Abstract
The conversion yield of d-psicose from d-fructose by a d-psicose 3-epimerase from Agrobacterium tumefaciens increased with increasing molar ratios of borate to fructose, up to a ratio of 0.6. The formation of the psicose-borate complex was the result of the higher binding affinity of borate for psicose than for fructose. The formed psicose-borate complex did not participate in the conversion reaction, acting instead as if the product had been removed. Thus, more fructose was converted to psicose in order to restore the equilibrium. The maximum conversion yield of psicose with borate was about twofold that obtained without borate and occurred at a 0.6 molar ratio of borate to fructose. Above this ratio, the conversion yield decreased with increasing ratios, because the amount of fructose available decreased through the formation of the initial fructose-borate complex. The structures of the two sugar-borate complexes, determined by nuclear magnetic resonance spectroscopy, were α-d-psicofuranose cis-C-3,4 diol borate and β-d-fructopyranose cis-C-4,5 diol borate.
d-Psicose (d-ribo-2-hexulose, or d-allulose), a carbon-3 epimer of d-fructose, is present as a nonfermentable constituent of cane molasses (2), a sugar moiety of the nucleoside antibiotic psicofuranine (9), and a free sugar in wheat (33) and Itea plants (15). The sugar is a noncaloric sweetener for weight reduction (31) based on its suppression of hepatic lipogenous enzyme activity (30). Psicose can be chemically synthesized from fructose by using a molybdate ion catalyst (1), by producing it from 1,2:4,5-di-o-isopropylidene-β-d-fructopyranose (32), or by boiling fructose in ethanol and triethylamine (8). The biological conversion of fructose to psicose has been studied in recent years using only two enzymes, Agrobacterium tumefaciens d-psicose 3-epimerase (23-25) and Pseudomonas cichorii d-tagatose 3-epimerase (16-18, 37).
In aqueous solution, boron exists as either boric acid [B(OH)3] or borate [B(OH)4−] with a pKa of 9.2 (22), although more borate than boric acid is formed (20). The formation of borate complexes with diol-containing compounds such as carbohydrates has been studied previously for its potential applications in various fields of science and technology (7, 41). The ketoses lactulose, maltulose, and cellobiulose are chemically synthesized from the aldoses lactose, maltose, and cellobiose, respectively, in high-yield isomerization reactions carried out in alkaline solutions containing borate (12, 13). Borate can be used to analyze mixtures of ribose, arabinose, and ribulose by high-performance liquid chromatography (HPLC) using an ion exclusion chromatography column (7). Initially, the sugar retention times are very close to one another, but ribose, arabinose, and ribulose are better separated by increasing the borate concentration in the eluent, owing to the different degrees with which borate forms complexes with the different carbohydrates. The migration of some carbohydrates during thin-layer chromatography (TLC) is decreased by solvent systems containing borate (29). Thus, carbohydrates with borate can be better separated by either HPLC or TLC than those without borate due to the unique capacity for complex formation by each carbohydrate with borate. Borate is used as an additive to enhance regio- or stereoselectivity in the enzyme modification of the glucosylation of pyridoxine (39). Recently, high yields of l-ribulose and d-tagatose have been obtained through complex formation by l-ribulose or d-tagatose with borate (11, 27). However, the mechanism for the conversion shift with added borate and the structure of the sugar-borate complex have not yet been investigated.
In this study, the conversion shift of fructose to psicose by d-psicose 3-epimerase from A. tumefaciens in the presence of added borate was explained by the greater capacity of borate for complex formation with psicose than with fructose. A mechanism for the conversion shift is proposed, and the chemical structures of the fructose-borate and psicose-borate complexes were determined.
MATERIALS AND METHODS
Bacterial strains, plasmid, and culture conditions.
A. tumefaciens ATCC 33970, Escherichia coli BL21(DE3), and pET-24a(+) plasmid (Novagen, Darmstadt, Germany) were used as the source of genomic DNA encoding d-psicose 3-epimerase, as host cells, and as the expression vector, respectively (23). The recombinant E. coli cells for the expression of the enzyme were cultivated in 500 ml of Luria-Bertani medium (1.0% tryptone, 0.5% yeast extract, and 1.0% sodium chloride) in a 2,000-ml flask containing 20 μg of kanamycin/ml at 37°C with shaking at 200 rpm. When the absorbance of the culture at 600 nm reached 0.5, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.1 mM to induce d-psicose 3-epimerase expression, and the culture was incubated at 16°C for 16 h.
Purification of d-psicose 3-epimerase.
The recombinant cells were harvested from the culture broth by centrifugation at 6,000 × g for 30 min at 4°C, washed twice with 0.85% NaCl, and then resuspended in a lysis buffer (50 mM NaH2PO4, 300 mM NaCl, pH 8.0) containing 1 mg of lysozyme/ml. The resuspended cells were disrupted using a sonicator (sonic dismembrator model 100; Fisher Scientific, Pittsburgh, PA) on ice for 2 min. The cell debris was removed by centrifugation at 13,000 × g for 20 min at 4°C, and the supernatant was filtered through a 0.45-μm-pore-size filter. All subsequent purification steps using column chromatography were carried out in a cold room at 4°C with a fast protein liquid chromatography system (Bio-Rad Laboratories, Hercules, CA). The filtrate was applied to a Profinity IMAC affinity chromatography column (Bio-Rad Laboratories) equilibrated with 50 mM sodium phosphate buffer containing 300 mM NaCl and 10 mM imidazole (pH 8.0). The column was washed extensively with the same buffer, and the bound protein was eluted with a linear gradient from 10 to 200 mM imidazole at a flow rate of 7 ml/min. The active fractions were collected and dialyzed against 50 mM N-(2-hyroxyethyl)piperazine-N′-(3-propanesulfonic acid) (EPPS) buffer (pH 8.0). After dialysis, the resulting solution was used as the purified enzyme.
Enzyme assay.
To bind the d-psicose 3-epimerase with Mn2+ and to remove unbound Mn2+, the enzyme was incubated at 20°C with 1 mM Mn2+ for 4 h and dialyzed at 4°C against 50 mM EPPS buffer (pH 8.0) overnight before the enzyme was used. Unless otherwise stated, all reactions were performed at 50°C with 50 mM borate buffer (pH 9.0) containing 100 mM d-fructose and 4 U of the enzyme/ml for 5 min. The control reaction without borate was carried out with 50 mM EPPS buffer (pH 8.0) instead of the borate buffer. The reaction was stopped by boiling the reaction mixture at 100°C for 5 min. One unit of d-psicose 3-epimerase activity was defined as the amount of the enzyme required to produce 1 μmol of d-psicose per min at pH 8.0 and 50°C.
Effects of pH and temperature on the conversion of fructose to psicose in the presence of borate.
To examine the fructose epimerization reaction by the enzyme with and without borate, the pH was varied between 6.0 and 10.0. For the reactions in the presence of borate, a 50 mM borate buffer system was used throughout the pH range. The reactions without borate were carried out with 50 mM concentrations of three different buffers: maleic acid-NaOH buffer for pH 6.0 to 7.0, EPPS buffer for pH 7.0 to 9.0, and glycine-NaOH buffer for pH 9.0 to 10.0. To investigate the effect of temperature, the temperature was varied from 40 to 60°C in 50 mM EPPS buffer (pH 8.0) or in 50 mM borate buffer (pH 9.0).
Bioconversion of fructose to psicose in the presence of borate.
The effect of the molar ratio of borate to fructose on the conversion of fructose to psicose was investigated using 100 mM fructose and 0 to 100 mM borate. The reactions were performed at pH 9.0 and 50°C for 3 h. The effect of the fructose concentration on the conversion in the presence of borate was studied by varying the concentrations of fructose and borate. The fructose concentrations were 50, 100, and 150 mM, and the molar ratio of borate to fructose was varied from 0 to 1.0.
Analysis of sugars and sugar-borate complexes using TLC.
TLC was used for the evaluation of sugars and sugar-borate complexes at room temperature using silica gel 60 F254 TLC plates (Merck Co., Whitehouse Station, NJ). One microliter of the reaction solution was spotted onto a silica gel plate, and the plate contents were developed using a solvent mixture of ethanol, chloroform, n-butanol, and 25% ammonia water (5:2:4:8, vol/vol/vol/vol). The developed plate contents were dried and visualized using a solvent mixture of methanol, H2SO4, and N-1-naphtylethylenediamine dihydrochloride (95:5:0.3, vol/vol/wt) and then subjected to heating for 10 min at 121°C (34). The visualized spots were measured using an NIH Image program (19).
For control samples, mixtures of 100 mM sugar and 0 to 100 mM borate were incubated for 30 min without the enzyme. The enzyme reaction products were obtained by incubating 100 mM fructose and 60 to 100 mM borate with d-psicose 3-epimerase at 50°C for 3 h. The mixtures and enzyme reaction products were applied to a TLC plate. As borate was not visualized by TLC, the concentration of the sugar-borate complex was calculated from the initial sugar concentration and the area of the sugar-borate complex relative to the total area.
Nuclear magnetic resonance (NMR) analysis of the sugar-borate complexes.
Each sugar-borate complex was obtained by mixing equal volumes of 100 mM borate and 100 mM fructose or psicose.
All NMR spectra were recorded at 303 K on a 500-MHz spectrometer at the Korea Basic Science Institute. The relevant spectral data for the sugar and sugar-borate complex in 100% D2O were acquired. Chemical shifts were expressed relative to the 4,4-dimethyl-4-silapentane-1-sulfonate signal in D2O at 0 ppm. Additional experiments with the sugar and sugar-borate complex in a dimethyl sulfoxide (DMSO) solution were performed, and chemical shifts were referenced to tetramethylsilane at 0 ppm. For the spectral assignments, we collected gradient-selected correlation spectroscopy (gCOSY) and total correlation spectroscopy spectra with a mixing time of 80 ms (5, 35). We also collected gradient-selected phase-sensitive 1H-13C heteronuclear single-quantum coherence (gHSQC) and gradient-selected absolute-value 1H-13C heteronuclear multiple-bond coherence spectra to aid in the spectral assignments (40). Two-dimensional 1H-1H phase-sensitive nuclear Overhauser effect spectroscopy and rotating-frame nuclear Overhauser effect spectroscopy experiments were performed with a mixing time of 600 ms (3, 28). All spectra were acquired with a spectral width of 2,000 Hz and with 1,024 t1 and 4,096 t2 data points as the size of free induction decay. Baseline correction was applied in both dimensions. The NMR spectra were processed with NMRPipe (6) and visualized with Sparky (10).
Analytical methods.
Protein concentrations were determined by the Bradford method using bovine serum albumin as a standard protein (4). Amberlite IRA-743 and Dowex X50X8 resins (4:1, vol/vol) were used to remove borate from the sugar-borate complex for all samples containing borate (14). After the removal of borate, each sample was chromatographed using an HPLC system (model SCL-10A; Shimadzu, Kyoto, Japan) equipped with a Shimadzu RID-10A detector and a BP-100 Ca2+ carbohydrate column (Benson Polymeric Inc., Reno, NV). The column contents were eluted with water at 80°C at a flow rate of 1 ml/min.
RESULTS AND DISCUSSION
Effects of pH and temperature on the conversion of fructose to psicose by A. tumefaciens d-psicose 3-epimerase in the presence of borate.
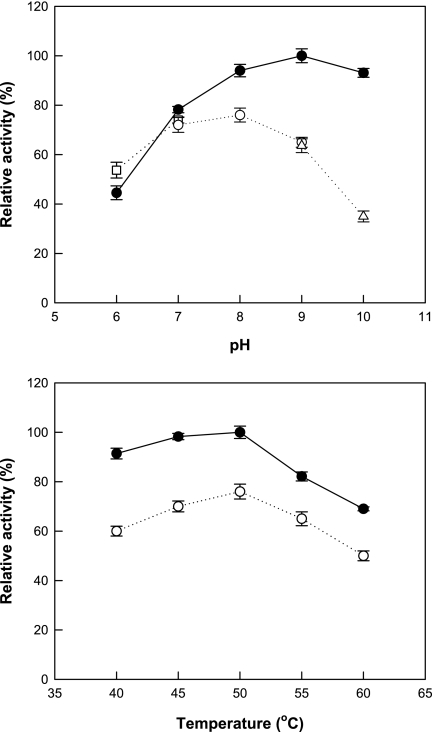
To investigate the effects of pH and temperature on the conversion of fructose to psicose in the presence of borate, the enzyme activities over the pH range of 6 to 10 and over the temperature range of 40 to 60°C were determined (Fig. 1). Under alkaline conditions above pH 8, the enzyme activity was higher with borate than without borate. Maximum d-psicose 3-epimerase activity was obtained at pH 8.0 and 50°C without borate and at pH 9.0 and 50°C with borate.
FIG. 1.
Effects of pH and temperature on the conversion of fructose to psicose in the presence of borate. For the pH effect, the reactions were performed with 50 mM borate buffer for pH 6.0 to 10.0 (•), 50 mM maleic acid-NaOH buffer for pH 6.0 to 7.0 (□), 50 mM EPPS buffer for pH 7.0 to 9.0 (○), and 50 mM glycine-NaOH buffer for pH 9.0 to 10.0 (▵). To determine the effects of temperature, the reactions were performed with 50 mM borate buffer at pH 9.0 (•) and with 50 mM EPPS buffer without borate at pH 8.0 (○). Data are the means of results from three experiments.
Conversion of fructose to psicose by A. tumefaciens d-psicose 3-epimerase in the presence of borate.
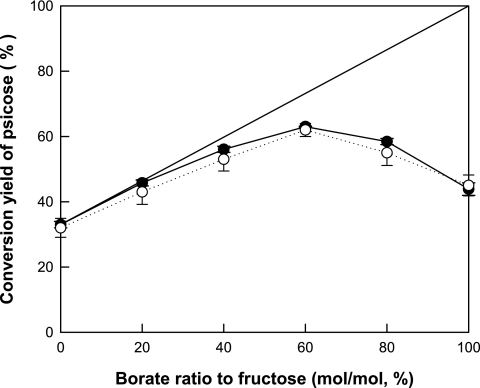
The effect of the molar ratio of borate to fructose on the conversion of fructose to psicose was investigated using 100 mM fructose and 0 to 100 mM borate (Fig. 2). The conversion yield of psicose from fructose increased with increases in the molar ratio of borate to fructose, up to a ratio of 0.6. At ratios above 0.6, the conversion yield decreased with increasing ratios of borate to fructose. The maximum conversion yield of psicose from fructose was 64% and occurred at a borate-to-fructose ratio of 0.6.
FIG. 2.
Effect of the molar ratio of borate to fructose on the conversion of fructose to psicose by A. tumefaciens d-psicose 3-epimerase. The reactions were performed at pH 9.0 and 50°C for 3 h using 100 mM fructose and 0 to 100 mM borate. The conversion yields of psicose from fructose obtained from experimental data (•) are the means of results from three experiments. The theoretical conversion yields of psicose (○) were calculated based on the amount of available fructose, excluding the initially formed fructose-borate complex, and are the means of results from 20 experiments. The line indicates the theoretical maximum level of conversion of fructose to psicose with the addition of borate.
The conversion of fructose to psicose was investigated at fructose concentrations from 50 to 200 mM. At the same molar ratio of borate to fructose, the conversion yields of psicose from fructose were almost the same at every fructose concentration, with a maximum conversion yield of 64.0 to 64.5% at a borate ratio of 0.6 in all cases (data not shown). These results suggest that the conversion yield of psicose from fructose was determined by the molar ratio of borate to fructose and not by the fructose concentration.
Structural analysis of the sugar-borate complexes.
The structures of the sugars and sugar-borate complexes were determined by NMR spectroscopy. The assignment of the 1H resonances of the sugars was achieved by gCOSY and total correlation spectroscopy experiments. The full assignment of both 13C and 1H resonances was completed with gHSQC and gradient-selected absolute-value 1H-13C heteronuclear multiple-bond coherence experiments. The compositions of epimers of psicose or fructose in water were calculated by volume integration of the H-5-C-5 peaks in the gHSQC spectra of the respective sugars. The conformational equilibrium was determined to be as follows: for psicose, an α-d-psicofuranose/β-d-psicofuranose/α-d-psicopyranose/β-d-psicopyranose ratio of 38:13:25:24, and for fructose, an α-d-fructopyranose/β-d-fructopyranose/α-d-fructofuranose/β-d-fructofuranose ratio of 3:71:5:21. These results are very similar to previously reported data: for psicose, α-d-psicofuranose, 39%; β-d-psicofuranose, 15%; α-d-psicopyranose, 22%; and β-d-psicopyranose, 24%, and for fructose, α-d-fructopyranose, 3%; β-d-fructopyranose, 72%; α-d-fructofuranose, 5%; and β-d-fructofuranose, 20% (26).
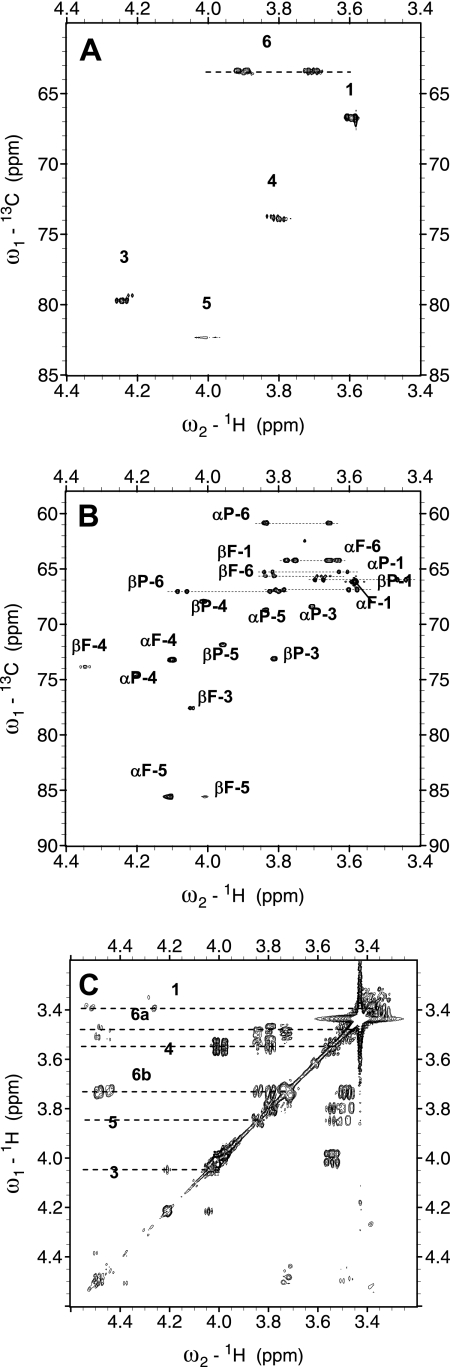
The gHSQC spectra of psicose and the psicose-borate complex revealed only one form of the complex (Fig. 3A) but four forms of psicose (Fig. 3B). The C-5 chemical shift of the psicose-borate complex (82.3 ppm) is typical of fructopyranose, and the 1H chemical shift of the complex (3.6 ppm) is typical of the α-epimer. Therefore, the psicose-borate complex exists as the α-d-psicofuranose-borate form. We observed the psicose-borate complex in DMSO to aid in identifying the position of the borate complex. As shown in Fig. 3C, the OH groups of the psicose-borate complex seen at C-3 and C-4 in the gCOSY spectrum were absent. Thus, the psicose-borate complex existed as a mixture of α-d-psicofuranose cis-C-3,4 diol borate and its boric acid. By using gHSQC and gCOSY experiments, the fructose-borate complex was similarly determined to be β-d-fructopyranose cis-C-4,5 diol borate (or boric acid) (data not shown). The chemical structures of the sugar-borate complexes are depicted in Fig. 4.
FIG. 3.
NMR spectra. (A) gHSQC spectrum of psicose-borate complex in D2O. (B) gHSQC spectrum of psicose in D2O. Chemical shifts are expressed relative to the 4,4-dimethyl-4-silapentane-1-sulfonate signal in D2O at 0 ppm. αF, α-d-psicofuranose; βF, β-d-psicofuranose; αP, α-d-psicopyranose; βP, β-d-psicopyranose. (C) gCOSY spectrum of the psicose-borate complex in DMSO. Chemical shifts were referenced to tetramethylsilane at 0 ppm. The OH groups at C-3 and C-4 of the psicose-borate complex were absent in the gCOSY spectrum.
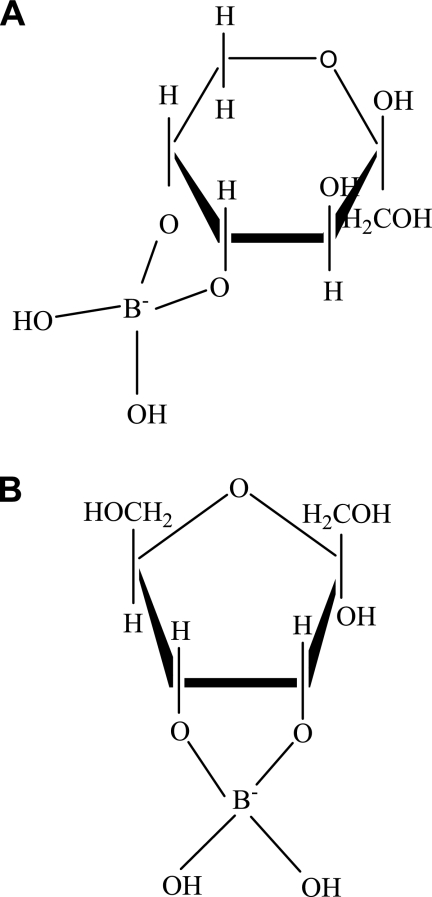
FIG. 4.
Chemical structures of the sugar-borate complexes. (A) Fructose as β-d-fructopyranose cis-C-4,5 diol borate. (B) Psicose as α-d-furanopsicose cis-C-3,4 diol borate.
Binding affinities of borate for fructose and psicose.
A sugar-borate complex forms when a cis-diol sugar moiety condenses with borate. Thus, borate can change the equilibrium of any enzyme reaction involving cis-diol sugars (36). A previous analysis (38) reported local association constants of borate (given in parentheses) for various sugars as follows: cis-diol furanose (2,500 to 45,000) ≫ exocyclic-diol pyranose (250 to 1,500) > exocyclic-diol furanose (50 to 100) > cis-diol pyranose (10 to 20) > exocyclic cis/trans-diol pyranose (3 to 6) ≫ trans-diol pyranose/furanose (0). The complexes that borate formed with fructose and psicose were cis-C-4,5 diol pyranose and cis-C-3,4 diol furanose, respectively. Thus, the binding affinity of borate for psicose was higher than that for fructose.
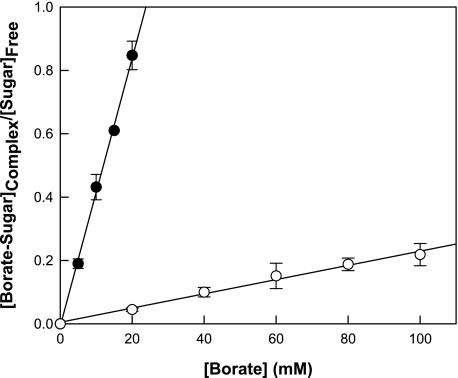
As an indicator of binding affinity, the association constant (KA) of each sugar was determined from the slope in Fig. 5 for the complex concentration/free-sugar concentration ratio plotted against the free-borate concentration by using the following equation: KA = [S·B]/[S][B], where [S·B], [S], and [B] are the concentrations of the sugar-borate complex, free sugar, and free borate, respectively (20, 21). The calculated KA of psicose (54.3 liters/mol) was higher than that of fructose (3 liters/mol).
FIG. 5.
KAs of fructose (○) and psicose (•) with various molar ratios of borate to sugar. The KA of each sugar was determined from the slope for the complex concentration/free-sugar concentration ratio plotted against the free-borate concentration. As borate was not visualized by TLC, the concentration of the sugar-borate complex was calculated from the initial sugar concentration and the area of the sugar-borate complex relative to the total area. Data presented are the means of results from 20 experiments.
Binding of borate to fructose with and without the enzyme.
The binding of borate to fructose at different molar ratios of borate to fructose with and without the enzyme was investigated using TLC (Fig. 6). Above the borate-to-fructose ratio of 0.6, fructose-borate complex formation increased with increasing molar ratios of borate to fructose. The sugar-borate complex, i.e., α-d-psicofuranose cis-C-3,4 diol or β-d-fructopyranose cis-C-4,5 diol borate, which exhibits a different structure at C-4 compared with the sugar, was not bound to the catalytic residues of the enzyme because C-2, C-3, and C-4 of fructose or psicose were tightly bound to the catalytic residues, in accordance with the catalytic mechanism (25). Therefore, the sugar-borate complex in the initial substrate solution could not participate in the conversion reaction and the increased amount of the fructose-borate complex at higher borate-to-fructose ratios resulted in a decreased amount of fructose available for conversion to psicose by A. tumefaciens d-psicose 3-epimerase. Thus, the production of psicose was decreased at borate-to-fructose ratios above 0.6.
FIG. 6.
TLC analysis of binding of borate to fructose without and with the enzyme. For control samples, mixtures of 100 mM fructose and 0 to 100 mM borate were incubated for 30 min without the enzyme. Lanes: 1, 100 mM fructose; 2, 100 mM psicose; 3, 100 mM fructose and 0 mM borate; 4, 100 mM fructose and 20 mM borate; 5, 100 mM fructose and 40 mM borate; 6, 100 mM fructose and 60 mM borate; 7, 100 mM fructose and 80 mM borate; and 8, 100 mM fructose and 100 mM borate. The enzyme reaction products were obtained by incubating 100 mM fructose and 60 to 100 mM borate with d-psicose 3-epimerase at pH 9.0 and 50°C for 3 h. Lanes: 9, 60 mM borate; 10, 80 mM borate; and 11, 100 mM borate.
As shown by the TLC results for the enzyme reaction products, the amounts of the fructose-borate complex were nearly the same in the presence and absence of the enzyme, whereas the amount of the psicose-borate complex was higher in the presence of the enzyme than in its absence. These results suggest that the amount of the fructose-borate complex formed during the enzyme reaction was negligible.
Proposed mechanism for the conversion of fructose to psicose by A. tumefaciens d-psicose 3-epimerase in the presence of borate.
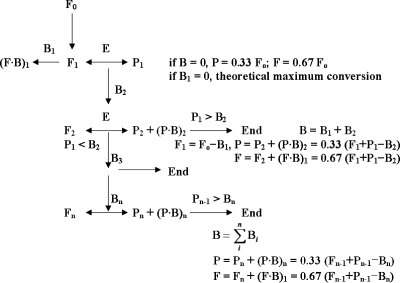
We propose a conversion shift mechanism for the theoretical conversion of psicose, considering the formation of the fructose-borate complex (Fig. 7). A theoretical maximum conversion of psicose was calculated on the assumption that the fructose-borate complex was not formed. Initially, d-psicose 3-epimerase produces 33% psicose from fructose at equilibrium. Borate binds to the newly produced psicose to form the psicose-borate complex. The psicose-borate complex, formed as α-d-psicofuranose cis-C-3,4 diol borate, does not participate in the conversion reaction, acting as if the product was removed. Thus, more fructose is converted to psicose to restore the equilibrium. The newly produced psicose subsequently binds to borate, and the process continues until all the borate is bound to psicose. As a result, the conversion of fructose to psicose increases with increasing borate levels. The theoretical maximum conversion is indicated by the line in Fig. 2.
FIG. 7.
Proposed mechanism for the conversion shift of fructose to psicose in the presence of borate by A. tumefaciens d-psicose 3-epimerase. E, enzyme; F, fructose; P, psicose; B, borate; P·B, psicose-borate complex; F·B, fructose-borate complex.
To calculate a theoretical conversion, the initially formed fructose-borate complex was excluded from the conversion reaction of fructose to psicose and the amount of the fructose-borate complex formed during the enzyme reaction was disregarded. Thus, only the available fructose, calculated as the initial amount of fructose minus the amount of the fructose-borate complex, participated in the conversion reaction. At borate-to-fructose ratios of less than 0.6, the conversion was shifted toward psicose owing to the formation of the psicose-borate complex, which acts as if the product was removed, and at the same time, a negligible effect of borate on the availability of fructose because of negligible formation of the fructose-borate complex. Therefore, psicose production increased with increasing borate ratios, up to a ratio of 0.6. However, at borate-to-fructose ratios above 0.6, the conversion to psicose decreased with increasing molar ratios of borate to fructose because greater fructose-borate complex formation markedly decreased the amount of available fructose. As shown in Fig. 2, the theoretical conversion yields of psicose agreed well with those obtained from the experimental data, confirming that the proposed mechanism is reasonable.
In the present study, the conversion of fructose to psicose by A. tumefaciens d-psicose 3-epimerase in the presence of borate was investigated. A conversion shift mechanism for the enzyme-catalyzed epimerization with borate was proposed, and the structures of the sugar complexes were determined. At borate-to-fructose molar ratios of less than 0.6, borate formed a complex primarily with the psicose product rather than with the fructose reactant, thus shifting the conversion toward psicose.
Acknowledgments
This study was supported by the Korea Science and Engineering Foundation (KOSEF) through the National Research Laboratory program funded by the Ministry of Science and Technology (R0A-2007-000-20015-0).
We appreciate Katharine J. Gibson for the suggestion that started this study.
Footnotes
Published ahead of print on 31 March 2008.
REFERENCES
- 1.Bilik, V., and K. Tihlarik. 1973. Reaction of saccharides catalyzed by molybdate ions. Chem. Zvesti 28:106-109. [Google Scholar]
- 2.Binkley, W. W. 1963. The fate of cane juice simple sugars during molasses formation. IV. Probable conversion of d-fructose to d-psicose. Int. Sugar J. 65:105-106. [Google Scholar]
- 3.Bothner-By, A. A., R. L. Stephens, J. Lee, C. D. Warren, and R. W. Jeanloz. 1984. Structure determination of a tetrasaccharide: transient nuclear Overhauser effects in the rotating frame. J. Am. Chem. Soc. 106:811-813. [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Braunschweiler, L., and R. R. Ernst. 1983. Coherence transfer by isotropic mixing: application to proton correlation spectroscopy. J. Magn. Reson. 53:521-528. [Google Scholar]
- 6.Delaglio, F., S. Grzesiak, G. Vuister, G. Zhu, J. Pfeifer, and A. Bax. 1995. A mutidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6:277-293. [DOI] [PubMed] [Google Scholar]
- 7.De Muynck, C., J. Beauprez, W. Soetaert, and E. J. Vandamme. 2006. Boric acid as a mobile phase additive for high performance liquid chromatography separation of ribose, arabinose and ribulose. J. Chromatogr. A 1101:115-121. [DOI] [PubMed] [Google Scholar]
- 8.Doner, L. W. 1979. Isomerization of d-fructose by base: liquid-chromatographic evaluation and the isolation of d-psicose. Carbohydr. Res. 70:209-216. [Google Scholar]
- 9.Eble, T. E., H. Hoeksema, G. A. Boyack, and G. M. Savage. 1959. Psicofuranine. I. Discovery, isolation, and properties. Antibiot. Chemother. 9:419-420. [PubMed] [Google Scholar]
- 10.Goddard, T., and D. G. Kneller. 2001. Sparky tutorial and reference manual, p. 8-23. University of California, San Francisco.
- 11.Helanto, M., K. Kiviharju, M. Leisola, and A. Nyyssölä. 2007. Metabolic engineering of Lactobacillus plantarum for production of l-ribulose. Appl. Environ. Microbiol. 73:7083-7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicks, K. B., E. V. Symanski, and P. E. Pfeffer. 1983. Synthesis and high-performance liquid chromatography of maltulose and cellobiulose. Carbohydr. Res. 112:37-50. [Google Scholar]
- 13.Hicks, K. B., and F. W. Parrish. 1980. A new method for the preparation of lactulose from lactose. Carbohydr. Res. 82:393-397. [Google Scholar]
- 14.Hicks, K. B., G. L. Simpson, and A. G. W. Bradbury. 1986. Removal of boric acid and related compounds from solutions of carbohydrates with a boron-selective resin (IRA-743). Carbohydr. Res. 147:39-48. [Google Scholar]
- 15.Hough, L., and B. E. Stacey. 1963. The occurrence of d-ribohexulose in Itea ilicifolia, Itea virginica, and Itea yunnanensis. Phytochemistry 2:315-320. [Google Scholar]
- 16.Ishida, Y., T. Kamiya, H. Itoh, Y. Kimura, and K. Izumori. 1997. Cloning and characterization of the d-tagatose 3-epimerase gene from Pseudomonas cichorii ST-24. J. Ferment. Bioeng. 83:529-534. [Google Scholar]
- 17.Itoh, H., H. Okaya, A. R. Khan, S. Tajima, S. Hayakawa, and K. Izumori. 1994. Purification and characterization of d-tagatose 3-epimerase from Pseudomonas sp. ST-24. Biosci. Biotechnol. Biochem. 58:2168-2171. [Google Scholar]
- 18.Itoh, H., T. Sato, and K. Izumori. 1995. Preparation of d-psicose from d-fructose by immobilized d-tagatose 3-epimerase. J. Ferment. Bioeng. 80:101-103. [Google Scholar]
- 19.Kim, D., J. F. Robyt, S. Y. Lee, J. H. Lee, and Y. M. Kim. 2003. Dextran molecular size and degree of branching as a function of sucrose concentration, pH, and temperature of reaction of Leuconostoc mesenteroides B-512FMCM dextransucrase. Carbohydr. Res. 338:1183-1189. [DOI] [PubMed] [Google Scholar]
- 20.Kim, D. H., K. F. Faull, A. J. Norris, and C. D. Eckhert. 2004. Borate-nucleotide complex formation depends on charge and phosphorylation state. J. Mass Spectrom. 39:743-751. [DOI] [PubMed] [Google Scholar]
- 21.Kim, D. H., S. Q. Hee, A. J. Norris, K. F. Faull, and C. D. Eckhert. 2006. Boric acid inhibits adenosine diphosphate-ribosyl cyclase non-competitively. J. Chromatogr. A 1115:246-252. [DOI] [PubMed] [Google Scholar]
- 22.Kim, D. H., B. N. Marbois, K. F. Faull, and C. D. Eckhert. 2003. Esterification of borate with NAD+ and NADH as studied by electrospray ionization mass spectrometry and 11B NMR spectroscopy. J. Mass Spectrom. 38:632-640. [DOI] [PubMed] [Google Scholar]
- 23.Kim, H. J., E. K. Hyun, Y. S. Kim, Y. J. Lee, and D. K. Oh. 2006. Characterization of an Agrobacterium tumefaciens d-psicose 3-epimerase that converts d-fructose to d-psicose. Appl. Environ. Microbiol. 72:981-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim, K., H. J. Kim, D. K. Oh, J. J. Cheong, and S. Rhee. 2006. Expression, purification, and crystallization of d-psicose 3-epimerase from Agrobacterium tumefaciens. J. Microbiol. Biotechnol. 16:647-650. [Google Scholar]
- 25.Kim, K., H. J. Kim, D. K. Oh, S. S. Cha, and S. Rhee. 2006. Crystal structure of d-psicose 3-epimerase from Agrobacterium tumefaciens and its complex with true substrate d-fructose: a pivotal role of metal in catalysis, an active site for the non-phosphorylated substrate, and its conformational changes. J. Mol. Biol. 361:920-931. [DOI] [PubMed] [Google Scholar]
- 26.Kopper, S., and S. Freimund. 2003. The composition of keto aldoses in aqueous solution as determined by NMR spectroscopy. Helv. Chim. Acta 86:827-843. [Google Scholar]
- 27.Lim, B. C., H. J. Kim, and D. K. Oh. 2007. High production of d-tagatose by the addition of boric acid. Biotechnol. Prog. 23:824-828. [DOI] [PubMed] [Google Scholar]
- 28.Macura, S., and R. R. Ernst. 1980. Elucidation of cross relaxation in liquids by two-dimensional NMR spectroscopy. Mol. Phys. 41:95-117. [Google Scholar]
- 29.Maloney, M. D. 2003. Carbohydrates, p. 445-470. In J. Sherma and B. Fried (ed.), Chromatographic science, vol. 89. Handbook of thin-layer chromatography. Marcel Dekker, New York, NY. [Google Scholar]
- 30.Matsuo, T., Y. Baba, M. Hashiguchi, K. Takeshita, K. Izumori, and H. Suzuki. 2001. Dietary d-psicose, a C-3 epimer of d-fructose, suppresses the activity of hepatic lipogenic enzymes in rats. Asia Pac. J. Clin. Nutr. 10:233-237. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo, T., H. Suzuki, M. Hashiguchi, and K. Izumori. 2002. d-Psicose is a rare sugar that provides no energy to growing rats. J. Nutr. Sci. Vitaminol. (Tokyo) 48:77-80. [DOI] [PubMed] [Google Scholar]
- 32.McDonald, E. J. 1967. A new synthesis of d-psicose (d-ribo-hexulose). Carbohydr. Res. 5:106-108. [Google Scholar]
- 33.Miller, B. S., and T. Swain. 1969. Chromatographic analyses of the free amino acids, organic acids and sugars in wheat plant extracts. J. Sci. Food Agric. 11:344-348. [Google Scholar]
- 34.Mukerjea, R., D. Kim, and J. F. Robyt. 1996. Simplified and improved methylation analysis of saccharides, using a modified procedure and thin-layer chromatography. Carbohydr. Res. 292:11-20. [Google Scholar]
- 35.Rance, M., O. W. Sφrenson, G. Bodenhausen, G. Wagner, R. R. Ernst, and K. Wüthrich. 1983. Improved spectral resolution in COSY 1H NMR spectra of proteins via double quantum filtering. Biochem. Biophys. Res. Commun. 117:479-485. [DOI] [PubMed] [Google Scholar]
- 36.Smith, K. W., and S. L. Johnson. 1976. Borate inhibition of yeast alcohol dehydrogenase. Biochemistry 15:560-565. [DOI] [PubMed] [Google Scholar]
- 37.Takeshita, K., A. Suga, G. Takada, and K. Izumori. 2000. Mass production of d-psicose from d-fructose by a continuous bioreactor system using immobilized d-tagatose 3-epimerase. J. Biosci. Bioeng. 90:453-455. [DOI] [PubMed] [Google Scholar]
- 38.van den Berg, R., J. A. Peters, and H. van Bekkum. 1994. The structure and (local) stability constants of borate esters of mono- and di-saccharides as studied by 11B and 13C NMR spectroscopy. Carbohydr. Res. 253:1-12. [DOI] [PubMed] [Google Scholar]
- 39.Wada, K., and Y. Asano. 2003. Use of borate to control the 5′-position-selective microbial glucosylation of pyridoxine. Appl. Environ. Microbiol. 69:7058-7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willker, W., D. Leibfritz, R. Kerssebaum, and W. Bermel. 1993. Inverse heteronuclear correlation spectroscopy. Magn. Reson. Chem. 31:287-292. [Google Scholar]
- 41.Yoshinari, T., R. T. Forbes, P. York, and Y. Kawashima. 2003. Crystallisation of amorphous mannitol is retarded using boric acid. Int. J. Pharm. 258:109-120. [DOI] [PubMed] [Google Scholar]