Abstract
Sulfite plays an important role in beer flavor stability. Although breeding of bottom-fermenting Saccharomyces strains that produce high levels of SO2 is desirable, it is complicated by the fact that undesirable H2S is produced as an intermediate in the same pathway. Here, we report the development of a high-level SO2-producing bottom-fermenting yeast strain by integrated metabolome and transcriptome analysis. This analysis revealed that O-acetylhomoserine (OAH) is the rate-limiting factor for the production of SO2 and H2S. Appropriate genetic modifications were then introduced into a prototype strain to increase metabolic fluxes from aspartate to OAH and from sulfate to SO2, resulting in high SO2 and low H2S production. Spontaneous mutants of an industrial strain that were resistant to both methionine and threonine analogs were then analyzed for similar metabolic fluxes. One promising mutant produced much higher levels of SO2 than the parent but produced parental levels of H2S.
The bottom-fermenting yeast Saccharomyces pastorianus is used to produce beer and has been proposed to be a natural hybrid between Saccharomyces cerevisiae and Saccharomyces bayanus (30). Bottom-fermenting yeasts have two types of genes, one set highly homologous (more than 90% identity) to those of S. cerevisiae and the other less so but highly homologous to S. bayanus (i.e., non-S. cerevisiae [Lg type]) (8, 14, 27, 33). One way in which S. pastorianus differs from baker's yeast (S. cerevisiae) is its tendency to produce higher levels of both sulfite (SO2) and hydrogen sulfide (H2S).
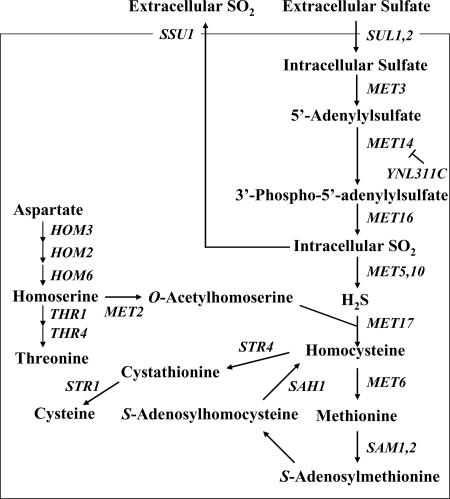
It is well known that sulfur compounds in beer make significant contributions to flavor and aroma. SO2, for example, acts as an antioxidant, which slows the development of oxidation haze and staling of flavors in beer. In contrast, H2S has an aroma of rotten eggs and is also a precursor of other compounds with undesirable sensory characteristics. SO2 and H2S are produced by yeast during reductive sulfate assimilation (Fig. 1). Inorganic sulfate is taken up through a sulfate permease and reduced to SO2 by enzymes encoded by MET3, MET14, and MET16. SO2 is then reduced to H2S by SO2 reductase encoded by MET5 and MET10 (29). The next intermediate, homocysteine, which is synthesized from H2S and O-acetylhomoserine (OAH) by OAH sulfhydrylase encoded by MET17, leads to the formation of cysteine, methionine, and S-adenosylmethionine (SAM). SAM transcriptionally represses all of the genes involved in sulfate assimilation. Park and Bakalinsky previously reported that SSU1 encodes an SO2 efflux pump that exports intracellular SO2 through the plasma membrane (18).
FIG. 1.
Schematic of the reductive sulfate assimilation pathway in yeast. YNL311C encodes an F-box protein (13). Ynl311c negatively regulates Met14 and degrades it via a ubiquitin-related pathway.
In the postgenomic era, systematic and high-throughput analyses of mRNA and proteins have become central to recent functional genomics initiatives. Metabolomics entails the analysis of all cellular metabolites and has become a powerful new tool for gaining insight into functional biology. Measurement of numerous metabolites within a cell and tracking concentration changes as a function of growth or environmental conditions not only provides direct information on metabolic phenotypes but also complements gene expression and proteomic studies. Current large-scale methods for the analysis of metabolites are based on gas chromatography (GC)-mass spectrometry (MS) (7), liquid chromatography-MS (31), nuclear magnetic resonance (20), and Fourier transform ion cyclotron resonance-MS (1). Recently, capillary electrophoresis-electrospray ionization (CE-ESI)-MS has emerged as a powerful analytical tool, and a number of CE-ESI-MS methods have been developed for the analysis of charged species such as carboxylic acids, phenolic compounds, amino acids, metal species, tetramines, and herbicides (25). While metabolite-profiling analysis of the glutathione synthesis pathway in baker's yeast was recently reported (15), to our knowledge, the metabolomic analysis of bottom-fermenting yeast has not yet been reported.
The physiology of sulfur metabolism in Saccharomyces yeasts, particularly in regard to SO2 and H2S production, has received significant attention (11, 17, 28). Although the biosynthesis of these two compounds is interconnected, it would be desirable to increase SO2 and to decrease H2S in beer. Yeast strains that produce lowered levels of H2S have been constructed or isolated by gene disruption, conventional mutagenesis, and cell fusion techniques. However, those treatments have also resulted in undesirable phenotypes (11). Mutants that produce higher levels of SO2 have been obtained by the overexpression of SSU1 and/or MET14 (4) and by the disruption of MET10 (11). However, bottom-fermenting yeast strains in which MET10 was disrupted were also found to have acquired undesirable phenotypes (11), possibly due to a reduction in intracellular methionine or slow growth. Furthermore, because these yeasts were obtained by the use of recombinant DNA technology, they cannot be used commercially.
In this paper, we report integrated metabolome and transcriptome analysis of reductive sulfate assimilation in baker's and bottom-fermenting yeasts. Based on these data, we proposed that OAH is one of the rate-limiting factors for SO2 and H2S production, and we constructed high-level SO2-producing mutants without increasing H2S levels by simultaneously increasing the flux from aspartate to OAH and increasing the flux from sulfate to SO2. One candidate mutant that produced much higher levels of SO2 than the parent, parental levels of H2S, and no undesirable changes in fermentation properties was isolated.
MATERIALS AND METHODS
Strains and media.
Bottom-fermenting yeast S. pastorianus strains used in this study were KBY011 (our laboratory stock), YMO106 (mutant selected from KBY011), and B43 (meiotic segregant of KBY011). Baker's yeast S. cerevisiae strains used were S288C (MATα) and SYT001 (MATa; derived from DBY7286). Strains were grown in YPD (1% yeast extract, 2% peptone, and 2% glucose), YPD10 (1% yeast extract, 2% peptone, and 10% glucose), SD10 (0.4% yeast nitrogen base without amino acids and ammonium sulfate, 0.2% ammonium sulfate, and 10% glucose), SD10(2) (0.2% yeast nitrogen base without amino acids and ammonium sulfate, 0.1% ammonium sulfate, and 10% glucose), or Brewer's wort. Strains and media used in each experiment are shown in Table 1. YPDL plates (0.5% yeast extract, 0.3% peptone, 4% glucose, 0.02% ammonium sulfate, 0.1% lead nitrate, and 2% agar) were used to test for H2S production (9). SDLE plates (0.17% yeast nitrogen base without amino acids and ammonium sulfate, 0.5% ammonium sulfate, 2% glucose, 0.1% lead nitrate, 10 mg/ml dl-ethionine, and 2% agar) and SDH medium (0.17% yeast nitrogen base without amino acids and ammonium sulfate, 0.5% ammonium sulfate, 2% glucose, and 100 mg/ml of dl-hydroxynorvaline) were used to isolate mutants. Geneticin (G418) at 200 μg/ml, blasticidin S at 50 μg/ml, or aureobasidin A at 1 μg/ml was added to YPD plates to select and screen transformants. Transformation of yeast strains was carried out by use of a modified gene pulser procedure (16).
TABLE 1.
Strains and media used in this study
| Expt | Strain(s) | Medium |
|---|---|---|
| 1 | S288C and KBY011 | SD10 |
| 2 | SYT001 | SD10(2) with or without threonine |
| 3 | B43 and derivatives | YPD10 + G418 |
| 4 | B43 and derivatives | YPD10 |
| 5 | KBY011 and YMO106 | Wort |
Isolation of meiotic segregant from bottom-fermenting yeast.
Meiotic segregants were obtained from KBY011 as described previously by Bilinski et al. (2). With respect to SO2 and H2S production, the meiotic segregant B43 is similar to KBY011.
Isolation of mutants resistant to amino acid analogs.
Wild-type cells were incubated in 5 ml of YPD at 20°C for 3 days. After centrifugation, cells were resuspended in 5 ml of distilled water. One hundred fifty microliters of cells was spread onto SDLE plates and incubated at 20°C for 10 days. Ethionine-resistant mutants were incubated in 5 ml of YPD at 20°C for 3 days. After centrifugation, the cells were resuspended in 10 ml of distilled water. One hundred microliters of cells was added to SDH and incubated at 20°C for 5 days. One hundred microliters of culture was spread onto YPDL plates, which were then incubated at 20°C for 7 days.
Construction of overexpression plasmids.
To construct pYES-G418GP, a 1.5-kb SalI fragment containing a GPD promoter and PGK1 terminator cassette derived from pSY114 (32) was inserted at the SalI site of pYES-G418 (34). To construct plasmids overexpressing S. cerevisiae MET14 (ScMET14), ScHOM3, LgHOM3, LgMET17, and LgTHR1, these genes were cloned by PCR using KBY011 genomic DNA and inserted at the BamHI site of pYES-G418GP. To construct the integration vector pAUR-HOM3, ScHOM3 was inserted into the BamHI site of pSY114, and a 3-kb GPD promoter-ScHOM3-PGK terminator cassette was inserted at the SalI site of pAUR101 (Takara Shuzo) carrying an aureobasidin A resistance marker. Integration of the ScHOM3 gene was confirmed by Southern hybridization analysis.
Construction of disruption plasmids.
To disrupt both Lg and S. cerevisiae types of YNL311C and HOM3 genes in B43, the LgYNL311C gene was cloned from a KBY011 cosmid library based on homology with the corresponding S. cerevisiae genes. To construct a vector harboring a blasticidin S resistance cassette, a 0.5-kb HindIII fragment carrying blasticidin S resistance was inserted at the HindIII site of pSY114 carrying a GPD promoter and a PGK terminator. This blasticidin S resistance expression cassette was then integrated into the open reading frames of the LgYNL311C and LgHOM3 genes, respectively. The corresponding S. cerevisiae-type genes were disrupted by DNA fragments amplified using genomic DNA derived from the yeast knockout strains (Open Biosystems). Disruption of both types of genes was confirmed by PCR.
Growth conditions for comprehensive analyses.
KBY011 and S288C were precultured with shaking at 20°C for 3 days in 500 ml of YPD10. Cells were harvested and diluted to a density of 0.5% (wt/vol) in 2 liters of fresh SD10 and grown with gentle stirring at 20°C for 3 days under anaerobic conditions produced by initial headspace exclusion and N2 flushing. Cells were then harvested, diluted to a density of 0.5% (wt/vol) in 2 liters of the fresh SD10, and grown at 20°C under anaerobic conditions with gentle stirring (experiment 1). To test the effect of the addition of threonine, SYT001 cells were harvested and diluted to a density of 0.5% (wt/vol) in 2 liters of fresh SD10(2) with or without 1 g/liter of threonine and grown at 20°C under anaerobic conditions with gentle stirring. Cells were then collected at different time points (0, 6, 24, and 48 h) and harvested for metabolome analysis (experiment 2).
Growth conditions for small-scale fermentation.
B43, KBY011, and YMO106 were precultured with shaking at 20°C for 3 days in 500 ml of YPD10 (200 mg/liter of G418 was added in experiment 3). Cells were harvested, diluted to a density of 0.5% (wt/vol) in 200 ml of fresh YPD10 (experiments 3 and 4) or 500 ml of wort (experiment 5), and grown at 20°C for 4 days under anaerobic conditions produced by initial headspace exclusion and N2 flushing. Cells were then harvested, diluted to a density of 0.5% (wt/vol) in 200 ml of fresh YPD10 or 500 ml of wort, and grown with gentle stirring at 20°C under anaerobic conditions.
DNA microarray analysis.
DNA microarray experiments were carried out using bottom-fermenting yeast oligoarrays (Agilent DNA microarray system). Oligonucleotide probes (60 bp) were spotted onto the array, which carries 3,181 probes derived from Lg-type genes and 6,637 probes derived from S. cerevisiae genes. Total RNA was extracted using glass beads (21) and purified using an RNeasy column (Qiagen), and 0.2 μg of RNA was labeled using the Agilent linear amplification/labeling kit (Agilent Technologies) according to the manufacturer's instructions. Hybridization of labeled cRNA to the arrays was performed using the manufacturer's hybridization protocol. Microarrays were washed, dried, and scanned on a dual-laser DNA microarray scanner (model G2565BA; Agilent Technologies). Feature Extraction and Image Analysis software programs were used. Normalization was carried out by the Lowess method. For each experiment, the data presented are hybridization means for two arrays in two-dye swap experiments (i.e., Cy3 and Cy5 dye-swapping experiments).
Extraction of intracellular metabolite.
Metabolites were extracted using a modification of a previously described procedure (25). Cells were harvested from a culture medium (optical density at 600 nm of 30) by filtration through a 0.45-μm-pore-size filter. Methionine sulfone and 2-morpholinoethanesulfonic acid (MES) were used as internal cationic and anionic standards, respectively. Lyophilized samples were dissolved in 50 μl of Milli-Q water before CE-ESI-MS analysis.
CE-ESI-MS conditions for metabolite analysis.
All CE-ESI-MS experiments were performed using an Agilent capillary electrophoresis system equipped with an air pressure pump, an Agilent 1100 series MSD mass spectrometer and an isocratic high-performance liquid chromatography (HPLC) pump, a G1603A Agilent CE-MS adapter kit, and a G1607A Agilent CE-ESI-MS sprayer kit (Agilent Technologies) as described previously (23, 24).
Other analyses.
The supernatants from filtered samples were assayed for free SO2 and organic acids by HPLC. H2S was detected quantitatively using a headspace GC-sulfur chemiluminescence detector system. Higher alcohols, esters, acetaldehyde, and diacetyl were measured by GC.
Statistical analysis.
Each metabolomic experiment (experiments 1 and 2) was done in triplicate in independent experiments, and data were analyzed by using mean values from triplicate samples, except for SO2 and H2S. The data below the detection limit were not included for statistical analysis. In experiments involving overproduction of Met14 and Hom3 in B43, significant differences in SO2 and H2S production levels between the two strains were compared using Student's t test (ystat2006.xls; S. Yamazaki, Igaku Tosho Press, Inc., Japan).
Microarray data accession number.
The microarray data are registered in the ArrayExpress (EBI) database under accession number E-MEXP-1086.
RESULTS
Metabolite and gene expression profiling analyses of sulfur metabolism in bottom-fermenting and baker's yeasts.
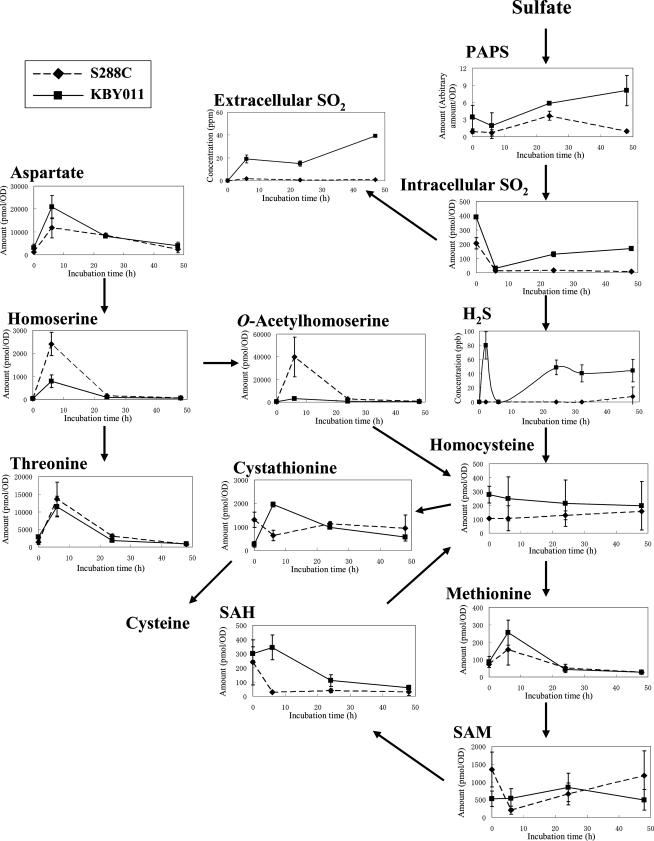
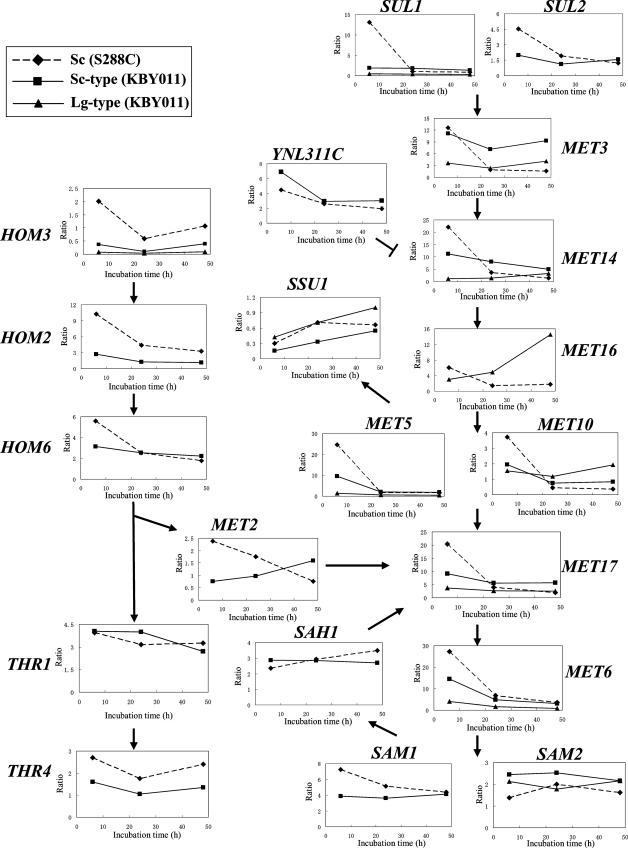
For unknown reasons, bottom-fermenting yeast produces high levels of SO2 and H2S under anaerobic conditions, while baker's yeast does not (26). To determine the basis for this difference, we carried out transcriptome and metabolome analyses. SD10 medium lacking amino acids was used to minimize complications resulting from amino acid uptake. Most intracellular metabolites related to sulfate assimilation were measured by CE-ESI-MS, while SO2 was analyzed by HPLC, and H2S was analyzed by GC. Figure 2 shows levels of sulfur metabolites in cell extracts and SO2 and H2S in media. Because a commercial 3′-phospho-5′-adenylylsulfate standard was not available, 3′-phospho-5′-adenylylsulfate was quantified relative to the internal standard MES. As reported above, the production of extracellular SO2 and H2S by bottom-fermenting strain KBY011 was much greater than that by baker's yeast S. cerevisiae S288C. In contrast, levels of intracellular OAH and homoserine were much lower in KBY011 than in S288C. Figure 3 shows the expression profiles of genes involved in sulfur metabolism in S288C and KBY011. We constructed the bottom-fermenting yeast microarray based on the expressed sequence tag data for KBY011 (33), because the whole-genome sequence of bottom-fermenting yeast was not available at the time of this writing. Graphs for which a KBY011 data set are missing indicate that the respective probes were unavailable in the expressed sequence tag data. The expression patterns of the MET2, MET14, and MET16 genes were found to differ between S288C and KBY011. Furthermore, the expression levels of both the Lg- and S. cerevisiae-type HOM3 genes were much lower in KBY011 than in S288C in the early stage of fermentation. Based on the metabolome and transcriptome data, we suggest that KBY011 has a lower level of OAH than S288C, probably due to the low level of expression of HOM3 or related enzyme activities. We presume that KBY011 may produce higher levels of SO2 and H2S than S288C due to limiting amounts of OAH, which reacts with H2S to form homocysteine.
FIG. 2.
Changes in metabolite pools related to reductive sulfate assimilation in bottom-fermenting and baker's yeasts. Extracellular SO2 and H2S levels are concentrations in the spent media. Concentrations of the other metabolites (pmol) are expressed per unit yeast biomass (an amount of cells equivalent to an optical density [OD] at 600 nm of 1). The data are means of three independent experiments, with the error bars indicating standard deviations. Diamonds and squares indicate baker's yeast (S288C) and bottom-fermenting yeast (KBY011), respectively (experiment 1). SAH, S-adenosylhomocysteine; PAPS, 3′-phospho-5′-adenylylsulfate.
FIG. 3.
Changes in expression levels of genes involved in reductive sulfate assimilation in bottom-fermenting and baker's yeasts. Diamonds, squares, and triangles indicate baker's yeast, bottom-fermenting yeast S. cerevisiae (Sc)-type, and bottom-fermenting yeast Lg-type genes, respectively (experiment 1). The y axis is the expression ratio relative to the zero time point. These microarray data are means of analyses taken from two independent fermentation experiments with very similar results (experiment 1).
OAH is rate limiting for H2S production.
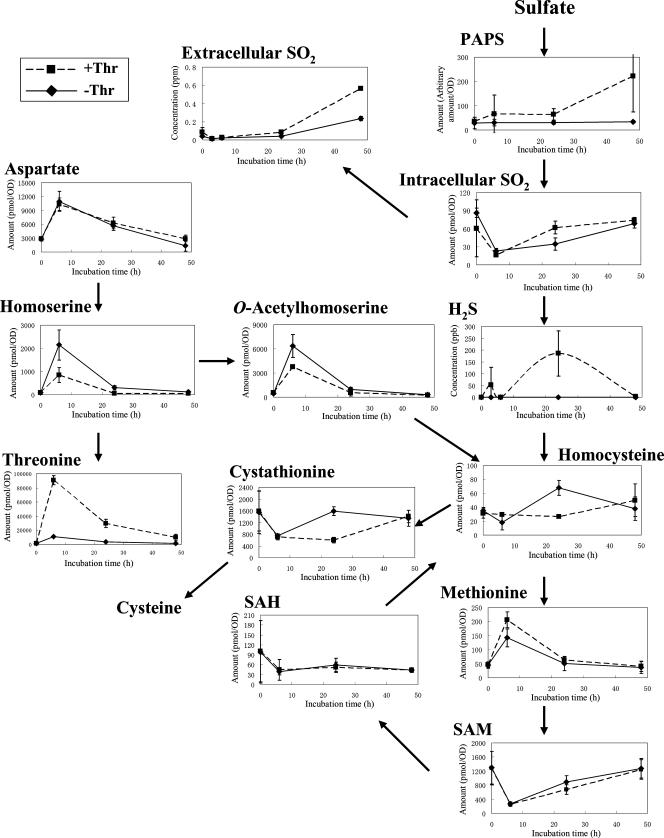
To test the hypothesis that OAH is a rate-limiting factor for SO2 and H2S production, we performed the experiments described below. It was reported previously that in yeast, threonine inhibits aspartate kinase encoded by HOM3 through feedback inhibition (6) and that the addition of threonine increases SO2 production (10), suggesting that the addition of threonine would shift the pattern of sulfate assimilation in baker's yeast to that observed in bottom-fermenting yeast. Therefore, metabolome analysis of baker's yeast strain SYT001 grown in SD10(2) was performed with and without added threonine. As expected, the addition of threonine caused elevated levels of SO2 and H2S production, especially 24 h after the addition (Fig. 4). The metabolome data show that homoserine and OAH levels were lower in the presence of added threonine than in the absence of threonine (Fig. 4). These results are consistent with threonine feedback-inhibiting aspartate kinase leading to a decrease in OAH production and a resulting increase in SO2 and H2S levels.
FIG. 4.
Changes in metabolite pools related to reductive sulfate assimilation in baker's yeast with or without threonine. Concentrations of metabolites are indicated as described in the Fig. 2 legend. The data represent mean values from three independent experiments, with error bars showing standard deviations. Squares and diamonds indicate baker's yeast with and without the addition of threonine, respectively (experiment 2). SAH, S-adenosylhomocysteine; PAPS, 3′-phospho-5′-adenylylsulfate; OD, optical density.
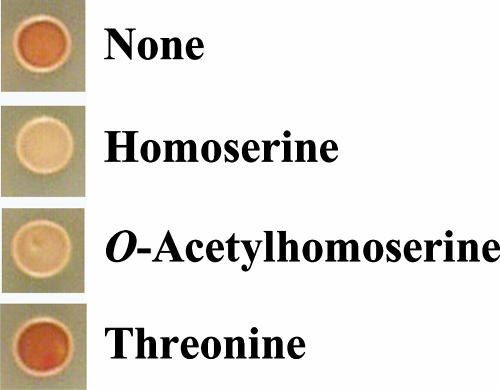
Moreover, the effect of the addition of OAH on H2S production was tested on YPDL plates using KBY011. Figure 5 shows that threonine increased H2S production, while homoserine and OAH decreased H2S production at a final concentration of 1 mM. These results strongly suggest that OAH is a rate-limiting factor for H2S production.
FIG. 5.
Effect of OAH on H2S production in bottom-fermenting yeast. All compounds shown were added to YPDL plates at a final concentration of 1 mM. Cells of bottom-fermenting yeast strain KBY011 were incubated at 25°C on YPDL plates for 4 days.
Effect of overexpression and disruption of genes involved in sulfate assimilation.
As noted above, OAH appears to be the rate-limiting factor for SO2 and H2S production. SO2 and H2S production are linked (5), as SO2 is the immediate biochemical precursor of H2S in the reductive sulfate assimilation pathway (Fig. 1), indicating that flux from sulfate to SO2 is important for both SO2 and H2S production. Therefore, the effects of genetically altering the flux from aspartate to OAH and the flux from sulfate to SO2 on SO2 and H2S production were investigated using relevant overexpressed and disrupted genes. As bottom-fermenting yeast is proposed to be a tetraploid, meiotic segregants expected to each have a set of S. cerevisiae-type and Lg-type genes from KBY011 were isolated to make the experiment more tractable. One of them, B43, exhibited sulfur metabolism and a fermentation profile similar to those of parent strain KBY011 (data not shown).
Initially, in order to regulate the flux from aspartate to OAH, a B43 strain overexpressing LgHOM3, LgMET17, or LgTHR1 was constructed. The levels of SO2 and H2S of the LgHOM3-overexpressing B43 strain were found to be 1.1- and 3.5-fold lower than those of the control, respectively (Table 2). A hom3 disruptant was also constructed and evaluated. SO2 and H2S levels in the hom3 disruptant were 2.6- and 2.8-fold higher than those of the parent, respectively (Table 3). These results indicate that the flux from aspartate to OAH has more significant effects on H2S production than on SO2 production.
TABLE 2.
Effect of gene overexpression on SO2 and H2S production in strain B43a
| Gene | SO2 level (ppm) | H2S level (ppb) |
|---|---|---|
| Vector | 2.51 | 118.3 |
| LgHOM3 | 2.27 | 33.5 |
| LgMET17 | 2.24 | 0 |
| LgTHR1 | 2.58 | 143.5 |
SO2 and H2S levels were measured after 23 h. The data represent means for two independent experiments (experiment 3).
TABLE 3.
Effect of gene disruption on SO2 and H2S production in strain B43a
| Genotype | SO2 level (ppm) | H2S level (ppb) |
|---|---|---|
| Parent | 1.55 | 102 |
| Δhom3 | 3.97 | 290 |
| Δynl311c | 3.89 | 166 |
SO2 and H2S levels were measured after 24 h (experiment 4).
To alter the flux from sulfate to SO2, a Met14-overproducing strain was constructed by disrupting YNL311C. Ynl311c has an F-box motif, interacts with Met14 (13), and is involved in its proteolysis via the ubiquitin pathway (S. Yoshida et al., unpublished results). The levels of SO2 and H2S in the ynl311c disruptant were found to be 2.5- and 1.6-fold higher, respectively, than those in the controls (Table 3). This indicates that the flux from sulfate to SO2 has a greater effect on SO2 production than on H2S production.
Based on these results, strains producing high SO2 and low H2S levels were sought by simultaneously increasing the flux from aspartate to OAH and increasing the flux from sulfate to SO2.
Breeding of bottom-fermenting yeast by genetic modification and spontaneous mutants resistant to both methionine and threonine analogs.
Strain B43, which overproduced both Hom3 and Met14, produced almost same amount of H2S as the parent strain on YPDL plates (data not shown). The levels of SO2 and H2S after the overproduction of Met14 and Hom3 were 2.68 ± 0.22 ppm and 165.4 ± 11.8 ppb, respectively, for the parent strain and 3.86 ± 0.50 ppm (P < 0.05) and 131.8 ± 13.3 ppb (P < 0.05), respectively, for the Δyn1311c ScHOM3 strain, as measured after 24 h (means and standard deviations from three independent experiments [experiment 3] are shown). Similarly, with respect to the simultaneous overexpression of both ScMET14 and ScHOM3, the levels of SO2 and H2S of the control strain were 1.57 ± 0.56 ppm and 113.1 ± 19.2 ppb, respectively, and those of the strain overexpressing both ScMET14 and ScHOM3 were 2.55 ± 0.65 ppm (P < 0.05) and 88.4 ± 17.4 ppb (P < 0.05), respectively, as measured after 23 h (values are means and standard deviations from five independent experiments [experiment 3]). Significant differences between the parent and genetically modified strains were determined by a Student's t test. These results indicate that high SO2 and low H2S production can be achieved by simultaneously increasing the flux from aspartate to OAH and increasing the flux from sulfate to SO2.
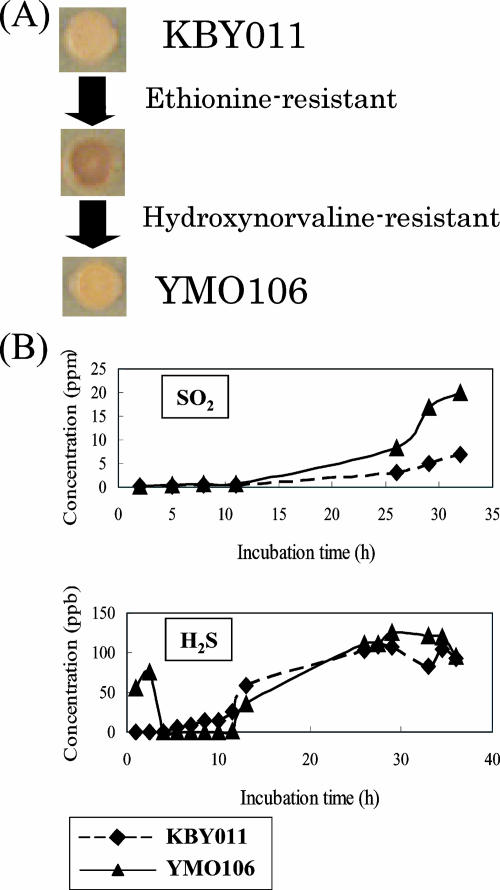
In order to isolate a spontaneous mutant exhibiting similar metabolic fluxes for commercial use, strains resistant to both methionine and threonine analogs were selected. Initially, we isolated ethionine-resistant mutants from KBY011, whose flux from sulfate to H2S would be expected to increase. From among 1.4 × 108 cells, 14 ethionine-resistant candidates that formed black colonies on YPDL plates were isolated (Fig. 6A). SO2 and H2S productivities were analyzed in three of them. One, YMO2, produced 2.2-fold more SO2 and 2.4-fold more H2S than did the parent strain in YPD10 medium. Hydroxynorvaline-resistant mutants of YMO2 were then selected in order to increase the flux from aspartate to OAH. From among 108 cells, 10 candidates that formed thin brown colonies were isolated (Fig. 6A). One candidate, YMO106, produced 2.7-fold more SO2 than did parent strain KBY011 but produced the same amount of H2S (1.04-fold more) in wort after 24 h of incubation (Fig. 6B). No differences in H2S accumulation were observed between KBY011 and YMO106 using YPDL plates (Fig. 6A). These results suggest that mutants that produce much higher SO2 levels but without a significant increase in H2S levels can be isolated by simultaneously increasing the flux from aspartate to OAH and increasing the flux from sulfate to SO2. An independently isolated ethionine- and hydroxynorvaline-resistant mutant from a different industrial strain was also found to produce a high level of SO2 and a low level of H2S (data not shown). This mutant exhibited almost the same SO2 and H2S productivities as the HOM3- and MET14-overexpressing strain, demonstrating that mutants that produce higher SO2 and lower H2S levels can be isolated by the selection of mutants that are resistant to the two-amino-acid analogs.
FIG. 6.
SO2 and H2S production in the spontaneous mutant. (A) Strategy for isolating mutant strain YMO106 from parental strain KBY011. Yeast cells were incubated at 20°C on YPDL plates for 5 days. (B) Extracellular SO2 and H2S concentrations in media are shown. Diamonds and triangles indicate parental strain KBY011 and mutant strain YMO106, respectively (experiment 5).
Lager fermentations using strains KBY011 and YMO106 in 200-liter batches of wort were performed. Chemical analysis of the resultant beers is summarized in Table 4. With respect to SO2, the beer produced by YMO106 (YMO106 beer) contained much higher levels of SO2 than that produced by KBY011 (KBY011 beer) at all sampling times. At the end of the main fermentation, the YMO106 beer contained less H2S than did the KBY011 beer, while the opposite was true after a period of maturation. It is more important to decrease H2S during the main fermentation than during beer maturation, because undesirable compounds derived from H2S are produced during the main fermentation. The increase in H2S in the YMO106 beer would be expected to disappear upon longer maturation. The YMO beer contained about the same amount of organic acids and volatile compounds as did the KBY011 beer (diacetyl, acetaldehyde, esters, and fusel alcohols), suggesting that the introduced mutations did not cause undesirable changes in the concentrations of compounds having a major sensory impact in beer (Table 4).
TABLE 4.
Levels of SO2, H2S, and selected aroma compounds in beer after the main fermentation and in bottled beer after maturation produced by strain KBY011 (parent) and derivative strain YMO106a
| Compound | Concn
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO2 (fermentation tank) (ppm) | SO2 (bottled beer) (ppm) | H2S (fermentation tank) (ppb) | H2S (bottled beer) (ppb) | Total diacetyl (ppm) | Acetaldehyde (ppm) | Ethyl acetate (ppm) | Amyl alcohol (ppm) | Isoamyl acetate (ppm) | Sulfate (ppm) | Maleic acid (ppm) | Citric acid (ppm) | Succinic acid (ppm) | Lactic acid (ppm) | Acetic acid (ppm) | |
| KBY-1 | 1.5 | 1.3 | 49.0 | 2.6 | 7.0 | 23.8 | 70.4 | 2.0 | 89 | 96 | 204 | 119 | 97 | 89 | |
| YMO-1 | 2.2 | 3.7 | 30.3 | 2.0 | 4.1 | 18.3 | 97.4 | 1.8 | 100 | 109 | 199 | 116 | 106 | 57 | |
| KBY-2 | 2.5 | 1.6 | 27.1 | 2.9 | 0.04 | 4.0 | 21.3 | 85.0 | 1.8 | 110 | 104 | 190 | 85 | 92 | 112 |
| YMO-2 | 7.1 | 4.2 | 25.7 | 3.3 | 0.04 | 5.0 | 23.9 | 78.2 | 2.2 | 99 | 92 | 207 | 96 | 107 | 133 |
KBY-1 and YMO-1 indicate beer produced in the first fermentation by KBY011 and YMO106, respectively. KBY-2 and YMO-2 indicate beer produced in the second fermentation by KBY011 and YMO106, respectively.
DISCUSSION
Metabolomics, the global analysis of cellular metabolites, is becoming a powerful new tool for gaining insight into biological functions. Analysis of a number of metabolites and tracking concentration changes under various physiological and genetic conditions should provide direct information on metabolic phenotypes complementary to gene expression. Metabolome and transcriptome approaches in this work revealed three main insights. First, OAH is the rate-limiting factor for the production of H2S. Second, the flux from aspartate to OAH has a greater impact on H2S production than on SO2 production, while flux from sulfate to SO2 has a greater impact on SO2 production than on H2S production. Third, the simultaneous increase in the flux from aspartate to OAH and the flux from sulfate to SO2 resulted in high levels of SO2 production with no increase in H2S formation relative to that of the parent.
In order to increase SO2 and to lower H2S production, we compared the formations of these metabolites in bottom-fermenting and baker's yeasts. Metabolome data indicated that the bottom-fermenting species produced less OAH, a homocysteine precursor, than did S. cerevisiae. One reason for this reduced amount of OAH is possibly due to lower expression levels of HOM3 in bottom-fermenting yeast, which causes higher SO2 and lower H2S productivity, suggesting that OAH is the rate-limiting factor in SO2 and H2S production.
Alternative approaches to developing yeast strains that produce higher SO2 and lower H2S levels have been proposed. One is the disruption of genes encoding SO2 reductase subunits or other proteins required for SO2 reductase function, including MET5, MET10, MET1, and MET8. Hansen and Kielland-Brandt reported previously that a MET10 disruption resulted in high levels of SO2 production (11). Considering the function of MET10, one would have expected lower H2S levels as well. The overexpression of SSU1, encoding a plasma membrane SO2 efflux pump, has also been proposed to be a means of reducing H2S levels. The use of a sulfite reductase mutant or a mutant that exports a significant amount of SO2 in raw materials such as wort that has a limited amino acid content is likely to lead to limited growth due to the depletion of methionine and cysteine. In contrast, in the HOM3- and MET14-overexpressing strain, intracellular methionine or cysteine was sufficient, as evidenced by the ability of this strain to grow in unsupplemented minimal SD medium. It is possible that the combined overexpression of SSU1 and MET14 would work as well as overexpression of HOM3 and MET14. Nonetheless, all the strains described above have been genetically manipulated and therefore are inappropriate for commercial use.
With commercial use in mind, we selected spontaneous mutants that were resistant to both ethionine (methionine analog) and hydroxynorvaline (threonine analog) to obtain a high-level SO2-producing strain that did not produce increased levels of H2S. Strain YMO106, which produced higher SO2 levels but parental levels of H2S, was selected in this screen. The bottom-fermenting yeast strain in which all MET10 genes were disrupted produced 13-fold more SO2 but 5-fold more acetaldehyde, 3-fold more 1-propanol, and 1.7-fold more dimethyl sulfide (DMS) than the parental strain (11). As shown in Table 4, strain YOM106 obtained in this study produced 2.8- and 2.6-fold more SO2 (fermentation tank and bottled beer produced in the second fermentation, respectively), 1.3-fold more acetaldehyde, and 0.9- and 1.1-fold more H2S than the parental strain (fermentation tank and bottled beer produced in the second fermentation, respectively). Beer produced by YMO106 was very similar to that produced by the parental strain except for the higher level of SO2. These results indicate that selection for resistance to amino acid analogs, based on the integration of the metabolome and transcriptome data, was a successful approach for obtaining a high-level SO2-producing strain. To our knowledge, this is the first report to integrate metabolome and transcriptome data for the development of improved industrial yeast strains.
It is possible that the mutation responsible for ethionine resistance in YMO106 is in SAM1 or SAM2, both of which are involved in the transcriptional regulation of methionine biosynthesis (3, 22), in the methionine permease MUP1 (12), or in STR4, which encodes cystathionine β-synthase (Fig. 1). Previous screening of the yeast gene knockout collection for H2S-overproducing mutants led to the identification of mup1 and str4 deletion mutants (Yoshida et al., unpublished). Mutations in SAM1 or SAM2 would also be expected to result in a loss of function because SAM represses the transcription of the methionine biosynthetic genes including MET3 and MET14. However, the sam1 and sam2 mutants were not found to overproduce H2S, possibly because wild-type SAM1 and SAM2 complemented sam2 and sam1, respectively, in the single mutants. Nonetheless, it is possible that SAM1 and SAM2 might be repressed simultaneously in YMO106. On the other hand, a mutation in HOM3 might be responsible for hydroxynorvaline resistance, because HOM3-R2 dominant mutants have been reported to exhibit hydroxynorvaline resistance in S. cerevisiae (19). However, as the hydroxynorvaline resistance of YMO106 is weak, it is possible that the mutation is not in HOM3. It is possible that the mutation responsible for hydroxynorvaline resistance is in genes involved in threonine uptake or in other genes including HOM2, HOM6, MET2, MET17, THR1, and THR4.
DMS, dimethyl disulfide, methional, and methionol were measured in bottled beers. Relative to KBY011, YMO106 was found to produce 1.6-fold more DMS, 0.6-fold less dimethyl disulfide, 0.6-fold less methional, and 1.5-fold more methionol, indicating minor consequences of the introduced mutation on the methionol synthesis and methionine degradation pathway. Significantly, these values are below taste threshold levels.
Finally, we report herein a method to identify rate-limiting factors in a metabolic pathway by the integration of transcriptome and metabolome data, followed by genetic and nongenetic metabolic engineering to increase desirable end products in yeast. This method should have general application to the problem of increasing the production of useful metabolites while minimizing the simultaneous production of undesirable but related compounds derived from a linked pathway.
Footnotes
Published ahead of print on 29 February 2008.
REFERENCES
- 1.Aharoni, A., C. H. R. de Vos, H. A. Verhoeven, C. A. Maliepaard, G. Kruppa, R. Bino, and D. B. Goodenowe. 2002. Nontargeted metabolome analysis by use of Fourier transform ion cyclotron mass spectrometry. Omics 6:217-234. [DOI] [PubMed] [Google Scholar]
- 2.Bilinski, C. A., I. Russell, and G. G. Stewart. 1987. Physiological requirements for induction of sporulation in lager yeast. J. Inst. Brew. 92:216-219. [Google Scholar]
- 3.Cherest, H., and Y. Surdin-Kerjan. 1978. S-Adenosylmethionine requiring mutants in Saccharomyces cerevisiae: evidences for the existence of two methionine adenosyl transferases. Mol. Gen. Genet. 163:153-167. [DOI] [PubMed] [Google Scholar]
- 4.Donalies, U. E., and U. Stahl. 2002. Increasing sulphite formation in Saccharomyces cerevisiae by overexpression of MET14 and SSU1. Yeast 19:475-484. [DOI] [PubMed] [Google Scholar]
- 5.Duan, W., F. A. Roddick, V. J. Higgins, and P. J. Rogers. 2004. A parallel analysis of H2S and SO2 formation by brewing yeast in response to sulfur-containing amino acids and ammonium ions. J. Am. Soc. Brew. Chem. 62:35-41. [Google Scholar]
- 6.Farfán, M.-J., L. Aparicio, and I. L. Calderón. 1999. Threonine overproduction in yeast strains carrying the HOM3-R2 mutant allele under the control of different inducible promoters. Appl. Environ. Microbiol. 65:110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiehn, O., J. Kopka, P. Dörmann, T. Altmann, R. N. Trethewey, and L. Willmitzer. 2000. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 18:1157-1161. [DOI] [PubMed] [Google Scholar]
- 8.Fujii, T., H. Yoshimoto, N. Nagasawa, T. Bogaki, Y. Tamai, and M. Hamachi. 1996. Nucleotide sequences of alcohol acetyltransferase genes from lager brewing yeast, Saccharomyces carlsbergensis. Yeast 12:593-598. [DOI] [PubMed] [Google Scholar]
- 9.Gregory, J. C., and J. D. Boeke. 1996. A useful colony colour phenotype associated with the yeast selectable/counter-selectable marker MET15. Yeast 12:939-941. [DOI] [PubMed] [Google Scholar]
- 10.Gyllang, H., M. Winge, and C. Korch. 1989. Regulation of SO2 formation during fermentation, p. 347-354. In European Brewery Convention, Proceedings of the 22nd Congress. Oxford University Press, Oxford, United Kingdom.
- 11.Hansen, J., and M. C. Kielland-Brandt. 1996. Inactivation of MET10 in brewer's yeast specifically increase SO2 formation during beer production. Nat. Biotechnol. 14:1587-1591. [DOI] [PubMed] [Google Scholar]
- 12.Isnard, A. D., D. Thomas, and Y. Surdin-Kerjan. 1996. The study of methionine uptake in Saccharomyces cerevisiae reveals a new family of amino acid permeases. J. Mol. Biol. 262:473-484. [DOI] [PubMed] [Google Scholar]
- 13.Ito, T., T. Chiba, R. Ozawa, M. Yoshida, M. Hattori, and Y. Sakaki. 2001. Comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kodama, Y., F. Omura, and T. Ashikari. 2001. Isolation and characterization of a gene specific to lager brewing yeast that encodes a branched-chain amino acid permease. Appl. Environ. Microbiol. 67:3455-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafaye, A., C. Junot, Y. Pereira, G. Lagniel, J. C. Tabet, E. Ezan, and J. Labarre. 2005. Combined proteome and metabolite-profiling analyses reveal surprising insights into yeast sulfur metabolism. J. Biol. Chem. 280:24723-24730. [DOI] [PubMed] [Google Scholar]
- 16.Meilhoc, E., J. M. Masson, and J. Teissie. 1990. High efficiency transformation of intact yeast cells by electric field pulses. Bio/Technology 8:223-227. [DOI] [PubMed] [Google Scholar]
- 17.Omura, F., and Y. Shibano. 1995. Reduction of hydrogen sulfide production in brewing yeast by constitutive expression of MET25 gene. J. Am. Soc. Brew. Chem. 53:58-62. [Google Scholar]
- 18.Park, H., and A. T. Bakalinsky. 2000. SSU1 mediates sulphite efflux in Saccharomyces cerevisiae. Yeast 16:881-888. [DOI] [PubMed] [Google Scholar]
- 19.Ramos, C., and I. S. Calderon. 1992. Overproduction of threonine by Saccharomyces cerevisiae mutants resistant to hydroxynorvaline. Appl. Environ. Microbiol. 58:1677-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reo, N. V. 2002. NMR-based metabolomics. Drug Chem. Toxicol. 25:375-382. [DOI] [PubMed] [Google Scholar]
- 21.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Shimoi, N., H. Fukuda, Y. Fukuda, K. Murata, and A. Kimura. 1991. Nucleotide sequence and characterization of a gene conferring resistance to ethionine in yeast Saccharomyces cerevisiae. J. Ferm. Bioeng. 71:211-215. [Google Scholar]
- 23.Soga, T., and D. N. Heiger. 2000. Amino acid analysis by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 72:1236-1241. [DOI] [PubMed] [Google Scholar]
- 24.Soga, T., Y. Ueno, H. Naraoka, Y. Ohashi, M. Tomita, and T. Nishioka. 2002. Simultaneous determination of anionic intermediates for Bacillus subtilis metabolic pathways by capillary electrophoresis electrospray ionization mass spectrometry. Anal. Chem. 74:2233-2239. [DOI] [PubMed] [Google Scholar]
- 25.Soga, T., Y. Ohashi, Y. Ueno, H. Naraoka, M. Tomita, and T. Nishioka. 2003. Quantitative metabolome analysis using capillary electrophoresis mass spectrometry. J. Proteome Res. 2:488-494. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi, T., M. Hojito, and K. Sakai. 1980. Genes controlling hydrogen-sulfide production in Saccharomyces cerevisiae. Bull. Brew. Sci. 26:29-36. [Google Scholar]
- 27.Tamai, Y., K. Tanaka, N. Umemoto, K. Tomizuka, and Y. Kaneko. 2000. Diversity of the HO gene encoding an endonuclease for mating-type conversion in the bottom fermenting yeast Saccharomyces pastorianus. Yeast 16:1335-1343. [DOI] [PubMed] [Google Scholar]
- 28.Tezuka, H., T. Mori, Y. Okumura, K. Kitabatake, and Y. Tsumura. 1992. Cloning of a gene suppressing hydrogen sulfide production by Saccharomyces cerevisiae and its expression in a brewing yeast. J. Am. Soc. Brew. Chem. 50:130-133. [Google Scholar]
- 29.Thomas, D., and Y. Surdin-Kerjan. 1997. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 61:503-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaughan-Martini, A., and A. Martini. 1998. A taxonomic study: Saccharomyces Myen ex Reessm, p. 358-371. In C. P. Kurtzman and J. W. Fell (ed.), The yeasts. Elsevier, Amsterdam, The Netherlands.
- 31.Wilson, I. D., J. K. Nicholson, J. Castro-Perez, J. H. Granger, K. A. Johnson, B. W. Smith, and R. S. Plumb. 2005. High resolution “ultra performance” liquid chromatography coupled to oa-TOF mass spectrometry as a tool for differential metabolic pathway profiling in functional genomic studies. J. Proteome Res. 4:591-598. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida, S., M. Suzuki, S. Yamano, M. Takeuchi, H. Ikenaga, N. Kioka, H. Sakai, and T. Komano. 1999. Expression and characterization of rat UDP-N-acetylglucosamine: α-3-D-mannnoside β-1,2-N-acetylglucosaminyltransferase I in Saccharomyces cerevisiae. Glycobiology 9:53-58. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida, S., K. Hashimoto, E. Shimada, T. Ishiguro, T. Minato, S. Mizutani, H. Yoshimoto, K. Tashiro, S. Kuhara, and O. Kobayashi. 2007. Identification of bottom-fermenting yeast genes expressed during lager beer fermentation. Yeast 24:599-606. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida, S., K. Hashimoto, K. Kanai-Tanaka, H. Yoshimoto, and O. Kobayashi. 2007. Identification and characterization amidase-homologous AMI1 genes of bottom-fermenting yeast. Yeast 24:1075-1084. [DOI] [PubMed] [Google Scholar]