Abstract
The transformation of 2-aminobenzothiazole (ABT) was studied under various conditions: (i) a photodegradation process at a λ of >300 nm in the presence of an Fe(III)-nitrilotriacetic acid complex (FeNTA), (ii) a biodegradation process using Rhodococcus rhodochrous OBT18 cells, and (iii) the combined processes (FeNTA plus Rhodococcus rhodochrous in the presence or absence of light). The transformation of ABT in the combined system, with or without light, was much more efficient (99% degradation after 25 h) than in the separated systems (37% photodegradation and 26% biodegradation after 125 h). No direct photolysis of ABT was observed. The main result seen is the strong positive impact of FeNTA on the photodegradation, as expected, and on the biotransformation efficiency of ABT, which was more surprising. This positive impact of FeNTA on the microbial metabolism was dependent on the FeNTA concentration. The use of UV high-performance liquid chromatography, liquid chromatography-electrospray ionization mass spectrometry, and in situ 1H nuclear magnetic resonance provided evidence of the intermediary products and thus established transformation pathways of ABT in the different processes. These pathways were identical whether the degradation process was photo- or biotransformation. A new photoproduct was identified (4OH-ABT), corresponding to a hydroxylation reaction on position 4 of the aromatic ring of ABT.
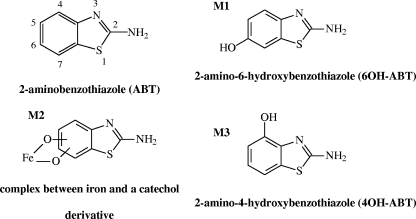
Benzothiazoles are a family of chemicals whose chemical skeleton is constituted from a benzene ring fused with a thiazole ring. They are used in a variety of industrial products and processes and are manufactured worldwide. Depending on the substituents on the benzothiazole moiety, they can have a wide range of biological properties and be used as fungicides in lumber and leather production (7, 29), antialgal agents (9), slimicides in the paper and pulp industry (27), herbicides (20, 32), or even drugs for lateral amyotrophic sclerosis (8) or chemotherapeutics (10, 13). Nonetheless, they also have industrial applications in the manufacture of azo dye (15), and their main production is for rubber manufacture, where they are used as catalysts in the vulcanization process. Released from rubber products or industrial effluents (tanneries, benzothiazole production plants, etc.), benzothiazoles have been detected in industrial wastewaters and also in various environmental media (21, 22, 29). They are of concern for the aquatic environment due to their limited biodegradability and potential toxicity (16, 28, 35).
Only a few papers report studies of the biodegradation of benzothiazole derivatives first with activated sludges from industrial effluent treatment plants and then with a few bacterial isolates (11). The biodegradation of several benzothiazoles with various Rhodococcus strains isolated from wastewater treatment plants, including R. erythropolis BTS1, R. rhodochrous OBT18, and R. pyridinovorans PA, has been described in detail (4, 11, 15, 17-19). Several metabolites have been identified, and biodegradative pathways have been proposed for different benzothiazole derivatives. In particular, it was shown that the 6-OH-benzothiazole derivative was an intermediate metabolite common to all the pathways and that a catechol 1,2 dioxygenase was present in R. pyridinovorans PA. These studies have shown that some benzothiazole derivatives, such as benzothiazole, were relatively quickly degraded even at a relatively high concentration (3 mM) but that others remained more persistent. 2-Aminobenzothiazole (ABT), a component of azo dye but also a metabolite of the herbicide methabenzthiazuron (MBTU) (36), is one of the examples of a poorly degradable benzothiazole (17).
The photochemical studies of this class of compounds were mainly focused on derivatives having pesticidal properties. The first research work emphasized the natural process of direct photolysis under solar light. Brownlee et al. (7) studied the fate of 2-(thiocyanomethylthio)benzothiazole (TCMTB) in the aquatic compartment and showed that that active molecule of the fungicide BUSAN was rapidly photolysed. The herbicide MBTU, on the contrary, had a very low photoreactivity (24), but the addition of nitrate ions (photoinductors) in water increased MBTU photodegradation by a factor of 10 (26). More recently, Malouki (25) studied in detail the photolysis of 2-mercaptobenzothiazole (MBT) in water. MBT appeared more photoreactive than the majority of benzothiazoles. In this study, the authors compared the phototransformation of MBT in Milli-Q and in lake water. In this last case, the disappearance of MBT was 4 times faster, showing that chromophoric components of the natural water of the lake strongly contributed to the phototransformation of MBT in the aquatic environment.
In conclusion, the photoinduced degradation of benzothiazoles was clearly more efficient than direct photolysis. As a consequence, to efficiently treat effluents contaminated with benzothiazoles, different advanced oxidation processes have been tested, including ozonation (14), ozone oxidation combined with activated carbon (33), and the photo-Fenton process involving an Fe(III) aquacomplex and H2O2 under irradiation (2, 3, 30). A complete elimination of the benzothiazole derivatives was observed with these treatments.
These different studies show that benzothiazoles are mostly recalcitrant to elimination by natural bio- or photodegradation, the best way to eliminate them in water being photoinduced processes. An original study on the sequential bio- and phototransformations was carried out on MBTU in water (24). As mentioned before, MBTU was very slowly phototransformed at wavelengths higher than 290 nm. Nevertheless, it was successfully oxidized into 6-hydroxymethabenzthiazuron by the fungal species Aspergillus niger. However, this first metabolite was not further metabolized by Aspergillus niger but was efficiently photooxidized with ring cleavage of the aromatic ring and photodimerized under irradiation at a λ of >290 nm. This work showed the complementarity of photo- and biodegradation approaches to study the fate or elimination of benzothiazoles in water.
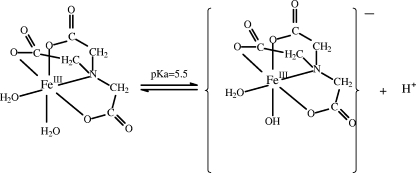
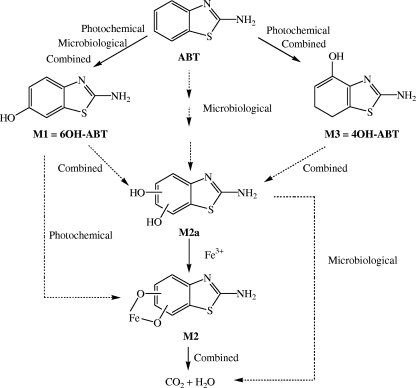
The main objective of this work was to propose a new approach combining simultaneous photo- and biodegradation processes for the treatment of water. This strategy could be very interesting from an economical point of view, as biotechnological processes and the use of solar light are very inexpensive compared to advanced oxidation processes. ABT was chosen due to its low biodegradation by Rhodococcus strains in water (17) and its very low photolysis rate under solar light. In this study, an iron complex with nitrilotriacetic acid (NTA) (FeNTA) was used as a photoinducer (Fig. 1). Moreover, this complex is considered to be a good model of iron complexes in organic matter. This paper focused on the efficiency of a combined system (photoinduction process with FeNTA and biodegradation with Rhodococcus rhodochrous OBT18 isolated from a wastewater treatment plant by De Wever et al. [12]) for ABT degradation. The identification of the main pathways involved in both ABT degradation processes and, as a consequence, of the structures of the intermediary products, was a particular goal of the study (Fig. 2).
FIG. 1.
Chemical structure of FeNTA.
FIG. 2.
Chemical structures of ABT and its derivatives.
MATERIALS AND METHODS
Chemicals and preparation of the solutions.
ABT, 2-amino-4-methoxybenzothiazole, NTA, benzoquinone (98%), thiourea, hydroxylamine chlorhydrate, and ferrozine [3-(2-pyridyl)-5,6-diphenyl-1,2,4-triazine-4,4′-disulfonic acid, sodium salt, 97%] were purchased from Aldrich. Fe(III) perchlorate [Fe(ClO4)3, 9H2O] was purchased from Fluka. The perchlorate is used as a counteranion because it is not photosensitive and it is not an oxidant, contrary to chlorate anion that can contribute strongly to the oxidation of the benzothiazole and interfere in our results. Tetradeuterated sodium trimethylsilylpropionate (TSP-d4) was purchased from Eurisotop (Saint Aubin, France).
FeNTA solution.
To an aqueous solution of 100 ml of 4 mM NTA at pH 4.0 was added, with stirring, 100 ml of a freshly prepared solution of 4 mM Fe(ClO4)3. This mixture was stirred for 1 h, and the pH was adjusted to 4.0 with 1 N NaOH. The total complexation of iron was controlled by UV-visible spectrophotometry (ɛ260, 6,000 M−1 cm−1). The analysis gave evidence for a 1:1 stoichiometry. The pH of the FeNTA solution (2 mM) was adjusted to 7.0 with 1 N NaOH just before the experiment.
Growth conditions and incubation with xenobiotic compounds.
Rhodococcus rhodochrous OBT18 was grown in 100-ml portions of Trypcase soy broth (bioMérieux, Marcy l'Etoile, France) in 500-ml Erlenmeyer flasks incubated at 27°C and 200 rpm. The cells (300 ml of the culture medium) were harvested after 24 h of culture and centrifuged at 8,000 rpm for 15 min at 4°C. The bacterial pellet was washed first with a NaCl solution (8 g liter−1) and then with Volvic mineral water. The resting cells (600 mg dry weight in 100 ml Volvic water) were incubated with ABT (0.5 mM) and/or FeNTA (1 mM) in 500-ml Erlenmeyer flasks (clear or brown borosilicate glass) at 27°C under agitation (200 rpm) and light irradiation. The initial ABT solution at 1.0 mM was prepared in Volvic mineral water. Samples (three, 1 ml) were regularly taken directly from the incubation medium and centrifuged at 12,000 rpm for 5 min. One milliliter of supernatant was used directly for iron measurement, and the two other 1-ml samples were frozen until analysis by high-performance liquid chromatography (HPLC) or nuclear magnetic resonance (NMR). The controls consisted of preparations incubated under the same conditions without the cells or without the substrate. Cells killed by heat were also tested with ABT.
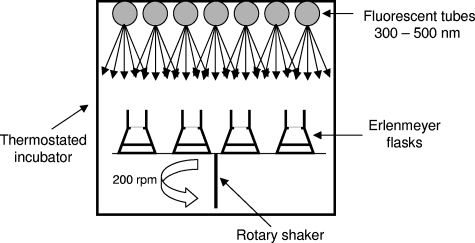
Incubator.
The incubator was equipped with 7 TLD Philips fluorescent tubes of 15 W, emitting within the range of wavelengths from 300 to 500 nm (polychromatic irradiation) with a maximum emission at 360 nm. The irradiated or incubated media were placed in 500-ml Erlenmeyer flasks at 27°C under agitation (200 rpm) to maintain the homogeneity and the oxygenation of the solution (Fig. 3). The polychromatic photon flux was measured and controlled at each location of the Erlenmeyer flasks with a Testo 545 luxmeter.
FIG. 3.
Diagram of incubator used to study photo- and biodegradation processes of ABT.
Analytical methods. (i) UV-visible spectroscopy.
UV-visible spectra were monitored on a Cary 3 or 13 spectrophotometer. The precision of the apparatus was ±0.002 for optical density.
(ii) HPLC analyses.
HPLC analyses were performed using a Waters 510 chromatograph fitted with a Waters UV-diode array detector 996 and a reversed-phase column (5-μm, 250- by 4.6-mm Interchrom Nucleosil C8; Interchim) at room temperature. The mobile phase was acetonitrile-water (25:75, vol/vol) with a flow rate of 1 ml min−1 and a λ range from 200 to 400 nm.
(iii) LC-ESI-MS analyses.
A Waters/Micromass LC-quadrupole time-of-flight mass spectrometer (MS) (Micromass, Manchester, United Kingdom), equipped with an orthogonal geometry Z-spray ion source, was used. The solution was introduced into the atmospheric-pressure ionization source after separation by LC and ionized by electrospray ionization (ESI) in positive mode (Vcapillary = 3 kV; Tsolvation = 120°C; Tdesolvation = 400°C; 0 to 1,000 m/z). A Waters Alliance 2695 HPLC was used to deliver acetonitrile-acidified water solutions. A reverse-phase column (3.5-μm, 100- by 2.1-mm Xtena MS C18,) with a flow rate of 0.25 ml min−1 was used. The mobile phase was composed of acetonitrile (solvent A) and acidified water (acetic acid, 0.1% vol/vol) (solvent B). The gradient was 0 to 15 min, 5 to 90% solvent A (linear); 15 to 20 min, 90% solvent A; 20 to 23 min, 90 to 5% solvent A; 23 to 32 min, 5% solvent A (equilibrium period).
(iv) 1H NMR spectroscopy.
All NMR spectra were recorded with a Bruker Avance 500 spectrometer at 298 K by using a 5-mm, triple-tuned 1H-13C-15N probe equipped with a z-gradient coil. Water resonance was suppressed by using the classical double pulsed-field gradient echo sequence WATERGATE. A total of 128 scans were collected (relaxation delay, 1 s; acquisition time, 4.67 s; spectral window, 7,002 Hz; 65,536 data points). A 1-Hz line-broadening procedure was applied before Fourier transformation, and baseline correction was performed on spectra before integration with Bruker software. TSP-d4 was used as an internal reference for chemical shift (0 ppm) and for quantification of degradation products. To 540 μl of supernatant was added 60 μl of a 5 mM TSP-d4 solution in D2O.
Titration of total iron (according to Stookey [31]).
The total iron concentration was determined after reduction of Fe(III) species with a solution of hydroxylamine chlorhydrate (3 mM) (104.25 mg of the salt dissolved into 200 ml 32% HCl in a 500-ml flask, completed with distilled water). Ferrozine forms a strong colored complex with Fe(II). Into a 5-ml flask were added 0.25 ml sample, 1.5 ml water, and 0.5 ml hydroxylamine solution (3 mM). After vigorous stirring and a 10-min wait, 0.5 ml ferrozine (20 mM), 1 ml 0.1 N ammonium acetate buffer, and water were added to a total volume of 5 ml. After the mixture was stirred, the optical density at 562 nm was measured. The accuracy of the method is ±0.1 μM.
SPE procedure.
An Oasis HLB solid-phase extraction (SPE) cartridge from Waters was first conditioned with methanol and water. The supernatant, filtered with a 0.22-μm filter (solution 1; 10 ml), was passed through the cartridge (solution 2). The solid phase was then washed with 4 ml Milli-Q water (solution 3), and the retained compounds were extracted with 4 ml methanol (solution 4). All the solutions were analyzed by HPLC and by NMR after evaporation of the solvent. The iron in each solution was titrated.
Synthesis of the degradation products of ABT. (i) Synthesis of 6OH-ABT.
2-Amino-6-hydroxybenzothiazole (6OH-ABT; M1) was synthesized according to the method of Lau and Gompf (23). To a solution of 1.9 g (0.025 mol) of thiourea in 50 ml ethanol and 3 ml concentrated HCl (37%) was added dropwise a hot solution of 1,4-benzoquinone (5.4 g, 0.05 mol) in 100 ml ethanol with stirring. At the beginning of the addition, the solution decolorized, but at the end, it remained red. After 24 h of stirring at room temperature, the solvent was removed under vacuum. The residue was treated with boiling acetonitrile, filtered under vacuum, and washed with small portions of cold ethanol. The slightly pink solid (6OH-ABT·HCl) was dried overnight. The solid was then dissolved in the minimum quantity of water and recrystallized with concentrated HCl. After filtration, the compound was obtained as a white solid (2.84 g; yield, 56%). The melting point [mp] was 263 to 265°C. To obtain the free amine, the salt was dissolved in water and neutralized with 4% sodium acetate. 6OH-ABT was recrystallized in a mixture of ethanol and H2O to 98% purity. The mp was 249 to 251°C. 1H NMR (500 MHz, D2O, TSP-d4) δ (ppm): 6.87 (dd, 3J = 8.4 Hz, 4J = 2.2 Hz, 1H); 7.18 (dd, 4J = 2.2 Hz, 1H); 7.31 (dd, 3J = 8.4 Hz, 1H).
(ii) Synthesis of 4OH-ABT.
The synthesis of 2-amino-4-hydroxybenzothiazole (4OH-ABT; M3) was adapted from the method of Vel'tman and Yanovskii (34). A solution of 2-amino-4 methoxybenzothiazole (1.35 g, 7.5 mmol) in 3 ml of chlorobenzene and 0.3 ml 47% HBr was refluxed for 8 h. After being cooled, this mixture was filtered, and the white solid obtained was dried. The filtrate was treated with NaHCO3, and a precipitate was observed. This precipitate was filtered and recrystallized. A 15% yield of 4OH-ABT was obtained after several recrystallizations. The mp was 185 to 186°C. 1H NMR (500 MHz, D2Ο, TSP-d4) δ (ppm): 7.04 (t, 3J = 8.0 Hz, 1H); 7.23 (dd, 3J = 7.6 Hz, 1H); 7.83 (dd, 3J = 7.6 Hz, 1H).
RESULTS
In the same set of experiments, both degradation processes of ABT (0.5 mM) have been studied separately (biodegradation by the bacterial strain Rhodococcus rhodochrous OBT18 and photodegradation at 365 nm in the presence or in the absence of FeNTA) and in combination (bacterial strain in the presence or absence of FeNTA with or without light). The starting pH in all the experiments was 7.0. The kinetics of ABT disappearance and degradation product formation were monitored by reverse-phase HPLC and 1H in situ NMR.
Kinetic study of photo- and/or biodegradation of ABT.
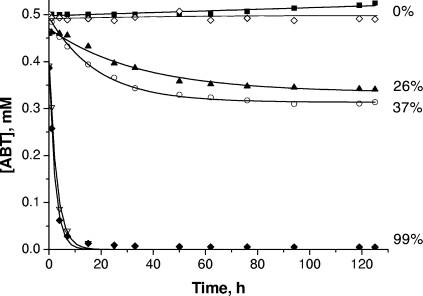
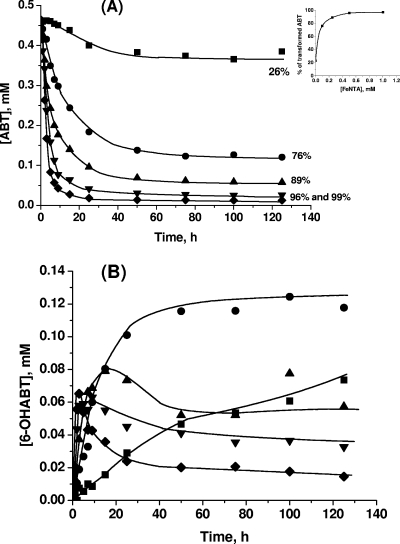
The kinetics of ABT degradation under the different conditions involving photo- and/or biodegradation processes are presented in Fig. 4. ABT is a relatively stable compound. No photodegradation after 125 h of irradiation by direct photolysis and only a low biotransformation (26% after 125 h of incubation) were observed. This biodegradation rate is in agreement with the results of the previous studies of Haroune et al. (17). The fact that ABT did not disappear with cells killed with heat was verified, showing that ABT was really biotransformed. In the case of biodegradation, the initial concentration of ABT measured was slightly lower than the theoretical concentration (0.5 mM). Two hypotheses can explain this phenomenon: (i) the addition of the bacterial suspension, leading to a dilution of around 2%, and (ii) adsorption of ABT on the surface of cells.
FIG. 4.
Time courses of ABT concentration during degradation under various conditions: direct photolysis (▪); Rhodococcus rhodochrous OBT18 (▴); FeNTA without irradiation (⋄); FeNTA under irradiation (○); R. rhodochrous and FeNTA (▿); and R. rhodochrous and FeNTA under irradiation (⧫). Values to the right are percentages of degradation after 125 h.
The presence of FeNTA (1 mM) strongly favored the photo- and biodegradation of ABT. In the absence of light or bacteria, no transformation or disappearance of ABT was observed. FeNTA had no direct effect on ABT degradation (data not shown) and did not interact with ABT. Nevertheless, the presence of FeNTA under irradiation leads to 37% degradation of ABT after 125 h of irradiation. This result points up the photochemical properties of this iron complex for the transformation of ABT. These properties have already been observed by Abida et al. (1) with 4-chlorophenol. A strong positive effect of FeNTA on the biodegradation process with or without light was also obtained. Under both of these conditions, 95% of ABT was degraded after 10 h of incubation. The iron complex seems to stimulate the biodegradation activity of the cells.
Identification of ABT metabolites or photoproducts.
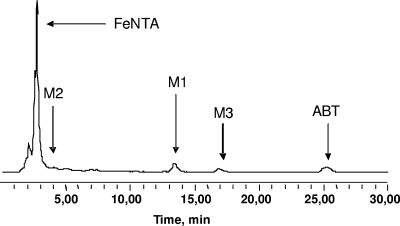
During ABT degradation under the different conditions tested, new peaks with shorter retention times than the parent molecule appeared on the chromatograms. The same three main degradation products (M1, M2, and M3) were observed in the course of the reactions where FeNTA was present with or without light (Fig. 5). In the absence of FeNTA, only two metabolites were observed under irradiation or not: M1 and M2a. This last degradation product was hardly detected in HPLC and had a retention time very close to that of M2 (chromatogram not shown).
FIG. 5.
HPLC chromatogram of a sample obtained after 12.5 h of incubation during ABT biodegradation with Rhodococcus rhodochrous OBT18 in the presence of FeNTA. The mobile phase was acetonitrile-water (25:75, vol/vol); the flow rate was 1 ml min−1. λdet = 265 nm, where det is detection.
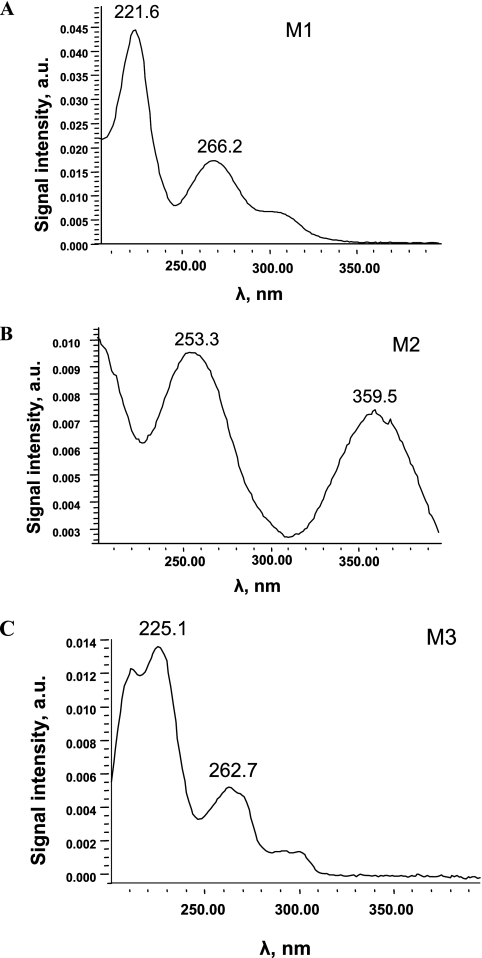
The UV-visible spectra of the three degradation products are presented in Fig. 6 and correspond to aromatic structures. The main compound, M1, was observed whatever the conditions of ABT degradation (photo- or biodegradation). M1 has been previously identified as 6OH-ABT (17).
FIG. 6.
UV-visible spectra of the degradation products of ABT. a.u., arbitrary units.
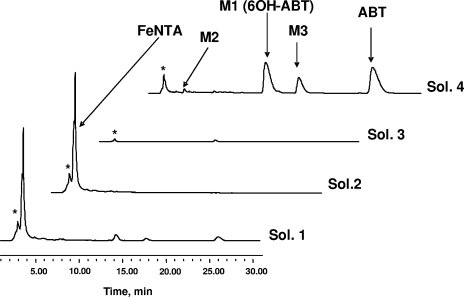
To identify the other degradation products, a specific experiment of ABT (0.5 mM) biodegradation in the presence of FeNTA (1 mM) was carried out on a quantitative scale. The reaction was stopped after 12.5 h of incubation, corresponding to the disappearance of 90% of ABT. Afterwards, 200 ml of supernatant was freeze-dried and then dissolved in D2O to obtain information by 1H NMR. The spectrum recorded under these conditions (spectrum not shown) cannot be easily analyzed: many poorly defined signals of between 0 and 5 ppm were present, issued from cells and FeNTA degradation. In the aromatic region, the signals were also very broad, a phenomenon that was certainly due to the presence of iron, a paramagnetic element, and salts. Therefore, a specific method had to be developed to purify the supernatant using SPE (see Materials and Methods). The HPLC chromatograms of the four solutions obtained before (solution 1) and after the different steps of SPE treatment (solutions 2, 3, and 4) are presented in Fig. 7.
FIG. 7.
HPLC chromatograms obtained after each step of SPE treatment. Sol. 1 (solution 1), supernatant before treatment; sol. 2, after the passage of the supernatant through the cartridge; sol. 3, after washing of the cartridge with H2O; sol. 4, after elution with methanol. *, cellular metabolites.
The SPE treatment efficiently removed most of the FeNTA and its degradation products. In the final solution (solution 4), obtained after elution with methanol, ABT, M1, and M3 were clearly detected by HPLC due to their concentration and purification during the process. However, M2 and polar products were present in very low concentrations. A quantitative assessment, obtained after analyses by HPLC, 1H NMR, and iron titration, is depicted in Table 1
TABLE 1.
Concentrations of different products after each step of SPE treatmenta
| Solution | Assay | Concn (mM) of:
|
Total Fe (mM) | ||
|---|---|---|---|---|---|
| 6OH-ABT | 4OH-ABT | ABT | |||
| 1 | HPLC | 0.036 | 0.011 | 0.027 | 0.662 |
| 2 | HPLC | 0 | 0 | 0 | 0.627 |
| 3 | HPLC | 0.007 | 0 | 0 | 0.007 |
| 4 (10× concn) | HPLC | 0.299 | 0.284 | 0.028 | |
| 1H NMRb | 0.292 | 0.130 | 0.282 | ||
The level of recovery of 6OH-ABT was 83%, that of 4OH-ABT was 117%, and that of ABT was 107%. After the last step (solution 4), 96% of iron was eliminated.
1H NMR was performed with internal standard TSP-d4 (0.5 mM).
The SPE treatment allowed at the same time the almost complete elimination of iron from the solution and a good recovery of the three products derived from benzothiazole. This treatment was very favorable for the NMR (sharp signals) and mass spectrometry analyses.
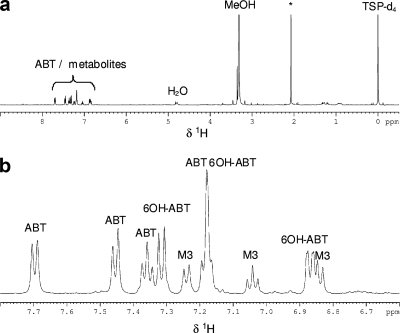
According to the 1H NMR spectrum of solution 4 (Fig. 8), M3 had a benzothiazole structure with only three aromatic protons (three multiplets in the aromatic region). Moreover, the shape of the multiplets and the coupling constants showed that these protons were vicinal. Therefore, a substitution occurred in the aromatic ring in either position 4 or 7. In order to identify the nature of the substituent, LC-(positive mode)-ESI-MS experiments were carried out. M3 had an m/z of 167 ([M+H]+), differing from the molecular weight of ABT by 16 and identical to that of 6OH-ABT. The substituent is a hydroxyl group. However, it was not possible to conclude from these data whether its position was on carbon atom 4 or 7. The synthesis of 4OH-ABT has been performed. Its 1H NMR spectrum was compared to that of M3 and was found to be the same. To confirm this assignment, the synthesized product was added to solution 4. The areas of the HPLC peak and the NMR signals of M3 were increased. M3 was assigned to 2-amino-4-hydroxybenzothiazole (4OH-ABT).
FIG. 8.
(a) 1H NMR spectrum of solution 4 obtained after SPE treatment. (b) Aromatic region of the 1H NMR spectrum of solution 4. *, cellular metabolites.
After the SPE treatment, the concentration of M2 remained too weak for identification by NMR. Moreover, during LC-ESI-MS analysis, this compound was unstable and was not detected in the mass spectrometer. The UV-visible spectrum of M2 (Fig. 6B) showed two absorption bands at 253 and 360 nm, confirming the presence of an aromatic ring. On the other hand, the band at 360 nm could correspond to the formation of an iron complex with a degradation product of ABT. Actually, M2 was observed only in the presence of iron. In the literature, Brand et al. (5, 6) reported the formation of a product with a similar spectrum (two bands near 265 and 396 nm) during the degradation of 4-octylphenol photoinduced by iron aquacomplexes. This product has been assigned to a complex between iron and 4-octylpyrocatechol. The hypothesis of the formation of a complex between iron and a catechol, formed after a second step of hydroxylation of ABT, can be proposed. The formation of catechols has already been reported during the biodegradation by Rhodococcus rhodochrous OBT18 of two other benzothiazole derivatives (2-hydroxybenzothiazole [OBT] and MBT) (18, 19). The noncomplexed catechol derivative of ABT could be the degradation product M2a, which was observed as traces only in the absence of FeNTA in the medium and which had a retention time very close to that of M2.
Effect of FeNTA concentration on ABT biodegradation.
As shown previously, FeNTA had a strong positive effect on the efficiency of ABT biodegradation by Rhodococcus rhodochrous, even without light. A study of the effect of the concentration of FeNTA in the range from 0.1 to 1.0 mM was carried out (Fig. 9A).
FIG. 9.
Kinetics of ABT (0.5 mM) biodegradation by Rhodococcus rhodochrous OBT18 (A) and 6OH-ABT (M1) formation (B) in the presence of different FeNTA concentrations (▪, 0 mM; •, 0.1 mM; ▴, 0.25 mM; ▾, 0.5 mM; ⧫, 1 mM) without light. Percentage values indicate the percentage of degradation after 125 h.
The results clearly showed a positive effect of FeNTA on ABT biodegradation whatever the concentration tested. However, this effect was not linear, as is shown in the inset in Fig. 9A. The kinetics of ABT degradation were very similar in the presence of 0.5 or 1.0 mM, an excess of iron complex having no real impact on the efficiency of ABT transformation. It is worth noting that even the addition of 0.1 mM of FeNTA allowed the efficiency of ABT degradation to increase by a factor higher than 3.
The impact of FeNTA concentration on the evolution of the main degradation product, M1 (6OH-ABT), was even more pronounced (Fig. 9B). If no difference in the rate of disappearance of ABT with FeNTA concentrations of 1.0 and of 0.5 mM was observed, the transformation of M1 was less efficient with a concentration of 0.5 mM. After 50 h of incubation, the remaining concentration of M1 was 2 times higher in the presence of 0.5 mM of iron complex than with 1.0 mM (0.04 mM instead of 0.02 mM, respectively).
Photo- and biodegradation of 6OH-ABT.
The same set of experiments was carried out with the major degradation product of ABT, 6OH-ABT (M1). The concentration of 6OH-ABT used was 0.12 mM due to its lower solubility in water. In the systems with bacteria and FeNTA in the presence or absence of light, the degradation of 6OH-ABT was very fast and 99% of 6OH-ABT was transformed within 10 h of incubation. FeNTA had again a strongly positive effect, as the biodegradation of 6OH-ABT in the absence of this iron complex leads to a low disappearance of the starting product (17% after 125 h of incubation) in the absence of light (data not shown). However, this compound was more photodegradable than ABT, and under irradiation, its degradation reached 52% after 125 h of incubation. These results were similar to those obtained with ABT. The positive impact of iron on the efficiency of degradation by Rhodococcus rhodochrous OBT18 was also obvious and impressive in the case of 6OH-ABT.
DISCUSSION
In this paper, the processes of photo- and biodegradation of a benzothiazole derivative, ABT, have been investigated separately and in combined systems. The results showed that ABT was not degraded by direct photolysis in our incubator and was weakly biotransformed by Rhodococcus rhodochrous OBT18 (26%). The presence of light had almost no effect on the bacterial cells under the conditions used. We clearly observed the formation of orange-red pigments when cells were irradiated that are likely to protect the cells against light.
(i) Comparison of the processes’ efficiencies.
Three degradation systems have been studied in detail: (a) a photochemical process (FeNTA [1.0 mM] with light), (b) a microbiological process (Rhodococcus rhodochrous OBT18), and (c) a combined system (Rhodococcus rhodochrous and FeNTA [1.0 mM] with or without light). The transformation of ABT in the combined system with or without light was much more efficient (99% degradation after 25 h) than in the separate systems (37% photodegradation and 26% biodegradation after 125 h). This observation points up the major effect of the iron complex on microbial activity. This positive impact of FeNTA on the microbial metabolism for the transformation of ABT was observed more or less strongly with different concentrations of FeNTA. This effect of FeNTA was observed not only for ABT but also for its main degradation product, 6OH-ABT (M1) (Fig. 10). Under our experimental conditions, from an FeNTA concentration of 0.5 mM, the further addition of FeNTA had no significant effect on ABT degradation but an important one on the kinetics of 6OH-ABT. After 125 h of incubation, its concentration in the presence of 0.5 mM of FeNTA (0.035 mM) was more than twice the concentration obtained in the presence of 1 mM of FeNTA (0.015 mM). This result shows that iron species present in solution were also able to activate the enzyme responsible for 6OH-ABT transformation. This result was in agreement with the fact that the biodegradation of 6OH-ABT used as the starting substrate was very strongly increased by the presence of iron.
FIG. 10.
Proposed ABT degradation pathways by photochemical (FeNTA under irradiation), microbiological (Rhodococcus rhodochrous OBT 18), and combined processes (Rhodococcus rhodochrous OBT18 and FeNTA with or without light).
(ii) Proposed degradation pathways.
In this paper, the pathways of degradation of ABT by different processes (photochemical, induced by FeNTA; microbial; and combined in the presence or absence of light) have been studied and are summarized in Fig. 10.
(a) The photoinduced degradation of ABT with FeNTA (37% in 125 h of irradiation) leads mainly to the formation of 6OH-ABT but also to 4OH-ABT as end products. Under these conditions, only a weak degradation of 6OH-ABT was observed. However, the experiments carried out with 6OH-ABT as the starting compound showed that 6OH-ABT was photodegraded in the presence of FeNTA. FeNTA is capable of photoinducing the degradation of organic compounds (1), but in the experiments with ABT, limiting conditions seemed to occur, impairing the progress of degradation. This result (the accumulation of 6OH-ABT) shows that FeNTA is not a photocatalyst and that there is no continuous formation of oxidative radicals.
In this photochemical process, another photoproduct (M2) was observed. Its UV-visible spectrum could correspond to an iron-catechol complex. This hypothesis is in agreement with the reactivity of hydroxyl radicals on aromatic structures. The process leads to two successive steps of hydroxylation, followed by the opening of the aromatic ring. Moreover, in the presence of iron, complexation between the catechol structure and iron has already been proposed (1, 6).
(b) The microbiological process (using Rhodococcus rhodochrous OBT18) of ABT degradation (26% in 125 h) leads to the accumulation of 6OH-ABT in the medium. 6OH-ABT was not further degraded even if it was used as the starting compound. The hydroxylation on position 6 on the aromatic ring is a common stage of benzothiazole biodegradation by Rhodococcus rhodochrous OBT18 (18, 19). 4OH-ABT was not formed in the microbiological process. Another metabolite containing an aromatic structure, M2a, was observed in trace amounts. Its retention time in HPLC was very close to that of M2. Our hypothesis is that M2a is a catechol form of ABT. The presence of a catechol 1,2-dioxygenase has already been evidenced in Rhodococcus pyridinovorans strain PA (18). It was also shown in Rhodococcus rhodochrous OBT18, as a diacid was observed during MBT biodegradation (19). This enzyme is known to transform catechol into diacid by the cleavage of a catechol ring.
(c) The combination of both processes (Rhodococcus rhodochrous OBT18 and FeNTA with or without light) had a strong effect on the transformation of ABT, but not on its biodegradative pathway. The same degradation products (6OH-ABT, 4OH-ABT, and M2) were obtained with or without light. It is important to note that 4OH-ABT was observed even in the experiments without light, which was not the case in the microbiological process. This difference could be due to the much more significant progress of the reaction in the presence of FeNTA.
In conclusion, the results of this study have shown that the transformation pathways of ABT are identical whatever the degradation process, photo- or biodegradation. A new degradation product was identified (4OH-ABT), corresponding to a hydroxylation reaction on position 4 of the aromatic ring of ABT, mainly observed when FeNTA was present in the medium. The main result is the strong positive impact of FeNTA on the photodegradation efficiency as expected and on the biodegradation efficiency of ABT, which was more surprising. The activation of iron-containing enzymes, such as mono- or dioxygenases, could explain this effect. Such enzymes have been shown to be involved in the biodegradative pathways of benzothiazole derivatives (18, 19). In any case, this positive effect of FeNTA may have an impact on the fate of ABT in wastewater treatment plants or in natural waters, as iron and iron complexes are present in these environments. Indeed, although ABT was shown to be hardly biodegradable, its concentration in wastewater treatment plants was always shown to be low (21, 22). Therefore, it could be interesting to check whether this mechanism of the activation of cellular metabolism by iron is a general feature. For that, not only will the effects of other forms of iron on the activity of R. rhodochrous cells be tested but also other pollutants and other microorganisms with biodegradative enzymes containing iron in their active site.
Acknowledgments
We thank the French Embassy in Moldova for A. Bunescu's Ph.D. fellowship and INTAS for its financial support.
Ion Dragalin is gratefully acknowledged for his technical help in the study of 6OH-ABT photodegradation.
Footnotes
Published ahead of print on 29 February 2008.
REFERENCES
- 1.Abida, O., G. Mailhot, M. Litter, and M. Bolte. 2006. Impact of iron-complex (Fe(III)-NTA) on photoinduced degradation of 4-chlorophenol in aqueous solution. Photochem. Photobiol. Sci. 5:395-402. [DOI] [PubMed] [Google Scholar]
- 2.Andreozzi, R., A. D'Apuzzo, and R. Marotta. 2000. A kinetic model for the degradation of benzothiazole by Fe3+-photo-assisted Fenton process in a completely mixed batch reactor. J. Hazard. Mater. 80:241-257. [DOI] [PubMed] [Google Scholar]
- 3.Andreozzi, R., V. Caprio, and R. Marotta. 2001. Oxidation of benzothiazole, 2-mercaptobenzothiazole and 2-hydroxybenzothiazole in aqueous solution by means of H2O2/UV or photoassisted Fenton systems. J. Chem. Technol. Biotechnol. 76:196-202. [Google Scholar]
- 4.Besse, P., B. Combourieu, G. Boyse, M. Sancelme, H. De Wever, and A.-M. Delort. 2001. Long range 1H-15N heteronuclear shift correlation at natural abundance: a tool to study benzothiazole biodegradation by two Rhodococcus strains. Appl. Environ. Microbiol. 67:1412-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand, N. 1998. Thèse de l'Université Blaise Pascal, no. d'ordre D.U.: 1063, Clermont Ferrand, France.
- 6.Brand, N., G. Mailhot, M. Sarakha, and M. Bolte. 2000. Primary mechanism in the degradation of 4-octylphenol photoinduced by Fe(III) in water-acetonitrile solution. J. Photochem. Photobiol. A 135:221-228. [Google Scholar]
- 7.Brownlee, B. G., J. H. Carey, G. A. Mac-Innis, and I. T. Pellizzari. 1992. Aquatic environmental chemistry of 2-(thiocyanomethylthio)benzothiazole and related benzothiazoles. Environ. Toxicol. Chem. 11:1153-1168. [Google Scholar]
- 8.Bryson, H., B. Fulton, and P. Benfield. 1996. Riluzole: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in amyotrophic lateral sclerosis. Drugs 52:549-563. [DOI] [PubMed] [Google Scholar]
- 9.Bujdakova, H., T. Kuchta, E. Sidoova, and A. Gvozdjakova. 1993. Anti-Candida activity of four antifungal benzothiazoles. FEMS Microbiol. Lett. 112:329-334. [DOI] [PubMed] [Google Scholar]
- 10.Choi, S. J., H. J. Park, S. K. Lee, S. W. Kim, G. Han, and H. Y. Choo. 2006. Solid phase combinatorial synthesis of benzothiazoles and evaluation of topoisomerase II inhibitory activity. Bioorg. Med. Chem. 14:1229-1235. [DOI] [PubMed] [Google Scholar]
- 11.De Wever, H., P. Besse, and H. Verachtert. 2001. Microbial transformations of 2-substituted benzothiazoles. Appl. Microbiol. Biotechnol. 57:620-625. [DOI] [PubMed] [Google Scholar]
- 12.De Wever, H., S. De Cort, I. Noots, and H. Verachtert. 1997. Isolation and characterization of Rhodococcus rhodochrous for the degradation of the wastewater component 2-hydroxybenzothiazole. Appl. Microbiol. Biotechnol. 47:458-461. [Google Scholar]
- 13.Dubey, R., P. K. Shrivastava, P. K. Basniwal, S. Bhattacharya, and N. S. Moorthy. 2006. 2-(4-Aminophenyl)benzothiazole: a potent and selective pharmacophore with novel mechanistic action towards various tumour cell lines. Mini-Rev. Med. Chem. 6:633-637. [DOI] [PubMed] [Google Scholar]
- 14.Fiehn, O., G. Wegener, J. Jochimsen, and M. Jekel. 1998. Analysis of the ozonation of 2-mercaptobenzothiazole in water and tannery wastewater using sum parameters, liquid- and gas chromatography and capillary electrophoresis. Water Res. 32:1075-1084. [Google Scholar]
- 15.Gaja, M. A., and J. S. Knapp. 1997. The microbial degradation of benzothiazoles. J. Appl. Microbiol. 83:327-334. [Google Scholar]
- 16.Gold, L. S., T. H. Slone, B. R. Stern, and L. Bernstein. 1993. Comparison of target organs of carcinogenicity for mutagenic and non-mutagenic chemicals. Mutat. Res. 286:75-100. [DOI] [PubMed] [Google Scholar]
- 17.Haroune, N., B. Combourieu, P. Besse, M. Sancelme, and A.-M. Delort. 2001. 1H NMR: a tool to study the fate of pollutants in the environment. C. R. Acad. Sci. 4:759-763. [Google Scholar]
- 18.Haroune, N., B. Combourieu, P. Besse, M. Sancelme, T. Reemtsma, A. Kloepfer, A. Diab, J. S. Knapp, S. Baumberg, and A.-M. Delort. 2002. Benzothiazole degradation by Rhodococcus pyridinovorans strain PA: evidence of a catechol 1,2-dioxygenase activity. Appl. Environ. Microbiol. 68:6114-6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haroune, N., B. Combourieu, P. Besse, M. Sancelme, A. Kloepfer, H. de Wever, and A.-M. Delort. 2004. Metabolism of 2-mercaptobenzothiazole by Rhodococcus rhodochrous. Appl. Environ. Microbiol. 70:6315-6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartley, D., and H. Kidd. 1987. The agrochemical handbook. The Royal Society of Chemistry, Nottingham, United Kingdom.
- 21.Kloepfer, A., R. Gnirss, M. Jekel, and T. Reemtsma. 2004. Occurrence of benzothiazoles in municipal wastewater and their fate in biological treatment. Water Sci. Technol. 50:203-208. [PubMed] [Google Scholar]
- 22.Kloepfer, A., M. Jekel, and T. Reemtsma. 2005. Occurrence, sources, and fate of benzothiazoles in municipal wastewater treatment plants. Environ. Sci. Technol. 39:3792-3798. [DOI] [PubMed] [Google Scholar]
- 23.Lau, P. T. S., and T. E. Gompf. 1970. Reaction of quinones with thiourea. A novel route to 2-amino-6-hydroxybenzothiazoles and 2-amino-5-hydroxynaphto[1,2-d]thiazoles. J. Org. Chem. 35:4103-4108. [Google Scholar]
- 24.Malouki, M., G. Giry, P. Besse, B. Combourieu, M. Sancelme, F. Bonnemoy, C. Richard, and A.-M. Delort. 2003. Sequential bio- and phototransformation of the herbicide methabenzthiazuron in water. Environ. Toxicol. Chem. 22:2013-2019. [PubMed] [Google Scholar]
- 25.Malouki, M. 2004. Thèse de l'Université Mentouri de Constantine, Constantine, République Algérienne Démocratique et Populaire.
- 26.Malouki, M. A., B. Lavédrine, and C. Richard. 2005. Phototransformation of methabenzthiazuron in the presence of nitrate and nitrite ions. Chemosphere 60:1523-1529. [DOI] [PubMed] [Google Scholar]
- 27.Meding, B., K. Toren, A. T. Karlberg, S. Hagberg, and K. Wass. 1993. Evaluation of skin symptoms among workers at a Swedish paper mill. Am. J. Ind. Med. 23:721-728. [DOI] [PubMed] [Google Scholar]
- 28.Nawrocki, S. T., K. D. Drake, C. F. Watson, G. D. Foster, and K. J. Maier. 2005. Comparative aquatic toxicity evaluation of 2-(thiocyanomethylthio)benzothiazole and selected degradation products using Ceriodaphnia dubia. Arch. Environ. Contam. Toxicol. 48:344-350. [DOI] [PubMed] [Google Scholar]
- 29.Reemtsma, T., O. Fiehn, G. Kalnowski, and M. Jekel. 1995. Microbial transformations and biological effects of fungicide-derived benzothiazoles determined in industrial wastewater. Environ. Sci. Technol. 29:478-485. [DOI] [PubMed] [Google Scholar]
- 30.Sarasa, J., T. Llabrés, P. Ormad, R. Mosteo, and J. L. Ovelleiro. 2006. Characterization and photo-Fenton treatment of used tires leachate. J. Hazard. Mater. 136:874-881. [DOI] [PubMed] [Google Scholar]
- 31.Stookey, L. L. 1970. Ferrozine: a new spectrophotometric reagent for iron. Anal. Chem. 42:779-781. [Google Scholar]
- 32.Takuzo, H., I. Masataka, T. Konosuke, and T. Masanobu. 1982. Synthesis of benzoaxazoles, benzothiazoles and benzimidazoles and evaluation of their antifungal, insecticidal and herbicidal activities. Chem. Pharm. Bull. 30:2996-3004. [Google Scholar]
- 33.Valdés, H., and C. A. Zaror. 2005. Advanced treatment of benzothiazole contaminated waters: comparison of O3, AC, and O3/AC processes. Water Sci. Technol. 52:281-288. [PubMed] [Google Scholar]
- 34.Vel'tman, R. P., and F. G. Yanovskii. 1955. Hydroxy derivatives of 2-aminobenzothiazole. Ukr. Khemich. Zh. 21:347-349. [Google Scholar]
- 35.Whittaker, M. H., A. M. Gebhart, T. C. Miller, and F. Hammer. 2004. Human health risk assessment of 2-mercaptobenzothiazole in drinking water. Toxicol. Ind. Health 20:149-163. [DOI] [PubMed] [Google Scholar]
- 36.Witte, E. G., H. Philipp, and H. Vereecken. 1998. Binding of 13C-labelled 2-aminobenzothiazole to humic acid as derived from 13C NMR spectroscopy. Org. Geochem. 29:1829-1835. [Google Scholar]