Abstract
We observed that Lactobacillus reuteri JCM1112 produces B12 and folate. However, the folate/B12 mass ratio found was far below that desired for human consumption (∼170:1). We used metabolic engineering applying genetic and physiological approaches to improve this ratio and developed a generic and natural process that significantly increases folate production.
Humans have an auxotrophic requirement for vitamin B12 and folate, and the recommended intakes of these nutrients for healthy adults are 2.4 and 400 μg/day, respectively (7). Suboptimal intake of either of these compounds has been linked to cardiovascular disease, neuropathy, birth defects, cancer, and different types of anemia, among other pathologies (4). Remarkably, the onset of vitamin B12 deficiency symptoms is often delayed by an increased intake of folate (20). This masking of B12 deficiency has resulted in the restriction of folate intake levels and prevented folate fortification in many countries (7). Strict vegetarian dietary regimens tend to be poor in vitamin B12 and rich in folic acid, increasing the risk of vitamin B12 deficiency masking. This has boosted the popularity of fortifying vegetarian foodstuffs with B12 (3).
Coenzyme B12 is synthesized by a few members of the bacterial and archaeal groups (13). In situ microbial B12 production is a convenient strategy to achieve natural enrichment of fermented foods, notably from vegetable sources. Lactobacillus reuteri is a gram-positive, heterofermentative lactic acid bacterium with a long history of safe use by the food industry (10). This microorganism ferments several sugars, and this flexibility leads to its capacity to thrive on several substrates of vegetable origin (14). Strain CRL1098 has been reported to produce different forms of B12 (18, 25), and the draft genome sequence of strain JCM1112 (accession no. CP000705) (http://www.jgi.doe.gov/) suggests that it is able to produce folate, as well as B12. In this study, we investigated the possibility of using L. reuteri for the combined production of both vitamins at a ratio desired for human consumption, ∼170:1 (wt/wt).
In silico analysis of the folate biosynthesis genes of L. reuteri JCM1112.
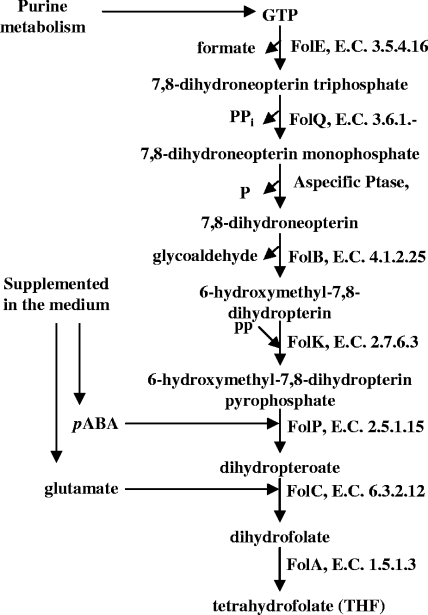
Folate is a tripartite molecule assembled from GTP, para-aminobenzoic acid (PABA), and one or more l-glutamate moieties. The biosynthesis pathway has been extensively characterized in several lactic acid bacteria, including Lactobacillus plantarum WCFS1 (Fig. 1). The predicted product of each folate biosynthesis gene of this bacterium was used to search the genome of L. reuteri JCM1112 using the BLAST algorithm (2). The sequence identity of the bidirectional best hit was calculated on the nucleotide and amino acid levels based on separate Needleman-Wunsch global alignments (15) determined using the needle script included in EMBOSS (European Molecular Biology Open Software Suite) (17) with default settings. Gene order was analyzed using the ERGO bioinformatics suite (http://ergo.integratedgenomics.com/ERGO/) (16). The two clusters are very similar, as expected from the close phylogenetic relationship of their hosts (Table 1). Sequence identity is high at both the amino acid and nucleotide levels (on average, 43 and 51%, respectively). Gene order is completely conserved throughout the entire length of the approximately 4.5-kb cluster composed of six genes.
FIG. 1.
Folate biosynthesis pathway in L. plantarum WCFS1.
TABLE 1.
Presence of folate biosynthesis genes in the genome of L. reuteri JCM1112 as determined by homology searches with L. plantarum WCFS1
| L. plantarum WCFS1 open reading frame | Gene | Length of protein (amino acids)a | Assigned function |
L. reuteri JCM1112 orthologue
|
|||
|---|---|---|---|---|---|---|---|
| Open reading frame | Length of protein (amino acids)a | % Identity
|
|||||
| Amino acid | Nucleotide | ||||||
| lp3299 | folB | 122 | Dihydroneopterin aldolase (EC 4.1.2.25) | Lreu1280 | 111 | 48 | 58 |
| lp3298 | folK | 170 | 2-Amino-4-hydroxy-6-hydroxymethyldihydropteridine pyrophosphokinase (EC 2.7.6.3) | Lreu1279 | 170 | 43 | 55 |
| lp3297 | folE | 189 | GTP cyclohydrolase I (EC 3.5.4.16) | Lreu1278 | 192 | 57 | 59 |
| lp3296 | folC2 | 454 | Folylpolyglutamate synthase (EC 6.3.2.17)/dihydrofolate synthase (EC 6.3.2.12) | Lreu1277 | 419 | 38 | 47 |
| lp3295 | xtp2 | 195 | XTP pyrophosphatase (EC 3.6.1.-) | Lreu1276 | 195 | 35 | 48 |
| lp3294 | folP | 263 | Dihydropteroate synthase (EC 2.5.1.15) | Lreu1275 | 387 | 37 | 39 |
Length based on the number of amino acid residues predicted in the gene product.
Characterization of B12 and folate production in CDM by L. reuteri JCM1112 and derivatives of this strain.
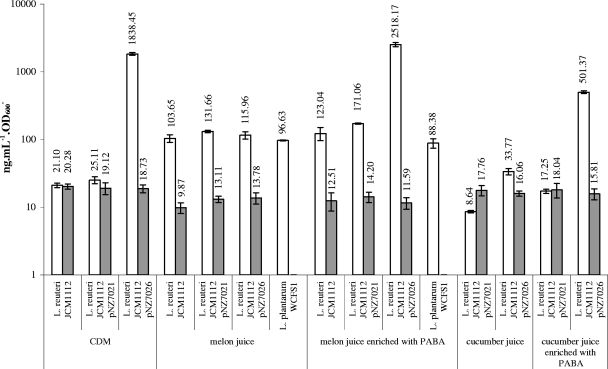
The human isolate L. reuteri JCM1112 (type strain) was obtained from the Japanese Collection of Microorganisms (Riken, Japan). It was cultured at 37°C in chemically defined medium (CDM) containing 10 mg/liter of PABA and lacking vitamin B12 and folic acid (26). Folate in stationary-phase cultures was quantified as described previously (8) with a bioassay using Lactobacillus casei ATCC 7469 as the indicator strain, which included enzymatic deconjugation of polyglutamate tails (23). The vitamin B12 content was determined as described in the Official Methods of Analysis of AOAC International, using the Lactobacillus delbrueckii subsp. lactis ATCC 7830 vitamin B12 assay (9). Cell extracts of stationary-phase cultures used for B12 analysis were prepared as previously described (18). In CDM L. reuteri JCM1112 produces around 20 μg of folate·liter−1·unit of optical density at 600 nm (OD600)−1 at an approximately 1:1 (wt/wt) ratio with B12 (Fig. 2).
FIG. 2.
Folate (open bars) and B12 (shaded bars) production by L. reuteri wild-type and derivative strains and by L. plantarum WCFS1 in different media. Plasmid pNZ7021 is the empty plasmid, and plasmid pNZ7026 contains the folate biosynthesis gene cluster of L. plantarum. Each bar represents the average of three biological replicates, and the error bars show standard deviations. All experiments were repeated with at least two different batches of media with similar results.
We used a metabolic engineering strategy as proof of principle for the possibility that the ratio of production of these two vitamins was influenced. We aimed at increasing folate production through the overexpression of the complete folate biosynthesis gene cluster, as described previously for other lactic acid bacteria (29, 30), ideally leaving the native B12 production unchanged. The constructs used in this study cannot be directly used by the food industry, but the use of food-grade alternatives is possible. A wide variety of food-grade systems have been developed for lactic acid bacteria (namely, for representatives of the genus Lactobacillus) (5). L. reuteri was transformed by electroporation as described elsewhere (27) with plasmids pNZ7021 (empty vector) and pNZ7026 harboring the folate biosynthesis gene cluster of L. plantarum WCFS1 under control of the pepN promoter (28). The derivatives of JCM1112 were cultured and analyzed for folate and B12 content in a fashion similar to that used for the parent strain. Chloramphenicol was used as a selection marker at a final concentration of 10 μg/ml. The constitutive overexpression of the folate biosynthesis genes of L. plantarum WCFS1 in cultures of L. reuteri JCM1112/pNZ7026 resulted in an almost 100-fold increase in folate levels (Fig. 2), while the control (L. reuteri JCM1112/pNZ7021) did not show any change in folate and B12 production. The overproduction of folate was found to have a very small effect on B12 production (<10% reduction), resulting in a folate/B12 ratio of approximately 100:1 (wt/wt), which was stable over five consecutive transfers (data not shown). The high levels of folate overproduction for the strain transformed with pNZ7026 were expected, provided that PABA was supplied in the medium. The same construct has been tested with L. plantarum, resulting in similar folate production levels (28), and similar results were obtained when the same strategy was applied to Lactococcus lactis (30) and Lactobacillus gasseri (29).
Characterization of B12 and folate production in fruit fermentations.
We assessed the applicability of the principle of improving folate/B12 ratios through genetic engineering to media other than CDM. Most (sub)tropical fruits are perishable and sensitive to chill damage, leading to losses of up to 40% in industrialized countries and far greater than 50% in less economically developed nations (6, 12). Fermentation is a secular process of food preservation, which in this case could increase the vitamin content of a raw material. Juice derived from two Cucumis spp. (melon and cucumber) was selected for natural enrichment, since this material is low in folate and deficient in B12 according to the USDA National Nutrient Database for Standard Reference (http://www.ars.usda.gov/ba/bhnrc/ndl.). Melon juice medium was made from Cucumis melo var. reticulatus after peeling and removal of seeds. The pulp was liquefied using a kitchen blender (Masterchef 370; Moulinex, France), and the resulting paste was squeezed through a cotton cloth. The flowthrough was centrifuged twice at 8,000 × g for 10 min using a Sorvall centrifuge (Newton, CT). The supernatant was stored at −20°C until it was used. Before inoculation, the melon juice was diluted at a 4:1 (vol/vol) ratio with potassium phosphate buffer (final concentration, 0.1 M; pH 5.8). Further dilution was found to result in growth impairment (data not shown). The final pH was adjusted to 6.0, and the melon juice medium was forced through a 0.22-μm filter to ensure sterility. Cucumber juice medium was prepared from intact cucumber (Cucumis sativus) and was sterilized using the procedure described above for melon medium with the following modifications: (i) an additional filtration step using a cellulose filter (0.15 mm) was used before centrifugation, and (ii) the cucumber juice was diluted in 1 volume of potassium phosphate buffer (final concentration, 0.1 M; pH 5.8). When mentioned below, both media were supplemented with 10 mg/liter PABA. This concentration of PABA does not conflict with exiting food legislation as PABA is listed as a generally regarded as safe compound with an upper intake limit of 30 mg/day (http://www.cfsan.fda.gov/∼dms/opa-appa.html). If appropriate, 10 μg/ml chloramphenicol was also added. Biomass formation in the different growth media is indicated in Table 2.
TABLE 2.
Biomass formation in the different growth media
| Strain | Final OD600 in:
|
||||
|---|---|---|---|---|---|
| CDM | Melon juice | Melon juice enriched with PABAa | Cucumber juice | Cucumber juice enriched with PABAa | |
| L. reuteri JCM1112 | 2.8 | 2.9 | 2.5 | NDb | ND |
| L. reuteri JCM1112/pNZ7021 | 2.8 | 2.3 | 2.0 | 1.4 | 1.2 |
| L. reuteri JCM1112/pNZ7026 | 2.5 | 1.9 | 2.9 | 1.6 | 1.1 |
| L. plantarum WCFS1 | 3.5 | 3.9 | 3.0 | ND | ND |
Supplemented with 10 mg/liter PABA.
ND, not determined.
Folate and B12 contents were determined for cultures of L. reuteri transformed with pNZ7026 and pNZ7021. The background folate levels in melon and cucumber media were found to be 22.5 ± 0.9 and 10.0 ± 0.4 μg/liter, respectively. As expected, B12 could not be detected in these media. The overexpression of the folate biosynthesis cluster of WCFS1 in L. reuteri JCM1112/pNZ7026 led to production of a high level of folate (2,518.2 ± 182.1 μg·liter−1·OD600 unit−1) and a folate/B12 ratio of ∼250:1 (wt/wt), but only when PABA was added (Fig. 2). PABA availability has been shown to limit folate biosynthesis in several lactic acid bacteria (24, 30). The control experiment using L. reuteri JCM1112 with the empty vector pNZ7021 resulted in the production, in melon medium, of 131.7 ± 5.5 μg·liter−1·OD600 unit−1 of folate, which is more than five times greater than the production in CDM (P < 0.001, pairwise t test). In cucumber medium, folate production by JCM1112/pNZ7021 was negatively affected compared to the production in CDM, regardless of the addition of PABA (Fig. 2). The overexpression of the folate biosynthesis genes had an effect similar to that described for CDM, but the final folate/B12 ratios were 1 order of magnitude lower than desired. The twofold reduction in B12 production observed for the melon juice fermentation can be attributed to the amount of sugars present, ∼1.5% glucose and ∼2% fructose as determined by high-performance liquid chromatography analyses performed as described elsewhere (21). Such concentrations have been shown in previous studies to repress B12 biosynthesis at the transcriptional level (1, 19).
The remarkable feature of melon fermentation in comparison to CDM and cucumber fermentation is the 5- to 10-fold-grater production of folate by the strain carrying the empty plasmid (pNZ7021). To establish the unique ability of melon juice to induce production of a high level of folate, we tested the parent strain, L. reuteri JCM1112, and another lactic acid bacterium, L. plantarum WCFS1 (11). Both L. reuteri and L. plantarum showed a 5- to 10-fold increase in folate production in melon juice medium compared to CDM (Fig. 2). Folate biosynthesis relies on three building blocks (Fig. 1) whose availability does not seem to explain this unsuspected observation. We have experimentally ruled out PABA and l-glutamate since both of these compounds are present in excess in CDM. Regarding the other building block, it has been shown that GTP is not the rate-limiting substrate in folate biosynthesis (24), which can be explained by the small flux from GTP to folate in comparison to the total GTP pool. This implies that an increase in GTP availability for folate synthesis cannot reasonably explain the increase in folate production observed in melon juice. Folate production is tightly regulated on both the transcriptional and translational levels (22, 23, 28). We suspect that there might be an interaction between a compound present in melon juice and one of these regulatory factors. However, the nature of the postulated interaction is unclear and remains to be elucidated.
In this study, we demonstrated that it is possible to combine the production of folate and the production of B12 in L. reuteri. We used, as proof of principle, a metabolic engineering strategy to optimize the ratio of production of these two vitamins and assessed its applicability to fruit fermentations. This resulted in the development of a natural fermentation process to increase folate production by lactobacilli to levels substantially higher than those previously described (24). The findings reported here may lead to the development of (fermented) foods based on perishable fruits, such as melons, with extended durability and higher nutritional value. A good-tasting fermented melon juice or melon squash containing high folate and vitamin B12 levels could be the start of a product line with a longer shelf-life that especially targets vitamin-deficient populations.
Footnotes
Published ahead of print on 14 March 2008.
REFERENCES
- 1.Ailion, M., T. A. Bobik, and J. R. Roth. 1993. Two global regulatory systems (Crp and Arc) control the cobalamin/propanediol regulon of Salmonella typhimurium. J. Bacteriol. 175:7200-7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Dietetic Association and Dietitians of Canada. 2003. Position of the American Dietetic Association and Dietitians of Canada: vegetarian diets. J. Am. Diet Assoc. 103:748-765. [DOI] [PubMed] [Google Scholar]
- 4.Carmel, R., R. Green, D. S. Rosenblatt, and D. Watkins. 2003. Update on cobalamin, folate, and homocysteine. Hematol. Am. Soc. Hematol. Educ. Program 2003:62-81. [DOI] [PubMed] [Google Scholar]
- 5.de Vos, W. M. 1999. Safe and sustainable systems for food-grade fermentations by genetically modified lactic acid bacteria. Int. Dairy J. 9:3-10. [Google Scholar]
- 6.Food and Agriculture Organization, World Health Organization. 2005. Bananas and tropical fruits. Report on the 4th Session of the FAO Committee on Commodity Losses, Guayaquil, Ecuador. World Health Organization, Geneva, Switzerland.
- 7.Food and Agriculture Organization, World Health Organization. 2004. Vitamin and mineral requirements in human nutrition, 2nd ed. World Health Organization, Geneva, Switzerland.
- 8.Horne, D. W., and D. Patterson. 1988. Lactobacillus casei microbiological assay of folic acid derivatives in 96-well microtiter plates. Clin. Chem. 34:2357-2359. [PubMed] [Google Scholar]
- 9.Horowitz, W. (ed.). 2006. Official methods of analysis of AOAC International, 18th ed. AOAC International, Gaithersburg, MD.
- 10.Kandler, O., and N. Weiss. 1986. Regular nonsporing gram positive rods, p. 1208-1234. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 11.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 100:1990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyons, J. M. 1973. Chilling injury in plants. Annu. Rev. Plant Physiol. 24:445-466. [Google Scholar]
- 13.Martens, J. H., H. Barg, M. J. Warren, and D. Jahn. 2002. Microbial production of vitamin B12. Appl. Microbiol. Biotechnol. 58:275-285. [DOI] [PubMed] [Google Scholar]
- 14.Meroth, C. B., J. Walter, C. Hertel, M. J. Brandt, and W. P. Hammes. 2003. Monitoring the bacterial population dynamics in sourdough fermentation processes by using PCR-denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 69:475-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Needleman, S. B., and C. D. Wunsch. 1970. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 48:443-453. [DOI] [PubMed] [Google Scholar]
- 16.Overbeek, R., N. Larsen, T. Walunas, M. D'Souza, G. Pusch, E. Selkov, Jr., K. Liolios, V. Joukov, D. Kaznadzey, I. Anderson, A. Bhattacharyya, H. Burd, W. Gardner, P. Hanke, V. Kapatral, N. Mikhailova, O. Vasieva, A. Osterman, V. Vonstein, M. Fonstein, N. Ivanova, and N. Kyrpides. 2003. The ERGO genome analysis and discovery system. Nucleic Acids Res. 31:164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice, P., I. Longden, and A. Bleasby. 2000. EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet. 16:276-277. [DOI] [PubMed] [Google Scholar]
- 18.Santos, F., J. L. Vera, P. Lamosa, G. F. de Valdez, W. M. de Vos, H. Santos, F. Sesma, and J. Hugenholtz. 2007. Pseudovitamin B12 is the corrinoid produced by Lactobacillus reuteri CRL1098 under anaerobic conditions. FEBS Lett. 581:4865-4870. [DOI] [PubMed] [Google Scholar]
- 19.Santos, F., J. L. Vera, R. van der Heijden, G. Valdez, W. M. de Vos, F. Sesma, and J. Hugenholtz. 2008. The complete coenzyme B12 biosynthesis gene cluster of Lactobacillus reuteri CRL1098. Microbiology 154:81-93. [DOI] [PubMed] [Google Scholar]
- 20.Stabler, S. P. 1999. B12 and nutrition, p. 343-365. In R. Banerjee (ed.), Chemistry and biochemistry of B12. John Wiley & Sons, Inc., New York, NY.
- 21.Starrenburg, M. J., and J. Hugenholtz. 1991. Citrate fermentation by Lactococcus and Leuconostoc spp. Appl. Environ. Microbiol. 57:3535-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sybesma, W. 2003. Metabolic engineering of folate production in lactic acid bacteria. Ph.D. thesis. Wageningen University, Wageningen, The Netherlands.
- 23.Sybesma, W., M. Starrenburg, M. Kleerebezem, I. Mierau, W. M. de Vos, and J. Hugenholtz. 2003. Increased production of folate by metabolic engineering of Lactococcus lactis. Appl. Environ. Microbiol. 69:3069-3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sybesma, W., M. Starrenburg, L. Tijsseling, M. H. Hoefnagel, and J. Hugenholtz. 2003. Effects of cultivation conditions on folate production by lactic acid bacteria. Appl. Environ. Microbiol. 69:4542-4548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taranto, M. P., J. L. Vera, J. Hugenholtz, G. F. De Valdez, and F. Sesma. 2003. Lactobacillus reuteri CRL1098 produces cobalamin. J. Bacteriol. 185:5643-5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teusink, B., F. H. van Enckevort, C. Francke, A. Wiersma, A. Wegkamp, E. J. Smid, and R. J. Siezen. 2005. In silico reconstruction of the metabolic pathways of Lactobacillus plantarum: comparing predictions of nutrient requirements with those from growth experiments. Appl. Environ. Microbiol. 71:7253-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter, J., N. C. Heng, W. P. Hammes, D. M. Loach, G. W. Tannock, and C. Hertel. 2003. Identification of Lactobacillus reuteri genes specifically induced in the mouse gastrointestinal tract. Appl. Environ. Microbiol. 69:2044-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wegkamp, A. 2008. Modulation of folate production in lactic acid bacteria. Ph.D. thesis. Wageningen University, Wageningen, The Netherlands.
- 29.Wegkamp, A., M. Starrenburg, W. M. de Vos, J. Hugenholtz, and W. Sybesma. 2004. Transformation of folate-consuming Lactobacillus gasseri into a folate producer. Appl. Environ. Microbiol. 70:3146-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wegkamp, A., W. van Oorschot, W. M. de Vos, and E. J. Smid. 2007. Characterization of the role of para-aminobenzoic acid biosynthesis in folate production by Lactococcus lactis. Appl. Environ. Microbiol. 73:2673-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]