Abstract
Shuttle vectors that replicate stably and express selectable phenotypes in both Thermococcus kodakaraensis and Escherichia coli have been constructed. Plasmid pTN1 from Thermococcus nautilis was ligated to the commercial vector pCR2.1-TOPO, and selectable markers were added so that T. kodakaraensis transformants could be selected by ΔtrpE complementation and/or mevinolin resistance. Based on Western blot measurements, shuttle vector expression of RpoL-HA, a hemagglutinin (HA) epitope-tagged subunit of T. kodakaraensis RNA polymerase (RNAP), was ∼8-fold higher than chromosome expression. An idealized ribosome binding sequence (5′-AGGTGG) was incorporated for RpoL-HA expression, and changes to this sequence reduced expression. Changing the translation initiation codon from AUG to GUG did not reduce RpoL-HA expression, but replacing AUG with UUG dramatically reduced RpoL-HA synthesis. When functioning as translation initiation codons, AUG, GUG, and UUG all directed the incorporation of methionine as the N-terminal residue of RpoL-HA synthesized in T. kodakaraensis. Affinity purification confirmed that an HA- plus six-histidine-tagged RpoL subunit (RpoL-HA-his6) synthesized ectopically from a shuttle vector was assembled in vivo into RNAP holoenzymes that were active and could be purified directly from T. kodakaraensis cell lysates by Ni2+ binding and imidazole elution.
Archaea, in common with Bacteria, have small circular genomes not encased in a nuclear compartment, with many genes organized and cotranscribed in operons. Archaeal genome replication and expression machineries, however, have many features more similar to their eukaryotic than to their bacterial counterparts (1, 5, 9, 15). Progress in understanding archaeal replication and gene expression has been made using purified components in vitro, but in vivo validation of the results so obtained has been limited by the lack of genetic systems. This shortcoming has been most pronounced for the thermophilic and hyperthermophilic Euryarchaea, many of which are the foci of physiological and biochemical investigations (5, 9). Fortunately progress is now being made, most notably with Thermococcus kodakaraensis (2, 8), since it was discovered that T. kodakaraensis is naturally competent for DNA uptake and incorporates donor DNA into its genome by homologous recombination (25, 27). Deletion and mutation of chromosomal genes have resulted in the identification of novel biochemical pathways and facilitated the dissection of several events in archaeal transcription in T. kodakaraensis (12-14, 17, 19, 22, 23, 26, 28). To overcome the need for homologous recombination, we have now constructed shuttle vectors that replicate and express genes in both T. kodakaraensis and Escherichia coli. By using plasmid expression, we have documented and quantified the roles of a ribosome binding sequence (RBS) and alternative initiation codons in archaeal translation in T. kodakaraensis. We have also established that if one subunit (RpoL) of the multisubunit archaeal DNA-dependent RNA polymerase (RNAP) is synthesized ectopically from a shuttle vector, it is incorporated into functional holoenzymes. Affinity tagging of this plasmid-encoded subunit has then been used to purify the 11-subunit RNAP directly from T. kodakaraensis cell lysates.
MATERIALS AND METHODS
Reagents and enzymes.
Except where otherwise noted, all chemicals were purchased from Sigma Chemicals (St. Louis, MO); nucleases, restriction enzymes, linear pCR2.1-TOPO plasmid DNA, and TOPO-TA cloning kits were from Invitrogen (Carlsbad, CA); plasmid and PCR product purification kits were from Qiagen Inc. (Valencia, CA); Ni2+-charged HiTrap chelating columns were from GE Healthcare (Piscataway, NJ); and 32P-labeled reagents were from MP Biomedicals (ICN, Irvine, CA).
Media and growth conditions.
T. kodakaraensis cultures were grown under anaerobic conditions at 85°C in Marine Arts (MA)-yeast extract tryptone (YT) or artificial seawater-amino acids-(AA) medium with or without added tryptophan (50 μg/ml), and cells competent for DNA uptake were prepared as described previously (2, 25). T. kodakaraensis KW128 (ΔpyrF ΔtrpE::pyrF) transformants capable of growth in the absence of tryptophan, or the presence of mevinolin, were selected on artificial seawater-AA plates or MA-YT plates containing 15 μM mevinolin, respectively, solidified by incorporation of 1% Gelrite. E. coli DH5α cultures were grown in LB medium at 37°C, and transformants were selected on LB plates that contained 100 μg carbenicillin/ml.
Construction of T. kodakaraensis-E. coli shuttle vectors.
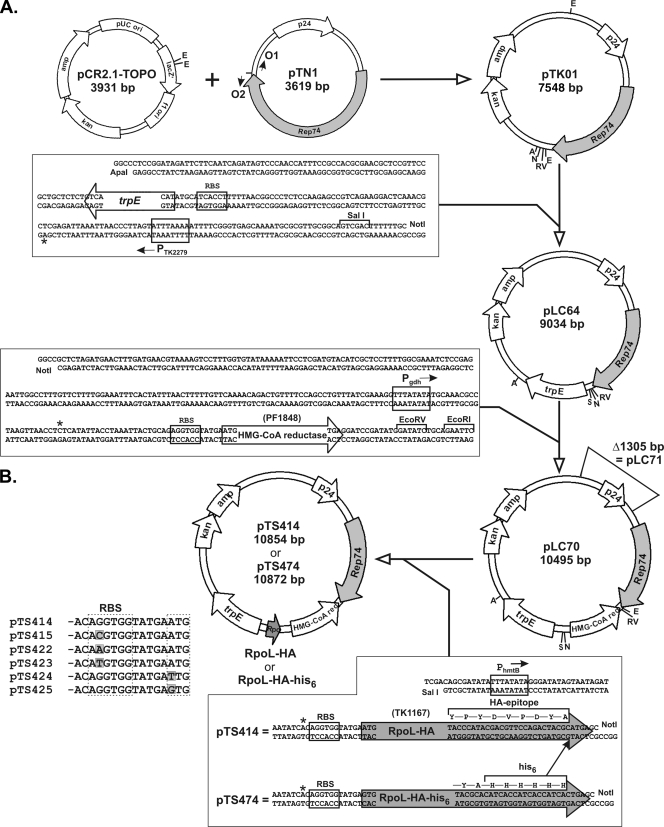
A sample of pTN1 was generously provided by N. Soler (29). A preparation of linear pTN1 DNA molecules was generated with 3′-A extensions by PCR amplification using oligonucleotide primers O1 and O2 (5′-TTTTCTGATATTCTGATTTTCTGATAATTTGCCAATTCTGGCAATTTGGC and 5′-TCAGGAATTTGGAATTTTCAGTTCTAACACATCTGGTGGGTCCTCCCAAG, respectively; Fig. 1A). A pTN1 molecule from this preparation was ligated with pCR2.1-TOPO linearized at position 293, resulting in the entire pTN1 sequence being cloned within, and flanked by, regions of the pCR2.1-TOPO multiple cloning site. The resulting plasmid, designated pTK01, was amplified in E. coli DH5α, and both strands were fully sequenced to confirm the construction (Fig. 1A).
FIG. 1.
Shuttle and expression plasmid constructions. (A) The pTN1 sequence (29) is available in GenBank (accession no. EF495263), and the pCR2.1-TOPO sequence is available at http://www.genomex.com/vector_sequence/PCR2.1_Topo.txt. To obtain pTK01, a linear pTN1 DNA molecule, generated by PCR amplification using primers O1 and O2, was ligated with pCR2.1-TOPO linearized at position 293 between the two EcoRI sites (E), as purchased from Invitrogen. The trpE-containing DNA sequence shown was ligated with ApaI (A)- plus NotI (N)-digested pTK01 to produce pLC64. The DNA sequence shown containing the HMG-CoA reductase-encoding gene (PF1848) was ligated with NotI- plus EcoRV (RV)-digested pLC64 DNA to produce pLC70. The asterisks identify the sites of transcription initiation. Deletion of the 1,305-bp region indicated from pLC70 generated pLC71. (B) The DNA sequences shown that encode RpoL-HA and RpoL-HA-his6 were ligated with SalI (S)- plus NotI-digested pLC70 to generate pTS414 and pTS474, respectively. The RBS regions immediately upstream and the initiation codons of the RpoL-HA-encoding gene in pTS414, pTS415, pTS422, pTS423, pTS424, and pTS425 are shown aligned with the single-nucleotide differences highlighted.
An ∼1.5-kbp DNA molecule (trpE cassette; Fig. 1A) that expresses the T. kodakaraensis trpE gene constitutively from the T. kodakaraensis PTK2279 promoter was constructed previously (23). As illustrated in Fig. 1A, this cassette was ligated into pTK01, resulting in plasmid pLC64, and aliquots of pLC64 DNA were used to transform T. kodakaraensis KW128 (ΔpyrF ΔtrpE::pyrF), with transformants selected by growth on plates in the absence of tryptophan. Cultures of several independent transformants were grown in MA-YT medium, and plasmid preparations were isolated from 3-ml aliquots. The cells were suspended in 0.5 ml of 100 mM Tris-HCl, 100 mM NaCl, 50 mM EDTA (pH 8) that contained 10 μg RNase A and lysed by the addition of 25 μl of 20% (wt/vol) sodium dodecyl sulfate (SDS), mixing, and addition of 20 μl of 5 M NaOH. After 5 min at room temperature, 350 μl of 5 M potassium acetate (pH 4.8) was added and the precipitated material was removed by centrifugation. The supernatant was loaded onto a minipreparation spin column, and bound DNA was washed with 500 μl phosphate buffer, followed by 750 μl phosphatidylethanolamine (Qiagen), and then eluted using 30 to 50 μl of 10 mM Tris-HCl (pH 8). Restriction enzyme digestion and sequencing confirmed that the plasmid isolated from several independent T. kodakaraensis prototrophic transformants was pLC64.
Expression of PF1848, a hydroxymethylglutaryl coenzyme A reductase (HMG-CoA reductase)-encoding gene cloned from Pyrococcus furiosus, in T. kodakaraensis confers resistance to simvastatin and mevinolin (17, 23). A ∼1.5-kbp DNA molecule (Mevr cassette; Fig. 1A) that has PF1848 transcribed from the constitutive T. kodakaraensis Pgdh promoter (8) was cloned from pTS429 (23) into pLC64 to generate pLC70 (Fig. 1A). Transformation of T. kodakaraensis KW128 with pLC70 resulted in complementation of the ΔtrpE::pyrF mutation and conferred resistance to >30 μM mevinolin.
Southern blot measurements of plasmid copy number in T. kodakaraensis.
Aliquots (1 to 7 μg) of total DNA isolated from T. kodakaraensis KW128(pLC70) cells, grown to mid-exponential phase, were digested twice with 20 U of SalI for 90 min at 37°C. The resulting restriction fragments were separated by electrophoresis through 1.1% (wt/vol) agarose gels and then transferred to Zeta-probe membranes (Bio-Rad, Oakland, CA). The membranes were incubated with two hybridization probes (∼400 bp), one specific for TK1280, a single-copy chromosomal gene (8, 22), and the other specific for trpE (carried on pLC70), 32P labeled by random priming using NEBlot kits (New England Biolabs, Ipswich, MA). The specific activities of the probes were established by direct 32P counting, and dilutions were spotted onto 3MM paper to provide standard curves. These were measured concurrently with the amounts of the 32P-labeled probes that hybridized to the SalI fragment that contained the complementary TK1280 or trpE sequence bound to Zeta-probe membrane.
Construction of plasmids that encode RpoL-HA and RpoL-HA-his6.
The gene that encodes RpoL, one of the 11 subunits of the T. kodakaraensis RNAP, was previously cloned (22). The coding sequence was extended in frame by ligation of oligonucleotide sequences that added either a hemagglutinin (HA) epitope (YPYDVPDYA; HA tag) or the HA tag plus six histidine residues (HA-his6) to the C terminus of RpoL (Fig. 1B). These DNA molecules were cloned into pLC70, generating pTS414 and pTS474, respectively. Derivatives of pTS414 were generated with the RBS (pTS415, pTS422, and pTS423) or translation initiation codon (pTS424 and pTS425) changed by using site-specific mutagenic oligonucleotides with QuikChange-XL mutagenesis kits (Stratagene, La Jolla, CA).
Western blot measurements of RpoL-HA synthesis in T. kodakaraensis.
Cultures of T. kodakaraensis KW128, T. kodakaraensis TS366 (22), and T. kodakaraensis strains containing plasmid pLC70, pTS414, pTS415, pTS422, pTS423, pTS424, or pTS425 were grown in MA-YT-pyruvate medium to mid-exponential phase. The cells were harvested and lysed, and the protein concentrations of the lysates were determined by Bradford assays. Identical quantities of protein from each lysate were subjected to SDS-polyacrylamide gel electrophoresis (PAGE), and the separated polypeptides were transferred to polyvinylidene difluoride membranes (GE Healthcare). The membranes were incubated, under Western blotting conditions, with anti-HA antibodies (Covance Research Products, Denver, PA), and the complexes formed were visualized by using the ECF system (GE Healthcare) and quantified by using a Storm 840 phosphorimager (GE Healthcare).
Purification of RpoL-HA and in vitro transcription using T. kodakaraensis components.
The column chromatographic protocol used to purify RNAP and RNAP containing the RpoL-HA subunit from T. kodakaraensis and the reagents and conditions used for in vitro transcription using T. kodakaraensis components have been described previously (22). The polypeptides in purified RNAP preparations were separated by SDS-PAGE, and the region of the gel that contained RpoL-HA was excised. Preparations of RpoL-HA so purified were fragmented and subjected to mass spectrometry (at Cornell University, Life Sciences Core Laboratories Center, Ithaca, NY) and to N-terminal sequencing by Edman degradation (at the Iowa State University, Biotechnology Services Facility, Ames, IA).
Ni2+ affinity purification of RpoL-HA-his6-tagged RNAP from T. kodakaraensis.
T. kodakaraensis KW128(pLC70) and T. kodakaraensis KW128(pTS474) cultures were grown to mid-exponential phase in MA-YT-pyruvate medium containing 5 μM mevinolin (23). The cells were harvested; resuspended in 25 mM Tris-HCl (pH 8), 1 M NaCl, 10% (vol/vol) glycerol; and lysed by freeze-thawing three times using liquid N2. Particulates were removed by centrifugation, and aliquots of the resulting clarified supernatants were loaded onto 10-ml Ni2+-charged HiTrap chelating columns. After being washed with 25 mM Tris-HCl (pH 8), 1 M NaCl, 10% glycerol, material that bound to the column was eluted using a linear gradient of 0 to 500 mM imidazole dissolved in 25 mM Tris-HCl (pH 8), 100 mM NaCl, and 10% glycerol. The polypeptides present in aliquots of the eluted material were separated by SDS-PAGE and visualized by Coomassie blue staining.
RESULTS
T. kodakaraensis-E. coli shuttle vectors.
Plasmid pTN1 was isolated by Soler et al. (29) from Thermococcus nautilus, a relative of T. kodakaraensis, and sequenced (3,619 bp), revealing the presence of two long open reading frames designated Rep74 and p24 (29). The Rep74-encoded protein (Rep74; ∼74 kDa) has a sequence ∼60% homologous to that of the replication protein Rep75 characterized from the Pyrococcus abyssi plasmid pGT5 (7, 16). The p24-encoded protein (p24; ∼24 kDa) has no clearly recognizable relatives but does bind and compact DNA (29). The entire pTN1 sequence was cloned within the multiple cloning site region of pCR2.1-TOPO. The resulting plasmid, designated pTK01 (Fig. 1A), retains all of the multiple cloning sites, replicates stably, and confers ampicillin and kanamycin resistance on E. coli. To provide a selectable phenotype for T. kodakaraensis KW128 (ΔpyrF ΔtrpE::pyrF) transformation, a trpE expression cassette (23) was ligated into pTK01, resulting in pLC64 (Fig. 1A). Transformation of T. kodakaraensis KW128 (ΔpyrF ΔtrpE::pyrF) with pLC64 resulted in tryptophan-independent growth, and the reisolation of intact pLC64 from representative transformants confirmed that this resulted from plasmid expression of trpE complementing the chromosomal ΔtrpE::pyrF mutation.
To add a second selectable phenotype, a cassette that expresses PF1848 (encoding HMG-CoA reductase) from P. furiosus in T. kodakaraensis (23) was cloned into pLC64, generating pLC70 (Fig. 1A). Transformation of T. kodakaraensis KW128 with pLC70 resulted in transformants that could be selected on the basis of either tryptophan-independent growth or resistance to mevinolin (Mevr). As mevinolin resistance could also be selected on plates containing nutrient-rich medium plus mevinolin, transformation with pLC70 was not limited to T. kodakaraensis strains that carry the ΔtrpE::pyrF mutation. Plasmid preparations were purified from several independent T. kodakaraensis transformants, and restriction enzyme digestions and sequencing confirmed that, in all cases, the plasmid isolated was intact pLC70. There were no detectable differences in the numbers of T. kodakaraensis transformants (∼1/108 recipient cells) or E. coli transformants (∼106/108 recipient cells) generated when the recipient cells were transformed with 1 μg of pLC70 DNA purified from T. kodakaraensis or from E. coli.
Shuttle vector stability and copy number in T. kodakaraensis.
Based on measurements of optical density at 600 nm, cultures of T. kodakaraensis KW128 and T. kodakaraensis KW128(pLC70) had identical growth rates in MA-YT-pyruvate medium, and cultures of T. kodakaraensis KW128(pLC70) grew at the same rates with or without mevinolin present. To determine if selective pressure were necessary to maintain pLC70 in T. kodakaraensis KW128(pLC70), cultures were grown in MA-YT-pyruvate medium without mevinolin, diluted 50-fold, and regrown through seven serial passages. Samples were then diluted and plated on nonselective medium to obtain single colonies. Cells from >80% of the isolated colonies so generated still contained pLC70.
To determine the shuttle vector copy number, Southern blots of SalI-digested total DNA isolated from exponentially growing T. kodakaraensis KW128(pLC70) cells were generated and quantified. Based on 32P-labeled probe hybridizations, the SalI restriction fragment that contained trpE (from pLC70) was present in these DNA preparations at an ∼3-fold-higher abundance than the SalI fragment that carried TK1280 (from the T. kodakaraensis chromosome). Given this result, although the number of chromosomes in a growing T. kodakaraensis cell remains to be established, pLC70 (and presumably all pTN1-based replicons) is present in such cells at a ratio of ∼3 per chromosome.
Replication of pLC70 in T. kodakaraensis requires Rep74 but not p24.
Derivatives of pLC70 were generated to determine if Rep74 and p24 were essential for pLC70 propagation in T. kodakaraensis. As shown in Fig. 1A, an ∼1.3-kbp region was deleted from pLC70 that removed the entire p24 coding sequence and a putative downstream ∼250-bp insertion sequence (29). The smaller plasmid generated, designated pLC71, replicated stably and conferred tryptophan-independent growth and Mevr on T. kodakaraensis KW128. In contrast, deleting a single base pair in the Rep74 gene resulted in a plasmid (pLC60) that was stable and replicated in E. coli but that could not be propagated in T. kodakaraensis. This deletion in codon 487 placed a nonsense codon in frame at position 490, resulting in an open reading frame that encodes a ∼56-kDa C-terminally truncated version of Rep74. If this Rep74 variant is synthesized, it does not support plasmid maintenance in T. kodakaraensis.
Measurement of RBS and initiation codon contributions to translation in T. kodakaraensis.
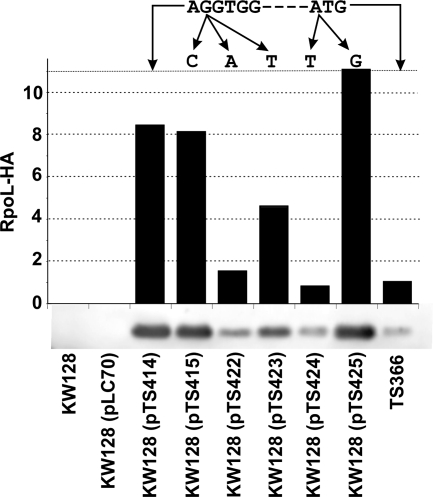
Western blot assays, using anti-HA antibodies, were used to measure and compare the levels of RpoL-HA synthesized in vivo in T. kodakaraensis strains. The RpoL-HA-encoding gene was either integrated into the chromosome (T. kodakaraensis TS366 [22]) or expressed from a plasmid (pTS414, pTS415, pTS422, pTS423, pTS424, or pTS425 [Fig. 1B]). As expected, there was no RpoL-HA detectable in lysates of the control cultures, T. kodakaraensis KW128 and T. kodakaraensis KW128(pLC70), but RpoL-HA was present in the lysates of all cells that contained an RpoL-HA-encoding gene (Fig. 2). Plasmid expression from pTS414 resulted in an ∼8-fold-higher abundance of RpoL-HA than did expression from the chromosome in T. kodakaraensis TS366 (22). Changing the first G in the RBS to C (pTS415; Fig. 1B) had no detrimental effect on plasmid-directed RpoL-HA synthesis, but replacing the G with A (pTS422) or T (pTS423) substantially reduced RpoL-HA synthesis. Changing the ATG initiation codon to GTG (pTS425) did not decrease, but rather marginally increased, RpoL-HA synthesis, while making TTG the initiation codon dramatically reduced RpoL-HA synthesis (Fig. 2).
FIG. 2.
Synthesis of RpoL-HA in T. kodakaraensis. The relative amounts of RpoL-HA present in extracts of T. kodakaraensis KW128, T. kodakaraensis TS366, and T. kodakaraensis KW128 strains containing the plasmids listed. A phosphorimager (Storm 840; GE Healthcare) was used to measure the fluorescence emissions from the Western blot shown generated using anti-HA antibodies. In three independent repetitions of this experiment, the results were essentially identical. The sequence of the RBS and the initiation codon of the RpoL-HA gene integrated into the chromosome of T. kodakaraensis TS366 (22) and present in pTS414 (Fig. 1) are provided above the figure with the sequence changes in pTS415 and pTS422 through pTS425 indicated.
RNAP preparations were purified (22) from T. kodakaraensis KW128 strains expressing pTS414, pTS424, or pTS425, and the RpoL-HA subunit was gel purified. Both N-terminal amino acid sequencing and mass spectrometry confirmed that, in every case, the N-terminal residue of the RpoL-HA was methionine. Therefore, not only AUG, but also GUG and UUG, directed methionine incorporation into RpoL-HA when functioning as translation initiation codons in T. kodakaraensis.
Plasmid expression, holoenzyme assembly, and affinity purification of HA-his6-tagged T. kodakaraensis RNAP.
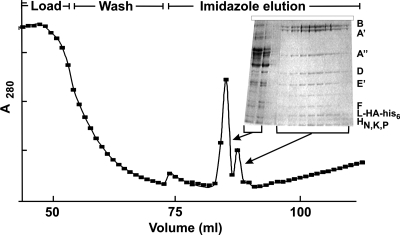
Purification of multisubunit enzymes intact, as well as the definitive identification of all of the polypeptides that constitute such enzymes in vivo, is often difficult. One approach to these issues is to construct strains that have a known subunit of the enzyme modified to facilitate immunoprecipitation or affinity matrix capture and so purification of the entire enzyme. Plasmid pTS474 was constructed (Fig. 1B) to evaluate this approach with T. kodakaraensis. Lysates of T. kodakaraensis KW128(pLC70) and T. kodakaraensis KW128(pTS474) were loaded onto Ni2+-charged HiTrap chelating columns and washed, and the bound proteins were then eluted using an imidazole gradient. Imidazole eluted only one peak of A280 absorbing material from columns loaded with T. kodakaraensis KW128(pLC70) proteins but eluted two distinct peaks of A280 absorbing material from columns bound by proteins from T. kodakaraensis KW128(pTS474) (Fig. 3). The second peak contained predominantly 10 polypeptides that migrated as previously established for the subunits of RNAP purified from T. kodakaraensis (22), plus RpoL-HA-his6, which migrated slightly slower than RpoL (Fig. 3). RpoL-HA-his6 was therefore assembled into RNAP holoenzymes in vivo, and this facilitated purification of the entire enzyme by affinity to the Ni2+-charged chelating matrix. The RNAP so purified was active in vitro and in in vitro transcription assays generated results (not shown) indistinguishable from those obtained using RNAP preparations that contained RpoL or RpoL-HA purified from T. kodakaraensis strains by the established column chromatography protocol (22).
FIG. 3.
Purification of RpoL-HA-his6-tagged RNAP. A280 measurements were made of fractions obtained from a Ni2+-charged chelating column, loaded with clarified T. kodakaraensis(pTS474) cell lysate, washed, and eluted using a 0 to 500 mM imidazole gradient. As shown, the material eluted by imidazole formed two discrete peaks of A280 absorbing material. The second peak contained almost exclusively polypeptides with the electrophoretic mobilities established previously (22) for the indicated subunits of purified T. kodakaraensis RNAP.
DISCUSSION
We have established that plasmids that contain both the ColE1 and pTN1 replication machineries are maintained stably in both E. coli and T. kodakaraensis, and by adding genes that provide selectable phenotypes, E. coli-T. kodakaraensis shuttle vectors have been constructed (Fig. 1). The minimal region(s) of pTN1 required for replication of these bacterial-archaeal shuttle vectors in T. kodakaraensis remains to be defined, but we have established that p24, one of the two proteins known to be encoded by pTN1 (29), is not essential. Rep74 is apparently essential, consistent with its ∼60% amino acid sequence homology and likely functional homology with Rep75, a replication protein encoded by plasmid pGT5 in P. abyssi (7, 16). In this regard, it does, however, seem noteworthy that pGT5 has an ∼10-fold-higher copy number in P. abyssi (16) than we estimate for pLC70 in T. kodakaraensis. We have constructed potential shuttle vectors by ligating pGT5 and pCR2.1-TOPO sequences, but none have replicated stably in T. kodakaraensis.
Expression of shuttle plasmid-carried genes in T. kodakaraensis has been shown to complement a chromosomal deletion (ΔtrpE) and confer antibiotic (Mevr) resistance. Plasmid expression has also been used to add a sequence to a multisubunit enzyme (HA- and/or His6 tag) that facilitates Western blot quantification (Fig. 2) and/or affinity purification together with any tightly associated proteins (Fig. 3). Plasmid overexpression should also facilitate more-rapid purification of any enzyme of interest directly from T. kodakaraensis. Furthermore, by providing the necessary complementing activity from a plasmid, it should now be possible to mutate and manipulate essential genes in the T. kodakaraensis genome. Ideally, complementation would be controlled by using a promoter that can be regulated by the addition or removal of a gratuitous reagent, and several candidate promoter, regulator, and reagent combinations have been evaluated but, to date, without success. As an alternative, we have established that very different concentrations of a plasmid-encoded protein can be logically obtained in vivo in T. kodakaraensis by manipulating the RBS and/or changing the initiation codon (Fig. 2).
Based on the translation factors encoded in archaeal genomes, in vitro structural and functional studies, and bioinformatics scrutiny of sequences (1, 3, 4, 6, 8, 11, 15, 18, 21, 24, 31, 33), archaeal translation initiation has both bacterial and eukaryotic features. Almost all studies of archaeal translation in vitro have employed components isolated from Sulfolobus solfataricus, and the results obtained argue that translation initiation in this species occurs by two mechanisms, one dependent on and one independent of an RBS (3, 6, 11, 15). Several examples of transcription initiation occurring so close to the translation initiation codon that the resulting mRNA cannot contain an upstream RBS support extrapolating this conclusion to other Archaea (1, 4, 15, 30, 31). To our knowledge, only two previous reports describe the results of in vivo investigations of archaeal translation initiation (24, 30), both of which took advantage of the availability of a transformation system for the mesophilic halophile Halobacterium salinarum. As documented here for the RBS variants in T. kodakaraensis, changes introduced into a sequence predicted to be a natural RBS did result in differences in the levels of protein synthesis in vivo in H. salinarum (24). The frequent use of GTG and TTG as initiation codons in Archaea resulted initially in many genome misannotations (20, 31, 32), but with this now well recognized, establishing how different initiation codons contribute to translation in Archaea is clearly of experimental interest (30). As predicted by structural studies (1, 11, 18, 33) and consistent with most (32) but not all (10) previous reports, all three initiation codons directed incorporation of methionyl in T. kodakaraensis as the N-terminal residue of RpoL-HA. The results obtained also argue that UUG directs translation initiation much less efficiently in T. kodakaraensis than does AUG or GUG, at least when substituted into a transcript in which AUG is the natural initiation codon.
Acknowledgments
This work was supported by research grants from the Department of Energy (DE-FG02-87ER13731) and National Institutes of Health (GM53185) to J.N.R. and a National Institutes of Health fellowship (1F32-GM073336-01) to T.J.S.
We are very grateful to N. Soler and P. Forterre for the gift of pTN1 and for the pTN1 sequence that was generously provided to us before publication.
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Allen, G. S., and J. Frank. 2007. Structural insights on the translation initiation complex: ghosts of a universal initiation complex. Mol. Microbiol. 63:941-950. [DOI] [PubMed] [Google Scholar]
- 2.Atomi, H., T. Fukui, T. Kanai, M. Morikawa, and T. Imanaka. 2004. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benelli, D., E. Maone, and P. Londei. 2003. Two different mechanisms for ribosome/mRNA interaction in archaeal translation initiation. Mol. Microbiol. 50:635-643. [DOI] [PubMed] [Google Scholar]
- 4.Brennels, M., O. Hering, C. Lange, and J. Soppa. 2007. Experimental characterization of cis-acting elements important for translation and transcription in halophilic Archaea. PLoS Genet. 3:2450-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavicchioli, R. 2007. Archaea. Molecular and cellular biology. ASM Press, Washington, DC.
- 6.Condò, I., A. Ciammaruconi, D. Benelli, D. Ruggero, and P. Londei. 1999. cis-acting signals controlling translational initiation in the thermophilic archaeon Sulfolobus solfataricus. Mol. Microbiol. 34:377-384. [DOI] [PubMed] [Google Scholar]
- 7.Erauso, G., S. Marsin, N. Benbouzid-Rollet, M. F. Baucher, T. Barbeyron, Y. Zivanovic, D. Prieur, and P. Forterre. 1996. Sequence of plasmid pGT5 from the archaeon Pyrococcus abyssi: evidence for rolling-circle replication in a hyperthermophile. J. Bacteriol. 178:3232-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fukui, T., H. Atomi, T. Kanai, R. Matsumi, S. Fujiwara, and T. Imanaka. 2005. Complete genome sequence of the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1 and comparison with Pyrococcus genomes. Genome Res. 15:352-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrett, R. A., and H.-P. Klenk. 2007. Archaea. Evolution, physiology, and molecular biology. Blackwell Publishing, Oxford, United Kingdom.
- 10.Hansen, T., B. Reichstein, R. Schmid, and P. Schönheit. 2002. The first archaeal ATP-dependent glucokinase, from the hyperthermophilic crenarchaeon Aeropyrum pernix, represents a monomeric, extremely thermophilic ROK glucokinase with broad hexose specificity. J. Bacteriol. 184:5955-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasenöhrl, D., D. Benelli, A. Barbazza, P. Londei, and U. Bläsi. 2006. Sulfolobus solfataricus translation initiation factor 1 stimulates translation initiation complex formation. RNA 12:674-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu, G.-Q., X. Zheng, Y.-F. Yang, P. Ortet, Z.-S. She, and H. Zhu. 2008. ProTISA: a comprehensive resource for translation initiation site annotation in prokaryotic genomes. Nucleic Acids Res. 36:D114-D119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imanaka, H., A. Yamatsu, T. Fukui, H. Atomi, and T. Imanaka. 2006. Phosphoenolpyruvate synthase plays an essential role for glycolysis in the modified Embden-Meyerhof pathway in Thermococcus kodakaraensis. Mol. Microbiol. 61:898-909. [DOI] [PubMed] [Google Scholar]
- 14.Kanai, T., J. Akerboom, S. Takedomi, H. J. van de Werken, F. Blombach, J. van der Oost, T. Murakami, H. Atomi, and T. Imanaka. 2007. A global transcriptional regulator in Thermococcus kodakaraensis controls the expression levels of both glycolytic and gluconeogenic enzyme-encoding genes. J. Biol. Chem. 282:33659-33670. [DOI] [PubMed] [Google Scholar]
- 15.Londei, P. 2005. Evolution of translation initiation: new insights from the archaea. FEMS Microbiol. Rev. 29:185-200. [DOI] [PubMed] [Google Scholar]
- 16.Lucas, S., L. Toffin, Y. Zivanovic, D. Charlier, H. Moussard, P. Forterre, D. Prieur, and G. Erauso. 2002. Construction of a shuttle vector for, and spheroplast transformation of, the hyperthermophilic archaeon Pyrococcus abyssi. Appl. Environ. Microbiol. 68:5528-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumi, R., K. Manabe, T. Fukui, H. Atomi, and T. Imanaka. 2007. Disruption of a sugar transporter gene cluster in a hyperthermophilic archaeon using a host-marker system based on antibiotic resistance. J. Bacteriol. 189:2683-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikonov, O., E. Stolboushkina, A. Nikulin, D. Hasenöhrl, U. Bläsi, D. Manstein, R. Fedorov, M. Garber, and S. Nikonov. 2007. New insights into the interactions of the translation initiation factor 2 from Archaea with guanine nucleotides and initiator tRNA. J. Mol. Biol. 373:328-336. [DOI] [PubMed] [Google Scholar]
- 19.Orita, I., T. Sato, H. Yurimoto, N. Kato, H. Atomi, T. Imanaka, and Y. Sakai. 2006. The ribulose monophosphate pathway substitutes for the missing pentose phosphate pathway in the archaeon Thermococcus kodakaraensis. J. Bacteriol. 188:4698-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poole, F. L., B. A. Gerwe, R. C. Hopkins, G. J. Schut, M. V. Weinberg, F. E. Jenney, Jr., and M. W. W. Adams. 2005. Defining genes in the genome of the hyperthermophilic archaeon Pyrococcus furiosus: implications for all microbial genomes. J. Bacteriol. 187:7325-7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ring, G., P. Londei, and J. Eichler. 2007. Protein biogenesis in Archaea: addressing translation initiation using an in vitro protein synthesis system for Haloferax volcanii. FEMS Microbiol. Lett. 270:34-41. [DOI] [PubMed] [Google Scholar]
- 22.Santangelo, T. J., L. Čuboňová, C. L. James, and J. N. Reeve. 2007. TFB1 or TFB2 is sufficient for Thermococcus kodakaraensis viability and for basal transcription in vitro. J. Mol. Biol. 367:344-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santangelo, T. J., L. Čuboňová, R. Matsumi, H. Atomi, T. Imanaka, and J. N. Reeve. 2008. Polarity in archaeal operon transcription in Thermococcus kodakaraensis. J. Bacteriol. 190:2244-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sartorius-Neef, S., and F. Pfeifer. 2004. In vivo studies on putative Shine-Dalgarno sequences of the halophilic archaeon Halobacterium salinarum. Mol. Microbiol. 51:579-588. [DOI] [PubMed] [Google Scholar]
- 25.Sato, T., T. Fukui, H. Atomi, and T. Imanaka. 2003. Targeted gene disruption by homologous recombination in the hyperthermophilic archaeon Thermococcus kodakaraensis KOD1. J. Bacteriol. 185:210-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato, T., H. Imanaka, N. Rashid, T. Fukui, H. Atomi, and T. Imanaka. 2004. Genetic evidence identifying the true gluconeogenic fructose-1,6-bisphosphatase in Thermococcus kodakaraensis and other hyperthermophiles. J. Bacteriol. 186:5799-5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sato, T., T. Fukui, H. Atomi, and T. Imanaka. 2005. Improved and versatile transformation system allowing multiple genetic manipulations of the hyperthermophilic archaeon Thermococcus kodakaraensis. Appl. Environ. Microbiol. 71:3889-3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato, T., H. Atomi, and T. Imanaka. 2007. Archaeal type III RuBisCOs function in a pathway for AMP metabolism. Science 315:1003-1006. [DOI] [PubMed] [Google Scholar]
- 29.Soler, N., A. Justome, S. Quevillion-Cheruel, F. Lorieux, E. LeCam, E. Marguet, and P. Forterre. 2007. The rolling-circle plasmid pTN1 from the hyperthermophilic archaeon Thermococcus nautilus. Mol. Microbiol. 66:357-370. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan, G., M. P. Krebs, and U. L. RajBhandary. 2006. Translation initiation with GUC codon in the archaeon Halobacterium salinarum: implications for translation of leaderless mRNA and strict correlation between translation initiation and presence of mRNA. Mol. Microbiol. 59:1013-1024. [DOI] [PubMed] [Google Scholar]
- 31.Torarinsson, E., H.-P. Klenk, and R. A. Garrett. 2005. Divergent transcriptional and translational signals in Archaea. Environ. Microbiol. 7:47-54. [DOI] [PubMed] [Google Scholar]
- 32.Yamazaki, S., J. Yamazaki, K. Nishijima, R. Otsuka, M. Mise, H. Ishikawa, K. Sasaki, S.-I. Tago, and K. Isono. 2006. Proteome analysis of an aerobic hyperthermophilic crenarchaeon, Aeropyrum pernix K1. Mol. Cell. Proteomics 5:811-823. [DOI] [PubMed] [Google Scholar]
- 33.Yatime, L., E. Schmitt, S. Blanquet, and Y. Mechulam. 2004. Functional molecular mapping of archaeal initiation factor 2. J. Biol. Chem. 279:15984-15993. [DOI] [PubMed] [Google Scholar]