exhibiting viral CPE were tested for the presence of adenoviruses and enteroviruses by PCR or reverse transcription-PCR. Eight percent of the samples exhibited CPE on BGM cells, and 57% showed CPE on PLC/PRF/5 cells. Only enteroviruses were detected on the BGM cells, while 30% and 52% of the samples were positive for enteroviruses and adenoviruses, respectively, on the PLC/PRF/5 cells. Thirty percent of the samples were positive for both adenoviruses and enteroviruses in chlorinated activated sludge effluent. Thirty percent of the samples were positive for adenoviruses in the UV treatment effluent, but no enteroviruses were detected. In conclusion, the PLC/PRF/5 cells were more susceptible than BGM cells to viruses found in treated sewage. The use of BGM cells for TCVA may underestimate viral concentration in sewage effluent samples. The PLC/PRF/5 cells were more susceptible to adenoviruses, which is important in the evaluation of UV disinfection systems because adenoviruses are highly resistant to UV inactivation.
Bacterial indicators are commonly used for monitoring the efficacy of water treatment facilities and water quality. The detection of human viral pathogens in the absence of bacterial indicators has called into question the relationship between bacterial indicators and the virological quality of the water (2, 3, 8). Enteroviruses and adenoviruses have also been suggested as indicators for monitoring the human fecal contamination of water and for determining the efficacy of disinfection treatments (7). Enteroviruses are single-stranded RNA nonenveloped viruses that include poliovirus, echovirus, coxsackievirus, and a number of enteroviruses. The symptoms of enterovirus infections are associated with gastroenteritis, flaccid paralysis, myocarditis, pericarditis, and diabetes (7). Human adenoviruses are double-stranded DNA nonenveloped viruses, with 52 serotypes organized in subgroups A to F; subgroup F is composed of the enteric adenovirus most associated with diarrhea in children (24). The symptoms associated with adenovirus infection include gastroenteritis, pharyngitis, pneumonia, conjunctivitis, and meningoencephalitis (13). Infection can be acquired by ingestion, direct contact (eyes), and inhalation of aerosols (13). Adenovirus resistance to UV inactivation and the virus's long persistence in the environment have increased the interest in monitoring adenoviruses from water (12).
Cell culture is the most commonly use method for the isolation of infectious viruses from water (18). The Buffalo green monkey kidney (BGM) cell line is very sensitive to infection by poliovirus and coxsackievirus B and is the most common cell line used for the detection of such viruses in water (6). The BGM cell line recommended for the total culturable viral assay (TCVA) is the standard method for the enumeration of viruses from water (26). The TCVA depends on the ability of the viruses to propagate in BGM cells and create morphological changes or cytopathic effects (CPE) that can be identified and quantified. The combination of the BGM cell line with other cell lines such as A549, human colon adenocarcinoma (Caco-2), and primary liver carcinoma (PLC/PRF/5) lines for the surveillance of viruses in water has been reported to increase the sensitivity for the detection of adenoviruses, coxsackie A viruses, echoviruses and astroviruses, or other viruses that do not grow effectively on BGM cells (4, 22, 23, 29).
PLC/PRF/5 is a more efficient cell line for the propagation of adenoviruses 40 and 41 than Graham 293, Chang, KB, and A549 (9). In addition, the PLC/PRF/5 cell line has been effectively used for the detection of adenoviruses in river water and treated drinking water (27, 28), and it is also susceptible to infection by many enteroviruses, including coxsackie B viruses and reoviruses (10). The selection of a sensitive cell line for the determination of culturable viruses in water is important because it decreases the cost and effort required by avoiding the use of multiple cell lines.
This study compares the use of the BGM cell line with the PLC/PRF/5 line during the evaluation of a high-rate treatment process for sewage. This process consists of a chemically enhanced flocculation followed by UV disinfection and was designed to treat wet-weather influents (WWI) to reduce sewage overflow and the release of untreated sewage. Samples from a wastewater treatment plant (WWTP) before and after chlorination, chemically enhanced flocculation of the effluent, and UV treatment were collected and subjected to a TCVA on both BGM and PLC/PRF/5 cell lines.
MATERIALS AND METHODS
Sampling.
The South Shore WWTP is an activated sludge WWTP located along Lake Michigan in the City of Oak Creek, Wisconsin. The South Shore WWTP is one of the two WWTPs in the Milwaukee Metropolitan Sewerage District (MMSD). The MMSD serves 1.8 million clients in 1,100 km2; 5% of the system is combined sewage and storm water collection. Samples were taken from the effluent of the secondary clarifier before and after disinfection with chlorine. High-rate chemical flocculation treatment is designed to treat the WWI during storm events. The two chemically enhanced clarification units used were DensaDeg (Infilco Degremont, Richmond, VA) and Actiflo (Krüger, Cary, NC). These units use alum as a coagulant at concentrations between 150 to 250 mg/liter. The effluent from these units was subjected to disinfection by exposure to UV light (40,000 μW/cm2). The samples were collected in the months of May and July of 2005.
TCVA. (i) Sample processing.
One-liter samples were shipped on ice from the WWTP to the laboratory and processed within 48 h after sampling. The samples were concentrated by organic flocculation as described previously (26). Sample concentrates were resuspended in 30 ml of 0.15 M sodium phosphate with a final pH after resuspension of 7.2, and 0.3 ml of 100× antibiotic mix containing 10,000 units of penicillin G, 10,000 units of streptomycin sulfate, 25 μg/ml of amphotericin B, 0.3 ml of kanamycin sulfate (10 mg/ml), and 0.3 ml of gentamicin (5 mg/ml) was added. The concentrates were frozen at −80°C until assayed on cell culture. The efficiency of the organic flocculation averaged 60% using poliovirus type 1 (LSC-2ab; obtained from Baylor College of Medicine).
(ii) Cell culture infection.
The PLC/PRF/5 cell line (passage level 26 to 36; ATCC CRL-8024) and the BGM cell line (passage level 121 to 140; Unites States Environmental Protection Agency, Cincinnati, OH) were used in this study. The concentrates were split into a 15-ml subsample and a 4.5-ml subsample. From the 15-ml subsample, volumes of 2.5 ml were inoculated into 75-cm2 flasks containing 3- to 4-day-old monolayers. If more than three flasks were positives for CPE, then from the 4.5-ml subsample volumes of 1 ml were inoculated into 25-cm2 flasks. In the case of toxicity or if all the samples were positive, the concentrate was diluted 1/10, and 1 ml was inoculated into 25-cm2 flasks. The cells were between 3 to 5 days old with a confluent monolayer at the time of infection. The flasks were incubated with the sample at 37°C with slow agitation for 2 h. The concentrate was then removed, and the flasks were covered with minimal essential medium (catalog item 11700-077; Gibco Invitrogen Corporation, Grand Island, NY) supplemented with 2% fetal bovine serum (HyClone, Logan, UT), 1 mM sodium pyruvate, and 2 mM GlutaMAX (35050-061; Invitrogen, Grand Island, NY) and incubated for 14 days at 37°C. The flasks were examined for CPE every day. The medium was changed every 4 days.
(iii) Confirmation of positive and negative flasks.
Flasks that were showing signs of CPE or were negative after 14 days of incubation were frozen at −20°C and thawed three times, filtered though a 0.22-μm-pore-size membrane filter, and inoculated onto flasks containing fresh cell monolayers (3 to 5 days old) to confirm viral CPE. The flasks were incubated for 14 days as described before.
The viral most probable number (MPN)/liter was determined using the MPN General Purpose Program by Hurley and Roscoe (11). The viruses were harvested from the confirmatory flasks (CPE-positive flasks) or from the second-passage flasks (CPE-negative flasks) by three freeze-thaw cycles and kept at −20°C until PCR and reverse transcription-PCR (RT-PCR) analysis.
One-step RT-PCR and nested-PCR for the detection of enteroviruses.
Prior to RT-PCR analysis, the cell lysate was heated to 97°C for 5 min and chilled on ice. Ten microliters of cell lysate was mixed with 40 μl of RT-PCR mixture. The reaction mixture contained 1.0× reaction buffer (10× buffer consisting of 100 mM Tris-HCl, pH 8.00, and 500 mM of KCl), 3.5 mM MgCl2, a 300 μM concentration of each deoxynucleoside triphosphate (dNTP), 1.2 μM random hexamer mixture (Applied Biosystems, Roche Molecular Systems Inc. Branchburg, NJ), 10 units of RNase inhibitor (Applied Biosystems, Roche Molecular Systems, Inc., Branchburg, NJ), 25 units of murine leukemia virus reverse transcriptase (Applied Biosystems, Roche Molecular Systems, Inc., Branchburg, NJ), a 0.5 μM concentration of each primer (P1 and P2), 1.2 units of Amplitaq GOLD (Applied Biosystems, Roche Molecular Systems, Inc., Branchburg, NJ), and nuclease-free water, for a final volume of 50 μl.
The nested PCR mixture consisted of 1× PCR buffer, 2.9 mM MgCl2, a 200 μM concentration of each dNTP, a 0.4 μM concentration of each primer (P1 and P33), 1.25 units of Amplitaq GOLD (Applied Biosystems, Roche Molecular Systems, Inc. Branchburg, NJ), and 2 μl of the one-step RT-PCR products, for a final reaction mixture volume of 50 μl. The primers specific for enteroviruses were P1 upstream (5′-CCTCCGGCCCCTGAATG-3′), P2 downstream (5′-ACCGGATGGCCAATCCAA-3′), and Ent 33 downstream (5′-CCCAAAGTAGTCGGTTCCGC-3′) (21). The first round of PCR used the set of primers P1 and P2, with an amplified fragment of 195 bp. The second round of PCR used the set of primers P1 and P33, with an amplified fragment of 105 bp.
The conditions for the RT-PCR were modified from the conditions previously published by Reynolds et al. (20). The PCR conditions for the one-step RT-PCR were as follows: 44°C for 60 min, 99°C for 10 min, 25 s at 50°C, and 45 s at 72°C; 35 cycles of 25 s at 94°C, 25 s at 55°C, and 45 s at 72°C; and a final extension of 72°C for 7 min. The PCR conditions for the second round were as follows: 10 min at 94°C, 25 s at 55°C, and 45 s at 72°C; 35 cycles of 25 s at 94°C, 25 s at 60°C, and 45 s at 72°C; and a final extension step of 7 min at 72°C. The PCR products were analyzed by gel electrophoresis with 2% agarose in 0.5× Tris-borate-EDTA buffer and 5 μg/ml of ethidium bromide. The voltage was set at 5V/cm, and the gel was visualized using an AlphaImager 2000 (Alpha Innotech Corporation, San Leandro, CA).
Detection of adenovirus by PCR.
The PCR procedure for adenovirus was obtained from Van Heerden et al. (27). The primers for adenovirus were obtained from Avellón et al. (1). The primers for the first round of PCR were ADHEX1F (5′-AACACCTAYGASTACATGAAC-3′) and ADHEX2R (5′-KATGGGGTARAGCATGTT-3′), with an amplified fragment of 473 bp. The primers for the second round of PCR were ADHEX2F (5′-CCCMTTYAACCACCACCG-3′) and ADHEX1R (5′-ACATCCTTBCKGAAGTTCCA-3′), with an amplified fragment of 168 bp. The PCR mixture consisted of 1× PCR buffer, 2.5 mM MgCl2, a 200 μM concentration of each dNTP, a 1 μM concentration of each primer, 1 U of Amplitaq GOLD polymerase, 10 μl of sample, and water to a total volume of 50 μl. The conditions for the first round and nested PCR were the following: 10 min at 94°C; 35 cycles of 30 s at 94°C, 30 s at 50°C, and 30 s at 72°C; and a final extension of 10 min at 72°C. The conditions for both PCRs were the same. The PCR products were analyzed as described above.
Quality control assurance.
The cell culture facility and incubators were physically separated from the PCR facility. One biological hazard type II hood was used for processing the concentrated samples, and another was used for cell supernatants. The PCR facility consisted of four rooms to physically separate activities related to reagent preparation, sample processing, mixing of the RT-PCR and PCR mixtures, the mixing of first-round PCR product with nested PCR mixture, and gel electrophoresis. All the RT and PCR reagents were mixed in the workstation in the room for reagent preparation. The first-round PCR product and nested PCR solution were mixed in a workstation used exclusively for this function. The PCR workstations had no forced airflow and UV light. All the reagents were stored in a separate room from the samples. The workstations were cleaned with 10% bleach solution and exposed to UV light for at least 20 min. The equipment used in each room was not used in other areas (i.e., pipettes, tips, and laboratory coats were exclusive to each room). The PCR thermocyclers were in another room outside the work area. RNA-free water was used as a negative control.
Statistical analysis.
The reduction in viral concentration was calculated using the following formula: log10 reduction = log10 (Nt/N0), where Nt is the concentration of virus at time t, and N0 is the initial concentration of virus. The difference in titer between both cell lines was analyzed using one-way analysis of variance (Minitab 14; Minitab Inc. Cary, NC).
RESULTS
The results of the TCVA are shown in Table 1. Only 3 samples out of 37 (8%) were positive for culturable virus on the BGM cell line. Two samples from the secondary clarified effluent and one sample from the chlorine contact effluent were positive. The maximum MPN value was 1.68 MPN/liter. Using PLC/PRF/5 cells, 21 out of 37 (57%) samples were positive for culturable viruses, with an average of 28.5 MPN/liter. The highest value obtained was 341 MPN/liter. The average TCVA result before chlorination (the secondary clarifier effluent) was 35.8 MPN/liter, and the highest value was 135 MPN/liter. The average TCVA result after chlorination was 15.7 MPN/liter, and the highest value was 34.8 MPN/liter. This is a 50% or 0.26 log10 reduction in the concentration of viruses.
TABLE 1.
Culturable virus concentrations obtained with BGM and PLC/PRF/5 cell lines and the detection of enterovirus and adenovirus genomes in the infected cells
| Treatment | Virus detection in BGM cellsa
|
Virus detection in PLC/PRF/5 cellsa
|
||||
|---|---|---|---|---|---|---|
| MPN/liter | Adenovirus | Enterovirus | MPN/liter | Adenovirus | Enterovirus | |
| Secondary clarifier effluent | ||||||
| 0 | − | − | 11.8 | − | − | |
| 1.58 | − | + | 70 | + | − | |
| 0 | − | − | 0 | NA | NA | |
| 1.48 | − | − | 8.69 | + | + | |
| 0 | − | − | 10.2 | + | − | |
| 0 | − | − | 8.24 | + | − | |
| 0 | − | − | 15.1 | − | − | |
| 0 | − | − | 127 | + | − | |
| 0 | − | − | 136 | − | + | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 6.8 | + | − | |
| Chlorine contact effluent | ||||||
| 0 | − | − | 11.8 | − | − | |
| 1.63 | − | − | 33 | + | − | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 28.4 | − | + | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 24.7 | − | + | |
| 0 | − | − | 20.3 | − | − | |
| 0 | − | 34.8 | − | + | ||
| 0 | − | 19.8 | + | − | ||
| 0 | − | − | 0 | NA | NA | |
| Enhanced primary treatment | ||||||
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 341 | + | + | |
| 0 | − | − | 114 | + | + | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 0 | NA | NA | |
| UV effluent (primary, treated) | ||||||
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 7.35 | + | − | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 15.8 | − | − | |
| 0 | − | − | 9.13 | − | − | |
| 0 | − | − | 0 | NA | NA | |
| 0 | − | − | 0 | NA | NA | |
The presence (+) or absence (−) of the virus genome in the cell lysate as detected by PCR specific for human adenoviruses or by RT-PCR specific for enteroviruses. NA, not analyzed by PCR or RT-PCR.
After flocculation of the WWI, the average TCVA was 75.8 MPN/liter, and the highest was 341 MPN/liter. After UV disinfection this was reduced to 3.6 MPN/liter, and the highest value was 15.8 MPN/liter. This was a reduction of 1.32 log10.
Eighteen of the samples that were positive using the PLC/PRF/5 cell line were negative using the BGM cell line. None of the culture-negative samples on PLC/PRF/5 cells were positive on the BGM cell line. The amount of virus detected on the PLC/PRF/5 cell line TCVA was up to 44 times greater than the values obtained using BGM cells. The difference in titers obtained between the PLC/PRF/5 and BGM cell lines was statistically significant (P value of 0.007).
All of the flasks, including monolayers negative for CPE, were tested by PCR for the presence of adenoviruses and enteroviruses to assess the presence of noncytopathic enteroviruses or adenoviruses on the BGM monolayer. None of the samples were positive for adenoviruses, and one sample from the secondary clarifier was positive for enteroviruses. The presence of adenoviruses and enteroviruses was confirmed by PCR for all PLC/PRF/5 monolayers showing CPE. Seven samples (33%) were positive for enteroviruses, and 11 samples (52%) were positive for adenoviruses out of 21 samples. Three samples were positive for both viruses. From the 18 samples that were CPE positive on the PLC/PRF/5 cell line and negative on the BGM cell line, 6 samples were positive for enteroviruses, and 8 samples were positive for adenoviruses.
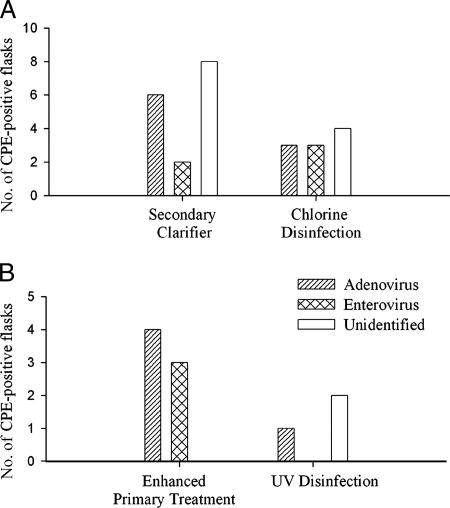
Figure 1A shows the adenoviruses and enteroviruses identified in CPE-positive flasks on the PLC/PRF/5 cell line of the WWTP effluent samples. Of the CPE-positive flasks from the prechlorination samples (secondary clarifier effluent), adenovirus was detected in five, and enteroviruses were detected in three. In the chlorine contact effluent, adenoviruses and enteroviruses were detected in equal numbers of samples (three each).
FIG. 1.
Detection of adenoviruses and enteroviruses in PLC/PRF/5 CPE-positive flasks after the secondary clarifier and chlorine disinfection (A) and after enhanced primary clarification and UV disinfection (B). Unidentified flasks are those that were negative for adenoviruses and enteroviruses but demonstrated viral CPE.
Figure 1B shows the adenoviruses and enteroviruses identified in CPE-positive flasks on the PLC/PRF/5 cell line of the treated WWI effluent samples. The effluent of the chemically enhanced flocculation system was directed to a UV chamber for disinfection prior to discharge. Two samples were positive before UV treatment: adenoviruses were detected in four of the CPE-positive flasks and enteroviruses were detected in three flasks. After UV light treatment, three samples were positive for infectious viruses: adenoviruses were detected in one flask and the other two CPE-positive flasks were negative for adenovirus and enterovirus.
DISCUSSION
Our results demonstrated that the PLC/PRF/5 cell line is more sensitive than the BGM cell line for the detection of culturable viruses in treated sewage. The findings of this study agree with the study of Grabow et al. (10) in which the numbers of samples that produced CPE on the PLC/PRF/5 cell line were double the numbers of samples that produced CPE on the BGM cell line. The investigators compared the use of the L20B mouse cell line, primary velvet monkey kidney (PVK) cell line, the BGM line, and the PLC/PRF/5 line for the detection of viruses from surface and reclamation water from different localities in South Africa. They found more samples producing CPE with the PVK cell line than with the PLC/PRF/5 cell line, but they did not detect any adenoviruses in the PVK cell line.
In our study, no adenoviruses were detected in the BGM cell line. Previous studies have demonstrated the detection of adenovirus on BGM cells from water samples by using a combination of cell culture and PCR, indicating that CPE was not produced in the BGM cells (16). Because we found a high number of samples positive for adenoviruses producing CPE on PLC/PRF/5 cells, we tested all of the second- and third-passage BGM flasks by PCR with specific primers for adenoviruses. For the detection of the adenovirus genome in cell supernatants by PCR, incubation times of at least 10 days may be needed at concentrations as low as 10−2 50% tissue culture infective doses in inoculums of concentrated samples used for cell culture assays (16). In our study, each passage was incubated for 14 days, and two to three passages for each sample were used; therefore, there was sufficient time for the slower-growing adenoviruses to be detected on the BGM cell line. In addition, adenovirus has been detected in cell lines such as A549 by PCR (14, 15) and PLC/PRF/5 by CPE (10) in samples that were negative using the BGM cell line. The combination of the PLC/PRF/5 cell line and PCR has been used to detect adenovirus without evidence of CPE after incubation periods of 7 days (28) to 14 days (27), suggesting the possibility of using the PLC/PRF/5 cell line in an integrated cell culture and PCR approach to detect adenoviruses in shorter periods than the 24-day incubation used in our study.
A surveillance of viruses in the sewage of Jones Island WWTP in the MMSD reported that of the viruses isolated, 68% were reoviruses, 28% were enteroviruses, and 4% were adenoviruses (23). The enteroviruses isolated from sewage included echovirus (80%), coxsackievirus B (14%), poliovirus (4%), and coxsackievirus A (1.1%) (21). Seven different cell lines were used in these studies, including the MK-1, HEP-2, HPS, HEL, BGM, RD, and Caco-2 lines. Fourteen percent of all the enteroviruses were isolated on BGM cells, which includes 83% of the coxsackievirus B and 1.9% of the echoviruses (22). Previous studies using clinical isolates have indicated that the BGM cell line is very sensitive to coxsackievirus B and not very sensitive to echovirus infection (5). There is no previous work describing the sensitivity of the PLC/PRF/5 cell line to echovirus infection. But the greater detection of enteroviruses observed in comparison with BGM and the proportion of echovirus previously reported in sewage (22) suggest that the PLC/PRF/5 cell line may be more sensitive to echovirus infection than the BGM cell line.
We found that the proportion of viruses isolated before disinfection changed in contrast to the proportion of viruses isolated postdisinfection. The proportion of enteroviruses increased after disinfection with chlorination, suggesting that chlorine disinfection produced at this plant during wet weather was not very effective for inactivating the enteroviruses detected in this study. This may be because the free chlorine in sewage combines with ammonia to produce chloramine (which is less effective than chlorine). In contrast, the UV treatment was very effective at inactivating enteroviruses but not adenoviruses. This result is in agreement with the observation that adenoviruses are very resistant to UV inactivation (17, 25).
The use of multiple cell lines will increase the probability of isolating the maximum number of viruses from wastewater (5, 22, 23). However, this drastically increases the cost per sample, and usually only one cell line is used. Currently, the recommended cell line in the United States for water and wastewater analysis is the BGM cell line. The advantage of using the PLC/PRF/5 cell line for the detection of viruses from treated sewage is not only that it had greater sensitivity but also that the viruses detected with this cell line were more resistant to disinfection and treatment. If only the BGM cell line had been used, then the effectiveness of the disinfectant treatment process would have been overestimated. In conclusion, our results demonstrate that the PLC/PRF/5 cell line is a more suitable cell line than the BGM cell line for the enumeration of total culturable virus from sewage. Other cell lines such as the human colon carcinoma cell line have the ability to propagate a wide range of viruses including adenovirus 40, astrovirus, enteroviruses, and hepatitis A virus (19), which may be useful to increase the types of infectious virus detected.
Acknowledgments
We especially thank Kelley R. Riley and Dave C. Love for their editorial support.
Footnotes
Published ahead of print on 7 March 2008.
REFERENCES
- 1.Avellón, A., P. Pérez, J. C. Aguilar, R. Lejarazu, and J. E. Echevarría. 2001. Rapid and sensitive diagnosis of human adenovirus infections by a generic polymerase chain reaction. J. Virol. Methods 92:113-120. [DOI] [PubMed] [Google Scholar]
- 2.Berg, G., D. R. Dahling, G. A. Brown, and D. Berman. 1978. Validity of fecal coliforms, total coliforms, and fecal streptococci as indicators of viruses in chlorinated primary sewage effluents. Appl. Environ. Microbiol. 36:880-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borchardt, M. A., P. D. Bertz, S. K. Spencer, and D. A. Battigelli. 2003. Incidence of enteric viruses in groundwater from household wells in Wisconsin. Appl. Environ. Microbiol. 69:1172-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapron, C. D., N. A. Ballester, J. H. Fontaine, C. N. Frades, and A. B. Margolin. 2000. Detection of astroviruses, enteroviruses, and adenovirus types 40 and 41 in surface waters collected and evaluated by the information collection rule and an integrated cell culture-nested PCR procedure. Appl. Environ. Microbiol. 66:2520-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chonmaitree, T., C. Ford, C. Sanders, and H. L. Lucia. 1988. Comparison of cell cultures for rapid isolation of enteroviruses. J. Clin. Microbiol. 26:2576-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahling, D. 1991. Detection and enumeration of enteric viruses in cell culture. Critical Rev. Environ. Contam. 21:237-263. [Google Scholar]
- 7.Fong, T. T., and E. K. Lipp. 2005. Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiol. Mol. Biol. Rev. 69:357-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerba, C. P., S. M. Goyal, R. L. LaBelle, I. Cech, and G. F. Bodgan. 1979. Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am. J. Public Health 69:1116-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grabow, W. O., D. L. Puttergill, and A. Bosch. 1992. Propagation of adenovirus types 40 and 41 in the PLC/PRF/5 primary liver carcinoma cell line. J. Virol. Methods 37:201-207. [DOI] [PubMed] [Google Scholar]
- 10.Grabow, W., K. L. Botma, J. C. Villiers, C. G. Clay, and B. Erasmus. 1999. Assessment of cell culture and polymerase chain reaction procedures for the detection of poliovirus in wastewater. Bull. W. H. O. 77:973-978. [PMC free article] [PubMed] [Google Scholar]
- 11.Hurley, M. A., and M. E. Roscoe. 1983. Automated statistical-analysis of microbial enumeration by dilution series. J. Appl. Bacteriol. 55:159-164. [Google Scholar]
- 12.Jiang, S. C. 2006. Human adenoviruses in water: occurrence and health implications: a critical review. Environ. Sci. Technol. 2006. 40:7132. [DOI] [PubMed] [Google Scholar]
- 13.Langley J. M. 2005. Adenovirus. Pediatr. Rev. 26:244-249. [DOI] [PubMed] [Google Scholar]
- 14.Lee, C., S. H. Lee, E. Han, and S. J. Kim. 2004. Use of cell culture-PCR assay based on combination of A549 and BGMK cell lines and molecular identification as a tool to monitor infectious adenoviruses and enteroviruses in river water. Appl. Environ. Microbiol. 70:6695-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee, H. K., and Y. S. Jeong. 2004. Comparison of total culturable virus assay and multiplex integrated cell culture-PCR for reliability of waterborne virus detection. Appl. Environ. Microbiol. 70:3632-3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee, S. H., C. Lee, K. W. Lee, H. B. Cho, and S. J. Kim. 2005. The simultaneous detection of both enteroviruses and adenoviruses in environmental water samples including tap water with an integrated cell culture-multiplex-nested PCR procedure. J. Appl. Microbiol. 98:1020-1029. [DOI] [PubMed] [Google Scholar]
- 17.Nwachuku, N., C. P. Gerba, A. Oswald, and F. D. Mashadi. 2005. Comparative inactivation of adenovirus serotypes by UV light disinfection. Appl. Environ. Microbiol. 71:5633-5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pepper, I. L., C. P. Gerba, and R. M. Maier. 2000. Environmental sample collection and processing, p. 177-194. In R. M Maier, I. L. Pepper, and C. P. Gerba (ed.), Environmental microbiology. Academic Press, San Diego, CA.
- 19.Pinto, R. M., J. M. Diez, and A. Bosch. 1994. Use of the colonic-carcinoma cell-line Caco-2 for in-vivo amplification and detection of enteric viruses. J. Med. Virol. 44:310-315. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds, K. A., C. P. Gerba, M. Abbaszadegan, and L. L. Pepper. 2001. ICC/PCR detection of enteroviruses and hepatitis A virus in environmental samples. Can. J. Microbiol. 47:153-157. [PubMed] [Google Scholar]
- 21.Schwab, K. J., R. Deleon, and M. D. Sobsey. 1996. Immunoaffinity concentration and purification of waterborne enteric viruses for detection by reverse transcriptase PCR. Appl. Environ. Microbiol. 62:2086-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedmak, G., D. Bina, and J. MacDonald. 2003. Assessment of an enterovirus sewage surveillance system by comparison of clinical isolates with sewage isolates from Milwaukee, Wisconsin, collected August 1994 to December 2002. Appl. Environ. Microbiol. 69:7181-7187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sedmak, G., D. Bina, J. Macdonald, and L. Couillard. 2005. Nine-year study of the occurrence of culturable viruses in source water for two drinking water treatment plants and the influent and effluent of a wastewater treatment plant in Milwaukee, Wisconsin (August 1994 through July 2003). Appl. Environ. Microbiol. 71:1042-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss, J. H., and E. G. Strauss. 2002. Viruses and human disease. Academic Press, San Diego, CA.
- 25.Thurston-Enriquez, J. A., C. N. Haas, J. Jacangelo, K. Riley, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Environmental Protection Agency. 1995. Virus monitoring protocols for the information collection requirements rule. EPA/814-B95-002. U.S. Environmental Protection Agency, Washington, DC.
- 27.Van Heerden, J., M. M. Ehlers, W. B. Van Zyl, and W. O. K. Grabow. 2003. Incidence of adenoviruses in raw and treated water. Water Res. 37:3704-3708. [DOI] [PubMed] [Google Scholar]
- 28.Van Heerden, J., M. M. Ehlers, W. B. van Zyl, and W. O. K. Grabow. 2004. Prevalence of human adenoviruses in raw and treated water. Water Sci. Technol. 50:39-43. [PubMed] [Google Scholar]
- 29.Vivier, J. C., M. M. Ehlers, and W. O. K. Grabow. 2004. Detection of enteroviruses in treated drinking water. Water Res. 38:2699-2705. [DOI] [PubMed] [Google Scholar]