Abstract
Exoelectrogenic bacteria have potential for many different biotechnology applications due to their ability to transfer electrons outside the cell to insoluble electron acceptors, such as metal oxides or the anodes of microbial fuel cells (MFCs). Very few exoelectrogens have been directly isolated from MFCs, and all of these organisms have been obtained by techniques that potentially restrict the diversity of exoelectrogenic bacteria. A special U-tube-shaped MFC was therefore developed to enrich exoelectrogenic bacteria with isolation based on dilution-to-extinction methods. Using this device, we obtained a pure culture identified as Ochrobactrum anthropi YZ-1 based on 16S rRNA gene sequencing and physiological and biochemical characterization. Strain YZ-1 was unable to respire using hydrous Fe(III) oxide but produced 89 mW/m2 using acetate as the electron donor in the U-tube MFC. Strain YZ-1 produced current using a wide range of substrates, including acetate, lactate, propionate, butyrate, glucose, sucrose, cellobiose, glycerol, and ethanol. Like another exoelectrogenic bacterium (Pseudomonas aeruginosa), O. anthropi is an opportunistic pathogen, suggesting that electrogenesis should be explored as a characteristic that confers advantages to these types of pathogenic bacteria. Further applications of this new U-tube MFC system should provide a method for obtaining additional exoelectrogenic microorganisms that do not necessarily require metal oxides for cell respiration.
Electricity generation in a mediatorless microbial fuel cell (MFC) is linked to the ability of certain bacteria, called exoelectrogens (“exo-” for exocellular and “electrogens” for the ability to transfer electrons to insoluble electron acceptors), to transfer electrons outside the cell to the anode in an MFC (23). Different genetic groups of bacteria have shown exoelectrogenic activity in MFCs, including β-Proteobacteria (Rhodoferax) (8), γ-Proteobacteria (Shewanella and Pseudomonas) (17, 18, 36), δ-Proteobacteria (Aeromonas, Geobacter, Geopsychrobacter, Desulfuromonas, and Desulfobulbus) (2, 3, 14, 15, 35), Firmicutes (Clostridium) (34), and Acidobacteria (Geothrix) (4). The mechanisms used for exocellular transport of electrons by these bacteria are still being studied. It has been demonstrated that cell-bound outer membrane cytochromes and conductive pili (nanowires) may play a key role in electron transfer for some Geobacter and Shewanella species (12, 27, 31, 37). Alternatively, some exoelectrogens, such as Pseudomonas aeruginosa (36) and Geothrix fermentans (4), excrete mediators to shuttle electrons to surfaces.
Many of the exoelectrogens that produce current in an MFC are dissimilatory metal-reducing bacteria (DMRBs) that were originally isolated based on their ability to reduce insoluble metals, such as Fe(III) or Mn(IV) oxides, in the natural environment (23, 25, 26). The mechanisms for electron transfer to metal oxides were originally assumed to be identical to those for electricity generation (26, 35). However, some new evidence suggests that the mechanisms for electron transfer to metal oxides and to MFC anodes are not always the same. Shewanella oneidensis MR-1 is an exoelectrogen capable of both electricity production and Fe(III) oxide reduction. Two mutants of S. oneidensis MR-1 (SO4144 and SO4572) were recently shown to be able to produce electricity but to have lost the ability to reduce Fe(III) oxide (5). Pelobacter carbinolicus was similarly found to be capable of Fe(III) reduction but was unable to produce current in an MFC (39). These results suggest that different genes may be involved in electron transfer to metal solids and in electron transfer to graphite electrodes.
Few exoelectrogens have been directly isolated from MFCs, and all of the previous methods used conventional plating techniques. However, the use of agar plates is not a selective method for electricity-producing bacteria. Clostridium butyricum and Aeromonas hydrophila were the first two microorganisms isolated from MFC anodes by plating with soluble Fe(III) citrate (34) or Fe(III) pyrophosphate (35). By using insoluble Fe(III) oxide as the electron acceptor, Geopsychrobacter electrodiphilus was isolated from a marine sediment fuel cell (15). Although these organisms have shown electricity generation in MFCs, Fe(III) plating methods eliminate the growth and isolation of other exoelectrogens that may not be able to respire with iron on the plates. General nutrient agar plates were also used for isolation of exoelectrogens from MFCs under aerobic and anaerobic conditions (36), but this method allowed nonselective growth of nonexoelectrogenic bacteria, making it difficult to choose which colonies should be used in further studies. Therefore, the current isolation methods used to obtain electricity-producing bacteria by plating are indirect and potentially biased, and they may not allow identification of the true diversity of the exoelectrogens functioning in MFCs.
In order to better understand the characteristics of bacteria capable of exoelectrogenic activity in MFCs, we developed a new method to enrich and isolate these bacteria that is independent of the need for metal oxide reduction. Our new device, called a U-tube MFC, was constructed to allow bacteria in suspension to directly settle on the anode, making it theoretically possible to eventually produce current from the initial growth of a single cell. Through repeated dilution to extinction, we showed that this U-tube MFC can be used to directly isolate exoelectrogens according to their electricity-generating ability and not their ability to reduce iron. This approach allows us to enrich and isolate additional exoelectrogenic strains that might not be obtainable by conventional plating techniques.
MATERIALS AND METHODS
U-tube MFC construction.
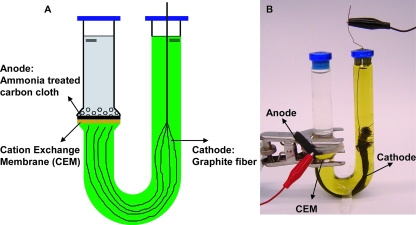
The U-tube MFC was constructed from a straight tube that formed the anode chamber (10 ml) and a U-shaped tube for the cathode chamber (30 ml). The two chambers were separated by a cation-exchange membrane (CEM) (1.77 cm2; CMI 7000; Membranes International Inc., United States) and were joined together by a C-type clamp (Fig. 1). Both the anode and cathode tubes were made from anaerobic culture tubes (Bellco Glass, United States) and sealed with butyl rubber stoppers. Placing the anode on the bottom of the vertically aligned anode chamber tube allowed bacteria to be readily deposited directly on the electrode surface. The U shape of the cathode chamber used hydrostatic pressure to keep the catholyte solution [100 mM K3Fe(CN)6 in a 100 mM phosphate buffer solution (PBS)] pressed against the cathode. Dissolved oxygen was not used as sparging would have resulted in gas collection on the cathode. The absence of oxygen in the cathode chamber was important during the growth of a small number of cells because oxygen could have diffused from the cathode chamber into the anode chamber through the membrane.
FIG. 1.
Schematic diagram (A) and photograph (B) of a U-tube MFC (with a carbon cloth anode and a graphite fiber cathode) used for isolation of an exoelectrogenic bacterium.
The anode was plain carbon cloth (type A; E-Tek, United States) pretreated using a high-temperature ammonia gas process (9). A piece of the anode (6-cm-long strip) extended outside the tube in order to make an electrical connection. The cathode was made of 15-cm-long plain graphite fibers (no. 292 carbon fiber tow; Fibre Glast, United States) wrapped at one end with a titanium wire, with the fiber bundle positioned close to the CEM (Fig. 1). The wire was extended through the top of the rubber stopper to complete the electrical connection. The graphite fiber cathode provided a much larger surface area (2,260 cm2) than the anode electrode (1.77 cm2) to reduce limitations on power generation by the cathode. The anode was connected to the cathode via a 1,000-Ω resistor, unless otherwise noted.
Isolation.
The initial inoculum was obtained from the anode of a single-chamber air-cathode cubic MFC (24) operated for more than 1 year (originally inoculated with primary clarifier overflow from a local wastewater treatment plant) fed with a PBS nutrient medium containing acetate (1 g/liter), NH4Cl (0.31 g/liter), KCl (0.13 g/liter), and metal salts (12.5 ml/liter) and vitamins (5 ml/liter) in a 50 mM PBS as described previously (22). The same medium with acetate (1 g/liter) was used in U-tube tests except as noted below for tests with different substrates. Standard anaerobic techniques were used throughout isolation procedures when possible. The medium was boiled under 1 atm of N2 before it was dispensed into anaerobic test tubes (Bellco Glass, United States) or the anode chamber of U-tube MFCs under N2. A ferricyanide solution was sparged with N2 and then put into the cathode chamber of the U-tube MFCs. All of the tubes and MFCs were sealed with rubber stoppers, crimped with aluminum caps, and sterilized by autoclaving. A piece (1 cm2) of the enriched anode from the single-chamber MFC was transferred to an anaerobic tube containing 10 ml of PBS (50 mM) and glass beads. The tube was vortexed, producing a suspension containing ∼3 × 108 cells/ml (measured by acridine orange direct counting using fluorescence microscopy). The cell suspension was then serially diluted in 10-fold steps to an end point dilution of 10−8 in anaerobic tubes. A sample (1 ml) from each tube was then transferred to the anode chamber of a U-tube MFC containing 9 ml PBS nutrient medium and acetate. A U-tube reactor without any cells (containing only sterile medium) was used as an uninoculated control.
The U-tube MFCs were incubated in a constant-temperature room at 30°C. Growth of exoelectrogens was monitored by determining current production. Electricity-producing cultures were incubated until the current peak was observed. The anode from the U-tube MFC containing the highest dilution that produced electricity was then transferred to an anaerobic tube containing sterile PBS, and the isolation procedure (vortexing and dilution) described above was repeated. Before reuse, the tubes were cleaned, reassembled, sterilized, and provided with a completely new carbon cloth anode, a new CEM, and a graphite fiber cathode. This procedure was repeated until the denaturing gradient gel electrophoresis (DGGE) profile used for community analysis showed a single band.
DNA extraction, PCR, DGGE, and sequence analysis.
One-half of the cell suspension extracted from each U-tube anode that represented the highest dilution with electricity production during each cycle was used to track community succession by DGGE. DNA was extracted using a PowerSoil DNA isolation kit (MO BIO Laboratories, United States) according to the manufacturer's instructions. A 16S rRNA gene fragment of the extracted DNA was then amplified by PCR using a 50-μl (total volume) mixture containing GoTaq Green Master Mix (Promega, United States), 1 μM of each primer, 100 ng of DNA template, and sterile deionized water (43a). For DGGE analysis, the primers used for PCR were GC968F (5′-CGCCCGCCGCGCCCCGCGCCCGGCCCGCCGCCCCCGCCCCAACGCGAAGAACCTTAC-3′) and 1401R (5′-CGGTGTGTACAAGACCC-3′) (38, 42). The samples were amplified using an iCycler iQ thermocycler (Bio-Rad Laboratories, United States) and the following thermal profile: 95°C for 4 min; 20 cycles of 30 s at 95°C, 30 s at 60°C, and 1 min at 72°C with a 0.1°C decrease in the annealing temperature per cycle to 58°C; 15 cycles of 30 s at 95°C, 30 s at 58°C, and 1 min at 72°C; and extension for 10 min at 72°C. DGGE was performed using a DCode universal mutation detection system (Bio-Rad Laboratories, United States) with a denaturing gradient ranging from 30 to 60% (100% corresponded to 7 M urea and 40% [vol/vol] deionized formamide). Electrophoresis was performed for 15 min at 30 V and for 13 h at 75 V at 60°C. The gels obtained were silver stained (38).
Once the DGGE analysis showed a single band, PCR was performed with primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1541R (5′-AAGGAGGTGATCCAGCC-3′) (43) to amplify the nearly complete bacterial 16S rRNA gene for sequencing the putative isolate. The DNA amplification was carried out under the following conditions: 95°C for 5 min; 35 cycles of 95°C for 1 min, 57°C for 30 s, and 72°C for 1.5 min; and finally 72°C for 7 min (43a). PCR products were purified with a QIAquick gel extraction kit (Qiagen, United States) and were ligated and cloned using a TOPO TA cloning kit (Invitrogen, United States) according to the manufacturer's instructions. Plasmids were isolated from randomly selected clone colonies with a QIAprep Spin Miniprep kit (Qiagen, United States), and nine plasmid inserts were then sequenced in both directions using an ABI 3730XL DNA sequencer (Applied Biosystems, United States) and found to be identical. The 16S rRNA gene sequence obtained was compared to the sequences of the most closely related strains in the GenBank database by using the BLAST program. A neighbor-joining phylogenetic tree was constructed using the Molecular Evolutionary Genetics Analysis package (MEGA, version 3) (20) with Kimura's two-parameter method (19). A bootstrap analysis was based on 1,000 resamplings.
Transmission and scanning electron microscopy.
For transmission electron microscope examination, a 5-μl cell suspension of the isolate was negatively stained using 2% aqueous uranyl acetate on a Formvar carbon-coated copper grid. The grid was air dried and then examined with a transmission electron microscope (JEM 1200 EXII; JEOL) at an accelerating voltage of 80 kV.
Selected MFC electrodes enriched with an isolate were examined using a scanning electron microscope. The electrode samples were fixed with 1.5% glutaraldehyde for 1 h and then postfixed with 1% osmium tetroxide for 30 min. After each fixation step, the samples were washed in 0.1 M cacodylate buffer three times. The fixed samples were dehydrated with ethanol and dried using a critical point drying process in liquid CO2 (BAL-TEC CPD030; Bal-Tec, United States). Samples were then sputter coated with Au/Pd and examined using a JSM 5400 scanning electron microscope (JEOL) at an accelerating voltage of 20 kV.
Physiological and biochemical characterization.
Carbon source utilization tests were performed with the isolate either using BIOLOG plates (Biolog Inc., United States) under aerobic conditions or using U-tube MFCs under anaerobic conditions. For all of the physiological and biochemical characterizations, the isolate was precultivated and enriched on Difco nutrient agar plates (for culturing nonfastidious microorganisms; BD Company, United States) or in Difco nutrient broth (BD Company, United States) at 30°C under aerobic conditions. Cells were washed three times with 50 mM PBS, and the concentration was adjusted to 5.0 × 108 ± 0.5 cells/ml (as determined by acridine orange direct counting) before tests. Duplicate BIOLOG GN2 MicroPlates containing 95 separate carbon sources were incubated with 150 μl of an isolate cell suspension in each well for 24 h at 30°C. To test the substrate utilization of the isolate for electricity production under anaerobic conditions, stationary-phase cultures of the isolate were inoculated (10/100, vol/vol) into U-tube MFCs containing propionate, butyrate, lactate, glucose, sucrose, cellobiose, ethanol, or glycerol (1 g/liter for all substrates) as the sole electron donor in 50 mM PBS nutrient medium (22), and the current production was measured using a 1,000-Ω resistor.
The denitrification activity of the isolate was determined in anaerobic tubes (in triplicate) containing 10 mM nitrate and 1 g/liter acetate at 30°C. One tube without cells was used as an abiotic control. Nitrate reduction was detected by the nitrite spot test using Griess reagents I and II [sulfanilamide and N-(1-naphthyl)-ethylenediamine dihydrochloride] and by measurement of the nitrate concentration at 620 nm with a Spectronic 20 spectrophotometer (Bausch and Lomb, United States).
The ability of cells to respire using hydrous ferric oxide (100 mM) (11) was investigated using 1 g/liter acetate in anaerobic tubes (in triplicate). One tube without cells was used as an abiotic control. All tubes were incubated at 30°C for 7 days. The reduction of Fe(III) was monitored using a ferrozine colorimetric method based on the production of HCl-extractable Fe(II) (28). Color changes in the ferrozine solution were measured at a fixed wavelength (562 nm).
Electricity production.
All U-tube MFCs were considered to be fully acclimated when the maximum voltage produced was repeated for at least three batch cycles. The reactor was refilled with fresh anode nutrient medium and cathode ferricyanide solution when the voltage dropped below ∼20 mV. The voltage (V) of U-tube MFC reactors was measured across a resistor using a data acquisition system (2700; Keithly, United States). Current (I) was calculated using the equation I = V/R, where R is resistance, and maximum current densities were normalized to the anode projected surface area. To obtain the polarization and power density curves and coulombic efficiency (CE) as a function of current, the external circuit resistance was varied from 250 to 5,000 Ω. One resistor was used for at least two separate full cycles of operation. The power (P = IV), power density (IV/Aan, where Aan is the anode surface area), and CE (defined as the fraction or percentage of electrons recovered as current in one batch cycle versus the total available electrons from the initial input substrate [e.g., 8 mol e− per mol acetate]) were calculated as previously described (44). For comparison of power densities achievable with this system, U tubes were inoculated with domestic wastewater (primary clarifier effluent) and then operated for multiple batch cycles until power generation was stable using the same substrate (acetate) and PBS nutrient medium.
Nucleotide sequence accession number.
The 16S rRNA gene sequence determined in this study has been deposited in the GenBank database under accession number EU275247.
RESULTS
U-tube isolation of exoelectrogens.
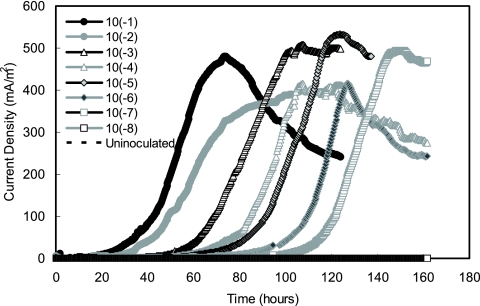
After the anode microorganisms from the acetate-enriched MFC were serially diluted and transferred to eight U-tube reactors containing acetate, current was produced with lag times increasing following the sequence of dilution (10−1 to 10−8). The lag phase ranged from 20 h long for the lowest dilution (10−1) to 100 h long for the second-highest dilution (10−7) (Fig. 2). The 10−8 dilution did not produce current after 160 h in this first round of dilutions. The lowest dilution (10−1) consistently produced the first current peak, and the highest current density, 481 mA/m2 (based on the anode area), was produced at ∼70 h. Within 150 h, all of the electricity-producing U tubes (10−1 to 10−7 dilutions) produced similarly high peak currents ranging from 412 to 532 mA/m2 (Fig. 2). Sequential current production was repeated for all eight of the dilution-to-extinction cycles, and no current was produced in the uninoculated control (abiotic) U-tube MFCs throughout the study.
FIG. 2.
Current generation (normalized to the anode surface area) as a function of time for eight sequential 10-fold dilutions (10−1 to 10−8) in eight U-tube MFC reactors using 1 g/liter acetate as the sole electron donor for the first cycle of dilution-to-extinction exoelectrogenic isolation of bacteria. A sterile U-tube system was used as an uninoculated control.
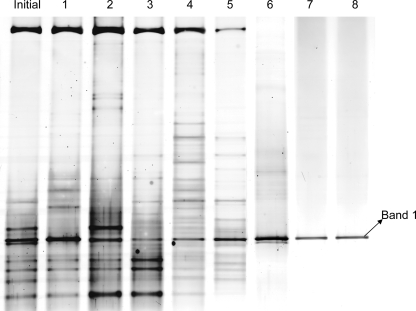
The DGGE profiles obtained over the eight enrichment and dilution cycles showed that the diversity of the microbial consortium significantly decreased after five isolation cycles (Fig. 3). Although the initial inoculum had relatively great genetic diversity based on the appearance of multiple bands, only a single band (band 1) remained after eight cycles, suggesting that only one bacterium was present in the system. The same band was observed for the other seven isolation cycles, indicating that one microbe accounted for a significant fraction of the community in all cycles.
FIG. 3.
DGGE profiles based on the 16S rRNA gene from the initial mixed culture and each of the highest cell dilutions capable of electricity production at the end of each cycle (cycles 1 to 8 [lanes 1 to 8, respectively]). The bacterial community dynamics indicated that strain YZ-1 (band 1) was isolated through successive cycles.
Sequence and phylogenic analysis.
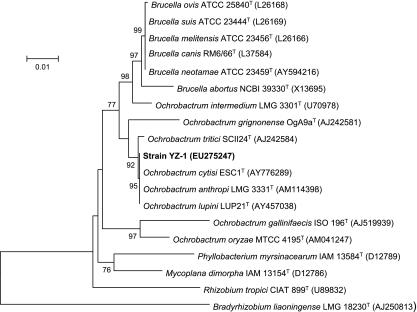
The nearly complete 16S rRNA gene sequence (1,446 nucleotides; GenBank accession number EU275247) of the isolate, designated strain YZ-1, was identical between positions 968 and 1401 (i.e., the target locations of primers 968F and 1401R, respectively) with the sequence obtained from band 1 of the DGGE gel. Phylogenetic analysis of 16S rRNA gene sequences of strain YZ-1 and closely related organisms in the GenBank database showed that strain YZ-1 belongs to the genus Ochrobactrum together with Ochrobactrum anthropi, Ochrobactrum cytisi, and Ochrobactrum lupini, with 100% identity to sequences from O. anthropi LMG3331T (= ATCC 49188T) and O. cytisi ESC1T (Fig. 4).
FIG. 4.
Phylogenetic tree of strain YZ-1 and closely related species based on 16S rRNA gene sequences. The tree was constructed using the neighbor-joining method. The numbers at nodes indicate the percentages of occurrence of the branching order in 1,000 bootstrapped trees for values greater than 50%. Scale bar = 1% divergence.
Physiological and biochemical characterization of YZ-1.
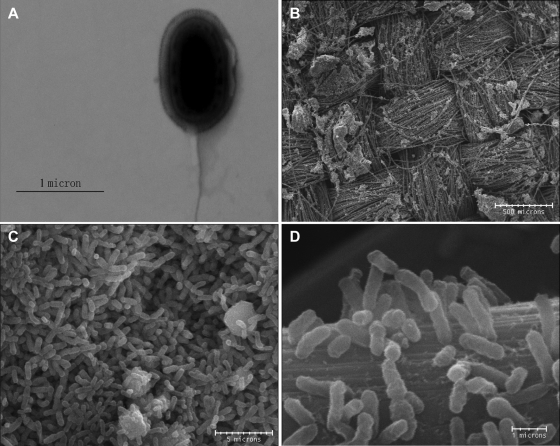
Strain YZ-1 is a gram-negative rod that is 1 to 1.5 μm long and 0.4 to 0.6 μm wide, and it is motile by means of a polar flagellum (Fig. 5A). Selected anodes enriched with strain YZ-1 were examined, and the results showed that cells formed a biofilm on the surface of carbon cloth fibers. In some places, cells colonized, formed multiple layers of biomass, and completely covered the electrode surface (Fig. 5B, C, and D).
FIG. 5.
Transmission electron micrograph of strain YZ-1 (A) and scanning electron micrographs of YZ-1 on the anode surface in U-tube MFC reactors fed with 1 g/liter acetate as the sole electron donor (B, C, and D). (B) Carbon cloth anode surface colonized by YZ-1 with cells attached. (C) Colony of YZ-1 completely covering the anode surface. (D) YZ-1 attached to a single carbon cloth fiber (D).
Carbon source versatility and nitrate reduction by strain YZ-1 were evaluated and compared to data reported previously for O. anthropi (13), O. cytisi (45), and O. lupini (41) (Table 1). Strain YZ-1 had more phenotypic characteristics of O. anthropi than of O. cytisi and O. lupini. Both strain YZ-1 and O. anthropi were able to reduce nitrate and aerobically utilize gluconate, galactose, d-fructose, acetate, propionate, butyrate, lactate, ethanol, glycerol, glucose, cellobiose, and sucrose, but they were unable to assimilate citrate (24 h), lactose, melibiose, and N-acetylglucosamine. Strain YZ-1 differed from O. cytisi in gluconate, citrate, and N-acetylglucosamine assimilation and differed from O. lupini in nitrate reduction and utilization of citrate, lactose, melibiose, galactose, d-fructose, turanose, N-acetylglucosamine, d-arabitol, glycerol, and cellobiose. Although strain YZ-1 showed exoelectrogenic activity by producing electricity in U-tube MFCs with acetate as the sole electron donor, it did not reduce iron oxide with the same carbon source. Strain YZ-1 was cultivated for 7 days at 30°C with acetate and poorly crystallized hydrous ferric oxide in anaerobic tubes. Samples were taken on days 1, 2, 3, and 7, but no detectable Fe(II) was found.
TABLE 1.
Physiological and morphological characteristics of strain YZ-1 and the most closely phylogenetically related species of the genus Ochrobactruma
| Characteristic | Strain YZ-1 | O. anthropi | O. cytisi | O. lupini |
|---|---|---|---|---|
| Cell length (μm) | 1-1.5 | ND | ND | 1.4-1.5 |
| Cell shape | Rod | Rod | Rod | Rod |
| Motility | Yes | Yes | Yes | No |
| Nitrate reduction | + | + | + | − |
| Use of carbon sources and electron donors | ||||
| Gluconate | + | + | − | + |
| Citrate (24 h) | − | − | + | + |
| Lactose | − | − | w | + |
| Melibiose | − | − | w | + |
| Galactose | + | + | w | − |
| d-Fructose | + | + | w | − |
| l-Arabinose | + | + | + | + |
| Turanose | + | + | + | − |
| N-Acetylglucosamine | − | −b | + | + |
| d-Arabitol | + | + | + | − |
| Acetate | + | + | ND | ND |
| Propionate | + | + | ND | ND |
| Butyrate | + | + | ND | ND |
| Lactate | + | + | ND | ND |
| Ethanol | + | + | ND | ND |
| Glycerol | + | + | w | − |
| Glucose | + | + | + | + |
| Cellobiose | + | + | w | − |
| Sucrose | + | + | + | ND |
The data for O. anthropi were obtained from reference 13, the data for O. lupini were obtained from reference 41, and the data for O. cytisi were obtained from reference 45. +, positive; −, negative; w, weak; ND, not done.
Data from this study for O. anthropi ATCC 49188T (= LMG 3331T), reported as positive by Holmes et al. (13).
Electricity production by strain YZ-1.
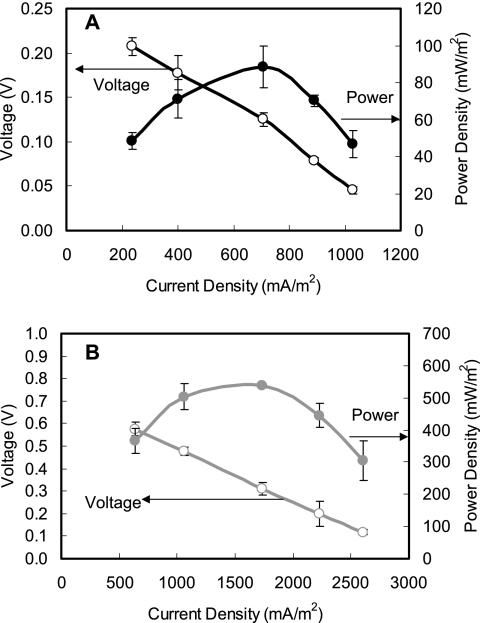
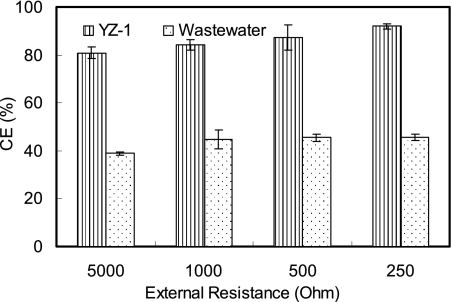
Electricity was rapidly generated in all U-tube reactors inoculated with strain YZ-1 within a few hours using acetate (1 g/liter) as the electron donor. After there was repeatable current production for at least three cycles, the electricity generation ability of strain YZ-1 was compared to that of a mixed-culture inoculum derived from domestic wastewater under the same conditions. Power density and polarization curves showed that YZ-1 produced less power than the mixed culture; the maximum power densities were 89 mW/m2 (at 1,000 Ω and 708 mA/m2) for strain YZ-1 and 539 mW/m2 (at 1,000 Ω and 1730 mA/m2) for the mixed culture (Fig. 6). However, strain YZ-1 showed a much higher electron recovery efficiency than the mixed culture. More than 80% of electrons from acetate were recovered as current using strain YZ-1 at 234 to 1,027 mA/m2, with a CE of 93% at 1,027 mA/m2. In contrast, only 39 to 46% of available electrons were recovered using the mixed culture over the current range from 639 to 2,603 mA/m2 (Fig. 7). O. anthropi type strain ATCC 49188 also generated electricity in the U-tube MFC, but the power produced (45 mW/m2, 1,000 Ω, and 502 mA/m2) was less than that obtained with strain YZ-1.
FIG. 6.
Power density (filled symbols) and voltage (open symbols) as a function of current density (normalized to the anode area) obtained by varying the external circuit resistance (250 to 5,000 Ω) for (A) strain YZ-1 and (B) domestic wastewater in U-tube MFCs using 1 g/liter acetate. The error bars indicate standard deviations based on averages measured during stable power output in two or more separate batch experiments.
FIG. 7.
CE obtained by varying the external circuit resistance (250 to 5,000 Ω) for strain YZ-1 and domestic wastewater in U-tube MFCs using 1 g/liter acetate. The error bars indicate standard deviations based on averages measured during stable power output in two or more separate batch experiments.
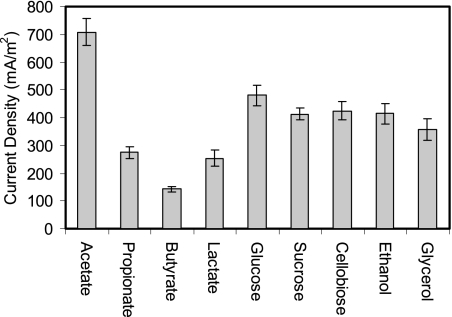
Strain YZ-1 used a greater diversity of carbon sources than most DMRBs. In U-tube MFCs, strain YZ-1 generated current (142 to 275 mA/m2) using propionate, butyrate, and lactate, although at densities that were 60 to 80% less than those produced with acetate (Fig. 8). However, YZ-1 showed higher current densities using sugars (monosaccharides and disaccharides) and alcohols, and the maximum current densities were 481 mA/m2 for glucose, 413 mA/m2 for sucrose, 425 mA/m2 for cellobiose, 414 mA/m2 for ethanol, and 357 mA/m2 for glycerol (Fig. 8).
FIG. 8.
Electricity generation (based on the anode surface area) by strain YZ-1 using different carbon sources (all at a concentration of 1 g/liter) in U-tube MFC reactors. The error bars indicate standard deviations based on averages measured during stable power output in two or more separate batch experiments.
DISCUSSION
By using a U-tube MFC system, we demonstrated that exoelectrogens can be isolated using dilution-to-extinction methods based directly on their ability to generate electricity. U-tube dual-chamber MFCs can be autoclaved and are well sealed so that a sterile and anaerobic environment can be obtained for exoelectrogen growth. Milliken and May (29) reported a similarly shaped MFC that was used for evaluation of electricity production by Desulfitobacterium hafniense based on use of a mediator (anthraquinone-2,6-disulfonate) and oxygen at the cathode. Instead of using two symmetrical curled tubes containing floating electrodes inside the tubes, as in the study of Milliken and May, the U-tube isolation device developed here uses a straight anode tube with a flat anode placed at the bottom. This configuration allows a small number of cells in the most diluted samples to settle onto an anode surface so that they can grow and produce current. The asymmetric U-shaped cathode chamber also provides a high ratio of cathode volume to anode volume and has a graphite fiber cathode with a large surface area to increase cathode efficiency, and a chemical catholyte solution is used to avoid oxygen contamination.
Most exoelectrogens that can produce power in an MFC are DMRBs initially isolated using agar plates containing Fe(III). However, strain YZ-1 obtained in this study produced electricity but was incapable of growth with acetate using hydrous ferric oxide [poorly crystallized Fe(III) oxide] in suspension or Fe(III) pyrophosphate on agar plates. Pham et al. (35) used various electron donor and acceptor combinations, including Fe(III) as the electron acceptor, for solid media for isolating exoelectrogens from MFCs. However, the isolates recovered from colonies accounted for less than 0.1% of the microbes estimated to be present by molecular analysis, indicating the selective limitation of traditional plating methods for isolating electricity-producing microorganisms.
The power densities generated from pure cultures of exoelectrogens are usually equal to or much lower than those obtained using a mixed culture under the same MFC architecture (type of reactor), circuit load (e.g., high internal resistance), or solution (i.e., conductivity) conditions (23). For example, Min et al. (30) reported similar maximum power densities for a wastewater inoculum (38 ± 1 mW/m2) and Geobacter metallireducens (36 to 40 mW/m2) in two-chamber MFC reactors with acetate as the substrate and dissolved oxygen as the electron acceptor. However, the internal resistance is extremely high for this type of reactor (1,286 Ω), and thus power is limited by the architecture and load and not by the ability of the bacteria to produce high current. When a single-chamber air-cathode MFC with an Mn4+ graphite anode and an estimated lower internal resistance (30 to 100 Ω) was used, Shewanella putrefaciens (32) produced a maximum power of 10.2 mW/m2. This was only 1.3% of the power output obtained using a sewage sludge inoculum in the same reactor (788 mW/m2) (33). An anaerobic sludge inoculum produced a maximum power density of 4,310 mW/m2 in a two-chamber MFC using ferricyanide with low internal resistance (3 Ω). In the same type of system, a pure culture of P. aeruginosa strain KRA3 produced only 28 mW/m2, which was <1% of the power generated with the mixed culture (36). Strain YZ-1 produced a maximum power density of 89 mW/m2 in U-tube MFC reactors (internal resistance, 69 ± 4 Ω), which was 17% of the power produced with the mixed consortium. However, strain YZ-1 had a high CE (up to 93%), similar to that observed with Geobacter sulfurreducens (95%) with acetate as the substrate (3).
Most DMRBs utilize a limited range of substrates for growth or electricity generation. S. putrefaciens, for example, oxidizes lactate to acetate, but it cannot oxidize acetate under anaerobic conditions (40). G. sulfurreducens (3, 6) completely oxidizes acetate for power generation, but it cannot utilize simple sugars. Rhodoferax ferrireducens oxidizes glucose and sucrose in MFCs, but it cannot use ethanol, glycerol, or butyrate (8, 10). Strain YZ-1 uses a much wider range of substrates as carbon sources (Table 1), including acetate, glucose, and cellobiose. These three substrates generated the following high current densities with strain YZ-1: 708 mA/m2 (acetate), 481 mA/m2 (glucose), and 425 mA/m2 (cellobiose). The versatile substrate utilization of strain YZ-1 indicates its potential relevance for use with wastewaters that have highly variable compositions.
Electricity generation is a newly identified characteristic of O. anthropi. However, Ochrobactrum species have been identified as members of anode microbial communities in other MFCs using glucose or acetate as a substrate (21, 36). Like another exoelectrogenic bacterium, P. aeruginosa, O. anthropi is an opportunistic pathogen belonging to the Proteobacteria. Both of these organisms are facultative bacteria that are capable of aerobic respiration and anaerobic denitrification (7, 13, 16). Furthermore, both of these bacteria are found in the rhizosphere of diverse plants, indicating an association with plant roots, and have the ability to degrade a wide range of environmental pollutants (1). Both microbes can produce exopolysaccharides, a factor which could be relevant to their colonization of the electrode in MFCs. These common characteristics of P. aeruginosa and O. anthropi suggest that exoelectrogenesis may be a selective property for opportunistic pathogens, a situation which should be further explored.
Although additional study is still needed to discover more details about the exocellular electron transfer mechanism(s) used by O. anthropi in MFCs, this is the first time that an Ochrobactrum species has been demonstrated to have the ability to produce electricity. It is suggested that a greater diversity of exoelectrogens may exist in anode communities and that isolates from these communities could be obtained using U-tube devices rather than traditional metal oxide plating methods. By using different substrates and using U-tube MFCs with lower internal resistances, these isolation devices may allow us to more fully study the diversity of exoelectrogenic bacteria in MFCs.
Acknowledgments
This project was funded by a grant from the U.S. Air Force Office of Scientific Research and by NSF grant CBET-0730359.
Footnotes
Published ahead of print on 21 March 2008.
REFERENCES
- 1.Berg, G., L. Eberl, and A. Hartmann. 2005. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 7:1673-1685. [DOI] [PubMed] [Google Scholar]
- 2.Bond, D. R., D. E. Holmes, L. M. Tender, and D. R. Lovley. 2002. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 295:483-485. [DOI] [PubMed] [Google Scholar]
- 3.Bond, D. R., and D. R. Lovley. 2003. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 69:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bond, D. R., and D. R. Lovley. 2005. Evidence for involvement of an electron shuttle in electricity generation by Geothrix fermentans. Appl. Environ. Microbiol. 71:2186-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bretschger, O., A. Obraztsova, C. A. Sturm, I. S. Chang, Y. A. Gorby, S. B. Reed, D. E. Culley, C. L. Reardon, S. Barua, M. F. Romine, J. Zhou, A. S. Beliaev, R. Bouhenni, D. Saffarini, F. Mansfeld, B. Kim, J. K. Fredrickson, and K. H. Nealson. 2007. Current production and metal oxide reduction by Shewanella oneidensis MR-1 wild type and mutants. Appl. Environ. Microbiol. 73:7003-7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caccavo, F., D. J. Lonergan, D. R. Lovley, M. Davis, J. F. Stolz, and M. J. McInerney. 1994. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl. Environ. Microbiol. 60:3752-3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson, C. A., and J. L. Ingraham. 1983. Comparison of denitrification by Pseudomonas stutzeri, Pseudomonas aeruginosa, and Paracoccus denitrificans. Appl. Environ. Microbiol. 45:1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhuri, S. K., and D. R. Lovley. 2003. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 21:1229-1232. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, S., and B. E. Logan. 2007. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 9:492-496. [Google Scholar]
- 10.Finneran, K. T., C. V. Johnsen, and D. R. Lovley. 2003. Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int. J. Syst. Evol. Microbiol. 53:669-673. [DOI] [PubMed] [Google Scholar]
- 11.Fredrickson, J. K., S. Kota, R. K. Kukkadapu, C. Liu, and J. M. Zachara. 2003. Influence of electron donor/acceptor concentrations on hydrous ferric oxide (HFO) bioreduction. Biodegradation 14:91-103. [DOI] [PubMed] [Google Scholar]
- 12.Gorby, Y. A., Y. S. Yanina, J. S. McLean, K. M. Rosso, D. Moyles, A. Dohnalkova, T. J. Beveridge, I. S. Chang, B. H. Kim, K. S. Kim, D. E. Culley, S. B. Reed, M. F. Romine, D. A. Saffarini, E. A. Hill, L. Shi, D. A. Elias, D. W. Kennedy, G. Pinchuk, K. Watanabe, S. Ishii, B. Logan, K. H. Nealson, and J. K. Fredrickson. 2006. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms. Proc. Natl. Acad. Sci. USA 103:11358-11363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes, B., M. Popoff, M. Kiredjian, and K. Kersters. 1988. Ochrobactrum anthropi gen. nov., sp. nov. from human clinical specimens and previously known as group Vd. Int. J. Syst. Bacteriol. 38:406-416. [Google Scholar]
- 14.Holmes, D. E., D. R. Bond, and D. R. Lovley. 2004. Electron transfer by Desulfobulbus propionicus to Fe(III) and graphite electrodes. Appl. Environ. Microbiol. 70:1234-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes, D. E., J. S. Nicoll, D. R. Bond, and D. R. Lovley. 2004. Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell. Appl. Environ. Microbiol. 70:6023-6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesseru, P., I. Kiss, Z. Bihari, and B. Polyák. 2002. The effects of NaCl and some heavy metals on the denitrification activity of Ochrobactrum anthropi. J. Basic Microbiol. 42:268-276. [DOI] [PubMed] [Google Scholar]
- 17.Kim, B. H., H. J. Kim, M. S. Hyun, and D. S. Park. 1999. Direct electrode reaction of Fe(III) reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 9:127-131. [Google Scholar]
- 18.Kim, H. J., H. S. Park, M. S. Hyun, I. S. Chang, M. Kim, and B. H. Kim. 2002. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzyme Microb. Technol. 30:145-152. [Google Scholar]
- 19.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 20.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2:150-163. [DOI] [PubMed] [Google Scholar]
- 21.Lee, J., N. T. Phung, I. S. Chang, B. H. Kim, and H. C. Sung. 2003. Use of acetate for enrichment of electrochemically active microorganisms and their 16S rDNA analyses. FEMS Microbiol. Lett. 223:185-191. [DOI] [PubMed] [Google Scholar]
- 22.Liu, H., and B. E. Logan. 2004. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 38:4040-4046. [DOI] [PubMed] [Google Scholar]
- 23.Logan, B. E. 2008. Microbial fuel cells. John Wiley & Sons, New York, NY.
- 24.Logan, B. E., S. Cheng, V. Watson, and G. Estadt. 2007. Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ. Sci. Technol. 41:3341-3346. [DOI] [PubMed] [Google Scholar]
- 25.Logan, B. E., and J. M. Regan. 2006. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 14:12-518. [DOI] [PubMed] [Google Scholar]
- 26.Lovley, D. R. 2006. Bug juice: harvesting electricity with microorganisms. Nat. Rev. Microbiol. 4:497-508. [DOI] [PubMed] [Google Scholar]
- 27.Lovley, D. R., D. E. Holmes, and K. P. Nevin. 2004. Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49:219-286. [DOI] [PubMed] [Google Scholar]
- 28.Lovley, D. R., and E. J. P. Philips. 1986. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl. Environ. Microbiol. 51:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milliken, C. E., and H. D. May. 2007. Sustained generation of electricity by the spore-forming, Gram-positive, Desulfitobacterium hafniense strain DCB2. Appl. Microbiol. Biotechnol. 73:1180-1189. [DOI] [PubMed] [Google Scholar]
- 30.Min, B., S. Cheng, and B. E. Logan. 2005. Electricity generation using membrane and salt bridge microbial fuel cells. Water Res. 39:1675-1686. [DOI] [PubMed] [Google Scholar]
- 31.Myers, C. R., and J. M. Myers. 1992. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciens MR-1. J. Bacteriol. 174:3429-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park, D. H., and J. G. Zeikus. 2002. Impact of electrode composition on electricity generation in a single compartment fuel cell using Shewanella putrefaciens. Appl. Microbiol. Biotechnol. 59:58-61. [DOI] [PubMed] [Google Scholar]
- 33.Park, D. H., and J. G. Zeikus. 2003. Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol. Bioeng. 81:348-355. [DOI] [PubMed] [Google Scholar]
- 34.Park, H. S., B. H. Kim, H. S. Kim, H. J. Kim, G. T. Kim, M. Kim, I. S. Chang, Y. K. Park, and H. I. Chang. 2001. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Clostridium butyricum isolated from a microbial fuel cell. Anaerobe 7:297-306. [Google Scholar]
- 35.Pham, C. A., S. J. Jung, N. T. Phung, J. Lee, I. S. Chang, B. H. Kim, H. Yi, and J. Chun. 2003. A novel electrochemically active and Fe(III)-reducing bacterium phylogenetically related to Aeromonas hydrophila, isolated from a microbial fuel cell. FEMS Microbiol. Lett. 223:129-134. [DOI] [PubMed] [Google Scholar]
- 36.Rabaey, K., N. Boon, S. D. Siciliano, M. Verhaege, and W. Verstraete. 2004. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl. Environ. Microbiol. 70:5373-5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reguera, G., K. D. McCarthy, T. Mehta, J. S. Nicoll, M. T. Tuominen, and D. R. Lovley. 2005. Extracellular electron transfer via microbial nanowires. Nature 435:1098-1101. [DOI] [PubMed] [Google Scholar]
- 38.Ren, N., D. Xing, B. E. Rittmann, L. Zhao, T. Xie, and X. Zhao. 2007. Microbial community structure of ethanol type fermentation in bio-hydrogen production. Environ. Microbiol. 9:1112-1125. [DOI] [PubMed] [Google Scholar]
- 39.Richter, H., M. Lanthier, K. P. Nevin, and D. R. Lovley. 2007. Lack of electricity production by Pelobacter carbinolicus indicates that the capacity for Fe(III) oxide reduction does not necessarily confer electron transfer ability to fuel cell anodes. Appl. Environ. Microbiol. 73:5347-5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott, J. H., and K. H. Nealson. 1994. A biochemical study of the intermediary carbon metabolism of Shewanella putrefaciens. J. Bacteriol. 176:3408-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trujillo, M. E., A. Willems, A. Abril, A. Planchuelo, R. Rivas, D. Ludeña, P. F. Mateos, E. Martínez-Molina, and E. Velázquez. 2005. Nodulation of Lupinus albus by strains of Ochrobactrum lupini sp. nov. Appl. Environ. Microbiol. 71:1318-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe, K., Y. Kodama, and S. Harayama. 2001. Design and evaluation of PCR primers to amplify bacterial 16S ribosomal DNA fragments used for community fingerprinting. J. Microbiol. Methods 44:253-262. [DOI] [PubMed] [Google Scholar]
- 43.Xing, D., N. Ren, Q. Li, M. Lin, A. Wang, and L. Zhao. 2006. Ethanoligenens harbinense gen. nov., sp. nov., isolated from molasses waste water. Int. J. Syst. Evol. Microbiol. 56:755-760. [DOI] [PubMed] [Google Scholar]
- 43a.Xing, D., Y. Zuo, S. Cheng, J. M. Regan, and B. E. Logan. Electricity generation by Rhodopseudomonas palustris DX-1. Environ. Sci. Technol., in press. [DOI] [PubMed]
- 44.Zuo, Y., P.-C. Maness, and B. E. Logan. 2006. Electricity production from steam exploded corn stover biomass. Energ. Fuel 20:1716-1721. [Google Scholar]
- 45.Zurdo-Piñeiro, J. L., R. Rivas, M. E. Trujillo, N. Vizcaíno, J. A. Carrasco, M. Chamber, A. Palomares, P. F. Mateos, E. Martínez-Molina, and E. Velázquez. 2007. Ochrobactrum cytisi sp. nov., isolated from nodules of Cytisus scoparius in Spain. Int. J. Syst. Evol. Microbiol. 57:784-788. [DOI] [PubMed] [Google Scholar]