Abstract
Two strains of Arcobacter butzleri, ATCC 49616 and an environmental isolate, became nonculturable in seawater microcosms at 4°C by 20 days and at room temperature by 14 days. Nonculturable cells were viable for up to 270 days of incubation in microcosms. Resuscitation of A. butzleri cells from microcosms at both temperatures was achieved 9 days after nutrient addition.
The genus Arcobacter is composed of pathogenic species (A. butzleri, A. cryaerophilus, A. skirrowii, and A. cibarius), nonpathogenic species (A. nitrofigilis and A. halophilus), and a number of species not yet established (such as “Candidatus Arcobacter sulfidicus”) (4, 6, 10, 20). The nonpathogenic species have been isolated from a variety of habitats, but no association with humans or animals has been reported yet. The other species, A. butzleri, A. cryaerophilus, A. skirrowii, and A. cibarius, are found as inhabitants of several animals, which can serve as natural reservoirs, and are also isolated from various environmental sources, including wastewater, surface water, seawater, and groundwater (5, 6, 9, 11, 16, 18). Arcobacter infections in humans were also described previously (14). A. butzleri and A. cryaerophilus have been isolated mainly from stool specimens from patients with diarrhea (8, 17, 21). The significance and prevalence of Arcobacter in human infections have probably been underestimated because of inappropriate detection and cultural methods for stool specimens (17).
Environmental studies have revealed differences in the arcobacter detection capabilities of PCR and culture methods with samples taken from marine environments (5). The viable but nonculturable (VBNC) state is a strategy developed by many gram-negative bacteria to survive under adverse environmental conditions (12). According to many studies, pathogenic bacteria in the VBNC state retain their virulence (1, 2, 7, 15). Knowledge of the survival strategies of arcobacters in the environment is very important for both control of water quality and understanding transmission of disease. So far, no reports have been published on the VBNC state of Arcobacter spp. in seawater.
The aim of the present study was to investigate the VBNC state of A. butzleri experimentally induced under starvation conditions, using a seawater microcosm at room temperature and at 4°C. Plate counts were compared with total direct counts (Live/Dead) to determine whether nonculturable cells of A. butzleri retain viability. Also, resuscitation experiments were conducted to establish the growth-limiting natural conditions of this species. Furthermore, a fluorescent in situ hybridization (FISH) approach for evaluating the viability of VBNC Arcobacter cells in seawater microcosms was also investigated and discussed.
The A. butzleri strains used in this study were ATCC 49616 and Ap-6, a strain isolated from coastal waters of the Strait of Messina (Italy).
Flasks containing autoclaved and filter-sterilized seawater were inoculated with each A. butzleri cell suspension to obtain a final concentration of 107 CFU ml−1. All the experiments were performed in duplicate: four flasks were incubated at 4°C and the other four at room temperature in the dark without shaking. To induce the VBNC state, flasks containing the cell suspensions were maintained at room temperature and 4°C until the bacterial cells in the microcosms did not yield growth when transferred to solid medium. Samples from every flask were taken every 3 days to determine culturability.
Time zero and subsequent samples were taken for plate count. Aliquots of 100 μl from a 10-fold serial dilution were placed onto plates of agarized Arcobacter medium (AM) (Oxoid) incubated under aerobic and microaerobic conditions at 30°C for 48 h. The platings were performed in triplicate, and the counts were expressed as mean values.
The nonculturability of cells was confirmed by concentrating a 10-ml microcosm sample on a 0.2-μm-pore-size filter placed on AM plates and incubated at 30°C for 48 h. Bacteria were considered to be VBNC forms when there were less than 0.1 CFU ml−1.
The viability of cells was determined using a Live/Dead BacLight bacterial viability kit (Molecular Probes) according to the manufacturer's instructions. Viability was evaluated every 3 days until entry into the VBNC state and then every 3 months for a period of 270 days.
Two independent resuscitation experiments were started 48 h after the culturable cell numbers were fixed at a level below 0.1 CFU ml−1. For resuscitation of the VBNC cells with the temperature upshifted, 5 ml of the VBNC cells incubated at 4°C was removed from the microcosm and transferred at room temperature for 24 h. The culturability of these cells was determined by plating them onto AM agar and onto tryptic soy agar (Oxoid) containing 5% sheep blood. Plates were incubated at 30°C for 96 h under both aerobic and microaerobic conditions.
To study the effect of nutrient addition on resuscitation, 5 ml from room temperature and 4°C microcosms containing VBNC cells was combined (1:1) with AM broth containing 5% laked horse blood (Oxoid). Cells were then incubated at room temperature for 24 h. One milliliter of each microcosm was also inoculated into thioglycolate broth (Becton Dickinson). All broths were incubated at 30°C in an aerobic atmosphere and monitored daily until the tubes became turbid and then subcultured. Both experiments were performed in triplicate. The resuscitation was also performed with 90-, 180-, and 270-day-old microcosms.
FISH analysis was carried out at day 0 and with 90-, 180-, and 270-day-old microcosms, according to the method of Pernthaler et al. (13). The Arcobacter-specific probe (ARC94 [TGCGCCACTTAGCTGACA]) synthesized and labeled with the cyanine dye Cy3 from MGW Biotech (Mannheim, Germany) has been used previously (11). Samples were then observed and photographed using a Zeiss LSM 5 DUO.
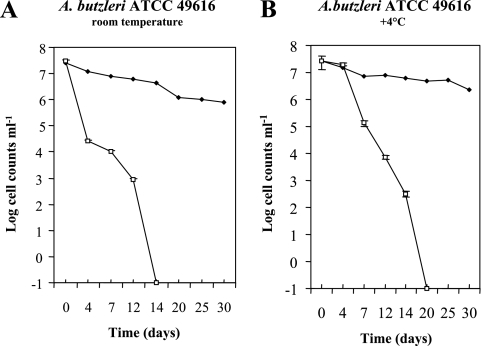
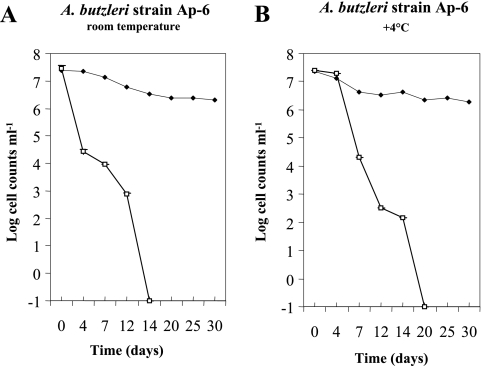
Our results showed that the numbers of culturable cells declined to <0.1 CFU ml−1 in all microcosms and that there was no difference in culturability between the two studied strains (Fig. 1 and 2). They became nonculturable in seawater microcosms at room temperature by 14 days (Fig. 1A and 2A) and at 4°C by 20 days (Fig. 1B and 2B). Contrary to results observed in 4°C microcosms, a sudden decrease in the culturability of cells was observed after 4 days in microcosms maintained at room temperature (Fig. 1A and 2A). To explain the retarded decline rates observed in microcosms maintained at 4°C, it is suggested that the low temperature may have slowed the metabolic activity. These results compare favorably to a study by Rice et al. (16) which reported that the A. butzleri population survived for up to 16 days in well water stored at 5°C.
FIG. 1.
Survival curves of Arcobacter butzleri ATCC 49616 in filter-sterilized seawater microcosms at room temperature (A) and 4°C (B). Culturable cells (numbers of CFU ml−1) (□) were counted on AM agar plates; viability (numbers of cells ml−1) (♦) was determined by a Live/Dead BacLight viability assay. Each point represents the mean of two repeated and independent experiments, and error bars for culturable cells indicate standard deviations of the distribution of the data.
FIG. 2.
Survival curves of Arcobacter butzleri strain Ap-6 in filter-sterilized seawater microcosms at room temperature (A) and 4°C (B). Culturable cells (numbers of CFU ml−1) (□) were counted on AM agar plates; viability (numbers of cells ml−1) (♦) was determined by a Live/Dead BacLight viability assay. Each point represents the mean of two repeated and independent experiments, and error bars for culturable cells indicate standard deviations of the distribution of the data.
The total live counts remained almost constant throughout the experiments, as demonstrated by the Live/Dead method. Despite the loss of culturability in all seawater microcosms, the A. butzleri cells showed intact, undamaged membranes for nearly 270 days, as indicated by the staining method.
The techniques used in this study to resuscitate VBNC A. butzleri were temperature upshift and nutrient addition. Resuscitation of a VBNC population of A. butzleri from microcosms was achieved only with nutrient addition. The VBNC cells from microcosms at both temperatures were recovered approximately 9 days after nutrient addition. Our experiments indicated that there was an advantage to using a microaerobic atmosphere for the recovery of seawater-stressed A. butzleri. Thioglycolate broth gave better recoveries than AM broth. Tryptic soy agar containing 5% sheep blood was the next-best medium for the recovery of VBNC A. butzleri. Confirmation of the identities of resuscitative, culturable arcobacters was done by using biochemical and molecular assays. Some resuscitated cells showed reduced cellular size and a change from spiral to coccoid morphology. Resuscitation was also obtained from the 90-, 180-, and 270-day-old microcosms. The induction of VBNC cells of A. butzleri appears to be linked more strongly with starvation than with temperature.
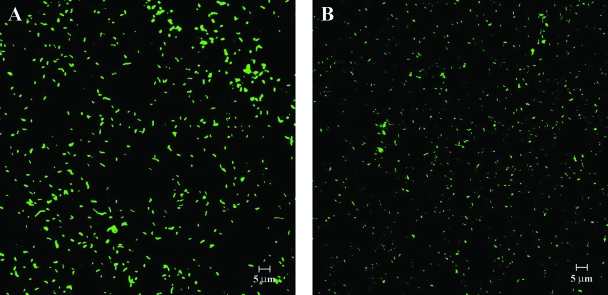
The presence of live cells in the absence of culturability was also confirmed by using FISH analysis. The FISH procedure showed homogeneous and high fluorescent hybridization signals from both nonstressed cells (at inoculation time) (Fig. 3A) and VBNC cells after 270 days (Fig. 3B). This indicated that ribosome degradation did not result in nonculturable cells of A. butzleri. The integrity of ribosomes and nucleic acids, however, may be maintained in a small number of VBNC cells during long-term starvation, and thus, these cells may be viable despite having no culturability (19). The recovery of A. butzleri cells may be the result of resuscitation from the VBNC state or regrowth of the few culturable cells in the microcosms. Our data suggest that the Live/Dead and FISH methods are useful tools for analyzing the viability of bacteria in seawater microcosms.
FIG. 3.
FISH micrographs of Arcobacter butzleri ATCC 49616 cells at inoculation time (A) and after 270 days of incubation (B) in a filter-sterilized seawater microcosm at room temperature.
This study shows for the first time the development of the VBNC state in A. butzleri after exposure to two different temperatures in nutrient-limiting seawater microcosms for prolonged periods of time. A. butzleri is able to survive as long as several months and to recover to the culturable state 270 days after nutrient addition. Our data suggest that A. butzleri can develop strategies that allow it to survive in seawater in the absence of nutrients and to persist in the environment in the VBNC state until it finds suitable conditions. The significance of the VBNC cells in the environmental transmission of A. butzleri to humans is not yet clear. Recently, we reported the isolation of toxigenic and adhesive strains of A. butzleri from a marine environment in Messina, Italy (3). Further studies are needed to demonstrate that starved cells of A. butzleri can also retain their virulence factors.
Acknowledgments
This study was supported by research grants (2004 financing) from the University of Messina, Italy.
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Baffone, W., B. Citterio, E. Vittoria, A. Casaroli, R. Campana, L. Falzano, and G. Donelli. 2003. Retention of virulence in viable but non-culturable halophilic Vibrio spp. Int. J. Food Microbiol. 89:31-39. [DOI] [PubMed] [Google Scholar]
- 2.Ben Kahla-Nakbi, A., A. Besbes, K. Chaieb, M. Rouabhia, and A. Bakhrouf. 2007. Survival of Vibrio alginolyticus in seawater and retention of virulence of its starved cells. Mar. Environ. Res. 64:469-478. [DOI] [PubMed] [Google Scholar]
- 3.Carbone, M., T. L. Maugeri, M. Giannone, C. Gugliandolo, A. Midiri, and M. T. Fera. 2003. Adherence of environmental Arcobacter butzleri and Vibrio spp. isolates to epithelial cells in vitro. Food Microbiol. 20:611-616. [Google Scholar]
- 4.Donachie, S. P., J. P. Bowman, S. L. W. On, and M. Alam. 2005. Arcobacter halophilus sp. nov., the first obligate halophile in the genus Arcobacter. Int. J. Syst. Evol. Microbiol. 55:1271-1277. [DOI] [PubMed] [Google Scholar]
- 5.Fera, M. T., T. L. Maugeri, C. Gugliandolo, C. Beninati, M. Giannone, E. La Camera, and M. Carbone. 2004. Detection of Arcobacter spp. in the coastal environment of the Mediterranean Sea. Appl. Environ. Microbiol. 70:1271-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houf, K., S. L. W. On, T. Coenye, J. Mast, J. Van Hoof, and P. Vandamme. 2005. Arcobacter cibarius sp. nov., isolated from broiler carcasses. Int. J. Syst. Evol. Microbiol. 55:713-717. [DOI] [PubMed] [Google Scholar]
- 7.Lleò, M. M., S. Pierobon, M. C. Tafi, C. Signoretto, and P. Canepari. 2000. mRNA detection by reverse transcription-PCR for monitoring viability over time in an Enterococcus faecalis viable but nonculturable population maintained in a laboratory microcosm. Appl. Environ. Microbiol. 66:4564-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansfield, L. P., and S. J. Forsythe. 2000. Arcobacter butzleri, A. skirrowii and A. cryaerophilus—potential emerging human pathogens. Rev. Med. Microbiol. 11:161-170. [Google Scholar]
- 9.Maugeri, T. L., C. Gugliandolo, M. Carbone, D. Caccamo, and M. T. Fera. 2000. Isolation of Arcobacter spp. from a brackish environment. New Microbiol. 23:143-149. [PubMed] [Google Scholar]
- 10.McClung, C. R., D. G. Patriquin, and R. E. Davis. 1983. Campylobacter nitrofigilis sp. nov., a nitrogen-fixing bacterium associated with roots of Spartina alterniflora Loisel. Int. J. Syst. Bacteriol. 33:605-612. [Google Scholar]
- 11.Moreno, Y., S. Botella, J. L. Alonso, M. A. Ferrús, M. Hernández, and J. Hernández. 2003. Specific detection of Arcobacter and Campylobacter strains in water and sewage by PCR and fluorescent in situ hybridization. Appl. Environ. Microbiol. 69:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver, J. D. 2005. The viable but nonculturable state in bacteria. J. Microbiol. 43:93-100. [PubMed] [Google Scholar]
- 13.Pernthaler, J., F. O. Glockner, W. Schonhuber, and R. Amann. 2001. Fluorescence in situ hybridization (FISH) with rRNA-target oligonucleotide probes. Methods Microbiol. 30:207-226. [Google Scholar]
- 14.Phillips, C. A. 2001. Arcobacters as emerging human foodborne pathogens. Food Control 12:1-6. [Google Scholar]
- 15.Pruzzo, C., R. Tarsi, M. M. Lleò, C. Signoretto, M. Zampini, L. Pane, R. R. Colwell, and P. Canepari. 2003. Persistence of adhesive properties in Vibrio cholerae after long-term exposure to sea water. Environ. Microbiol. 5:850-858. [DOI] [PubMed] [Google Scholar]
- 16.Rice, E. W., M. R. Rodgers, I. V. Wesley, C. H. Johnson, and S. A. Tanner. 1999. Isolation of Arcobacter butzleri from ground water. Lett. Appl. Microbiol. 28:31-35. [DOI] [PubMed] [Google Scholar]
- 17.Vandenberg, O., A. Dediste, K. Houf, S. Ibekwem, H. Souayah, S. Cadranel, N. Douat, G. Zissis, J. P. Butzler, and P. Vandamme. 2004. Arcobacter species in humans. Emerg. Infect. Dis. 10:1863-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Driessche, E., K. Houf, J. van Hoof, L. De Zutter, and P. Vandamme. 2003. Isolation of Arcobacter species from animal feces. FEMS Microbiol. Lett. 229:243-248. [DOI] [PubMed] [Google Scholar]
- 19.Weichart, D., D. McDougald, D. Jacobs, and S. Kjelleberg. 1997. In situ analysis of nucleic acids in cold-induced nonculturable Vibrio vulnificus. Appl. Environ. Microbiol. 63:2754-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wirsen, C. O., S. M. Sievert, C. M. Cavanaugh, S. J. Molyneaux, A. Ahmad, L. T. Taylor, E. F. DeLong, and C. D. Taylor. 2002. Characterization of an autotrophic sulfide-oxidizing marine Arcobacter sp. that produces filamentous sulfur. Appl. Environ. Microbiol. 68:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wybo, I., J. Breynaert, S. Lauwers, F. Lindenburg, and K. Houf. 2004. Isolation of Arcobacter skirrowii from a patient with chronic diarrhea. J. Clin. Microbiol. 42:1851-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]