Abstract
Vacuum cooling is a common practice in the California leafy green industry. This study addressed the impact of vacuum cooling on the infiltration of Escherichia coli O157:H7 into lettuce as part of the risk assessment responding to the E. coli O157:H7 outbreaks associated with leafy green produce from California. Vacuum cooling significantly increased the infiltration of E. coli O157:H7 into the lettuce tissue (2.65E+06 CFU/g) compared to the nonvacuumed condition (1.98E+05 CFU/g). A stringent surface sterilization and quadruple washing could not eliminate the internalized bacteria from lettuce. It appeared that vacuuming forcibly changed the structure of lettuce tissue such as the stomata, suggesting a possible mechanism of E. coli O157:H7 internalization. Vacuuming also caused a lower reduction rate of E. coli O157:H7 in stored lettuce leaves than that for the nonvacuumed condition.
The first produce-associated Escherichia coli O157:H7 outbreak, in 1991, was traced to fresh-pressed apple cider (10). Since then, fresh produce has been associated with more O157:H7 outbreaks, while incidents associated with foods of animal origin have decreased proportionally (22). Since 1995, a total of 19 outbreaks directly associated with O157:H7-contaminated lettuce have been reported, with two additional outbreaks related to another leafy green, spinach (5). During 2005-2006, there were three large multistate outbreaks of O157:H7 and four Salmonella contaminations associated with fresh produce (6-9), which further emphasized the need for a systematic risk assessment to identify the risk factors contributing to food-borne outbreaks.
Vacuum cooling is widely used in the California fresh produce industry, as described in the investigation reports of the O157:H7 outbreaks associated with California produce during 2002-2006 (2-4, 6-8). Under vacuum, the temperature of lettuce can be reduced efficiently from the field temperature of ∼28°C to ∼0°C as a result of the energy consumption from water evaporation at low pressure (27). For most leafy green produce, to avoid moisture loss from evaporation, water from a recirculation reservoir is sprayed on produce during vacuuming (2, 3, 6, 7, 27). Vacuum cooling is considered a very efficient approach to extending the shelf life of fresh produce in terms of decay reduction and physiological disorder control through efficient storage temperature management (1, 13). Most studies on the impact of vacuum cooling have focused on vegetable quality, using sensory attributes—general appearance, wilting, and decay—as criteria (1). Its impact on the fate of pathogens such as O157:H7 has never been studied thoroughly. One obvious reason for the lack of studies on the food safety aspect of this process is that the proportion of produce-associated outbreaks among all reported food-borne outbreaks was very low in the past, i.e., barely 0.7% in the 1970s, in contrast to 6% in the 1990s (22, 25).
Under vacuum, the balance between lettuce cell interior turgor pressure, the cell wall, and the atmosphere is disrupted. Water in the vacuoles will evaporate and escape into the atmosphere through the stomata, which may increase cell osmotic pressure and forcibly open the stomata. The hypotheses of this study are as follows: (i) the vacuum cooling process changes the microstructure of lettuce tissue to favor infiltration of O157:H7 and (ii) the transition of contaminated lettuce from vacuum to atmospheric pressure creates a suction force that facilitates O157:H7 infiltration. It is our objective to understand whether vacuuming promotes the infiltration of microorganisms into lettuce tissue and, furthermore, to determine its impact on produce safety.
MATERIALS AND METHODS
Lettuce.
Freshly harvested romaine lettuce (Lactuca sativa) was purchased from a local producer in Salinas, CA (anonymous company), and was used on the day of arrival. The outer layers and core of the lettuce were removed.
Bacterial strains and medium.
A derivative of E. coli O157:H7 ATCC 43888 (no stx1 or stx2 production) carrying a green fluorescent protein (GFP) marker (kanamycin resistant) was used in experiments using laser scanning confocal microscopy. A derivative of strain ATCC 43888 resistant to tetracycline was used for all other experiments. All bacteria were grown at 37°C in tryptic soy broth with shaking or on tryptic soy agar (TSA) plates. Kanamycin and tetracycline were added at 50 and 15 μg/ml, respectively, when appropriate.
Inoculation of bacteria.
Before inoculation, the lettuce was incubated at 28°C for 30 min to mimic the field temperature. Strain ATCC 43888 (tet) was grown for 14 h. Two hundred microliters of culture was transferred onto the abaxial surface (lower surface away from the plant's axis) of lettuce leaves and spread with a cotton swab within a 9-cm2 area. Each leaf was inoculated at two 9-cm2 areas located on opposite sides of the mid-rib and loosely wrapped with Parafilm. One set of the prepared leaves underwent the vacuum cooling process. The other set was kept at 4°C as the control set until both sets were ready for further experiments.
Vacuum cooling protocol.
The experimental set of the inoculated lettuce leaves underwent vacuuming at ∼4.6 Torr (a pressure used by standard commercial vacuum cooling units) (27) for 5 min in an AAS-11 vacuum chamber (Alloy Products Corporation, Waukesha, WI) with a 7.5 HP vacuum engine (Toshiba International Corporation Inc., Houston, TX). The surface temperatures of the leaves after the vacuuming process were measured with an AutoPro ST25 infrared thermometer (Raytek, Williston, VT), and the temperatures reached ∼10 to 12°C.
Lettuce leaf surface sterilization and efficacy verification.
Each leaf was immersed in 1.2% sodium hypochlorite (20% bleach; Clorox Inc., Oakland, CA) for 30 s and kept for 10 min in a biosafety hood at room temperature. Residual hypochlorite was removed by washing samples four times in 500-ml volumes of sterile distilled water. The leaf was then exposed to 30-mW/cm2 germicidal UV light (∼254 nm) for 10 min per side. Surface sterilization was confirmed by gently pressing the sterilized lettuce on TSA plates that were subsequently incubated at 37°C for 48 h. The surface sanitation was considered effective when the sterilization efficacy reached an 8-log reduction, which was estimated by the ratio of the number of residual colonies detected to the total number of cells inoculated onto the surfaces of the leaves. The total cells inoculated on the leaf surfaces were enumerated by plate counts of the homogenized lettuce leaves before sterilization.
Sampling plan and plate counts.
In an effort to improve the detection limit, both the vacuum and control groups were incubated at 37°C for 2 h before the sterilization treatment and sampling. To obtain the total cell counts of O157:H7 both on the surface and in the tissue, 10 spots within the inoculated area (9 cm2) were randomly collected into a stomacher bag by use of an Acu-Punch sampler with an 8-mm diameter (Acuderm, Inc., Fort Lauderdale, FL). Following a homogenization process using a Bioreba AG homogenizer (STA Laboratories, Gilroy, CA), the liquid extract was diluted in 10-fold steps with peptone water. Bacteria were enumerated on duplicate plates of TSA (with tetracycline). To obtain internal cell counts, bacterial enumeration was done after surface sterilization of lettuce leaves.
Laser confocal microscopy analysis.
The lettuce leaves were inoculated with washed or unwashed cells from an overnight culture of ATCC 43888 (no stx1 or stx2, but with GFP), with or without the vacuuming process. The samples were sliced (0.3 × 0.3 cm), mounted with immersion oil or water, and then covered with a cover glass. The slides were examined using an Olympus FV1000 laser scanning confocal microscope (Olympus America Inc., Center Valley, PA) with a 40× oil immersion objective (Nikon, Japan) and a 15-mW argon laser. A 488-nm filter block was used, with a gain value of 1.375 and an offset percentage of 10%. Signal collection was set from 3,071 to 4,095 square pixels to exclude the signal of chlorophylls. The background lettuce structure was captured in gray image without an excitation light source.
Each image was processed and reconstructed from 20 optical sections, using Olympus Fluoview, version 1.4a. The lengths and widths of all stomata from three representative figures were measured for both experimental and control groups with a built-in ruler of the software.
Survival experiment.
A 2-week kinetic experiment with triplicate samples was done by total plate counts with duplicate plates for both vacuum and control samples stored at 4°C. For total E. coli O157:H7 cell counts, samples were taken on days 0, 1, 3, 7, and 14. For internal cell counts, the lettuce leaves were surface sterilized, and the samples were processed on days 0, 1, 3, and 7; the day 14 sample was omitted because the lettuce texture appeared a bit rubbery when pressed with the sampler and was not suitable for surface sterilization.
Statistical analysis.
Reported plate count data represented the mean value obtained from at least two independent experiments with triplicate samples at each time, except where otherwise mentioned. Photomicrographs were selected to represent typical results. Student t test statistical analysis was performed for both plate counts and stoma measurements, with a significance criterion of P values of ≤0.05, using SAS software, version 9 (SAS Institute Inc., Cary, NC).
RESULTS
Vacuum cooling and infiltration of E. coli O157:H7 into the tissue of surface-intact lettuce leaves.
In order to study bacterial internalization, it was necessary to validate the method to differentiate the internalized bacterial count from the total count. The sterilization method combining 1.2% sodium hypochlorite solution immersion and UV exposure yielded an efficiency of approximately 99.999997% (∼8-log reduction). Plate counts of bacteria from the surface-sterilized samples represented the internalized bacterial counts.
A comparison of the internal cell counts between vacuum and control groups, based on nine independent experiments with triplicate samples each time, indicated that vacuum cooling had a significant impact on bacterial cell infiltration into lettuce tissue (P = 0.02) (Fig. 1). With vacuuming, a significantly larger portion of cells (5.4%; 2.65E+06 CFU/g) were internalized in the lettuce tissue than that for the control group (4.2%; 1.98E+05 CFU/g), while the total cell counts were similar for both groups (Fig. 1).
FIG. 1.
Effect of vacuum cooling on infiltration of E. coli O157:H7 into fresh lettuce. The plate counts for the internalized cells were performed with surface-sterilized lettuce samples. The total E. coli O157:H7 cell counts were performed as a control for equal inoculums between the vacuuming and control groups. All samples were triplicates in nine repeated experiments.
Mechanism of infiltration of E. coli O157:H7 into lettuce tissue under vacuum cooling.
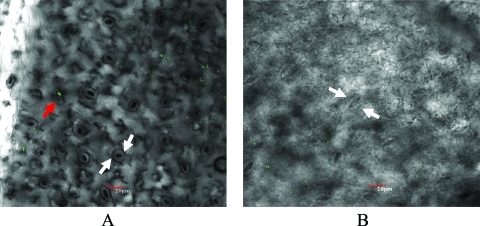
Since such a significant increase in internalized bacterial count due to vacuuming occurred on the intact surfaces of lettuce leaves and a previous report showed that bacteria gathered around stomata when Arabidopsis was exposed to a pathogen (20), a hypothesis was developed that vacuum cooling promoted the ability of O157:H7 cells to infiltrate the tissue via the stomata or cut edges, the major openings on the leaf. To test this theory, laser scanning microscopy was used for image analysis. The three-dimensional images clearly showed that O157:H7 cells were able to infiltrate under both vacuuming (Fig. 2A) and nonvacuuming (Fig. 2B) conditions, while under vacuuming the bacteria showed deeper penetration (unpublished data). A cluster of O157:H7 cells inside the stomata was captured (Fig. 2A). After the vacuuming procedure (Fig. 2A), the stomata appeared open and the guard cells were rounder and shorter than those in the control lettuce leaves (Fig. 2B). To quantify the openness of the stomata, over 30 stomata were measured in three representative figures from each group. The average width and length of the stomata of the vacuumed group were significantly different from those of the control group (Table 1). The ratio of width to length of all the stomata after vacuuming was significantly (P = 0.0002) higher than that of the nonvacuumed leaves. The increased ratio of width to length of the stomata in the vacuumed leaves reflected the enlarged vacuoles of the guard cells, which suggested that the vacuuming process forcibly opened the stomata (19, 21, 23). Moreover, although O157:H7 cells gathering inside the stomata were observed in both groups (Fig. 3), scattered bacterial cell clusters around the locations of many guard cells and other random subsurface locations were observed for the vacuumed group but not the control group (Fig. 3A).
FIG. 2.
Photomicrographs of lettuce tissue infiltrated with E. coli O157:H7 (GFP) cells under a laser scanning microscope (×400). The lettuce leaves were inoculated with a washed and diluted (∼104 CFU/cm2) overnight culture. (A) Compiled image from the surface to 20 μm beneath of a slice of lettuce leaf with vacuum treatment. (B) Compiled image from the surface to 20 μm beneath of a sliced lettuce leaf without vacuuming treatment. The locations of the two guard cells of a stoma are indicated with white arrows. The bacteria entrapped inside the stomata are indicated with a red arrow. Bar, 20 μm.
TABLE 1.
Measurements of stomata in vacuum and control groups (a total of >30 stomata in three micrographs for each group)
| Treatment | Stoma measurementa
|
||
|---|---|---|---|
| Width (μm) | Length (μm) | Ratio of width to length | |
| Vacuum | 15.6 ± 3.3 | 17.5 ± 2.9 | 0.9 ± 0.22 |
| Control | 17.7 ± 2.4 | 24.8 ± 3.1 | 0.7 ± 0.12 |
P values between treatments were 0.004, 3.38 × 10−13, and 0.0002 for width, length, and ratio, respectively.
FIG. 3.
Snapshots of E. coli O157:H7 (GFP) cell clusters internalized in lettuce tissue, 12 μm beneath the leaf surface. The lettuce was directly inoculated with an overnight culture (∼8.4 × 106 CFU/cm2) of E. coli O157:H7 (GFP). (A) Sample was applied with vacuum treatment. (B) Control without vacuum treatment. Dispersed bacterial cells around the two guard cells of a stoma are indicated with white arrows in panel A. Stomata filled with bacterial colonies are indicated with red arrows. Bar, 20 μm.
Impact of vacuuming on the survival of E. coli O157:H7 associated with lettuce leaves.
Considering that the nutrient availability on the lettuce surface is certainly different from that inside (16), it is possible that the internalization of O157:H7 caused by vacuuming was able to promote a longer survival of O157:H7. To test the impact of vacuuming on O157:H7 survival, a kinetic experiment was done for both vacuum and control samples stored at 4°C by enumerating O157:H7 cells both on the lettuce surface and inside the tissue. The vacuum group demonstrated a significantly lower reduction rate of total cell counts (P = 0.03) (Fig. 4), suggesting greater survival than that of the control group.
FIG. 4.
Comparison of E. coli O157:H7 survival rates between contaminated lettuce with and without vacuum treatment. The total cell counts for lettuce from the vacuuming (□; dashed line) and control (•; dashed line) groups were collected on days 0, 1, 3, 7, and 14; the internal cell counts for the vacuuming (□; intact line) and control (•; intact line) groups were collected on days 0, 1, 3, and 7.
If the reduced reduction rate was due to more available nutrients inside the lettuce tissue, the survival curves of the internalized O157:H7 cells in both the vacuumed and control groups should show similar patterns. However, when we compared the plate counts of O157:H7 cells from the surface-sterilized lettuce samples with and without vacuum treatment, it was found that after 1 week of storage at 4°C, 2 log more cells were recovered from the vacuum group than from the control group, in contrast to the initial, approximately 1-log difference between these two groups (Fig. 4). In other words, even with a similar nutrient availability, O157:H7 in the vacuumed group exhibited greater survival than did that in the control group. Moreover, the internalized cells in both groups demonstrated a more rapid decline in numbers than the total cell count of both surface and internalized bacteria (Fig. 4), which suggested that the greater survival of the vacuumed group was not due to greater nutrient availability.
DISCUSSION
This study yielded the first report that vacuum cooling significantly increases the infiltration of E. coli O157:H7 into lettuce tissue in terms of both cell numbers and the depth of penetration. Vacuuming enhanced the level of O157:H7 contamination, as stringent sterilization and a quadruple wash (applied to differentiate the internal cells from those on the surface in our study) could not eliminate the internalized bacteria. The current industry practice of a triple wash is apparently not adequate to alleviate the risk.
The internalization of E. coli O157:H7 and other pathogens has been studied previously with lettuce and other produce (17, 18). Transmissions of pathogen through intact roots or cut open surfaces were the primary routes studied. When the roots were experimentally damaged, the population size of internalized Salmonella cells in the hypocotyls-cotyledons, stems, and leaves of a tomato plant grown in a contaminated hydroponic system was similar to that in plants with intact roots (15). This suggested that damage to the root was not a prerequisite for the transmission of pathogen into a mature plant via the root. However, when bacteria were introduced to the damaged lettuce leaves, it appeared that E. coli O157:H7 preferentially attached to cut edges compared to the intact leaf surface (24). Experimentally damaged stem tissues also caused infiltration of Salmonella into the scar tissues of stems of tomato plants (14).
Mechanical damage frequently occurs during harvest, both intentionally (harvest-cutting the root and peeling off the outer leaves) and unintentionally (bruises), which exposes lettuce to a high risk of taking up pathogens from the environment and personnel, especially via leaves with surface damage. In our study, using a cotton swab for inoculation might unintentionally break trichomes (plant hairs) and crack the cuticle (waxy) layer mechanically, leading to a relatively larger number of cells penetrating the lettuce tissue than would penetrate any perfectly intact lettuce surface. However, the very limited damage on the surface resembled the typical conditions of lettuce during pre- and postharvest, when leaf breakage and bruising occur easily.
The internalization of O157:H7 under a normal atmosphere was visualized in previous studies, consistent with our finding that with no vacuuming treatment O157:H7 cells were able to penetrate into lettuce tissue, but via paths different from those under the vacuumed condition. Seo and Frank observed the entrapment of O157:H7 cells in the vacuole of a stoma, as well as attachment to the cut edge (20). Solomon et al. further provided visual evidence that O157:H7 cells aggregated around the intercellular space rather than being distributed randomly inside lettuce tissue (26). In our study, after vacuum treatment, it was found that bacterial cell clusters were scattered around the locations of many guard cells, the vacuoles of stomata, and other random subsurface locations. Previous leaf hydraulic studies using a vacuum pump method had demonstrated that water was able to move through leaf lamina to the evaporation sites, stomata, under the impact of a vacuum (negative pressure) to increase the apertures of stomata (19, 23). This observation suggested that infiltration of O157:H7 cells into lettuce during the vacuuming process was more likely a passive event. Additionally, the positive force from releasing the vacuum to the atmosphere might further facilitate O157:H7 infiltration into lettuce tissue.
Vacuuming also promoted the survival of bacteria in lettuce, but not because of greater nutrient availability (Fig. 4). The readings were higher for both the total and internal O157:H7 cell counts in the vacuum group than in the control group during storage. If the internal environment provided more nutrients for bacteria, then the total internal O157:H7 cell counts should show a slower decrease in time than the total cell counts for both groups. In contrast, the number of internal cells declined faster than the number of total cells for both groups, suggesting that the internal cells were likely facing a more stringent environment that was less favorable for bacterial survival due to plant defense responses, such as the wound reaction, hydrogen peroxide accumulation, etc. (11, 12). Therefore, the difference in survival between these two groups indicated that factors other than nutrient availability played roles.
In all, vacuum cooling is an efficient approach to improving fresh produce quality through efficient storage temperature management. However, this study yielded the first report that vacuum cooling increases the infiltration and survival of O157:H7 in lettuce. It would be unwise to abandon vacuum cooling for this reason, but if this risk is shown to apply to commercial vacuum cooling, it will be urgent to find means to eliminate the adverse effect of vacuum cooling and to enhance the safety of the system.
Acknowledgments
We thank Dean Cliver for reviewing the manuscript. Linda Harris, Jerry Gillespie, James Cullor, Mysore Sudarshana, and Wayne Smith are thanked for their scientific comments. Trevor Suslow and Glenn Young are thanked for sharing the E. coli O157:H7 (GFP) and E. coli O157:H7 Tetr strains, respectively. We appreciate James Thompson for his invaluable help with the vacuum system. Yihua Xu and Thomas Farver are thanked for help in statistical analysis.
The Western Institute for Food Safety and Security and the California Department of Food and Agriculture are thanked for funding this project.
Footnotes
Published ahead of print on 14 March 2008.
REFERENCES
- 1.Aharoni, N., and B. Yehosua. 1973. Delaying deterioration of romaine lettuce by vacuum cooling and modified atmosphere produced in polyethylene packages. J. Am. Soc. Hortic. Sci. 98:464-468. [Google Scholar]
- 2.Anonymous. 2002. E. coli O157:H7 illnesses in Washington. California Department of Health Services, Food and Drug Branch, Sacramento, CA.
- 3.Anonymous. 2004. Investigation of E. coli O157:H7 illnesses in San Diego and Orange counties. California Department of Health Services, Food and Drug Branch, Sacramento, CA.
- 4.Anonymous. 2004. Investigation of E. coli O157:H7 outbreak in San Mateo retirement facility. California Department of Health Services, Food and Drug Branch, Sacramento, CA.
- 5.Anonymous. 2005. Letter to California firms that grow, pack, process, or ship fresh and fresh-cut lettuce. Food and Drug Administration/CFSAN, College Park, MD.
- 6.Anonymous. 2005. Investigation of an E. coli O157:H7 outbreak associated with consumption of Dole brand prepackaged salads. California Department of Health Services, Food and Drug Branch, Sacramento, CA.
- 7.Anonymous. 2007. Investigation of the 2006 E. coli O157:H7 outbreak associated with Taco Bell restaurants in the northeastern United States. California Department of Health Services, Food and Drug Branch, Sacramento, CA.
- 8.Anonymous. 2007. Investigation of the E. coli O157:H7 outbreak associated with consumption of Dole brand pre-packaged baby spinach manufactured by Natural Selection Foods: September 13, 2006. California Department of Health Services, Food and Drug Branch, Sacramento, CA.
- 9.Anonymous. 2007. Multistate outbreaks of Salmonella infections associated with eating raw tomatoes in restaurants—United States, 2005-2006. Centers for Disease Control and Prevention, Atlanta, GA.
- 10.Besser, R. E., S. M. Lett, J. T. Weber, M. P. Doyle, T. J. Barrett, J. G. Wells, and P. M. Griffin. 1993. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157:H7 in fresh-pressed apple cider. JAMA 269:2217-2220. [PubMed] [Google Scholar]
- 11.Bestwick, C. S., I. R. Brown, and J. W. Mansfield. 1998. Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiol. 118:1067-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaquis, P. J., A. Wen, P. M. Toivonen, and K. Stanich. 2006. Evidence of an antilisterial factor induced by wounding of iceberg lettuce tissues. Lett. Appl. Microbiol. 42:289-295. [DOI] [PubMed] [Google Scholar]
- 13.Gorris, L. G. M., Y. de Witle, and E. J. Smid. 1994. Storage under moderate vacuum to prolong the keepability of fresh vegetables and fruits. Acta Hortic. 368:474-486. [Google Scholar]
- 14.Guo, X., J. Chen, R. E. Brackett, and L. R. Beuchat. 2001. Survival of salmonellae on and in tomato plants from the time of inoculation at flowering and early stages of fruit development through fruit ripening. Appl. Environ. Microbiol. 67:4760-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, X., M. W. van Iersel, J. Chen, R. E. Brackett, and L. R. Beuchat. 2002. Evidence of association of salmonellae with tomato plants grown hydroponically in inoculated nutrient solution. Appl. Environ. Microbiol. 68:3639-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassan, A. N., and J. F. Frank. 2003. Influence of surfactant hydrophobicity on the detachment of Escherichia coli O157:H7 from lettuce. Int. J. Food Microbiol. 87:145-152. [DOI] [PubMed] [Google Scholar]
- 17.Jablasone, J., K. Warriner, and M. Griffiths. 2005. Interactions of Escherichia coli O157:H7, Salmonella typhimurium and Listeria monocytogenes plants cultivated in a gnotobiotic system. Int. J. Food Microbiol. 99:7-18. [DOI] [PubMed] [Google Scholar]
- 18.Johannessen, G. S., G. B. Bengtsson, B. T. Heier, S. Bredholt, Y. Wasteson, and L. M. Rorvik. 2005. Potential uptake of Escherichia coli O157:H7 from organic manure into crisphead lettuce. Appl. Environ. Microbiol. 71:2221-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martre, P., G. B. North, and P. S. Nobel. 2001. Hydraulic conductance and mercury-sensitive water transport for roots of Opuntia acanthocarpa in relation to soil drying and rewetting. Plant Physiol. 126:352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melotto, M., W. Underwood, J. Koczan, K. Nomura, and S. Y. He. 2006. Plant stomata function in innate immunity against bacterial invasion. Cell 126:969. [DOI] [PubMed] [Google Scholar]
- 21.Nardini, A., M. T. Tyree, and S. Salleo. 2001. Xylem cavitation in the leaf of Prunus laurocerasus and its impact on leaf hydraulics. Plant Physiol. 125:1700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangel, J. M., P. H. Sparling, C. Crowe, P. M. Griffin, and D. L. Swerdlow. 2005. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982-2002. Emerg. Infect. Dis. 11:603-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sack, L., P. J. Melcher, M. A. Zwieniecki, and N. M. Holbrook. 2002. The hydraulic conductance of the angiosperm leaf lamina: a comparison of three measurement methods. J. Exp. Bot. 53:2177-2184. [DOI] [PubMed] [Google Scholar]
- 24.Seo, K. H., and J. F. Frank. 1999. Attachment of Escherichia coli O157:H7 to lettuce leaf surface and bacterial viability in response to chlorine treatment as demonstrated by using confocal scanning laser microscopy. J. Food Prot. 62:3-9. [DOI] [PubMed] [Google Scholar]
- 25.Sivapalasingam, S., C. R. Friedman, L. Cohen, and R. V. Tauxe. 2004. Fresh produce: a growing cause of outbreaks of foodborne illness in the United States, 1973 through 1997. J. Food Prot. 67:2342-2353. [DOI] [PubMed] [Google Scholar]
- 26.Solomon, E. B., S. Yaron, and K. R. Matthews. 2002. Transmission of Escherichia coli O157:H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 68:397-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson, J. F., F. G. Mitchell, T. R. Rumsey, R. F. Kasmire, and C. H. Crisosto. 1998. Commercial cooling of fruits, vegetables, and flowers. University of California, Davis, CA.