Abstract
Batch solar disinfection (SODIS) inactivation kinetics are reported for suspensions in water of Campylobacter jejuni, Yersinia enterocolitica, enteropathogenic Escherichia coli, Staphylococcus epidermidis, and endospores of Bacillus subtilis, exposed to strong natural sunlight in Spain and Bolivia. The exposure time required for complete inactivation (at least 4-log-unit reduction and below the limit of detection, 17 CFU/ml) under conditions of strong natural sunlight (maximum global irradiance, ∼1,050 W m−2 ± 10 W m−2) was as follows: C. jejuni, 20 min; S. epidermidis, 45 min; enteropathogenic E. coli, 90 min; Y. enterocolitica, 150 min. Following incomplete inactivation of B. subtilis endospores after the first day, reexposure of these samples on the following day found that 4% (standard error, 3%) of the endospores remained viable after a cumulative exposure time of 16 h of strong natural sunlight. SODIS is shown to be effective against the vegetative cells of a number of emerging waterborne pathogens; however, bacterial species which are spore forming may survive this intervention process.
Batch-process solar disinfection (SODIS) involves storing microbially contaminated drinking water in transparent containers such as plastic bags or plastic or glass bottles. These are placed in direct sunlight for periods of up to 8 h before consumption (9).
Over the past 15 years there has been a steady increase in the number of species that have been tested for their sensitivity to SODIS. However, several important species remain untested. Among these are Campylobacter jejuni and Yersinia enterocolitica, both of which have been recently described as emerging diarrheal pathogens in the developing world (20, 42). The biocidal effect of sunlight is due to optical and thermal processes, and a strong synergistic effect occurs for water temperatures exceeding 45°C (15) and UV discharge tubes (18); validation of the inactivation kinetics of some of the previously tested pathogens using natural rather than simulated sunlight is warranted.
The aims of this study were (i) to identify the limits of disinfection for SODIS of drinking water by examining inactivation kinetics using natural sunlight for a variety of bacterial species and (ii) to determine whether the previously untested waterborne diarrheal pathogens C. jejuni and Y. enterocolitica are susceptible to SODIS.
Five bacterial species were examined in this study: enteropathogenic Escherichia coli (EPEC), Y. enterocolitica, C. jejuni, Bacillus subtilis, and Staphylococcus epidermidis. EPEC, Y. enterocolitica, and C. jejuni are gastrointestinal pathogens which cause diarrhea and enteritis in humans.
EPEC remains a major cause of infantile diarrhea worldwide, though particularly in developing countries (41). Outbreaks of EPEC-associated gastroenteritis most often affect infants, especially those who are bottle fed, suggesting that contaminated water is often used to rehydrate infant formulae in underdeveloped countries (27, 38). Y. enterocolitica is most commonly contracted following ingestion of food or water contaminated with the bacterium (40). Research indicates that Y. enterocolitica is capable of surviving for several weeks in natural river water (37). Campylobacters have been associated with a number of outbreaks from contaminated drinking water (3, 39). The rate of Campylobacter infections worldwide has been increasing and in certain reports exceeds the incidence of salmonellosis and shigellosis (12, 19). Campylobacters have been associated with a number of outbreaks from contaminated drinking water (8).
Unlike the other species studied in this research, neither B. subtilis nor S. epidermidis can be considered to be a waterborne pathogen. The inactivation studies of S. epidermidis reported here were required as part of a baseline comparison for an unrelated optical inactivation study of the prebiofilmic planktonic phase of this species and are included here solely for reasons of comparison and completeness. Endospores of B. subtilis exhibit a high degree of resistance to inactivation by various physical treatments including wet and dry heat, UV and gamma radiation, chemical oxidants, extreme desiccation, vacuum, and acceleration (28, 29).
MATERIALS AND METHODS
Test organisms.
The bacterial species used were Campylobacter jejuni (NCTC 11168), Yersinia enterocolitica WA314 serotype O:8, S. epidermidis ATCC 35984 (RP62A), and non-verotoxin-producing Escherichia coli O157 (nontoxigenic eae-positive wild-type clinical isolate provided by the Irish Health Service Executive Public Health Laboratories, Cherry Orchard, Dublin, Ireland; laboratory strain identifier 05-E9-25). All were stored at −80°C as a bacterial stock on beads (TSC Ltd., Lancashire, United Kingdom).
Growth media and growth conditions.
Procedures for the preparation of Y. enterocolitica and E. coli suspensions for SODIS inactivation were identical except for the time and temperature of incubation, which were 48 h at 22°C for Y. enterocolitica and 24 h at 37°C for E. coli. Suspensions were prepared by transferring frozen bacterial strains onto Luria-Bertani agar plates (L61746; Sigma, United Kingdom). Single colonies from incubated plates were inoculated into Luria broth (L3522; Sigma, United Kingdom) and incubated under the appropriate conditions with shaking at 200 rpm. S. epidermidis suspensions were prepared in the same manner as for E. coli except that single colonies were inoculated from brain heart infusion agar (CM0375; Oxoid Ltd., United Kingdom) into brain heart infusion broth (CM0225; Oxoid Ltd., United Kingdom). Frozen C. jejuni cells were inoculated on Campylobacter blood-free selective agar plates (CM0739; Oxoid Ltd., United Kingdom). Inoculated plates were incubated at 37°C for 48 h in a microaerophilic atmosphere using an anaerobic gas generating kit (BR0038B; Oxoid Ltd., United Kingdom). One colony from an incubated plate was transferred to Brucella broth (B3051; Sigma, United Kingdom) and incubated as noted above with shaking.
Bacterial suspensions in stationary phase were harvested by centrifugation at 2,000 × g for 10 min. Pelleted bacteria were suspended in predistilled Milli-Q water. Centrifugation and suspension were repeated three times to facilitate complete removal of the growth medium. Finally, the pellet was resuspended in sterile predistilled Milli-Q filtered water to a final concentration of approximately 106 CFU/ml. The upper range of fecal contamination in the highly contaminated waters of the Kenyan Rift Valley is 106 CFU/ml with an average population of 8.6 × 103 (95% confidence limit of 5.3 × 101 to 1.2 × 104) CFU/ml (17). This value included all fecal bacteria regardless of virulence or origin. We wished to simulate the worst-case scenario in this study and thus used a bacterial concentration of 106 CFU/ml in most cases.
Bacterial enumeration technique.
Samples were serially diluted in sterile distilled water and plated on their respective media using the Miles and Misra drop count technique (25). In this study 20 μl of the approximately diluted sample was dropped onto a sterile agar plate in triplicate. Agar plates were completely free of surface moisture prior to plating of C. jejuni to avoid swarming. Plates were incubated at the optimal growth temperature for each species and counted the next day. In the case of Y. enterocolitica and C. jejuni the plates were counted after 2 days. Only those plates which produced discrete colonies in the drop area, preferably fewer than 40 colonies per drop, were selected and counted. The count was divided by the number of drops, multiplied by 50 to convert to 1 ml, and then divided by the dilution itself to give the number of CFU/ml. The detection limit of this technique was 17 CFU/ml. Antibacterial efficiency was evaluated by calculating the absolute value of log10N/N0, where N was the residual bacterial count after treatment and N0 was the initial count in CFU/ml added to the sample prior to treatment.
Spore preparation.
B. subtilis (ATCC 6633) was reactivated from frozen stock (15% glycerinated Trypticase soy broth) in a 100-ml flask containing 60 ml of Trypticase soy broth (Biolife) and was incubated at 37°C for 24 h under aerobic conditions with a magnetic stirrer, thus obtaining vegetative bacteria in the stationary phase. Afterwards, dilutions of up to 10−2 were prepared using phosphate buffer containing MnCl2 (20 mg/liter). The surface of the culture medium was seeded with 1 ml of the 10−2 dilution in preparation for sporulation (Trypticase soy agar [TSA] enriched with 0.25 mg/liter MgSO4) and then subjected to 37°C for 15 days for growing and sporulation. In order to obtain the spore inocula, the surface of TSA (sporulation medium) was rinsed with sterile water and scraped with a spatula. Five rinsing procedures were carried out in 50-ml tubes: the first rinse was made with 1% Tween 80 (1% Tween 80; Merck, Germany) and the next four were made with distilled water, at 12,000 rpm for 5 min at ambient temperature.
A quantity of spore suspension (200 ml) was poured into sterile 500-ml Erlenmeyer flasks and placed in a water bath at 80°C for 15 min in order to eliminate vegetative cells (10, 16). Afterwards they were kept at 4°C for subsequent bottle inoculation. Optical density of the cell suspension was measured with a spectrophotometer at a 546-nm wavelength. Sporulation was confirmed by phase-contrast microscopy using malachite green to confirm the presence of endospores and counterstaining with safranin to show the presence of vegetative cells. The described procedure resulted in suspensions with a spore concentration of 3.9 × 108 CFU/ml.
SODIS using natural sunlight. (i) Bolivia.
SODIS of B. subtilis endospores using real sunlight was carried out in the Center for Water and Environmental Sanitation at San Simon University in Cochabamba, in the central part of Bolivia (latitude, 17°27′S; longitude, 66°09′W; altitude, 2,500 m). Spring water was used in the B. subtilis studies because these formed part of a much larger public health study which was investigating several other waterborne species. At times as many as seven or eight different sets of samples were exposed simultaneously, requiring on some occasions as much as 300 liters of water to be available. It was not feasible to autoclave such large volumes of water in situ beforehand. Consequently the decision was made to use SODIS on the starting water. Untreated groundwater was obtained (from a depth of 50 m) from a company producing bottled water (Chacaltaya and Co., Cochabamba, Bolivia). Polyethylene terephthalate (PET) bottles of 2 liters in volume were filled with this water and transported to the laboratory 3 days before each assay. These bottles were placed on the roof of the laboratory, in full sunshine, on a sheet of corrugated iron inclined at 22° to the horizontal and facing due north for 48 h in order to remove any naturally occurring bacteria or other organisms which might interfere with the assays. Absence of total coliform, fecal coliform, and heterotrophic bacteria from the samples prior to the start of the B. subtilis studies was confirmed using standard plate count techniques (7). Subsequently, each bottle was inoculated with 1 ml of the cell suspension, giving an initial population density of 2.8 × 107 CFU/ml. Bottles were stored overnight to allow the endospores to acclimate to the conditions. Test bottles were exposed to sunshine on the roof, while the control bottles were kept in the dark at ambient temperature. Both control and test assays were prepared in duplicate. After solar exposure, the bottles were stored at room temperature (22°C) in the dark. After 48 h the water in each bottle was tested for regrowth using the plating techniques described previously. During the 2 days of the assays, microbiological population, water temperature, and radiation (global and UVA) measurements were taken at regular intervals during daylight hours. Radiation was measured using a pyranometer (LI DataLogger; Licor, Lincoln, NE). The weather conditions were mostly sunny; the accumulated global irradiation dose over the 2 days varied between 31.1 MJ m−2 and 48.8 MJ m−2. The accumulated global irradiation dose is the global energy per unit of surface area received. The maximum local-noon irradiance recorded in this time was 1,059 W m−2, and samples were exposed for a maximum of 8 h in each day.
(ii) Spain.
SODIS of the nonsporulating bacteria took place under real sunlight conditions on the roof of the laboratory at the Plataforma Solar de Almería solar research facility in Almería, in southern Spain (latitude, 37°05′N; longitude, 2°21′W; altitude, 500 m). The UV radiation measured is from 295 to 385 nm (UVA and UVB) (total UV radiometer; Eppley Laboratories, RI). Placed horizontally, the radiometer measures all incoming UV radiation (direct plus diffuse radiation from all directions). Measurements of global solar exposure were made using a CMP 21 pyranometer (Kipp and Zonen, The Netherlands) for shortwave global solar radiation measurements in the spectral range from 310 nm to 2,800 nm during the experiments.
Volumes of 1,500 ml of the bacterial cell preparation were placed in cleaned and rinsed 1,500-ml PET bottles. Samples were shaken vigorously by hand for 10 seconds to ensure maximum aeration before being sealed and left to stand for 15 min prior to solar exposure to let cells adapt to the water. All samples were placed on the roof of the laboratory, in full sunshine. During the experiments water temperature was monitored. The weather conditions during these studies were sunny and cloudless; the average daily accumulated global irradiation dose was 30.6 MJ m−2 (standard deviation, 2.4 MJ m−2). The maximum local-noon irradiance recorded in this time was 1,041 W m−2. In total each container was exposed to approximately 8 h of sunshine.
Modeling with GInaFiT.
The results of the SODIS experiments were analyzed using the Geeraerd and Van Impe Inactivation Model Fitting Tool (GInaFiT) (14). This model has been used previously for the fitting of solar (1) and photocatalytic disinfection (34, 35) studies. The following models were used: log-linear regression (2), log-linear plus tail (13), Weibull model (22), biphasic model (5), and Weibull model (14). All models were applied to each inactivation curve, and the values of the root mean sum of squared errors were compared. The model with the smallest root mean sum of squared errors was considered the best fit for the respective inactivation curve. T90 and F90 values (time and fluence to reduce plate counts by 90%, respectively) were calculated using the best-fit model of GInaFiT.
Comparison of variables was performed using an unpaired t test or a two-way analysis of variance (InStat; Graphpad, San Diego, CA), depending on the number of variables examined.
RESULTS
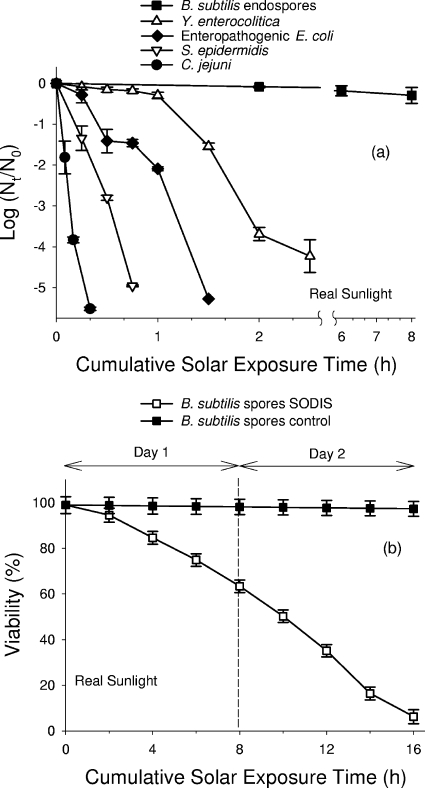
The sensitivity to natural-sunlight SODIS of the five bacterial species reported in this study is shown in Fig. 1a. The T90, F90, and F99 for each of the bacterial species were calculated from the best fit curves using GInaFiT (Table 1). The fluence values were calculated based on the wavelength between 295 and 385 nm in order to compare the experiments with each other. No reduction in population was detected for any of the control samples of the species examined (data not included but available on request). The order of sensitivity (from most sensitive to least sensitive) to batch-process SODIS was: C. jejuni > S. epidermidis > EPEC > Y. enterocolitica > B. subtilis endospores. In this study E. coli was the most resistant to the treatment; although it had a smaller T90, it required a larger amount of UV to achieve the same level of inactivation as those for the other bacteria (Table 1). One-way analysis of variance was performed on the results and revealed that the differences with regard to T90 were extremely significant (P < 0.001) between all populations, except in the case of Campylobacter jejuni and S. epidermidis, where the difference is significant (P < 0.05). In relation to fluence, no statistical difference was found between Yersinia enterocolitica and E. coli at F90 but there was a highly significant difference at F99 (P < 0.001). The table includes only the planktonic cells as it was difficult to obtain such data for the endospores due to the level of inactivation achieved. Complete inactivation (i.e., reductions greater than 4 orders of magnitude and final population below the limit of detection, which is 17 CFU/ml) was achieved within 3 h of exposure to sunlight for all the species studied except B. subtilis endospores, which experienced a reduction of only approximately 0.5 log unit by the end of the first day's exposure of eight consecutive hours. These B. subtilis samples were reexposed on the following day. After a cumulative exposure time of 16 h of natural sunlight (Fig. 1b), the maximum reduction observed for the endospores of B. subtilis was 96.3% (standard error, 3.0%), which corresponds to a 1.3-log-unit reduction for a cumulative global received dose of 79.9 MJ m−2.
FIG. 1.
(a) Inactivation kinetics of bacterial populations exposed to real sunlight conditions expressed in units of log reduction. (b) Inactivation kinetics of endospores of Bacillus subtilis samples exposed to real sunlight over two consecutive days and expressed in units of percent viability. Error bars indicate standard errors of triplicate measurements.
TABLE 1.
T90, F90, and F99 values of four different enterobacterial speciesa
| Strain | T90 (min) | F90 (kJ/m2) | F99 (kJ/m2) |
|---|---|---|---|
| C. jejuni NTCT 11168c | 2.1 ± 1.2 | 7.0 ± 3.0 | 14.5 ± 3.8 |
| E. coli O157b | 33.4 ± 3.7 | 78.7 ± 1.2 | 125.6 ± 7.8 |
| S. epidermidis RP62Ab | 12.0 ± 3.4 | 31.5 ± 9.2 | 52.9 ± 6.3 |
| Y. enterocolitica WA314 serotype O:8b | 78.6 ± 4.1 | 72.8 ± 1.2 | 89.9 ± 2.4 |
T90, time until 90% of the population is inactivated from sunlight exposure; F90 and F99, fluence until 90 and 99%, respectively, of the population is inactivated from sunlight exposure. The values, shown as means ± standard deviations, were derived from best-fit curves modeled with the program GInaFiT. Standard deviations were calculated from three independent measurements. Fluence was calculated from solar irradiation data for the wavelength range between 295 and 385 nm. GInaFiT fitted curves were used to obtain data.
Log linear and shoulder curves.
Linear curves.
DISCUSSION
A complete range of microbial sensitivities to SODIS is reported in this study. Neither Y. enterocolitica nor C. jejuni has been previously reported to be challenged by SODIS. C. jejuni was the most susceptible to the treatment (T90, 2.1 min), as it is microaerophilic and would theoretically be more susceptible to oxidative stresses. A previous study (30) reports that the pathogen is extremely sensitive to disinfection under simulated sunlight using a UVB irradiance of approximately 0.75 W m−2, which was comparable with a sunny day in Lancaster, United Kingdom (54°N). Y. entercolitica appears to be more resistant to the SODIS treatment, with a T90 of 78.6 min. Butler et al. in 1987 (4) exposed Y. entercolitica serotype O:3 and clinical isolates of C. jejuni and E. coli to UVC radiation and observed a 99.9% inactivation of C. jejuni, Y. enterocolitica, and E. coli at UVC doses of 18, 27, and 50 J/m2, respectively. S. epidermidis was included in this study for comparative reasons as it has a T90 of 12 (±3.4) min. Despite the difference in cell wall composition, this species was more susceptible to radiation than were the gram-negative bacteria Y. enterocolitica and EPEC. A study by Khaengraeng and Reed (18) used a nontoxigenic strain of E. coli O157 and simulated sunlight; using aerobic recovery methods, their T90 inactivation was 38 min, which compares well with this study's inactivation time of 33 min.
For all SODIS experiments performed in this study, where the treatment was effective, no regrowth was observed after 48 h, which is in good agreement with the study by Berney et al. (1) (5 days posttreatment).
Direct comparisons between experiments carried out in different locations under different local conditions are fraught with difficulties. Global irradiances in Spain and Bolivia were recorded using photometric apparatus of differing spectral sensitivities. Consequently, even though all experiments were conducted under conditions of strong sunshine and the observed maximum irradiances recorded for each location were similar (1,040 W m−2 and 1,059 W m−2), the comparisons were made using exposure time rather than received global dose. The Spanish group used 1.5-liter PET containers while the Bolivian group preferred 2-liter PET bottles. Smith et al. reported (36) that there was no significant difference between inactivation rates between samples exposed in 0.5-liter and 1.5-liter bottles for E. coli. It is therefore not unreasonable to expect minimal difference in inactivation kinetics for samples of 1.5-liter and 2-liter volumes.
It is clear from recent contributions of Sichel et al. (34) and Rincón and Pulgarin (32) that the presence of ions and dissolved organic matter in the water of the bacterial suspension has important consequences for the efficacy of the inactivation process. It is reasonable to expect the chemical and trace ion signature of the natural spring water used by the Bolivian group to differ from the Milli-Q water used by the Spanish group. Germination of dormant B. subtilis endospores can be triggered by elevated (but nonlethal) temperatures, the presence of organic molecules in the surrounding environment, or exposure to strong oxidizing agents (6, 11)—all of which could be expected to occur during SODIS under the conditions prevalent in the Bolivian portion of this study.
Despite the 5-log-unit reduction in population, a possible concern is the survival of a small population of viable bacteria below the detection limit (17 CFU/ml) for the experiments described. It is mathematically possible that ∼2,550 viable cells could remain in the 1.5-liter SODIS container and still escape detection. However, the infectious doses for Y. enterocolitica (31) and EPEC (26) at approximately 106 and 108 to 1010 cells, respectively, are at least 3 orders of magnitude higher than the proposed residual viable population. The infectious dose for C. jejuni is reported to be only 500 cells (33). C. jejuni appears more susceptible to SODIS treatment than any previously reported microbiological species, with a 5-log-unit reduction from the starting population to below the detection limit occurring after only 20 min of exposure to full sunlight. The standard SODIS exposure protocol recommends a further 5 h 40 min of full sunlight. Previous studies have also demonstrated that cells that remain viable after incomplete SODIS are much less infective than are unexposed cells (24, 36). Furthermore, no regrowth was detected in the exposed samples after 48 h. Consequently it is unlikely that a residual viable C. jejuni population either exists or poses a significant risk to the end user.
No significant germination or activation of the dormant spores was observed. As might be expected, the endospore stage of B. subtilis was quite resistant to the inactivating effects of the hostile environment established within the SODIS batch reactor during prolonged exposure to strong natural sunlight. The outer membrane of vegetative bacterial species, however, is less capable of withstanding such conditions, and hence, we observe their rapid inactivation under similar circumstances. In addition to the optical and oxidative conditions to which the cells were exposed, maximum water temperatures within the water samples during exposure between 28°C and 39°C were recorded in both Spain and Bolivia. In light of the well-documented resistance of B. subtilis to heat and pressure sterilization (23), it is remarkable that SODIS was able to inactivate as much as ∼96% of the B. subtilis endospores at all. These results are supported by the recent findings of Mendez-Hermida et al., whose SODIS studies of the highly resistant oocysts of the protozoan pathogen Cryptosporidium parvum showed that exposures over two consecutive days of strong sunshine were typically required for their complete inactivation (24). The real sunlight conditions used in the present study did not produce an endospore inactivation of magnitude equal to the 1.7-log-unit reduction previously reported by Lonnen et al. (21). A slightly lower simulated solar irradiance of 870 W m−2 was used by these researchers, compared to natural sunlight irradiance values of ∼1,040 W m−2 recorded in Almería for the current research. However, in the study by Lonnen et al., the simulated sunlight samples were maintained at a constant 40°C throughout the 8-h xenon arc light exposure, rather than the minimum-maximum-minimum temperature profile experienced by the samples exposed to natural sunlight. Typically in our studies water temperatures would start around 22°C and peak at 38°C for only a short period of about 2 h after the sun had achieved its zenith. This is likely to be the basis of the observed disparity between simulated and natural sunlight results. On the basis of field measurements obtained for 2-liter reactor volumes exposed to natural sunlight (in both Spain and Bolivia), at least 4 h is required for water samples to go from their starting temperature of ∼20°C to maximum temperature (usually between 35°C and 40°C; data not included but available on request). For those SODIS research groups without the benefit of reliable strong natural sunlight who must depend on solar simulation protocols, we would advise that water temperature be adjusted regularly to follow a profile obtained from realistic field experiments such as those, for example, reported by Joyce et al. (17).
In conclusion, we report for the first time the results of a study of SODIS using natural sunlight, for a range of microbial species, some of which are important waterborne diarrheal pathogens. SODIS is an effective and appropriate intervention against the previously untested diarrheal pathogens EPEC, C. jejuni, and Y. enterocolitica. Also the limits of SODIS have been clearly delineated. While bacterial species in their vegetative state are easily inactivated, those microbial species that form spores to survive in unfavorable environments are less sensitive to the harsh optical, thermal, and oxidative conditions established within the batch reactors during SODIS. SODIS is observed to inactivate these waterborne pathogens under real sunlight conditions and is an appropriate short-term emergency intervention against waterborne disease until more-permanent solutions can be put in place. Finally, simulated SODIS studies may be overestimating the degree of inactivation if the water temperature is maintained at a constant maximum temperature rather than following a natural temperature profile that one expects to observe for fixed volumes exposed to natural sunlight.
Acknowledgments
The research in Spain was funded by the Irish Health Research Board (grant no. NS-2003-07), the European Union (grant no. FP6-2004-INCO-DEV-3-031650-SODISWATER), and the Short Term Study Mission Programme of the European Science Foundation COST Action P9. The research in Bolivia was funded by the Swiss Federal Institute for Aquatic Science and Technology (EAWAG/SANDEC).
Footnotes
Published ahead of print on 21 March 2008.
REFERENCES
- 1.Berney, M., H. U. Weilenmann, A. Simonetti, and T. Egli. 2006. Efficacy of solar disinfection of Escherichia coli, Shigella flexneri, Salmonella typhimurium and Vibrio cholerae. J. Appl. Microbiol. 101:828-836. [DOI] [PubMed] [Google Scholar]
- 2.Bigelow, W. D. 1920. The thermal death point in relation to typical thermophilic organisms. J. Infect. Dis. 27:602-617. [Google Scholar]
- 3.Blaser, M. J., D. N. Taylor, and R. A. Feldman. 1983. Epidemiology of Campylobacter jejuni infections. Epidemiol. Rev. 5:157-176. [DOI] [PubMed] [Google Scholar]
- 4.Butler, R. C., V. Lund, and D. A. Carlson. 1987. Susceptibility of Campylobacter jejuni and Yersinia enterocolitica to UV radiation. Appl. Environ. Microbiol. 53:375-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerf, O., and F. Metro. 1977. Tailing of survival curves of Bacillus licheniformis spores treated with hydrogen peroxide. J. Appl. Bacteriol. 42:405-415. [DOI] [PubMed] [Google Scholar]
- 6.Chada, V. G., E. A. Sanstad, R. Wang, and A. Driks. 2003. Morphogenesis of Bacillus spore surfaces. J. Bacteriol. 185:6255-6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC.
- 8.Coker, A. O., R. D. Isokpehi, B. N. Thomas, K. O. Amisu, and C. L. Obi. 2002. Human campylobacteriosis in developing countries. Emerg. Infect. Dis. 8:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conroy, R. M., M. Elmore-Meegan, T. Joyce, K. G. McGuigan, and J. Barnes. 1996. Solar disinfection of drinking water and diarrhoea in Maasai children: a controlled field trial. Lancet 348:1695-1697. [DOI] [PubMed] [Google Scholar]
- 10.Driedger, A., E. Staub, U. Pinkernell, B. Marinas, W. Koster, and U. Von Gunten. 2001. Inactivation of Bacillus subtilis spores and formation of bromate during ozonation. Water Res. 35:2950-2960. [DOI] [PubMed] [Google Scholar]
- 11.Enright, M. R., J. O. McInerney, and C. T. Griffin. 2003. Characterization of endospore-forming bacteria associated with entomopathogenic nematodes, Heterorhabditis spp., and description of Paenibacillus nematophilus sp. nov. Int. J. Syst. Evol. Microbiol. 53:435-441. [DOI] [PubMed] [Google Scholar]
- 12.Frost, J. A., I. A. Gillespie, and S. J. O'Brien. 2002. Public health implications of Campylobacter outbreaks in England and Wales, 1995-9: epidemiological and microbiological investigations. Epidemiol. Infect. 128:111-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geeraerd, A. H., C. H. Herremans, and J. F. Van Impe. 2000. Structural model requirements to describe microbial inactivation during a mild heat treatment. Int. J. Food Microbiol. 59:185-209. [DOI] [PubMed] [Google Scholar]
- 14.Geeraerd, A. H., V. P. Valdramidis, and J. F. Van Impe. 2005. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 102:95-105. [DOI] [PubMed] [Google Scholar]
- 15.Heaselgrave, W., N. Patel, S. C. Kehoe, S. Kilvington, and K. G. McGuigan. 2006. Solar disinfection of poliovirus and Acanthamoeba polyphaga cysts in water—a laboratory study using simulated sunlight. Lett. Appl. Microbiol. 43:125-130. [DOI] [PubMed] [Google Scholar]
- 16.Huertas, A., B. Barbeau, C. Desjardins, A. Galarza, M. A. Figueroa, and G. A. Toranzos. 2003. Evaluation of Bacillus subtilis and coliphage MS2 as indicators of advanced water treatment efficiency. Water Sci. Technol. 47(3):255-259. [PubMed] [Google Scholar]
- 17.Joyce, T. M., K. G. McGuigan, M. Elmore-Meegan, and R. M. Conroy. 1996. Inactivation of fecal bacteria in drinking water by solar heating. Appl. Environ. Microbiol. 62:399-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khaengraeng, R., and R. H. Reed. 2005. Oxygen and photoinactivation of Escherichia coli in UVA and sunlight. J. Appl. Microbiol. 99:39-50. [DOI] [PubMed] [Google Scholar]
- 19.Koenraad, P., F. M. Rombouts, and S. H. W. Notermans. 2005. Epidemiological aspects of thermophilic Campylobacter in water-related environments: a review. Water Environ. Res. 69:52-63. [Google Scholar]
- 20.Leclerc, H., L. Schwartzbrod, and E. Dei-Cas. 2002. Microbial agents associated with waterborne diseases. Crit. Rev. Microbiol. 28:371-409. [DOI] [PubMed] [Google Scholar]
- 21.Lonnen, J., S. Kilvington, S. C. Kehoe, F. Al-Touati, and K. G. McGuigan. 2005. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Water Res. 39:877-883. [DOI] [PubMed] [Google Scholar]
- 22.Mafart, P., O. Couvert, S. Gaillard, and I. Leguerinel. 2002. On calculating sterility in thermal preservation methods: application of the Weibull frequency distribution model. Int. J. Food Microbiol. 72:107-113. [DOI] [PubMed] [Google Scholar]
- 23.Margosch, D., M. G. Ganzle, M. A. Ehrmann, and R. F. Vogel. 2004. Pressure inactivation of Bacillus endospores. Appl. Environ. Microbiol. 70:7321-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendez-Hermida, F., E. Ares-Mazas, K. G. McGuigan, M. Boyle, C. Sichel, and P. Fernandez-Ibanez. 2007. Disinfection of drinking water contaminated with Cryptosporidium parvum oocysts under natural sunlight and using the photocatalyst TiO2. J. Photochem. Photobiol. B Biol. 88:105-111. [DOI] [PubMed] [Google Scholar]
- 25.Miles, A. A., and S. S. Misra. 1938. The estimation of the bactericidal power of the blood. J. Hyg. 38:732-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naylor, S. W., A. J. Roe, P. Nart, K. Spears, D. G. Smith, J. C. Low, and D. L. Gally. 2005. Escherichia coli O157: H7 forms attaching and effacing lesions at the terminal rectum of cattle and colonization requires the LEE4 operon. Microbiology 151:2773-2781. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson, W. L., and P. Fajardo-Cavazos. 1997. DNA repair and the UV resistance of bacterial spores: from the laboratory to the environment. Recent Res. Dev. Microbiol. 1:125-140. [Google Scholar]
- 29.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Obiri-Danso, K., N. Paul, and K. Jones. 2001. The effects of UVB and temperature on the survival of natural populations and pure cultures of Campylobacter jejuni, Camp. coli, Camp. lari and urease-positive thermophilic campylobacters (UPTC) in surface waters. J. Appl. Microbiol. 90:256-267. [DOI] [PubMed] [Google Scholar]
- 31.Public Health Agency of Canada: Office of Laboratory Safety. 1 January 2007, posting date. Material safety data sheet: Yersinia enterocolitica. http://www.phac-aspc.gc.ca/msds-ftss/msds168e.html.
- 32.Rincon, A.-G., and C. Pulgarin. 2004. Effect of pH, inorganic ions, organic matter and H2O2 on E. coli K12 photocatalytic inactivation by TiO2: implications in solar water disinfection. Appl. Catal. B Environ. 51:283. [Google Scholar]
- 33.Robinson, D. A. 1981. Infective dose of Campylobacter jejuni in milk. BMJ 282:1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sichel, C., J. Blanco, S. Malato, and P. Fernández-Ibáñez. 2007. Effects of experimental conditions on E. coli survival during solar photocatalytic water disinfection. J. Photochem. Photobiol. B Chem. 189:139-246. [Google Scholar]
- 35.Sichel, C., M. de Cara, J. Tello, J. Blanco, and P. Fernández-Ibáñez. 2007. Solar photocatalytic disinfection of agricultural pathogenic fungi: Fusarium species. Appl. Catal. B Environ. 74:152-160. [Google Scholar]
- 36.Smith, R. J., S. C. Kehoe, K. G. McGuigan, and M. R. Barer. 2000. Effects of simulated solar disinfection of water on infectivity of Salmonella typhimurium. Lett. Appl. Microbiol. 31:284-288. [DOI] [PubMed] [Google Scholar]
- 37.Snelling, W. J., M. Matsuda, J. E. Moore, and J. S. G. Dooley. 2005. Campylobacter jejuni. Lett. Appl. Microbiol. 41:297-302. [DOI] [PubMed] [Google Scholar]
- 38.Spears, K. J., A. J. Roe, and D. L. Gally. 2006. A comparison of enteropathogenic and enterohaemorrhagic Escherichia coli pathogenesis. FEMS Microbiol. Lett. 255:187-202. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, D. N., K. T. McDermott, J. R. Little, J. G. Wells, and M. J. Blaser. 1983. Campylobacter enteritis from untreated water in the Rocky Mountains. Ann. Intern. Med. 99:38-40. [DOI] [PubMed] [Google Scholar]
- 40.Tersieva, S. I., and G. A. McFeters. 1991. Survival and injury of E. coli, C. jejuni and Y. enterocolitica in stream water. Can. J. Microbiol. 37:785-790. [DOI] [PubMed] [Google Scholar]
- 41.Trabulsi, L. R., R. Keller, and T. A. Tardelli Gomes. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8:508-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Younes, M., and J. Bartram. 2001. Waterborne health risks and the WHO perspective. Int. J. Hyg. Environ. Health 204:255-263. [DOI] [PubMed] [Google Scholar]