Abstract
The present study shows that feces samples of 14 human volunteers and isolated gut segments of mice (small intestine, cecum, and large intestine) are able to transform metals and metalloids into volatile derivatives ex situ during anaerobic incubation at 37°C and neutral pH. Human feces and the gut of mice exhibit highly productive mechanisms for the formation of the toxic volatile derivative trimethylbismuth [(CH3)3Bi] at rather low concentrations of bismuth (0.2 to 1 μmol kg−1 [dry weight]). An increase of bismuth up to 2 to 14 mmol kg−1 (dry weight) upon a single (human volunteers) or continuous (mouse study) administration of colloidal bismuth subcitrate resulted in an average increase of the derivatization rate from approximately 4 pmol h−1 kg−1 (dry weight) to 2,100 pmol h−1 kg−1 (dry weight) in human feces samples and from approximately 5 pmol h−1 kg−1 (dry weight) to 120 pmol h−1 kg−1 (dry weight) in mouse gut samples, respectively. The upshift of the bismuth content also led to an increase of derivatives of other elements (such as arsenic, antimony, and lead in human feces or tellurium and lead in the murine large intestine). The assumption that the gut microbiota plays a dominant role for these transformation processes, as indicated by the production of volatile derivatives of various elements in feces samples, is supported by the observation that the gut segments of germfree mice are unable to transform administered bismuth to (CH3)3Bi.
The transformation of metals and metalloids [metal(loid)s] into volatile derivatives by methylation or hydridization plays an important role in spreading and cycling these elements in our natural and anthropogenetically modified environment (6, 25). These transformations are catalyzed to a large part by organisms, mainly by microorganisms growing under anaerobic conditions. Several elements such as arsenic, antimony, bismuth, selenium, tellurium, and mercury are known or thought to be susceptible to these biotransformations (2, 5, 9, 17-22, 25).
Since many of these volatile derivatives are more toxic than their (mostly inorganic) precursors, such as Sb, Te, and Bi (1, 11, 14, 24), these processes may have an impact on human health. A high risk can be expected from scenarios in which these derivatives are accumulated in closed systems such as compartments of living organisms. The preliminary observation that feces from human volunteers emit volatile derivatives of metal(loid)s following incubation of feces samples under in vivo-like conditions (strict anaerobiosis, 37°C) suggests that the human gut microbiota is able to catalyze these transformation reactions in situ (12). Therefore, such processes may occur within the human body.
In the present study, we focused on the transformation of bismuth (administered to human volunteers and mice as colloidal bismuth subcitrate [CBS]) into its volatile derivatives monomethylbismuthdihydride [(CH3)BiH2], dimethylbismuthhydride [(CH3)2BiH], trimethylbismuth [(CH3)3Bi], and bismuthine (BiH3), since bismuth is widely used in a variety of applications: e.g., in pharmaceuticals (for example, in the treatment of peptic ulcers and of hemorrhoids), cosmetics (e.g., pigments), catalysts, industrial pigments, alloys, and ceramical additives (8). Especially the use of bismuth in cosmetics and pharmaceutical products such as the pearlescent pigment bismuth oxychloride (BiOCl) in cosmetics and the use of bismuth tartrate, aluminate, carbonate, subgallate, subcitrate, nitrate, or subsalicylate in pharmaceutical products leads to an intimate exposure of humans to bismuth. Poisonings by bismuth and bismuth compounds have occurred mainly during prolonged medical therapy, causing renal failures or mental disorders. Some hundred cases of encephalopathy—some of them fatal—were reported in France and Australia after prolonged intake of bismuth subgallate and subnitrate in high dosages in the 1970s and 1980s (3, 4). However, the clinical use of CBS does not appear to cause neurotoxicity (23).
To check how widespread the processes of metal(loid) derivatization are in the human population, we extended our study to a larger number of human volunteers (14 male subjects); to investigate if the gut microbiota is responsible for these derivatization processes, conventionally raised as well as germfree mice were used. The mouse model was also used to determine the transformation capabilities of various segments of the mammalian gut (small intestine, cecum, and large intestine).
MATERIALS AND METHODS
Human volunteer study.
Feces samples from 14 healthy human volunteers (male Caucasians, ages 25 to 31 years, nonsmokers, nonvegetarians, and body mass indexes of 19 to 26 kg m−2) were collected to study the metabolism of CBS. All human volunteers gave consent, and the study was approved by the ethics committee of the Medical Faculty of the University of Duisburg-Essen. Each human volunteer ingested two CBS-containing tablets (De-Noltab; Yamanouchi Europe B.V., The Netherlands), equivalent to a total amount of 215 mg bismuth. Before and after the administration of CBS, feces samples were examined for the production of volatile bismuth and other volatile metal(loid) derivatives by a purge-and-trap gas chromatographic (PT-GC) system coupled to an inductively coupled plasma mass spectrometer (ICP-MS; Fisons VG, PlasmaQuad II) (22). The production of methane was checked by gas chromatography with a flame ionization detector (GC-FID) (21), and the total metal(loid) content was determined by microwave digestion following ICP-MS analysis (12).
Sampling and incubation studies.
The first feces samples were collected from the human volunteers before the ingestion of the tablet (control), and up to six samples were taken over the following 5 days (65 samples in total). The human volunteers transferred 10 to 50 g feces into argon-filled, autoclaved (121°C, 20 min) 300-ml gas-tight-sealed glass bottles. To remove oxygen, the bottles were purged with helium after sample transfer. Subsequently, the samples were suspended in an autoclaved phosphate-buffered saline (PBS) buffer (1 ml g−1 feces; 8 g liter−1 NaCl, 0.2 g liter−1 KCl, 1.8 g liter−1 Na2PO4·2H2O, 0.24 g liter−1 KH2PO4 [pH 7.4]), which was equilibrated in an anaerobic (95% N2-5% H2) chamber (CoyLab Inc., Grass Lake, MI) before being added to the sample with a sterile syringe. The bottles were mantled with aluminum foil to darken and thus to prevent decomposition of the produced volatile metal(loid) derivatives by light and were incubated at 37°C while being shaken (200 rpm). The gas phases were analyzed for the production of volatile metal(loid) compounds in intervals of 5 to 60 h by PT-GC/ICP-MS over a time period of up to 4 weeks. Methane production was monitored by GC-FID. The frequency of the measurements depended on the amount of volatile metal(loid) compounds produced.
Mouse study.
To investigate the role of the gut microbiota in bismuth transformation, we conducted feeding experiments with conventionally raised and germfree C3H/HeJ mice (Mus musculus, males, ages 12 to 17 weeks). All experiments were carried out in accordance with the German guidelines for animal studies. Animal husbandry for conventionally raised mice was performed by the Central Animal Laboratory of the University Hospital Essen, and germfree mice were bred in the Central Animal Laboratory of the University Hospital Hannover and kept under sterile conditions.
The animals were housed individually in standard Macrolone cages type III (38 by 22 by 15 cm) with sawdust as bedding material under standard laboratory conditions (12-h light-12-h dark cycle with lights on at 8 a.m.; temperature, 21 ± 1°C; relative humidity, 55% ± 10%). The mice were fed either with a commercial standard mouse diet (SNIFF V1534) or a gamma radiation-sterilized, CBS-enriched standard diet (SNIFF V1534Bi; bismuth content of 35 mg kg−1) (SNIFF GmbH, Soest, Germany). The diet and bottled tap water were available ad libitum. The bismuth uptake of mice on the bismuth-enriched diet was calculated to be approximately 7 mg kg−1 day−1. For reinfection experiments, each germfree male mouse was housed together with a conventionally raised female mouse for 10 days to allow a recolonization of the germfree mouse gut by uptake of feces from the conventionally raised mouse before the feeding experiments.
The feeding experiments were conducted for 7 days in four groups: a control group (seven individuals of conventionally raised mice) fed with standard diet, a bismuth group (eight conventionally raised mice fed with a bismuth-enriched diet), a germfree bismuth group (three germfree mice fed with bismuth-containing chow), and a reinfected bismuth group (five reinfected conventionally raised mice fed with bismuth-containing diet).
Sampling and incubation studies.
After 7 days of feeding with the standard diet or the bismuth-containing diet, the mice were dissected under sterile and anaerobic conditions (sterile cages filled with argon before dissection). Their colon segments (divided into cecum and large and small intestine) were extracted and transferred into autoclaved, argon-filled, and gas-tight-sealed 10-ml brown glass tubes, and oxygen-free sterile filtrated PBS buffer was added (10 ml g−1 sample).
All samples were incubated at 37°C in a darkened shaker (200 rpm) and examined at intervals of 12 to 60 h for the production of volatile metal(loid) derivatives by PT-GC/ICP-MS and for the production of methane by GC-FID during a time period of up to 3 weeks, depending on the production of volatile metal(loid) compounds observed in each sample.
Biotransformation in mouse feces.
To further investigate the role of the gut microbiota in bismuth transformation, feces samples from conventionally raised and germfree mice fed with CBS-containing chow as well as feces samples from germfree mice, which were infected with feces from conventionally raised mice, were examined for biotransformation. Feces samples (0.5 to 1 g) were transferred into autoclaved, argon-filled, and gas-tight-sealed 10-ml brown glass tubes, and anaerobic sterile filtrated PBS buffer was added (10 ml g−1 sample). For the infection of germfree mouse feces, samples were inoculated with 20% of a feces suspension from conventionally raised mice. All samples were incubated at 37°C in a darkened shaker (200 rpm) and examined after 3 days for the production of volatile bismuth derivatives by PT-GC/ICP-MS.
Analytical methods.
The volatile metal(loid) compounds in the headspace of the samples were analyzed using a modified PT-GC system coupled to an ICP-MS as an element-specific detector as described previously (22). The different volatile species were detected by chromatographic retention and the mass/charge ratios of the respective metal(loid)s (m/z 75 As, m/z 120 Sn, m/z 121 Sb, m/z 126 Te, m/z 208 Pb, m/z 209 Bi). Limits of detection (LOD) of the analytes were calculated with the 3σ criterion: i.e., 57.9 fmol for antimony, 149.1 fmol for arsenic, 11 fmol for bismuth, 8.7 fmol for lead, 317.6 fmol for tellurium, and 4.3 fmol for tin.
For the determination of the total metal(loid) content of the feces and the colon segments, samples of 0.2 to 0.7 g were suspended in 4 ml HNO3 (65%, subboiled) and 2 ml H2O2 (30%, Suprapur; Merck, Darmstadt, Germany), digested in a microwave-accelerated reaction system (MARS 5; CEM, Kamp Lintfort, Germany), and analyzed by ICP-MS-analysis (12). All determinations were calculated per kg (dry weight) as determined by heating the samples at 110°C until a constant weight was achieved.
The methane concentration in the sample headspace was analyzed by withdrawing a defined gas sample with a gas-tight syringe and injecting it into a Hewlett Packard gas chromatograph (5890 II) equipped with a J&W Scientific capillary column (DB 5; 30 m by 0.25-mm inside diameter, coated with 5% phenylsilicon-95% methylsilicon) and an FID, as described previously (21).
Statistics.
The nonparametric Mann-Whitney-Wilcoxon U test was used (significance level, P < 0.05) for statistical analysis of the data.
RESULTS
The aim of the present study was to assess the capability of the microbiota of the human distal gut as well as of the murine gut segments (small intestine, cecum, and large intestine) to transform metal(loid)s into volatile derivatives by analyzing the microbial production of these derivatives in anaerobically incubated samples of feces or gut segments, respectively.
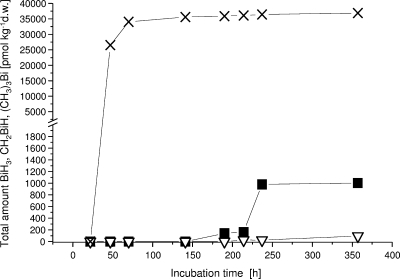
Metal(loid) transformation in the human distal gut was analyzed by means of feces samples collected from 14 male human volunteers prior to and after a single administration of 215 mg bismuth as CBS. To elucidate the transformation capabilities of the various segments of the mammalian gut (small intestine, cecum, and large intestine), conventionally raised mice were continuously fed with chow containing or devoid of CBS. To find additional evidence that the mammalian gut microbiota is mainly responsible for these derivatization processes, germfree mice were analyzed in parallel for the production of the volatile derivatives. Analyses of the transformation processes were performed by anaerobic incubation of the samples ex situ. To get as direct as possible a measure of transformation in vivo, we determined the initial rates of transformation immediately after sampling, which represents generally also the maximal rates, to avoid restriction of the reactions by substrate limitation or by interfering products of the microbial metabolism accumulated over a prolonged period of incubation. Long-term incubation, however, was also performed to detect slowly evolving derivatives. Methane production was monitored to check for correlation with the metal(loid) derivatization. As an example, Fig. 1 shows a representative time course for the production of (CH3)3Bi, (CH3)BiH2, and BiH3 in an anaerobically incubated feces sample collected from participant 1, 23 h after a single administration of 215 mg bismuth as CBS.
FIG. 1.
Production of volatile bismuth derivatives in a feces sample delivered by human volunteer 1 after 23 h of CBS administration. ▪, BiH3; ▿, CH3BiH2; and X, (CH3)3Bi. d.w., dry weight.
Single-bismuth administration to human volunteers: effects on the transformation of metal(loid)s into volatile derivatives in feces samples.
As shown in Table 1, some feces samples collected from the participants prior to the administration of CBS already showed production rates of the volatile bismuth derivatives CH3BiH2, (CH3)2BiH, (CH3)3Bi, and BiH3 in the range of 0.6 to 3.8 pmol h−1 kg−1 (dry weight), while the bismuth content of the samples was as low as 0.2 μmol kg−1 (dry weight) (Table 2).
TABLE 1.
Maximum production rates of volatile metal(loid) derivatives in the headspace of incubated feces samples from human volunteers before and after administration of CBS
| Volatile metal(loid) derivative | Mean (range) of maximum production rate in pmol h−1 kg−1 (dry wt):
|
|
|---|---|---|
| Before CBS administration | After CBS administrationa | |
| BiH3 | 1.4 (LOD-9.1) | 4.4 (LOD-94.9) |
| CH3BiH2 | 2.5 (LOD-17.1) | 3.6 (LOD-78.3) |
| (CH3)2BiH | 0.6 (LOD-6.5) | 0.07 (LOD-0.8) |
| (CH3)3Bi | 3.8 (0.3-17.1) | 2,108 (120.4-28,740) |
| AsH3 | LOD | 1.4 (LOD-46) |
| CH3AsH2 | 0.03 (LOD-0.3) | 5 (LOD-132.8) |
| (CH3)2AsH | LOD | 0.9 (LOD-18.1) |
| (CH3)3As | LOD | 0.7 (LOD-13.5) |
| SbH3 | LOD | 0.02 (LOD-0.3) |
| CH3SbH | 0.1 (LOD-1.1) | 0.5 (LOD-8.4) |
| (CH3)3Sb | 0.4 (LOD-2.0) | 4.6 (LOD-96.5) |
| (CH3)2Te | 54.3 (LOD-375) | 40 (LOD-657) |
| (CH3)4Pb | 0.03 (LOD-0.3) | 1.3 (LOD-17.2) |
| (CH3)4Sn | LOD | 0.05 (LOD-1.1) |
Samples containing >500 μmol Bi kg−1 (dry weight).
TABLE 2.
Element content in feces samples from human volunteers before and after administration of CBS
| Metal(loid) | Mean (range) element content in μmol kg−1 (dry wt):
|
|
|---|---|---|
| Before CBS administration | After CBS administrationa | |
| Bi | 0.2 (LOD-2.0) | 13,700 (1,420-60,700) |
| As | 1.5 (0.3-4.2) | 1.3 (0.2-4.1) |
| Sb | 0.4 (0.1-0.9) | 0.5 (0.1-1.3) |
| Pb | 1.5 (0.5-4.4) | 2.5 (0.8-8.3) |
Samples containing >500 μmol Bi kg−1 (dry weight).
Considerable production of volatile species of tellurium, and to a lesser extent of volatile derivatives of arsenic, antimony, and lead, was detected in preadministration samples, indicating also high-affinity derivatization mechanisms for these elements (Table 1).
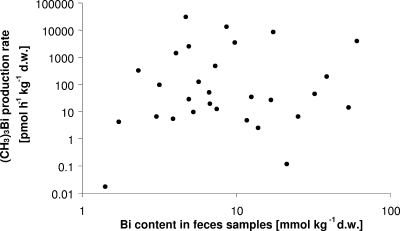
In postadministration samples containing more than 1 mmol Bi kg−1 (dry weight), the maximum production rates increased, being highest for (CH3)3Bi (approximately 500-fold). However, no correlation was found between the bismuth content of the samples containing bismuth at concentrations of 1.4 to 60.7 mmol Bi kg−1 (dry weight) and the production of volatile bismuth derivatives (Fig. 2).
FIG. 2.
Relationship between total Bi content and maximum (CH3)3Bi production rate in incubated human feces samples. d.w., dry weight.
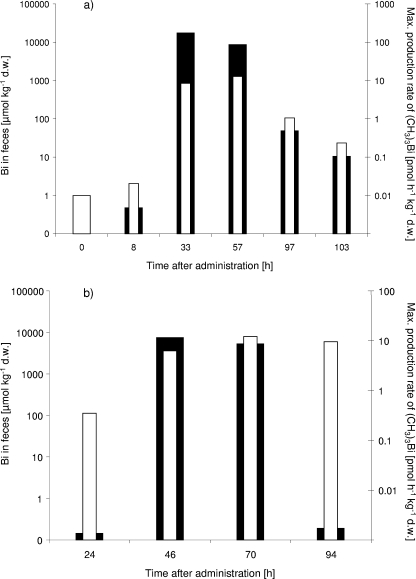
Although no general correlation between the bismuth content of samples taken from postadministration feces and the production rate of volatile bismuth derivatives could be established, a relationship between the production of volatile bismuth derivatives and the respective bismuth content was observed in individual participants. An example is shown in Fig. 3a, which depicts the bismuth content and the (CH3)3Bi production rate of the feces samples taken from participant 2 over 94 h after CBS administration. In contrast, the (CH3)3Bi production rate in the feces sample of participant 3 is independent of bismuth content (103 h after CBS administration; Fig. 3b).
FIG. 3.
Total Bi content (solid bars) and production rates of (CH3)3Bi (open bars) in feces samples from human volunteers 2 (a) and 3 (b) delivered before and after administration of CBS. d.w., dry weight.
This heterogeneous response to the CBS administration, which might be due in part to food composition or peristalsis, and, perhaps more probably, to physiologically based differences between the participants, is also reflected by the high variance of the time courses of the transformation productivity after CBS administration. Thus, the highest production rates of (CH3)3Bi were observed in a rather broad time interval of 20 to 80 h after CBS administration. The earliest sample exhibiting elevated production of (CH3)3Bi was collected from participant 4, already after 5 h of CBS administration. The latest sample revealing elevated (CH3)3Bi production was collected from participant 2, 129 h after administration of CBS.
Remarkably, not only the production of volatile bismuth derivatives increased in the postadministration samples, but also the production rates of volatile derivatives of the elements arsenic, antimony, tin, and lead (Table 1), although the contents of these metal(loid)s in the feces samples remained virtually unchanged upon the administration of CBS (Table 2).
Continuous bismuth administration to conventionally raised and germfree mice: effects on the metal(loid) derivatization in the various gut segments (small intestine, cecum, and large intestine) and the role of the gut microbiota in biovolatilization of metal(loid)s.
To investigate the influence of bismuth on the transformation of metal(loid)s into volatile derivatives in mice, the animals were fed with CBS-enriched chow. After adaptation for 7 days, the production of volatile derivatives was monitored in the small intestine, the cecum, and the large intestine by incubating the dissected gut parts separately under anaerobic conditions. Conventionally raised mice fed without CBS were used as a control. Similar to the preadministration feces samples of the human volunteers, the samples of the large intestine from the control mice showed significant production of several volatile derivatives of metal(loid)s present only at rather low concentrations (such as bismuth, tellurium, and antimony). This indicates high-affinity transformation activities of the murine gut (Tables 3 and 4). Contrary to the preadministration feces of the human volunteers, however, (CH3)3Bi represents the only transformation product of bismuth in the large intestine of the control mice (this finding applies also to the cecum and the small intestine) (Table 5). As exemplified in the case of the large intestine of mice and in accordance with the studies of the human feces samples, the production of volatile bismuth derivatives [besides (CH3)3Bi] is significantly stimulated by the increased concentration of bismuth in mice fed with CBS-enriched chow (Tables 3 and 4). In mice fed with CBS-enriched chow, the highest production rates of (CH3)3Bi were found in the cecum, whereas the small intestine showed the lowest productivity of (CH3)3Bi (Table 5).
TABLE 3.
Maximum production rates of volatile metal(loid) derivatives in the headspace of incubated large intestine samples from conventionally raised mice fed with standard or CBS-containing chow
| Volatile metal(loid) derivative | Mean (range) of maximum production rate in pmol h−1 kg−1 (dry wt)
|
|
|---|---|---|
| Standard chow | CBS-containing chowa | |
| BiH3 | LOD | 0.5 (LOD-2.3) |
| CH3BiH2 | LOD | 0.1 (LOD-0.8) |
| (CH3)3Bi | 5.0 (LOD-20.1) | 121.8 (0.6-378.1) |
| (CH3)2Te | 7.5 (LOD-52.4) | 16.4 (LOD-105) |
| (CH3)4Pb | LOD | 3.0 (LOD-12.3) |
| (CH3)3Sb | 3.5 (LOD-23.8) | 0.2 (LOD-1.6) |
Bi content of 35 mg kg−1.
TABLE 4.
Element content in feces samples from large intestine samples from conventionally raised mice fed with standard or CBS-containing chow
| Metal(loid) | Mean (range) element content in μmol kg−1 dry wt
|
|
|---|---|---|
| Standard chow | CBS-containing chowa | |
| Bi | 1.0 (LOD-4.9) | 350 (69.7-623.9) |
| Te | 0.4 (LOD-1.5) | 0.4 (0.2-0.6) |
| Pb | 2.3 (1-3.8) | 3.5 (0.6-6.3) |
| Sb | 1.9 (0.1-6.2) | 2.3 (0.1-4.5) |
Bi content of 35 mg kg−1.
TABLE 5.
Production of (CH3)3Bi and CH4 in incubated gut segments from conventional, germfree, and reinfected mice fed with standard or CBS-containing chow
| Mouse group and chow typea | Gut segment | Mean (range) Bi content in μmol kg−1 (dry wt) | Mean (range) of maximum (CH3)3Bi production rate in pmol h−1 kg−1 (dry wt) | Mean (range) CH4 production rate in mmol h−1 kg−1 (dry wt) |
|---|---|---|---|---|
| Conventional | ||||
| Standard chow | Small intestine | 0.4 (LOD-1.9) | 2.9 (1.4-5.5) | 0.3 (0.02-2.03) |
| Large intestine | 1.0 (LOD-4.9) | 5.0 (LOD-20.1) | 0.9 (LOD-6.03) | |
| Cecum | 0.4 (LOD-1.5) | 2.0 (0.1-5.4) | 1.8 (0.04-11) | |
| CBS-containing chow | Small intestine | 296.4 (97.3-498.7) | 1.9 (0.07-6.0) | 0.2 (0.01- 0.6) |
| Large intestine | 346.8 (69.7-623.9) | 121.8 (0.6-378.1) | 1.4 (0.06-2.8) | |
| Cecum | 95.6 (59.4-131.9) | 689.2 (10.4-2411.2) | 0.3 (0.1-0.5) | |
| Germfree | ||||
| CBS-containing chow | Small intestine | 144.0 (91.9-236.8) | LOD | LOD |
| Large intestine | 194.9 (113.7-277.8) | LOD | LOD | |
| Cecum | 283.2 (210.7-322.3) | LOD | LOD | |
| Reinfected | ||||
| CBS-containing chow | Small intestine | 51.9 (26.2-98.2) | 11.0 (1.2-38.4) | 0.05 (0.02-0.07) |
| Large intestine | 230.6 (93.9-321.3) | 422.6 (142.3-820.1) | 0.1 (LOD-0.5) | |
| Cecum | 286.7 (179.2-407.3) | 266.4 (12.1-496.5) | 0.06 (LOD-0.3) |
The Bi content of CBS-containing chow was 35 mg kg−1 for each group.
Increased production of the volatile derivatives of other elements simultaneous with an increased bismuth concentration in the murine gut was only observed in the case of tellurium and lead (Table 3), but only the increase of (CH3)4Pb proved to be significant (P = 0.04).
To find further support for the assumption that the gut microbiota plays a crucial or even the essential role in the production of these volatile metal(loid) derivatives, we investigated the production of these compounds by gut segments of germfree mice fed with CBS-containing chow. In three independent experiments, none of the dissected gut segments was able to produce (CH3)3Bi (Table 5) or any other volatile metal(loid) derivatives. In contrast, three other germfree mice fed with CBS-containing chow, which were reinfected by common housing with conventionally raised mice for 10 days to recolonize their gut, showed transformation activities in the dissected gut sections which are comparable to those of conventionally raised mice fed with CBS-containing chow (Table 5). Further support for the assumption that the gut microbiota plays an essential role in the production of volatile metal(loid) derivatives comes from the restoration of (CH3)3Bi production in germfree mouse feces samples which were inoculated with a suspension of feces from conventionally raised mice fed without CBS. The (CH3)3Bi production rates in these samples (0.12 ± 0.09 pmol h−1 kg−1) were comparable to feces samples from conventionally raised mice fed with CBS-containing chow (0.16 ± 0.06 pmol h−1 kg−1), whereas in incubated feces samples from germfree mice no production of (CH3)3Bi was detectable.
Volatilization of metal(loid)s and methane production in the gut: hints for a correlation?
A recent study (17) suggested that members of methanoarchaea in general play a pivotal role in processes transforming ionic species of metal(loid)s into volatile derivatives. Since methanoarchaeal strains of the human gut such as Methanosphaera stadtmanae and Methanobrevibacter smithii also showed a higher derivatization potential in comparison to bacterial gut isolates, it is reasonable to assume that methanoarchaea play a notable role in these processes in the human gut as well. A further argument for their general involvement in metal(loid) derivatization is their obligatory presence in the human gut, as shown by DNA analyses of Eckburg et al. (7), which is confirmed by our present analyses detecting methane production in the feces samples of all 14 human volunteers (mean value of methane production, 650 μmol h−1 kg−1 [dry weight]). Also, the gut samples of all conventionally raised mice used in the present study produced methane in the same order of magnitude (Table 5). However, no relationship between the maximum rates of methane production and (CH3)3Bi formation of all feces samples containing more than 0.5 mmol Bi kg−1 dry weight (to avoid bismuth limitations) could be found (data not shown).
DISCUSSION
As indicated by the presented ex situ experiments, transformation of metals and metalloids into volatile derivatives is a common feature of the human and murine gut. Previous studies only hinted at the existence of processes in the mammalian gut which lead to the transformation of metal(loid)s to nonvolatile metal(loid) derivatives, such as the transformation of arsenate or arsenite to mono- and dimethylarsonate (10) or the metabolization of dimthylarsonate to a still unknown but also nonvolatile arsenic derivative (26) by the cecum contents of rats.
The transformations of metal(loid)s into volatile hydride and methyl metal(loid) derivatives in humans and mice described in the present study are characterized by a similar precursor/product spectrum comprising inorganic species of antimony, bismuth, tellurium, and lead as precursors and the permethylated derivatives of these elements as the main products. Similarly, in both humans and mice, the reactions exhibit a remarkably high affinity to inorganic metal(loid) species. In our experiments with bismuth, we found derivatization in feces and gut at concentrations of 0.2 to 1.0 μmol kg−1 (dry weight) only. Furthermore—as shown for bismuth—the addition of an element susceptible to transformation is answered in both systems not only by an increase of its own transformation but also by enhanced production of derivatives of other elements, although not firmly statistically supported in every case. Both effects—the strikingly high affinity of the transformation reactions and their synergistic interaction—point to a risk of poisoning by these processes either directly [by their high general susceptibility to metal(loid)s at low concentrations] or indirectly [by the production of toxic metal(loid) derivatives through ingestion of other metal(loid) species].
We are very close to assuming that the observed similarities are mainly determined by the gut microbiota involved in both systems. Its leading role in the described transformation processes can be deduced from the transformation activity of feces samples, which consists for the overwhelming part of prokaryotic cells without noteworthy counts of viable epithelial cells, and from the observation that the gut parts of germfree mice are unable to transform administered bismuth into (CH3)3Bi. As found by Ley et al. (15), the compositions of the gut microbiota of mice and humans seem to be comparable; this would support our assumption concerning the main responsibility of the microbiota for the similar transformation phenotypes in humans and mice. Despite everything, we cannot explicitly exclude that epithelial cells also contribute to the transformation processes under in vivo conditions, assuming that the presence of an intact gut microbiota is required for such activity. Such interactions have been verified by several investigations which showed that gut microbiota can have considerable influence on mucosal tissues resulting in strong modulations of the host cell metabolism (13, 15, 16).
The observation that all incubation assays for measuring the production of volatile metal(loid) derivatives showed methane production—often in parallel—and the generally high capability of methanoarchaea to transform metal(loid)s into volatile derivatives (17) led to the suggestion that methanoarchaea play an essential role in these transformation processes also in the mammalian gut. As shown by statistical analyses, however, the production of volatile metal(loid) derivatives does not correlate with methane production, indicating that such a simple monofactorial correlation does not exist in our rather heterogeneous reaction assays.
The fact that the methane production by the intestine of reinfected mice was strikingly lower than that of “normal” conventionally raised mice despite comparable (CH3)3Bi production (Table 5), which could be due to an incomplete adaptation of the gut microbiota to the previously sterile gut environment, may be a hint that microorganisms other than methanoarchaea contribute to the metal(loid) derivatization of the intestine.
Acknowledgments
This research was supported by the Deutsche Forschungsgemeinschaft (Forschergruppe FOR415/3).
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Andrewes, P., K. T. Kitchin, and K. Wallace. 2004. Plasmid DNA damage caused by stibine and trimethylstibine. Toxicol. Appl. Pharmacol. 194:41-48. [DOI] [PubMed] [Google Scholar]
- 2.Bentley, R., and T. G. Chasteen. 2002. Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol. Mol. Biol. Rev. 66:250-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buge, A., G. Rancurel, M. Poisson, and H. Dechy. 1974. Encephalopathies myocloniques par les sels de bismuth. Nouv. Presse Méd. 3:2315-2320. [PubMed] [Google Scholar]
- 4.Burns, R., D. W. Thomas, and V. J. Barrow. 1974. Reversible encephalopathy possibly associated with bismuth subgallate ingestion. Br. Med. J. 1:220-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig, P. J. (ed.) 2003. Organometallic compounds in the environment, 2nd ed. John Wiley & Sons, Chichester, United Kingdom.
- 6.Dopp, E., L. M. Hartmann, A. M. Florea, A. W. Rettenmeier, and A. V. Hirner. 2004. Environmental distribution, analysis, and toxicity of organometal(loid) compounds. Crit. Rev. Toxicol. 34:301-333. [DOI] [PubMed] [Google Scholar]
- 7.Eckburg, P. B., E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 308:1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fickling, M. 1999. Review of bismuth in 1998. Bull. Bismuth Inst. 74:3-4. [Google Scholar]
- 9.Gadd, G. M. 1993. Microbial formation and transformation of organometallic and organometalloid compounds. FEMS Microbiol. Rev. 11:297-316. [Google Scholar]
- 10.Hall, L. L., S. E. George, M. J. Kohan, M. Styblo, and D. J. Thomas. 1997. In vitro methylation of inorganic arsenic in mouse intestinal cecum. Toxicol. Appl. Pharmacol. 147:101-109. [DOI] [PubMed] [Google Scholar]
- 11.Hathaway, G. J., N. H. Proctor, J. P. Hughes, and M. L. Fischman. 1991. Proctor and Hughes' chemical hazards of the workplace, 3rd ed. Van Nostrand Reinhold, New York, NY.
- 12.Hirner, A. V., L. M. Hartmann, J. Hippler, J. Koesters, K. Michalke, M. Sulkowski, and A. W. Rettenmeier. 2004. Organometal(loid) compounds associated with human metabolism, p. 181-203. In A. V. Hirner and H. Emons (ed.), Organic metal and metalloid species in the environment: analysis, distribution, processes and toxicological evaluation. Springer-Verlag, Heidelberg, Germany.
- 13.Hooper, L. V. 2004. Bacterial contributions to mammalian gut development. Trends Microbiol. 12:129-134. [DOI] [PubMed] [Google Scholar]
- 14.Laden, B. P., and T. D. Porter. 2001. Inhibition of human squalene monooxygenase by tellurium compounds: evidence of interaction with vicinal sulfhydryls. J. Lipid Res. 42:235-240. [PubMed] [Google Scholar]
- 15.Ley, R. E., F. Bäckhed, P. Turnbaugh, C. A. Lozupone, R. D. Knight, and J. I. Gordon. 2005. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 102:11070-11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, M., B. Wang, M. Zhang, M. Rantalainen, S. Wang, H. Zhou, Y. Zhang, J. Shen, X. Pang, M. Zhang, H. Wei, Y. Chen, H. Lu, J. Zuo, M. Su, Y. Qiu, W. Jia, C. Xiao, L. M. Smith, S. Yang, E. Holmes, H. Tang, G. Zhao, J. K. Nicholson, L. Li, and L. Zhao. 2008. Symbiotic gut microbes modulate human metabolic phenotypes. Proc. Natl. Acad. Sci. USA 105:2117-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer, J., K. Michalke, T. Kouril, and R. Hensel. Volatilisation of metals and metalloids: an inherent feature of methanoarchaea? Syst. Appl. Microbiol., in press. [DOI] [PubMed]
- 18.Meyer, J., A. Schmidt, K. Michalke, and R. Hensel. 2007. Volatilization of metals and metalloids by the microbial population of an alluvial soil. Syst. Appl. Microbiol. 30:229-238. [DOI] [PubMed] [Google Scholar]
- 19.Michalke, K., and R. Hensel. 2004. Microbial biotransformations of metal(loid)s, p. 285-293. In A. V. Hirner and H. Emons (ed.), Organic metal and metalloid species in the environment: analysis, distribution, processes and toxicological evaluation. Springer-Verlag, Heidelberg, Germany.
- 20.Michalke, K., J. Meyer, and R. Hensel. 2007. Methylation of metal(loid)s by methanoarchaea: production of volatile derivatives with high ecotoxicological impact and health concern. In R. Garrett and H. P. Klenk (ed.), Archaea—physiology, molecular biology and evolution. Blackwell, Oxford, United Kingdom. doi: 10.1002/9780470750865.ch25. [DOI]
- 21.Michalke, K., J. Meyer, A. V. Hirner, and R. Hensel. 2002. Biomethylation of bismuth by the methanogen Methanobacterium formicicum. Appl. Organometal. Chem. 16:221-227. [Google Scholar]
- 22.Michalke, K., E. B. Wickenheiser, M. Mehring, A. V. Hirner, and R. Hensel. 2000. Production of volatile derivatives of metal(loid)s by microflora involved in anaerobic digestion of sewage sludge. Appl. Environ. Microbiol. 66:2791-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noach, L. A., J. L. Eekhof, L. J. Bour, F. E. Posthumus Meyjes, G. N. Tytgat, and B. W. Ongerboer de Visser. 1995. Bismuth salts and neurotoxicity. A randomised, single-blind and controlled study. Hum. Exp. Toxicol. 14:349-355. [DOI] [PubMed] [Google Scholar]
- 24.Sollmann, T., and J. Seifter. 1939. The pharmacology of trimethyl bismuth. J. Pharmacol. Ther. 67:17-49. [Google Scholar]
- 25.Thayer, J. S. 2002. Biological methylation of less studied elements. Appl. Organometal. Chem. 16:677-691. [Google Scholar]
- 26.Yoshida, K., K. Kuroda, X. Zhou, Y. Inoue, Y. Date, H. Wanibuchi, S. Fukushima, and G. Endo. 2003. Urinary sulfur-containing metabolite produced by intestinal bacteria following oral administration of dimethylarsinic acid to rats. Chem. Res. Toxicol. 16:1124-1129. [DOI] [PubMed] [Google Scholar]