Abstract
Culture supernatants of Lactobacillus reuteri ATCC 55730 repressed ler expression in Escherichia coli O157:H7 cells, but neither the strain's isogenic luxS mutant nor the L. reuteri 100-23C wild-type strain and its luxS mutant elicited a comparable effect. Furthermore, the epinephrine-mediated induction of ler expression was repressed by secreted substance(s) of L. reuteri ATCC 55730.
Bacterial communication by secreted signaling molecules, known as quorum sensing (QS), impacts diverse cellular processes such as virulence gene expression (reviewed in references 12 and 33). Enterohemorrhagic Escherichia coli (EHEC) strains of serotype O157:H7, the causative agents of bloody diarrhea and hemolytic uremic syndrome in humans (11, 15), harbor QS-regulated virulence genes on a pathogenicity island termed the locus of enterocyte effacement (LEE) (23, 27) that are organized mainly into the five polycistronic operons LEE1 to LEE5 (6, 11). The first gene in LEE1, LEE-encoded regulator (ler), encodes the principal transcriptional activator of the LEE genes (5). The expression of ler was shown to be controlled by the bacterial QS molecule autoinducer 3 (AI-3) and the eukaryotic catecholamine hormones epinephrine and norepinephrine; thus, virulence properties such as the attachment to host cells are influenced by the cross talk between the bacteria and the host organism (24, 25). EHEC senses both AI-3 and catecholamines through the adrenergic two-component systems QseBC and QseEF, which in turn regulate LEE and flagellum gene expression via signal transduction cascades that are complex and not fully understood (3, 20, 26). An active LuxS synthase is the precondition for the formation of AI-3, as well as yet another QS signal, AI-2 (25). LuxS is a metabolic enzyme involved in the regeneration of the methyl donor S-adenosylmethionine in the activated methyl cycle of methionine metabolism (21). While AI-2, a furanosyl borate diester, is the actual product of LuxS activity, the molecular structure and synthesis of AI-3 are unknown (25). Although LuxS does not produce AI-3 directly, marked reduction of AI-3 activity of an EHEC luxS mutant has been observed, which could be restored by the expression of amino acid transporters and the addition of aspartate (31). LuxS homologues are widespread among both gram-negative and gram-positive bacteria, and AI-2 and AI-3 are, therefore, viewed as key mediators of intra- and interkingdom QS (12, 29). Recently, the LuxS-dependent induction of EHEC O157:H7 LEE genes by Lactobacillus reuteri was demonstrated (28). The stationary-phase supernatant of L. reuteri 100-23C, a rodent isolate (13), induced ler expression, which was abolished in an isogenic luxS mutant. Thus, it is very likely that the induction was mediated by AI-3-like molecules. In contrast to these findings, a recent publication showed that molecules secreted by probiotic Lactobacillus acidophilus La-5 led to reduced virulence gene expression of EHEC O157:H7, but whether this repression was LuxS dependent remained undetermined (14).
The aim of the present study was to investigate the LuxS/AI-3-dependent regulation of EHEC O157:H7 virulence genes by substances secreted by L. reuteri strains. We constructed an isogenic luxS mutant from the probiotic strain L. reuteri ATCC 55730 (BioGaia AB, Stockholm, Sweden) and compared the effects of supernatants of the wild type and the luxS mutant on EHEC ler transcription with the effects of supernatants of the corresponding strains of L. reuteri 100-23C. For this purpose, a novel fluorescence bioassay was developed by transcriptionally fusing the ler promoter with a green fluorescent protein (gfp) gene. The assay was validated by using supernatants of EHEC O157:H7 (the AI-3 producer) and E. coli DH5α (a natural luxS mutant), as well as growth medium with and without epinephrine.
Development and validation of a fluorescence bioassay that detected ler promoter activity.
To measure the influence of external signals on ler transcription, we fused the ler promoter of EHEC O157:H7 EDL933 with the promoterless gfp(ASV) gene located on pJBA89 (1), a plasmid originally constructed to measure acyl homoserine lactone QS signals. To do this, we amplified the promoter sequence by using the primers PlerF (CTGAGAATTCTTAGAGATACTGGCTTTCAGGAAAC [the EcoRI recognition site is underlined]) and PlerR (CTGACGCATGCTTTAATATTTTAAGCTATTAGC [the SphI recognition site is underlined]). A fragment of pJBA89 containing luxR and the promoters PluxR and PluxI was removed and replaced with a 0.9-kb PCR product of the ler promoter by digestion with EcoRI and SphI and ligation. The correct insertion was verified by sequencing the resulting plasmid, pIJ001 (5.3 kb) (data not shown). E. coli MG1655 (8) was transformed with pIJ01 by electroporation (4), yielding E. coli IJ01. The E. coli K-12 derivative MG1655 was chosen as the host on the basis of the successful utilization of K-12 strains in gene expression studies using reporter fusions of EHEC genes (10, 18, 22, 23, 25), although the EHEC-specific transcription regulators GrlA/GrlR, EtrA/EivF, and Pch, which affect LEE gene expression, are not present in the K-12 background (32).
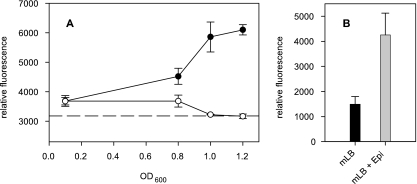
The applicability of E. coli IJ01 as the AI-3/epinephrine-responsive reporter organism in a fluorescence bioassay was established by using culture supernatants of EHEC O157:H7 strain EDL933 (with LuxS) (16) and E. coli strain DH5α (lacking LuxS; Promega), as well as growth medium with epinephrine (positive control) and without epinephrine (negative control). Supernatants were prepared by taking samples at several time points from the E. coli strains growing in modified LB (mLB) medium (with 4 g liter−1 NaCl) at 37°C, then removing cells by centrifugation and sterile filtering, and by adjusting the pH to 7.0 with 1 M NaOH. Prior to the start of fluorescence assays, E. coli IJ01 was inoculated in mLB medium containing 100 μg ml−1 ampicillin and grown aerobically at 30°C to an optical density at 600 nm (OD600) of ≤0.2. After two further subculturing steps at 30°C, the culture was diluted 1:20 in fresh mLB medium, in mLB containing 50 μM l-epinephrine, or in E. coli supernatants, all preheated to 30°C. Aliquots (200 μl) were transferred to a microtiter plate, which was then incubated with shaking at 30°C. Fluorescence (515 nm) and OD600 values were measured for up to 5 h by using a Cary-Eclipse fluorescence reader (Varian) and a model 450 microplate reader (Bio-Rad). The regulation of ler was expressed as the relative fluorescence obtained by dividing the absolute fluorescence signals by the OD600 value of the E. coli reporter strain IJ01. For this and for all following experiments, comparable amounts of growth of E. coli strain IJ01 in the different supernatants were checked (OD600) to eliminate cell density-dependent effects. As shown in Fig. 1A, the EDL933 supernatants of early to late stationary phase (OD600, 0.8 to 1.2) significantly induced the relative fluorescence (P < 0.05) of the reporter strain IJ01 compared to those of strains grown in the mLB medium (negative control) and the DH5α supernatants. Similar results have already been demonstrated for EHEC, but maximum AI-3-mediated induction of the LEE genes was shown for mid-exponential culture supernatants (32). In addition, mLB with epinephrine significantly induced relative fluorescence compared to mLB without epinephrine (P < 0.024) (Fig. 1B). These results confirmed the applicability of the fluorescence assay for the detection of ler expression.
FIG. 1.
Validation of the E. coli IJ01 fluorescence bioassay measuring ler promoter regulation. (A) Relative fluorescence induction obtained with culture supernatants of EHEC O157:H7 EDL933 (filled circles) and E. coli DH5α (open circles) of different growth phases and by mLB medium (negative control, dashed line). (B) Relative fluorescence values obtained with mLB medium (black bar) and mLB supplemented with 50 μM l-epinephrine (Epi, gray bar). Relative fluorescence values were calculated by dividing absolute fluorescence by the corresponding cell density (OD600) values. Error bars denote standard deviations of three independent experiments performed in duplicate.
Construction of the L. reuteri ATCC 55730 luxS mutant strain L. reuteri LTH6560.
An isogenic luxS mutant of L. reuteri strain ATCC 55730 was constructed by insertional inactivation, using the suicide vector pORI28 as described previously (30). An internal sequence of the luxS gene (bp 46 to 260 of lr0628; GenBank accession no. DQ233673) was amplified by using the primers luxSFor (TGACGAATTCTAAGCACCTTACGTTCGTTTAATTACC [the EcoRI recognition site is underlined]) and luxSRev (TGACGGATCCGTAATTAAGTGGAAACCAGTCGG [the BamHI recognition site is underlined]), cloned into pORI28 by using EcoRI and BamHI, and inserted in the chromosomal luxS open reading frame by homologous recombination. The correct localization of pORI28 in the luxS open reading frame and the singular insertion event in the chromosome of the obtained mutant strain L. reuteri LTH6560 were verified by PCR using the primers LuxCoF (GCACCTTACGTTCGTTTAATTACC) and LuxCoR (TCCCTTCATCAAGAATCTTC), flanking the insertion site, and by Southern blot hybridization, respectively (data not shown).
Influence of L. reuteri supernatants on ler expression.
Culture supernatants of the L. reuteri strains ATCC 55730, LTH6560, and 100-23C (13) and the luxS mutant of strain 100-23C (28) were tested for the ability to influence ler expression in the E. coli IJ01 bioassay. L. reuteri strains were grown anaerobically in modified MRS (mMRS) medium (9) at 37°C. Supernatants were prepared from cultures of different growth phases (OD600 of 0.1 to 2.5) by centrifugation, with pH adjustment to 7.0 and sterile filtration. mMRS medium (pH 7.0) was used as an AI-3-negative control. Prior to the start of the bioassay, E. coli IJ01 was cultured as described above and inoculated at a 1:20 dilution into L. reuteri supernatants, and the mMRS medium was adjusted to pH 7.0. Aliquots (200 μl) were transferred to a microtiter plate, and incubation and measurements were conducted as described above for the testing of E. coli supernatants.
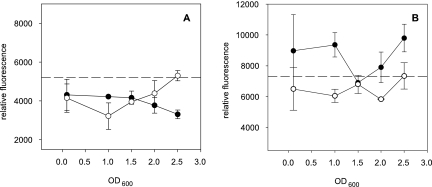
The supernatants of the L. reuteri strains ATCC 55730 and 100-23C and the luxS mutants exhibited different effects on ler expression (Fig. 2). The L. reuteri ATCC 55730 (wild type) exponential-phase supernatants (OD600, 0.1) caused lower relative fluorescence than the negative control mMRS medium and led to a constant decrease in fluorescence with increasing OD600 (Fig. 2A). At the stationary phase (OD600, 2.5), ler expression was significantly repressed compared to that of the luxS mutant LTH6560 (P < 0.005), as well as that of the mMRS medium (P < 0.0001). Supernatants of strain LTH6560 caused levels of ler expression similar to that of the wild type at an OD600 of 0.1 and lower expression at an OD600 of 1.0; but in the stationary phase (OD600 of 2.5), the fluorescence exceeded the corresponding wild-type values, equalling the mMRS medium values. In contrast, the L. reuteri 100-23C (wild type) supernatants of exponential and early stationary phases (OD600 0.1 and 1.0, respectively) induced levels of ler expression comparable to that of the luxS mutant and the mMRS medium, caused a minimum expression at an OD600 of 1.5, and induced ler expression at an OD600 of 2.5 compared to that of the luxS mutant and the mMRS medium (Fig. 2B). The 100-23C luxS mutant supernatants did not induce fluorescence above the negative control with mMRS medium.
FIG. 2.
Influence of culture supernatants of L. reuteri ATCC 55730 strains (A) and L. reuteri 100-23C strains (B) on ler expression, detected by applying the E. coli IJ01 fluorescence bioassay. Relative fluorescence inductions obtained with supernatants of the wild-type strains (filled circles) and of the corresponding isogenic luxS mutants (open circles). Dashed lines indicate the mean of the relative fluorescence induction of the negative control mMRS medium. Error bars denote standard deviations of three independent experiments performed in duplicate.
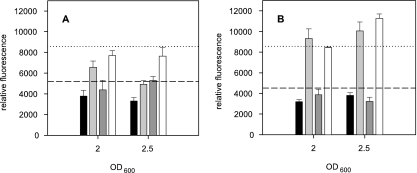
Surprisingly, the supernatants of L. reuteri ATCC 55730 exhibited a negative regulatory effect on ler expression compared to those of the luxS mutant and growth medium alone, whereas L. reuteri 100-23C supernatants induced ler transcription as described previously (28). These results indicated a LuxS-dependent interference with AI-3-mediated QS. To further investigate the nature of repression, we added epinephrine to supernatants of L. reuteri grown to OD600 values of 2.0 and 2.5 and investigated ler regulation. mMRS medium (pH 7.0) containing 50 μM l-epinephrine and without epinephrine served as positive and negative control, respectively. The results depicted in Fig. 3 indicated a similar fluorescence induction for spiked 100-23C wild-type and luxS mutant supernatants compared to that of the positive control mMRS medium with epinephrine (Fig. 3B) but reduced fluorescence caused by spiked ATCC 55730 wild-type supernatant (Fig. 3A). Evidently, the full extent of epinephrine-mediated ler induction was constrained by secreted substances of L. reuteri ATCC 55730 but not by those of its isogenic luxS mutant nor by those of the L. reuteri 100-23C wild type and the luxS mutant.
FIG. 3.
Impact of the addition of 50 μM l-epinephrine to stationary-phase supernatants of L. reuteri on ler expression. (A) Relative fluorescence obtained with L. reuteri ATCC 55730 (wild-type) supernatants without epinephrine (black bar) and with epinephrine (light gray bar) and with L. reuteri LTH6560 (luxS mutant) supernatants without epinephrine (dark gray bar) and with epinephrine (white bar). (B) Relative fluorescence obtained with L. reuteri 100-23C (wild-type) supernatants without epinephrine (black bar) and with epinephrine (light gray bar), and with L. reuteri 100-23C luxS mutant supernatants without epinephrine (dark gray bar) and with epinephrine (white bar). Dashed lines indicate the mean relative fluorescence of the negative control mMRS medium, and dotted lines mean relative fluorescence of mMRS medium supplemented with epinephrine used as positive control. Error bars denote standard deviations of two independent experiments performed in duplicate.
On the basis of the results obtained, we propose that LuxS of L. reuteri ATCC 55730 is responsible, either directly or indirectly, for the production and/or secretion of molecules that negatively regulate ler transcription in the stationary phase (OD600, 2.5). Since LuxS affects the central metabolism, a mutation consequently leads to pleiotropic effects; thus no conclusion can be drawn about the nature of these molecules. Interestingly, the LuxS activity differs among strains of the same species, leading to opposite effects on EHEC virulence gene transcription. A possible explanation of the mode of action would be that the secreted molecules of L. reuteri ATCC 55730 share structural homology with AI-3 or epinephrine and bind to the sensor kinase QseE or QseC, blocking the phosphorylation of the cognate response regulators QseF and QseB. This could abolish the signal transduction cascades analogous to alpha- and beta-adrenergic antagonists (25). However, a negative regulation of ler expression via these two-component systems has not been described to date. The facts that ler expression is significantly reduced by L. reuteri ATCC 55730 compared to that of medium and of the luxS mutant supernatant and that the epinephrine stimulus does not result in a full induction of ler transcription support the hypothesis of competing antagonistic molecules.
The in vivo relevance of EHEC virulence gene repression by L. reuteri ATCC 55730 and the underlying principles remain to be investigated. Nevertheless, it seems a promising approach for anti-QS-based therapeutic strategies for the treatment of infectious diseases that recently have gained considerable research interest (2, 7), for example, the inhibition of Pseudomonas aeruginosa or Staphylococcus aureus infections by QS interference (17, 19). Furthermore, the virulence gene repression was shown for the two strains L. reuteri ATCC 55730 and L. acidophilus La-5, which are proven probiotics, whereas the nonprobiotic L. reuteri 100-23C does not display this ability. Whether the observed repression is a characteristic of probiotic lactobacilli should be the subject of further investigation.
Acknowledgments
We thank K. Riedel and L. Eberl for generously supplying pJBA89 and A. Schaller for permission to use the Cary Eclipse fluorescence reader.
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Andersen, J. B., A. Heydorn, M. Hentzer, L. Eberl, O. Geisenberger, B. B. Christensen, S. Molin, and M. Givskov. 2001. gfp-based N-acyl homoserine-lactone sensor systems for detection of bacterial communication. Appl. Environ. Microbiol. 67:575-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cegelski, L., G. R. Marshall, G. R. Eldridge, and S. J. Hultgren. 2008. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 6:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clarke, M. B., D. T. Hughes, C. Zhu, E. C. Boedeker, and V. Sperandio. 2006. The QseC sensor kinase: a bacterial adrenergic receptor. Proc. Natl. Acad. Sci. USA 103:10420-10425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dower, W. J., J. F. Miller, and C. W. Ragsdale. 1988. High efficiency transformation of Escherichia coli by high voltage electroporation. Nucleic Acids Res. 16:6127-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott, S. J., V. Sperandio, J. A. Giron, S. Shin, J. L. Mellies, L. Wainwright, S. W. Hutcheson, T. K. McDaniel, and J. B. Kaper. 2000. The locus of enterocyte effacement (LEE)-encoded regulator controls expression of both LEE- and non-LEE-encoded virulence factors in enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 68:6115-6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garmendia, J., G. Frankel, and V. F. Crepin. 2005. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 73:2573-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez, J. E., and N. D. Keshavan. 2006. Messing with bacterial quorum sensing. Microbiol. Mol. Biol. Rev. 70:859-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guyer, M. S., R. R. Reed, J. A. Steitz, and K. B. Low. 1981. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harbor Symp. Quant. Biol. 45(Pt. 1):135-140. [DOI] [PubMed] [Google Scholar]
- 9.Hüfner, E., T. Markieton, S. Chaillou, A. M. Crutz-Le Coq, M. Zagorec, and C. Hertel. 2007. Identification of Lactobacillus sakei genes induced during meat fermentation and their role in survival and growth. Appl. Environ. Microbiol. 73:2522-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iyoda, S., and H. Watanabe. 2004. Positive effects of multiple pch genes on expression of the locus of enterocyte effacement genes and adherence of enterohaemorrhagic Escherichia coli O157 : H7 to HEp-2 cells. Microbiology 150:2357-2571. [DOI] [PubMed] [Google Scholar]
- 11.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 12.Kaper, J. B., and V. Sperandio. 2005. Bacterial cell-to-cell signaling in the gastrointestinal tract. Infect. Immun. 73:3197-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McConnell, M. A., A. A. Mercer, and G. W. Tannock. 1991. Transfer of plasmid pAMβ1 between members of the normal microflora inhabiting the murine digestive tract and modification of the plasmid in a Lactobacillus reuteri host. Microb. Ecol. Health Dis. 4:343-355. [Google Scholar]
- 14.Medellin-Peña, M. J., H. Wang, R. Johnson, S. Anand, and M. W. Griffiths. 2007. Probiotics affect virulence-related gene expression in Escherichia coli O157:H7. Appl. Environ. Microbiol. 73:4259-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien, A. D., T. A. Lively, T. W. Chang, and S. L. Gorbach. 1983. Purification of Shigella dysenteriae 1 (Shiga)-like toxin from Escherichia coli O157:H7 strain associated with haemorrhagic colitis. Lancet 2:573. [DOI] [PubMed] [Google Scholar]
- 17.Park, J., R. Jagasia, G. F. Kaufmann, J. C. Mathison, D. I. Ruiz, J. A. Moss, M. M. Meijler, R. J. Ulevitch, and K. D. Janda. 2007. Infection control by antibody disruption of bacterial quorum sensing signaling. Chem. Biol. 14:1119-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porter, M. E., P. Mitchell, A. Free, D. G. Smith, and D. L. Gally. 2005. The LEE1 promoters from both enteropathogenic and enterohemorrhagic Escherichia coli can be activated by PerC-like proteins from either organism. J. Bacteriol. 187:458-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasmussen, T. B., M. E. Skindersoe, T. Bjarnsholt, R. K. Phipps, K. B. Christensen, P. O. Jensen, J. B. Andersen, B. Koch, T. O. Larsen, M. Hentzer, L. Eberl, N. Hoiby, and M. Givskov. 2005. Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology 151:1325-1340. [DOI] [PubMed] [Google Scholar]
- 20.Reading, N. C., A. G. Torres, M. M. Kendall, D. T. Hughes, K. Yamamoto, and V. Sperandio. 2007. A novel two-component signaling system that activates transcription of an enterohemorrhagic Escherichia coli effector involved in remodeling of host actin. J. Bacteriol. 189:2468-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schauder, S., K. Shokat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41:463-476. [DOI] [PubMed] [Google Scholar]
- 22.Sharp, F. C., and V. Sperandio. 2007. QseA directly activates transcription of LEE1 in enterohemorrhagic Escherichia coli. Infect. Immun. 75:2432-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperandio, V., J. L. Mellies, W. Nguyen, S. Shin, and J. B. Kaper. 1999. Quorum sensing controls expression of the type III secretion gene transcription and protein secretion in enterohemorrhagic and enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 96:15196-15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperandio, V., A. G. Torres, J. A. Giron, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 183:5187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sperandio, V., A. G. Torres, B. Jarvis, J. P. Nataro, and J. B. Kaper. 2003. Bacteria-host communication: the language of hormones. Proc. Natl. Acad. Sci. USA 100:8951-8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sperandio, V., A. G. Torres, and J. B. Kaper. 2002. Quorum sensing Escherichia coli regulators B and C (QseBC): a novel two-component regulatory system involved in the regulation of flagella and motility by quorum sensing in E. coli. Mol. Microbiol. 43:809-821. [DOI] [PubMed] [Google Scholar]
- 27.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 95:7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tannock, G. W., S. Ghazally, J. Walter, D. Loach, H. Brooks, G. Cook, M. Surette, C. Simmers, P. Bremer, F. Dal Bello, and C. Hertel. 2005. Ecological behavior of Lactobacillus reuteri 100-23 is affected by mutation of the luxS gene. Appl. Environ. Microbiol. 71:8419-8425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vendeville, A., K. Winzer, K. Heurlier, C. M. Tang, and K. R. Hardie. 2005. Making “sense” of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat. Rev. Microbiol. 3:383-396. [DOI] [PubMed] [Google Scholar]
- 30.Walter, J., P. Chagnaud, G. W. Tannock, D. M. Loach, F. Dal Bello, H. F. Jenkinson, W. P. Hammes, and C. Hertel. 2005. A high-molecular-mass surface protein (Lsp) and methionine sulfoxide reductase B (MsrB) contribute to the ecological performance of Lactobacillus reuteri in the murine gut. Appl. Environ. Microbiol. 71:979-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walters, M., M. P. Sircili, and V. Sperandio. 2006. AI-3 synthesis is not dependent on luxS in Escherichia coli. J. Bacteriol. 188:5668-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walters, M., and V. Sperandio. 2006. Autoinducer 3 and epinephrine signaling in the kinetics of locus of enterocyte effacement gene expression in enterohemorrhagic Escherichia coli. Infect. Immun. 74:5445-5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waters, C. M., and B. L. Bassler. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21:319-346. [DOI] [PubMed] [Google Scholar]