Abstract
Aurora kinases are highly conserved proteins with important roles in mitosis. Metazoans contain two kinases, Aurora A and B, which contribute distinct functions at the spindle poles and the equatorial region respectively. It is not currently known whether the specialized functions of the two kinases arose after their duplication in animal cells or were already present in their ancestral kinase. We show that Dictyostelium discoideum contains a single Aurora kinase, DdAurora, that displays characteristics of both Aurora A and B. Like Aurora A, DdAurora has an extended N-terminal domain with an A-box sequence and localizes at the spindle poles during early mitosis. Like Aurora B, DdAurora binds to its partner DdINCENP and localizes on centromeres at metaphase, the central spindle during anaphase, and the cleavage furrow at the end of cytokinesis. DdAurora also has several unusual properties. DdAurora remains associated with centromeres in anaphase, and this association does not require an interaction with DdINCENP. DdAurora then localizes at the cleavage furrow, but only at the end of cytokinesis. This localization is dependent on DdINCENP and the motor proteins Kif12 and myosin II. Thus, DdAurora may represent the ancestral kinase that gave rise to the different Aurora kinases in animals and also those in other organisms.
Universal to all eukaryotes, Aurora kinases are known to function during multiple stages of cell division. The activity of Aurora kinases regulates almost every crucial stage of M phase, from chromosome condensation and separation to the very end of cytokinesis (10). Most animal cells contain two related kinases, Aurora A and B, which have distinct localizations and specialized roles during mitosis (1). Aurora A is localized primarily at the spindle poles and is implicated in the regulation of entry into mitosis, centrosome maturation, and spindle assembly (3, 28, 52). Aurora B forms the well-known chromosome passenger complex together with INCENP, survivin, and borealin (60). This complex associates with centromeres early in mitosis and then redistributes to the central spindle at the metaphase/anaphase transition (2, 53, 57). Accordingly, Aurora B is required for correct chromosome segregation and cytokinesis (31). Vertebrates contain an additional Aurora C kinase that is expressed exclusively in the testis, where it may play a role similar to that of Aurora B (35, 40). In contrast to animals, yeast cells contain only one Aurora kinase (Ipl1 in budding yeast and Ark1 in fission yeast) (12, 48). The yeast kinases have been shown to be mainly involved in chromosome segregation and cytokinesis (22, 39, 45, 47), processes that are usually regulated by animal Aurora B kinase. Similarly to animal Aurora B, yeast Auroras also associate with homologues of INCENP and survivin and localize to the centromeres and spindle (9, 34, 51). Unlike animal Aurora A, Ark1 mutant cells exhibited only a minor defect in spindle formation and Ark1 kinase does not seem to be localized on the spindle pole body (48).
To determine the separate roles of Aurora A and B kinases, it is important to consider their evolutionary origin. The high sequence similarity between these proteins suggests that they arose by gene duplication. Phylogenetic analysis suggests that the duplication of Aurora genes has occurred independently in vertebrates, invertebrates, and plants (8). However, it is not clear whether the original ancestral kinase had the properties and functions of Aurora A, Aurora B, or both. Given that, by most criteria, the yeast Aurora kinases resemble Aurora B, it would appear that the specialized features of Aurora A may have been a subsequent invention in animal cells after the duplication of the ancestral Aurora gene. Since fungi have a closed mitosis and a reduced set of spindle and cytoplasmic microtubules, it is possible that yeasts may have dispensed with the specialized activity of an Aurora A kinase. On the other hand, only the kinase domain of human Aurora A, but not of Aurora B, can partially complement the function of Saccharomyces cerevisiae Ipl1 when fused to the N terminus of Ipl1 (5). To better understand the similarities and differences among different Aurora kinases and to better appreciate what parallels can be drawn among them, it is important to analyze the function of Aurora kinases in other eukaryotes.
Other eukaryotes in which Aurora kinases have been identified include plants and the parasite trypanosomes, but the function of these kinases has not yet been characterized in great detail. In higher plants, Aurora kinases have diversified into two major groups, alpha and beta, that are not related to the animal Aurora A and B kinase groups (18). Both types of plant Aurora proteins localize on centromeres and spindle fibers and do not appear to be required for spindle formation (18). Given that plant mitotic spindles do not have centrosomes, it would seem reasonable to postulate that plants may not need an Aurora A-like kinase. In trypanosomes, the Aurora kinases have diversified into three closely related proteins that seem most similar in sequence and function to animal Aurora B (58). However, no homologues of INCENP, survivin, or borealin seem to be present in the genomes of any plants or trypanosomes. Therefore, it is not clear whether the mechanism of regulation of Aurora kinases in these organisms shares any similarity to that in animal cells.
Dictyostelium is an excellent model system to study mitosis and cytokinesis in eukaryotic cells. In addition to the many molecular tools available to study these cells, the morphology of their mitotic apparatus is amenable to detailed microscopy analysis (43, 44, 50). Dictyostelium cells contain relatively few interphase microtubules that are rearranged into a well-defined intranuclear spindle during mitosis. A large effort to identify and characterize the function of centrosomal proteins is well under way (25, 49), and a large collection of mutants that affect mitosis or cytokinesis have been gathered over the years (16, 20, 36, 38, 46). Most importantly, Dictyostelium discoideum contains a protein homologous to animal INCENP, the activating partner of Aurora B (15). This protein, DdINCENP, displays the dynamic behavior of chromosomal passenger proteins and is important for mitosis and cytokinesis. Since analysis of the Dictyostelium genome suggests that this organism has retained many of the ancestral properties of the last common ancestor of plants, animals, and fungi (19), it is an ideal system to dissect the original properties of Aurora kinases. Our findings presented here demonstrate that the single Dictyostelium Aurora kinase displays properties found in both Aurora A and B kinases and thus suggest that the ancestral Aurora kinase played both roles in primitive cells.
MATERIALS AND METHODS
Cloning of DdAurora and construction of GFP-DdAurora.
A 1.6-kb sequence corresponding to the full length of DdAurora gene was cloned from Dictyostelium genomic DNA by using the 5′ and 3′ primers AO-499 (5′-CGAGCTCATGAGTTATCCAAATAATAAAGAAAATAGTAACAATATTGGTG-3′) and AO-500 (5′-CGGATCCTTAATAAGTCATTTGAGATGGTAATGGAAGACCC-3′), respectively. The full-length DdAurora was further cloned into pTX-GFP (green fluorescent protein) vector.
Cell culture and transformation.
Dictyostelium discoideum AX2 (wild-type) cells were grown in axenic HL-5 medium supplemented with penicillin and streptomycin at 19°C. DdINCENP-null cells were grown with additional 5 μg/ml blasticidin S hydrochloride. Cells carrying pTX-based plasmids were grown in medium with additional 10 μg/ml G418. For stationary culture, cells were grown in plastic petri dishes. Cells in suspension culture were grown in conical flasks on a rotating shaker at ≈200 rpm. The constructs were introduced into cells with electroporation, and the transformants were selected in the HL-5 medium with 10 μg/ml G418.
Fluorescence microscopy of live cells.
For live microscopy of mitotic cells, cells in active log-phase growth were first resuspended to a concentration of 2 × 106 cells/ml. Cell suspension (0.5 ml) was plated on a small petri dish with a coverslip mounted at the bottom (MatTek, Ashland, MA). After cells attached to the coverslip, HL-5 medium was removed and replaced with low-fluorescence medium for at least 20 min before observation (7). The live imaging of the cells was conducted by using a Nikon Eclipse TE200 microscope (Nikon Instruments, Dallas, TX) equipped with a ×100 1.4 NA PlanFluor objective, shuttered illumination, and a Quantix 57 camera (Roper Scientific, Tucson, AZ) controlled by Metamorph (Universal Imaging Corp., West Chester, PA). The exposure time for the GFP fluorescence was 100 ms or less, with the interval time being at least 10 s.
Immunostaining and microscopy of mitotic cells.
For immunostaining of mitotic cells, cells in active log-phase growth were harvested from stationary culture and resuspended to a concentration of 2 × 106 cells/ml. Cell suspension (200 μl) was put on each coverslip and allowed to sit still for at least 20 min. For fixation, cells were first fixed in 1× PDF buffer (20 mM KCl, 11 mM K2HPO4, 13.2 mM KH2PO4, 1 mM CaCl2, 2.5 mM MgSO4 [pH 6.4]) with 2% formaldehyde and 0.01% Triton X-100 for 15 min at room temperature and then in dehydrated methanol with 1% formaldehyde at −20°C for 5 min. The primary antibody was affinity-purified anti-DdAurora or monoclonal anti-DdCP224. The second antibody was Texas red-conjugated goat anti-rabbit or fluorescein isothiocyanate-conjugated goat anti-mouse antibody (Molecular Probes, Eugene, OR). For DAPI (4′,6′-diamidino-2-phenylindole) staining of DNA, coverslips were immersed in 1× PDF buffer with 0.1 μg/ml DAPI for 10 min before the final wash step.
Antibody generation and affinity purification.
A full-length cDNA of DdAurora was cloned from Dictyostelium cDNA library (Stratagene, La Jolla, CA) by using the 5′ and 3′ primers AO-554 (5′-CGAATTCATGAGTTATCCAAATAATAAAGAAAATAGTAACAATATTGGTG-3′) and AO-500 (5′-CGGATCCTTAATAAGTCATTTGAGATGGTAATGGAAGACCC-3′), respectively. The PCR product was further cloned into the pMal-c2X vector (New England Biolabs, Ipswich, MA). A maltose-binding protein (MBP)-DdAurora fusion protein was expressed in Escherichia coli and purified according to the provided protocol. Five milliliters of amylose resin (New England Biolabs, Ipswich, MA) was used for 1 liter of Escherichia coli culture. The purified fusion protein was injected into rabbits to raise polyclonal anti-DdAurora antibodies (Cocalico Biologicals, Reamstown, PA). To purify polyclonal anti-DdAurora antibodies by affinity binding, purified MBP-DdAurora was further cross-linked to AminoLink coupling gel (Pierce, Rockford, IL) according to the provided protocol. Tandem-affinity purification (TAP)-tagged GFP was purified from Dictyostelium cell culture and used to generate polyclonal anti-GFP antibodies by the same procedure.
Affinity purification of polyclonal anti-DdAurora antibodies.
One milliliter of AminoLink coupling gel (Pierce, Rockford, IL) cross-linked with more than 10 mg of MBP-DdAurora was used to purify polyclonal anti-DdAurora antibodies from 2 ml of crude serum. The crude serum was first precleaned by centrifugation at 13,000 rpm in a desktop centrifuge for 10 min. The supernatant was diluted to 10 ml with 0.1 M sodium phosphate buffer (pH 7.4) and incubated with prewashed MBP-DdAurora gel for 2 h at 4°C in a 15-ml Poly-Prep chromatography column (Bio-Rad, Hercules, CA). The antibody-gel mixture was then packed by gravity in the column and washed extensively with 0.1 M sodium phosphate buffer. Bound anti-DdAurora antibodies were finally eluted by 100 mM glycine (pH 2.5) and equilibrated to pH 7.4 immediately with 1 M Tris-HCl (pH 8.0).
Coimmunoprecipitation with DdAurora antibodies.
Affinity-purified polyclonal anti-DdAurora antibodies were used in all immunoprecipitation experiments. Cells expressing target GFP-fusion protein were cultured in suspension in a 250-ml flask. When the concentration reached ∼2 × 106 cells/ml, actively dividing cells are harvested by centrifugation at 1,500 rpm for 5 min. They were washed in 1× PDF buffer once and then resuspended in 3 ml of 0.1 M sodium phosphate buffer (pH 7.4) with fresh addition of 1:100 protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) and 1 mM dithiothreitol. Cells were lysed by sonication six times for 15 s each with a 15-s rest in between. The lysate was then centrifuged at 13,000 rpm for 15 min at 4°C twice to clean up cell debris. Forty microliters of purified anti-DdAurora antibodies was added to 1 ml of supernatant after centrifugation. Either unspecific rabbit immunoglobulin G or no addition of antibody was used as a negative control. The mixture was rotated at 4°C for 1 h, and then 20 μl of prewashed protein A beads (Amersham Pharmacia, Piscataway, NJ) was added to the suspension. After another 30-min incubation, the antibody-protein A complex was spun down at 3,000 rpm for 1 min and washed by 6× 1 ml 0.1 M sodium phosphate buffer. Finally, bound proteins were eluted by boiling the protein-bead complex in 100 μl SDS-sample buffer at 95°C for 10 min. The elutions were resolved on a 10% polyacrylamide gel and examined by Western blot analysis with anti-GFP antibodies.
In vitro pull-down with MBP-DdAurora.
Because different DdINCENP truncations have very different expression levels in DdINCENP-null cells, an in vitro assay was used instead of coimmunoprecipitation to keep the amount of DdAurora input at the same level. Cells were cultured and harvested in the same way as that of the coimmunoprecipitation experiment. After sonication, GFP-DdINCENP-ΔC lysate was diluted 10 times before centrifugation. By this dilution, the input levels of GFP-fusion proteins were adjusted to the same level too. After centrifugation, 200 μl of MBP-DdAurora-saturated amylose beads was added to the supernatant and incubated at 4°C for 2 h. After extensive wash, the bound proteins were boiled off from the beads by 100 μl SDS-sample buffer. The elutions were also resolved on a 10% polyacrylamide gel and examined by Western blot analysis with anti-GFP antibodies.
RESULTS
Dictyostelium has a single protein with similarities to both Aurora A and Aurora B kinases.
A search of the Dictyostelium genome database with Aurora kinase sequences from other organisms yielded only one related gene, which has been named aurK (entry DDB0216254 in http://dictybase.org; GenBank accession no. XP_641803). The sequence of the aurK gene encodes a serine-threonine protein kinase of 43 kDa that has a high degree of sequence similarity to Aurora kinases from other organisms, including both Aurora A and Aurora B kinases (Fig. 1). Therefore, we will refer to this protein simply as Dictyostelium Aurora, or DdAurora.
FIG. 1.
Alignment of DdAurora with Aurora kinases from other organisms. DdAurora has high sequence similarity to both Aurora A and Aurora B kinases. DdAurora has the KEN sequence commonly found at the N terminus of Aurora kinases. DdAurora also has an N-terminal A box (PSXXXQRVXXQ; framed in dashed box), which is important for Aurora A destruction. Within the A box, the conserved phosphorylation site, serine 22 (boldface in the A-box sequence [asterisk on the figure]) is known to be phosphorylated in vertebrates to block Cdh1-dependent destruction of Aurora A (41). DdAurora also contains the Aurora signature motif (DFGWSXXXXXXXRXTXCGTXDYLPPE) within the activation loop (framed) and a D box (LLXXXPXXRXXLXXXXXHPW) near its C terminus (underlined in the figure). All Aurora kinases have these two motifs to regulate their activation and degradation. H.s., Homo sapiens; X.l. Xenopus laevis; S.c. Saccharomyces cerevisiae.
DdAurora contains the signature motifs found in all Aurora kinases, including a KEN box at its N terminus, the activation loop in the catalytic domain (DFGWSXXXXXXXRXTXCGTXDYLPPE), and a D2-type destruction box (LLXXXPXXRXXLXXXXXHPW), near its C terminus (11, 13, 54). In addition, DdAurora has a potential A box (PSXXXQRVXXQ) near its N terminus, which is a specific feature found in vertebrate Aurora A kinases. The A box has been shown to be important to regulate the Cdh1-dependent destruction of Aurora A. Phosphorylation of the consensus serine site (in boldface) in this motif is able to block destruction of Aurora A (41).
To explore the relationship between DdAurora and Aurora kinases from other organisms, we constructed a phylogenetic tree with protein sequences from many organisms (Fig. 2). Vertebrate proteins group into three distinct clades: the known Aurora A, B, and C kinases (8). Plant proteins form two clades, the Aurora alpha and beta kinase groups. The Aurora proteins from different fungi also form a clearly defined group. The Dictyostelium kinase branches off near the base of the animal and plant groups as it is about equally similar to the animal, plant, and fungus kinases (57.5% to human Aurora A, 56% to human Aurora B, and 61.5% to Arabidopsis ATAur-1 and 47.3 to budding yeast Ipl1). The protein most similar to DdAurora is an uncharacterized Aurora protein from the cycad Cycas rumphii (71.2%). Since it is generally thought that Dictyostelium diverged early in the evolution of eukaryotes, our analysis suggests that the archetype Aurora kinase in the common ancestor of plants, fungi, and animals already had characteristics of both Aurora A and Aurora B kinases. Importantly, this kinase had the A-box signature of Aurora A kinases.
FIG. 2.
Phylogenetic tree of Aurora kinases from different species. The core kinase domains of Aurora sequences from the indicated species were aligned by ClustalW, and the corresponding phylogenic tree was generated using MegAlign. Aurora kinases in vertebrates are divided into three groups of Aurora A, B, and C subfamilies. Plants have two groups of Aurora kinases: the alpha and beta subfamilies. Most fungi have a single Aurora kinase, similar to other members within this clade. Caenorhabditis elegans and Drosophila contain divergent Aurora A and B kinases that probably arose through an independent duplication from that giving rise to vertebrate kinases (8). The Dictyostelium DdAurora kinase (asterisk) is about equally similar to the kinases of animals, plants, and fungi.
Dictyostelium Aurora has the dynamic distribution of both Aurora A and Aurora B kinases.
To determine whether DdAurora behaves like Aurora A or Aurora B, we examined the dynamic localization of GFP-tagged DdAurora in live Dictyostelium cells. GFP-DdAurora accumulated to levels similar to that of the endogenous protein and did not affect the growth of Dictyostelium cells (see Fig. S1 in the supplemental material). In interphase, GFP-DdAurora was diffuse throughout the entire cytoplasm, with no discernible accumulation in the nucleus (Fig. 3a). When cells entered mitosis, GFP-DdAurora was readily detectable at spindle poles during prometaphase (Fig. 3b). GFP-DdAurora formed bright foci at both spindle poles, making it easy to identify mitotic cells among many interphase cells. Subsequently, GFP-DdAurora was visible at the metaphase plate, probably in association with centromeres (Fig. 3c). With the onset of anaphase, GFP-DdAurora localized at the central spindle and remained there during telophase and early cytokinesis (Fig. 3d to f). The Dictyostelium spindle is known to dismantle during late telophase or early cytokinesis (50). Accordingly, we observed that GFP-DdAurora disappeared from the region of the central spindle during early cytokinesis (Fig. 3f). At the end of cytokinesis, GFP-DdAurora localized at the cytoplasmic bridge that connects the two daughter cells (Fig. 3g). When this bridge finally severed, GFP-Aurora persisted at the breaking point for a short time (Fig. 3i and see Movies S1 and S2 in the supplemental material). From anaphase to the end of cytokinesis, GFP-DdAurora could also be constantly observed near the spindle pole regions (Fig. 3d to f; not shown in panels g to i because of the focus plane).
FIG. 3.
GFP-DdAurora localization in different stages of M phase. Shown are fluorescence images of live wild-type cells expressing GFP-DdAurora. In interphase, GFP-DdAurora was diffuse in the cytoplasm (a). During prometaphase, GFP-DdAurora concentrated at spindle poles (arrows) (b). At metaphase, GFP-DdAurora localized to the metaphase plate (arrowhead) in addition to spindle poles (c). GFP-DdAurora localized to the central spindle (arrowhead) from early anaphase and faded from this location as the cell progressed through cytokinesis (d to f). Notice that GFP-DdAurora did not accumulate at the cleavage furrow early in cytokinesis (e) or even when the furrow was very advanced (f). In contrast, DdINCENP has been shown to localize at the cleavage furrow early in cytokinesis (15). Near the end of cytokinesis, GFP-DdAurora accumulated at the cytoplasmic bridge formed between the two daughter cells (g and h). GFP-DdAurora persisted at the breaking point of the cytoplasm bridge for a time after cytokinesis (i). Bar, 5 μm. (See Movies S1 and S2 in the supplemental material.)
Our observations indicate that DdAurora displays the distribution of both Aurora A (polar localization) and Aurora B (central spindle localization). DdAurora also has novel properties not previously reported for any other Aurora kinase (localization at the cytoplasmic bridge in the absence of midbody microtubules). As expected for a protein participating in multiple steps during mitosis, we were not able to generate a knockout of the DdAurora gene (data not shown).
Polar localization of DdAurora.
To investigate the localization of DdAurora in greater detail, we raised polyclonal antibodies against DdAurora. The anti-DdAurora antibodies specifically recognized DdAurora as a single band in Western blot analysis of whole-cell lysates (see Fig. S1 in the supplemental material). Immunofluorescence microscopy with affinity-purified anti-DdAurora antibodies revealed a distribution of endogenous DdAurora similar to that observed with GFP-DdAurora (Fig. 4). The only difference between GFP-DdAurora and endogenous DdAurora distribution was their relative intensity on centromeres during prometaphase and metaphase plate. Endogenous DdAurora appeared brighter on centromeres than at the poles (Fig. 3a to b), whereas GFP-DdAurora was brighter at the poles than on centromeres (Fig. 4b and c). This might indicate a weaker association of GFP-DdAurora with the centromeres at these stages or a stronger association with the poles.
FIG. 4.
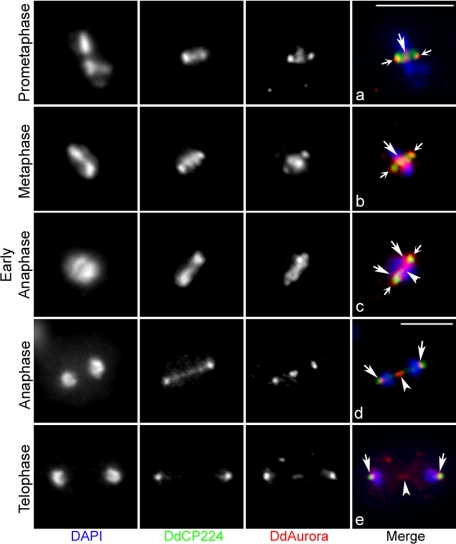
DdAurora localizes at the spindle poles, centromeres, and central spindle. Shown is colocalization of endogenous DdAurora and DdCP224 (a centrosomal and spindle marker) (24). Wild-type cells were fixed and stained with antibodies against DdAurora and DdCP224. In the merged images, DNA is shown in blue, DdAurora is shown in red, and DdCP224 is shown in green. (a and b) In prometaphase and metaphase, DdAurora localized to spindle poles (small arrows) and the metaphase plate (large arrows). DdAurora localized in close proximity but distal to DdCP224 at the poles. The metaphase plate localization corresponds to centromeric localization (Fig. 5). (c) In early anaphase, DdAurora localized simultaneously to spindle poles (small arrows), centromeres (large arrows), and the central spindle (arrowhead). (d and e) In late anaphase and telophase, DdAurora was lost from the distal portion of the spindle poles and was instead found on the inner side of the poles. Compare the locations of the polar DdAurora (red) in panels a and d in relation to the DdCP224-labeled centrosome (green). The DdAurora on the inner side of the poles represents protein associated with centromeres (Fig. 5). The staining of DdAurora at the central spindle was very bright in anaphase and faded during telophase (arrowheads). Bars, 5 μm.
We then used double-immunofluorescence microscopy to determine the localization of DdAurora in relation to that of the centrosomal protein DdCP224 (24). In Dictyostelium, the nuclear envelope is not dismantled during mitosis (44). Instead, the centrosome duplicates and becomes embedded in the nuclear envelope early in mitosis (59). The embedded centrosomes subsequently nucleate an intranuclear spindle. DdCP224, an ortholog of XMAP215, regulates microtubule dynamics (26) and is colocalized with γ-tubulin exactly at the mitotic spindle poles (24).
Close observation of the double-stained wild-type cells revealed that, rather than displaying an overlapping colocalization with DdCP224, DdAurora localized in close proximity but either distal to DdCP224 during prometaphase and metaphase (Fig. 4a to c) or proximal to DdCP224 during anaphase and telophase (Fig. 4d and e). Therefore, our images suggested that DdAurora was localized on the outer (cytosolic) side of the spindle poles during prometaphase and metaphase (Fig. 4a to c). Upon entry into anaphase, the outer polar DdAurora localization disappeared, to be replaced by DdAurora on the inner (intranuclear) side of the spindle poles (Fig. 4d and e).
DdAurora remains bound to centromeres after anaphase.
Dictyostelium centromeres migrate in close proximity to spindle poles in anaphase and remain associated with spindle poles through the rest of cell division (44). Thus, the presence of DdAurora on the inner side of the spindle poles, in close apposition with the segregated chromosomes (Fig. 4d), suggested the possibility that DdAurora remains associated with centromeres after metaphase. To test this possibility, we compared the localization of endogenous DdAurora with that of HcpA, a centromeric protein homologous to mammalian HP1 (32). Examination of cells expressing HcpA-GFP and immunostained with anti-DdAurora antibodies revealed that DdAurora colocalized with HcpA-GFP at centromeres in metaphase (Fig. 5a). During metaphase, Dictyostelium cells contain all centromeres congregated as a single dot at the center of the metaphase spindle and the chromosome arms spread outward perpendicularly to the spindle (50). At this stage, DdAurora was also present on the outer (cytosolic) side of the spindle poles (Fig. 4b).
FIG. 5.
DdAurora remains bound to centromeres even after anaphase. Wild-type cells expressing the centromeric marker HcpA-GFP (32) were stained with antibodies against DdAurora. In the merged images, DNA is shown in blue, DdAurora in red, and HcpA-GFP in green. (a) In metaphase, DdAurora localized to spindle poles (small arrows) and the metaphase plate. DdAurora colocalized with HcpA-GFP at the metaphase plate (large arrows), suggesting that DdAurora localizes to the centromeres at this stage. (b) In anaphase, DdAurora was found at the central spindle (arrowhead) in addition to the spindle poles (small arrows) and centromeres (large arrows). HcpA-GFP was diffusely distributed in the nucleus in anaphase, possibly by being displaced from centromeres by activated DdAurora (Fig. 6). (c) In telophase, HcpA-GFP could be observed again at the centromeres and colocalized extensively with centromeric DdAurora (large arrows). DdAurora also localized to the central spindle (shown by arrowhead) at this stage. Bars, 5 μm.
We confirmed the observation that the localization of HcpA-GFP changes early in anaphase (33). HcpA-GFP was bound to centromeres at metaphase but became diffuse inside the nucleus of cells in early anaphase (Fig. 5b). This suggests that HcpA-GFP dissociates from the centromeres at this time. HP1, the mammalian homologue of HcpA, is known to be displaced from mitotic chromosomes by Aurora B kinase during mitosis (21, 29). Later in telophase, HcpA-GFP was again localized at centromeres (Fig. 5c), which are closely apposed to the inner side of the spindle poles (32). At this stage, DdAurora clearly colocalized with HcpA-GFP at the centromeres of the telophase cells (Fig. 5c). The spindle poles of these cells, as marked by DdCP224 or γ-tubulin, are located farther away from the center of the spindle (Fig. 4e). These observations indicate that DdAurora remained bound to the centromeres of the separated chromatids during anaphase and telophase. HcpA is known to remain associated with the centromeres through interphase (33). The retention of Aurora kinase on centromeres after chromosomes have separated is unusual and has not been observed in any other species.
The Aurora B-like properties of DdAurora are dependent on its interaction with the IN-box domain of DdINCENP.
We have shown that DdAurora displays the distribution characteristic of both Aurora A and Aurora B kinases. Interestingly, the chromosomal passenger protein DdINCENP was found at some, but not all, of these locations in a mitotic cell (15). DdINCENP localized on centromeres during prometaphase and metaphase and on the central spindle during anaphase and telophase. These observations suggest the possibility that the DdAurora protein that colocalizes with DdINCENP may be part of a chromosomal passenger complex that has the properties of the similar complex formed by animal Aurora B kinase. On the other hand, the DdAurora found at the spindle poles would not be part of the chromosomal passenger complex and would behave as an Aurora A kinase. If this scenario is correct, then DdINCENP should be important only for the Aurora B-like localization of DdAurora. To test this hypothesis, we examined the localization of DdAurora in DdINCENP-null cells expressing the centromeric marker HcpA-GFP (32).
The localization of DdAurora on the spindle poles of metaphase cells was not influenced by the absence of DdINCENP (Fig. 6a and see Movie S3 in the supplemental material). This observation supports the idea that the polar localization of DdAurora is analogous to the polar localization of Aurora A and therefore does not require DdINCENP. In contrast, DdAurora failed to localize to the central spindle of DdINCENP-null cells during anaphase and telophase (Fig. 6b and c). The requirement of DdINCENP for the central spindle localization of DdAurora strengthens the model that these two proteins form a complex at the central spindle. However, DdINCENP was not required for the centromeric localization of DdAurora. DdAurora colocalized with HcpA-GFP on the centromeres of DdINCENP-null cells from metaphase to telophase (Fig. 6a, b, and c). Remarkably, HcpA-GFP was not displaced from the centromeres of DdINCENP-null cells during early anaphase (Fig. 6b) as it was displaced in wild-type cells (Fig. 5b). We interpret this observation as indicative that the loss of DdINCENP renders the centromeric DdAurora inactive and unable to displace HcpA from the centromere at the onset of anaphase. This is also consistent with the observation that DdINCENP-null cells have defects in chromosome segregation (15), which may be caused by failure to activate DdAurora at the centromeres.
FIG. 6.
DdINCENP is essential for DdAurora localization at the central spindle but not on centromeres. DdINCENP-null cells expressing the centromeric marker HcpA-GFP were stained with DdAurora antibodies. In the merged images, DNA is shown in blue, endogenous DdAurora is shown in red, and HcpA-GFP is shown in green. (a) In metaphase, DdAurora localized normally to the centromeres (large arrows) and spindle poles (small arrows). DdAurora colocalized with HcpA-GFP on the centromeres at the metaphase plate. (b) In anaphase, DdAurora localized to the spindle poles (small arrows) and centromeres (large arrows) but not at the central spindle. In contrast to wild-type cells (Fig. 5), HcpA was not displaced from the centromeres of DdINCENP-null cells. In these cells, HcpA-GFP remained associated with the centromeres and colocalized with DdAurora (large arrows) during anaphase. (c) In telophase, DdAurora continued its association with centromeres and colocalized with HcpA-GFP (large arrows). Bars, 5 μm.
To verify that the loss of DdAurora localization at the central spindle was caused by the absence of DdINCENP, we expressed GFP-DdINCENP in DdINCENP-null cells. We have shown previously that GFP-DdINCENP can fully rescue the mitosis and cytokinesis defects observed in DdINCENP-null cells (15). Similarly, we found that the localization of DdAurora to the central spindle was rescued by GFP-DdINCENP and both proteins colocalized extensively at the central spindle (Fig. 7). This result strongly suggests that DdINCENP and DdAurora formed a chromosomal passenger complex that localized at the central spindle at the metaphase/anaphase transition. To confirm the interaction between these two proteins we precipitated endogenous DdAurora or TAP-tagged DdAurora and found that endogenous DdINCENP or GFP-DdINCENP coprecipitated with DdAurora (see Fig. S2 in the supplemental material).
FIG. 7.
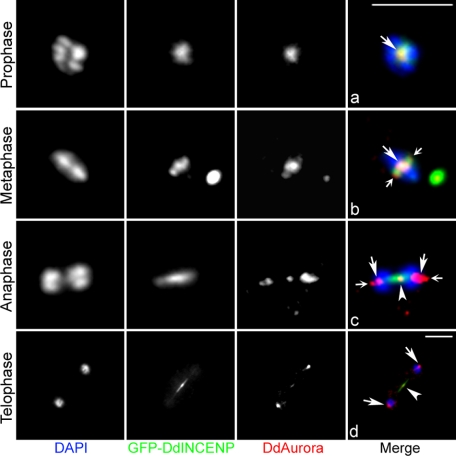
GFP-DdINCENP rescues the localization of DdAurora to the central spindle in DdINCENP-null cells. DdINCENP-null cells expressing GFP-DdINCENP were stained with anti-DdAurora antibodies. In the merged images, DNA is shown in blue, GFP-DdINCENP is shown in green, and endogenous DdAurora is shown in red. (a) In prophase, DdAurora and DdINCENP colocalized at the centromeres (arrow). (b) In metaphase, DdAurora colocalized with GFP-DdINCENP at the centromeres (large arrow). GFP-DdINCENP also labels the spindle poles (small arrows). (c) In anaphase, a portion of DdAurora redistributed to the central spindle. GFP-DdINCENP labeled most of the anaphase spindle and colocalized with DdAurora at the central spindle (arrowhead). (d and e) During telophase, DdAurora colocalized with GFP-DdINCENP at the central spindle (arrowhead). Bars, 5 μm.
The conserved C-terminal IN-box domain of INCENP is known to be essential for its interaction with Aurora B and activation of the kinase (6, 30). In addition, the N-terminal domain of DdINCENP is necessary and sufficient to localize DdINCENP to the cleavage furrow (14). To investigate the contribution of these domains to the localization of DdAurora, we determined the distribution of endogenous DdAurora in DdINCENP-null cells expressing two different DdINCENP truncation mutants (Fig. 8A). The localization of DdAurora at the central spindle of DdINCENP-null cells was rescued by expression of DdINCENP-ΔN but not of DdINCENP-ΔC (Fig. 8B). Even though DdAurora was absent from the central spindle, DdINCENP-ΔC was clearly visible at the central spindle, indicating that DdINCENP can localize at the central spindle independently of DdAurora. In all cases, DdAurora localized normally to the spindle poles in metaphase (data not shown) and to the centromeres of the separated chromosomes in telophase (Fig. 8B).
FIG. 8.
The IN-box domain of DdINCENP is required for the localization of DdAurora to the central spindle. (A) Schematic diagram of different truncation mutants of DdINCENP. The conserved IN-box domain, which is essential for Aurora B binding and activation, is shown as a gray box. (B) The truncated GFP-DdINCENP proteins were expressed in DdINCENP-null cells, and the central spindle localization of endogenous DdAurora was examined by immunofluorescence microscopy. During anaphase, DdAurora localized at the central spindle of cells expressing GFP-DdINCENP and GFP-DdINCENP-ΔN (arrowheads), but not in those expressing GFP-DdINCENP-ΔC. As shown in Fig. 6, the centromeric localization of DdAurora near the spindle poles (arrows) was independent of DdINCENP. Bar, 5 μm.
To test whether the localization results described above are mediated by the interaction between various DdINCENP truncations and DdAurora, we performed pull-down experiments on all different cell lines. We found that the proteins that contained the IN-box domain (full-length DdINCENP and DdINCENP-ΔN) coprecipitated with DdAurora (see Fig. S3 in the supplemental material). In contrast, the truncated form of DdINCENP lacking the IN-box domain (DdINCENP-ΔC) could not be pulled down with DdAurora. These results indicate that the C-terminal IN-box domain of DdINCENP is critical for its interaction with DdAurora and is required for the recruitment of DdAurora to the central spindle.
The motor protein Kif12 is important for the localization of DdAurora at the central spindle.
In mammalian cells, the kinesin 6-like protein MKLP2 is essential for the localization of the chromosomal passenger complex to the central spindle (27). In Dictyostelium, Kif12 was identified as a kinesin 6-like protein required for myosin localization to the furrow (37). In Kif12-null cells, GFP-DdINCENP can still localize to the central spindle but fails to localize at the cleavage furrow (14). To test whether Kif12 influences the distribution of DdAurora, we determined the localization of endogenous DdAurora in Kif12-null cells expressing HcpA-GFP. We found that DdAurora localized normally at the spindle poles and colocalized with HcpA-GFP at the centromeres of Kif12 mutant cells (Fig. 9a). However, the localization of DdAurora at the central spindle was abrogated in the Kif12 mutant cells (Fig. 9b and c and see Movie S4 in the supplemental material). Similar to our observations in wild-type cells (Fig. 5b), HcpA-GFP was diffusely distributed in the nucleus of Kif12-null cells during early anaphase (Fig. 9b). This finding suggests that Kif12 is not required for the activation of DdAurora at the centromeres in metaphase/anaphase.
FIG. 9.
Kif12 is essential for the localization of DdAurora at the central spindle. The localization of endogenous DdAurora in Kif12-null cells expressing HcpA-GFP was determined by immunofluorescence microscopy. In the merged images, DNA is shown in blue, DdAurora is shown in red, and the centromeric marker HcpA-GFP is shown in green. (a) In metaphase, DdAurora colocalized with HcpA-GFP at the centromeres (large arrows). DdAurora also localized at the spindle poles (small arrows). (b) In early anaphase, DdAurora localized to the spindle poles (small arrows) and centromeres (large arrows), but was absent from the central spindle. Again, HcpA was diffusely distributed in the nucleus of Kif12-null cells during anaphase, similar to its distribution in wild-type cells at this stage (Fig. 5b). (c) In telophase, DdAurora continued to be associated with centromeres and colocalized with HcpA-GFP at the congregated centromeres near the spindle poles (arrows). Bars, 5 μm.
Intriguingly, while we found that DdAurora failed to localize at the central spindle of Kif12-null cells, GFP-DdINCENP did localize at the central spindle in these mutant cells (14). This discrepancy could reflect different mechanisms of localization of these two proteins at the central spindle or may be due to the overexpression of GFP-DdINCENP. To distinguish between these possibilities, we stained Kif12-null cells expressing GFP-DdINCENP for endogenous DdAurora localization (Fig. 10). We found that DdAurora was restored at the central spindle of these cells indicating that overexpression of GFP-DdINCENP overcomes the defect caused by loss of Kif12. Immunoprecipitation assays also showed that GFP-DdINCENP interacted with DdAurora in Kif12-null cells (see Fig. S4 in the supplemental material).
FIG. 10.
Overexpression of GFP-DdINCENP in Kif12-null cells rescues translocation of DdAurora to the central spindle. The localization of endogenous DdAurora in Kif12-null cells expressing GFP-DdINCENP was determined by immunofluorescence microscopy. In anaphase and telophase, the central spindle localization (arrowheads) of DdAurora was rescued by the overexpression of GFP-DdINCENP. Bars, 5 μm.
Localization of DdAurora at the cleavage furrow.
Aurora B and INCENP are known to localize at the cleavage furrow and midbody of dividing animal cells but not at the mother-bud neck of yeast cells (9, 42, 57) Accordingly, Aurora B is required for cytokinesis in animal cells but the requirement is not so stringent in yeast cells (4, 48, 57). In Dictyostelium, DdINCENP localizes at the cleavage furrow early in cytokinesis and concentrates at the cytoplasmic bridge connecting the two daughter cells (15). DdINCENP is also required for the scission of the cytoplasmic bridge at the end of cytokinesis (15). Live imaging of GFP-DdAurora and immunofluorescence microscopy of endogenous DdAurora demonstrated that DdAurora localized at the region of the cleavage furrow, but only late in cytokinesis (Fig. 4f to h and see Movie S2 in the supplemental material). DdAurora was visible at the cleavage furrow only near the end of cytokinesis when the furrow becomes a thin cytoplasmic bridge between the two daughter cells (Fig. 4 h). When this bridge finally severed, GFP-Aurora persisted at the breaking point for a short time (Fig. 4i).
Since little is known about how Aurora B localizes at the cleavage furrow, we determined the ability of GFP-DdAurora to localize at the furrow of different mutant cell lines. We found that GFP-DdAurora failed to localize at the cleavage furrow of DdINCENP-null cells (see Movie S3 in the supplemental material). Given that DdINCENP is required for the localization of DdAurora at the central spindle, it is reasonable to expect a similar requirement for DdAurora localization at the cleavage furrow. It seems likely that the absence of DdAurora at the cleavage furrow is the basis of the cytokinesis defect observed in DdINCENP-null cells (15).
Similarly, GFP-DdAurora did not localize at the cleavage furrow of Kif12-null cells (see Movie S4 in the supplemental material). Since DdINCENP does not localize at the cleavage furrow in these mutant cells (14), it is likely that this is the cause for the failure to recruit DdAurora to the cleavage furrow.
We have shown previously that the distribution of DdINCENP at the cleavage furrow was disturbed by the absence of myosin II (15). In myosin II mutant cells, GFP-DdINCENP localized as a narrow band at the furrow instead of the broad cleavage furrow cortex localization observed in wild-type cells (15). To determine whether myosin II also influences the distribution of DdAurora, we determined the localization of endogenous DdAurora and of GFP-DdAurora in myosin II heavy-chain-null cells. The localization of endogenous DdAurora to the spindle poles, centromeres, and central spindle was normal in these mutant cells (Fig. 11A). In contrast, the localization of GFP-DdAurora at the cytoplasmic bridge during late cytokinesis was completely abrogated (Fig. 11B). Thus, it appears that an intact contractile ring is essential for the proper organization of the chromosomal passenger protein complex at the cleavage furrow.
FIG. 11.
Localization of GFP-DdAurora in myosin II-null cells. (A) Localization of endogenous DdAurora in myosin II-null cells was determined by immunostaining. In the merged images, DNA is shown in blue and endogenous DdAurora is shown in red. The polar and centromeric (arrow) localization during metaphase was normal in these cells. In addition, the central spindle localization of DdAurora (arrowheads) in anaphase was normal. Bar, 5 μm. (B) Live images of myosin II (myosin heavy chain)-null cells expressing GFP-Aurora during cytokinesis. The central spindle localization of GFP-DdAurora could still be observed at 01:19 (arrowhead). GFP-DdAurora failed to localize at the cytoplasmic bridge (arrows) formed between the two daughter cells. Bar, 5 μm.
DISCUSSION
We have presented here the characterization of the single Aurora kinase from Dictyostelium. Among the several Aurora kinases that have been studied to date, DdAurora is the first kinase with demonstrable characteristics of both Aurora A and Aurora B kinases. The similarity of DdAurora to both Aurora kinase subfamilies is shown not only by sequence homology, but also by subcellular localization at different stages of mitosis and by interactions with DdINCENP. These studies offer an evolutionary perspective on the properties of Aurora kinases in the common ancestor of animals, fungi, and plants. We suggest that the ancestral Aurora kinase already had the dual role of Aurora A and B kinases and that these roles were subsequently separated in the animal Aurora kinases. Our studies also revealed novel properties of the Dictyostelium kinase that have not been recognized in Aurora kinases from other organisms. Given the evolutionary conservation of these kinases, it seems likely that similar properties will be found in other Aurora kinases.
DdAurora has properties of Aurora A kinase.
Aurora A kinases are distinct from Aurora B kinases in having a longer N-terminal domain that contains an A-box sequence important for the proteolytic regulation of these proteins. The Dictyostelium DdAurora kinase contains an A-box sequence within its N-terminal domain similar to that of Aurora A kinases. A second property characteristic of Aurora A kinases is their localization at the spindle poles, where they play an important role in the maturation of centrosomes and spindle assembly. We have shown that DdAurora is also localized at the spindle poles from the initiation of mitosis until the beginning of anaphase. Given its sequence similarity to Aurora A kinases and similar localization during mitosis, we conclude that DdAurora plays the role of Aurora A during Dictyostelium mitosis. It will be interesting to identify proteins that may regulate the localization and function of DdAurora at the spindle poles. Since the vertebrate Aurora A regulators Aip1 and TPX2 do not appear to have orthologs in invertebrates, fungi or protists, it is likely that other proteins yet to be discovered regulate the activity of this kinase at the spindle poles.
Colocalization of DdAurora with the centrosomal marker DdCP224 revealed that DdAurora is localized on the outer side of the spindle poles. In Dictyostelium, the centrosome is embedded in the nuclear envelope during mitosis (44). Thus, one centrosomal surface remains in the cytosol while the other resides inside the nucleus. Our observations suggest that there may be functional differences between these two surfaces and that DdAurora may play a role on the cytosolic side of the centrosome during prometaphase and metaphase. In mammalian cells, Aurora A kinase was found to localize to centrosomes and the adjacent spindle microtubules (55). It is possible that this pericentrosomal localization of Aurora A is equivalent to the localization of DdAurora on the outer portion of the spindle poles.
DdAurora has properties of Aurora B kinase.
Aurora B kinases are characterized by their association with the proteins of the chromosomal passenger complex and dynamic sequential localization at centromeres, central spindle, and midbody. We have shown that DdAurora displays all these properties of Aurora B kinases. DdAurora is found on centromeres as early as prometaphase; it localizes at the central spindle during anaphase and at the cytoplasmic bridge at the end of cytokinesis. DdAurora also interacts with the chromosomal passenger protein DdINCENP and this interaction requires the conserved IN-box domain at the C terminus of DdINCENP. By all of these criteria, it is clear that DdAurora plays the role of Aurora B in Dictyostelium mitosis.
We demonstrated that DdAurora is found on centromeres by colocalization with the centromeric protein HcpA. In contrast with Aurora B from other organisms, we found that DdAurora remains associated with the centromeres even during anaphase and telophase. In Dictyostelium, the centromeres of the daughter chromatids congregate closely to the spindle poles during anaphase (44), and therefore, DdAurora and HcpA are visible as a single dot on the inner surface of the spindle poles. In contrast, animal Aurora B leaves the centromeres at the onset of anaphase and relocates to the central spindle (2, 53, 57). Interestingly, one of the Aurora isoforms (AtAur-1) found in Arabidopsis appears to move with centromeres during anaphase (18). While this localization has not been proven to be centromeric, it opens the possibility that Aurora kinases play an additional centromeric function during anaphase, at least in plants and Dictyostelium.
In contrast to DdAurora, its binding partner, DdINCENP, does not remain associated with anaphase centromeres. Like its animal counterpart, DdINCENP redistributes to the central spindle during anaphase (15). Therefore, the association of DdAurora with the centromere appears to be independent of DdINCENP. We confirmed this possibility by showing that DdAurora is still found on centromeres in DdINCENP-null cells.
The localization of Aurora B at the centromeres has been shown to be important for the regulation of proper microtubule attachment to kinetochores (17, 56). This activity is dependent on the proteins of the chromosomal passenger complex (51). Aurora B is also known to be required for the dissociation of human HP1 proteins from chromosomes during mitosis (21, 29). We found that Dictyostelium HcpA, the ortholog of human HP1, also dissociates from centromeres, but only during early anaphase. Importantly, we observed that HcpA failed to dissociate from anaphase centromeres in the DdINCENP-null mutants. We interpret these observations as indicative that the activity of the DdAurora/DdINCENP complex at the centromeres dissociates HcpA at the onset of anaphase. Concomitantly, as in other organisms, DdINCENP dissociates from the centromeres and the DdAurora that remains associated with the anaphase centromeres would not have Aurora B-like activity. DdINCENP and a portion of DdAurora redistribute to the central spindle and eventually to the cleavage furrow.
DdAurora forms a chromosomal passenger complex with DdINCENP.
We have shown that DdAurora interacts with DdINCENP and that this interaction requires the IN-box domain at the carboxyl terminus of DdINCENP. Therefore, it appears that Dictyostelium has a chromosomal passenger complex similar to that described in other organisms. However, the Dictyostelium genome does not encode any protein with similarity to the other chromosomal passenger complex proteins survivin/Bir1 and borealin. Elucidation of whether other proteins participate in the Dictyostelium chromosomal passenger complex will require the purification of this complex.
While it is clear that DdAurora and DdINCENP form a complex, we also provide evidence that their interaction must be transient and spatially regulated. The central spindle is a location where these two proteins clearly interact. DdAurora depends on DdINCENP to localize to the central spindle, although the reverse is not true. Similarly, both proteins are enriched at the cytoplasmic bridge connecting the two daughter cells at the end of cytokinesis. The localization of DdAurora at this location also requires the presence of DdINCENP. However, DdINCENP localizes to the invaginating cleavage furrow from the beginning of cytokinesis, whereas DdAurora does not. This would suggest that DdINCENP associates with the cleavage furrow and later recruits DdAurora. It seems likely that the late cytokinesis defect observed in DdINCENP-null cells is caused by a lack of DdAurora recruitment to the cytoplasmic bridge.
The differences in DdAurora and DdINCENP localization also suggest that, instead of being solely a DdAurora cofactor, DdINCENP may play additional roles in mitosis and cytokinesis by interacting with other proteins. For example, it has been shown recently that phosphorylated INCENP can bind to Polo-like kinase (23), another important mitotic kinase essential for mitotic entry, spindle formation, and cytokinesis (61). DdINCENP may also regulate mitosis by interacting with a Polo-like kinase protein in Dictyostelium.
The cytoskeletal proteins Kif12 and myosin II modulate a subset of DdAurora localizations.
Similarly to the role of the animal motor protein MKLP2, Dictyostelium Kif12 is essential for the localization of DdAurora to the central spindle at the metaphase/anaphase transition. However, when GFP-DdINCENP is overexpressed in Kif12-null cells, it rescues the localization of DdAurora to the central spindle. It seems likely that Kif12 is also important for the localization of DdINCENP to the central spindle but that overexpression of GFP-DdINCENP overcomes the loss of Kif12. Unfortunately, the antibodies raised against DdINCENP have not been useful for immunofluorescence studies to determine the distribution of endogenous DdINCENP. Nonetheless, while the overexpression of GFP-DdINCENP rescues the localization of DdAurora to the central spindle, it is not sufficient to recover the localization of either protein to the cleavage furrow of Kif12-null cells. This indicates that this motor protein is responsible, directly or indirectly, for the localization of the chromosomal passenger complex to the cleavage furrow.
The localization of proteins at the cleavage furrow is a poorly understood process. We showed previously that the distribution of DdINCENP at the cleavage furrow is abnormal in myosin II-null cells (15). We showed here that the localization of DdAurora was also affected by the absence of myosin II. Although GFP-DdAurora was still localized at the central spindle, it was absent from the cleavage furrows of myosin mutant cells. This suggests that the absence of myosin II not only affects localization of DdINCENP at the contractile furrow, but also disrupts the furrow localization of DdAurora. Since Aurora kinase activity is important for the completion of cytokinesis, the cytokinesis defect of myosin II-null cells could be explained by both failure of contraction and the absence of Aurora kinase activity at the furrow during late cytokinesis.
Supplementary Material
Acknowledgments
We would like to thank the members of the De Lozanne and O'Halloran labs for their comments and help throughout the development of this project. We also thank J. A. Spudich and G. S. Lakshmikanth for providing us with the Kif12-null cell line and D. A. Larochelle for an unpublished small interfering RNA construct.
This research was supported by grant GM48745 from the National Institutes of Health to A.D. and a grant from the Deutsche Forschungsgemeinschaft (SPP 1129) to W.N.
Footnotes
Published ahead of print on 7 March 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Adams, R. R., M. Carmena, and W. C. Earnshaw. 2001. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 1149-54. [DOI] [PubMed] [Google Scholar]
- 2.Adams, R. R., D. M. Eckley, P. Vagnarelli, S. P. Wheatley, D. L. Gerloff, A. M. Mackay, P. A. Svingen, S. H. Kaufmann, and W. C. Earnshaw. 2001. Human INCENP colocalizes with the Aurora-B/AIRK2 kinase on chromosomes and is overexpressed in tumour cells. Chromosoma 11065-74. [DOI] [PubMed] [Google Scholar]
- 3.Berdnik, D., and J. A. Knoblich. 2002. Drosophila Aurora-A is required for centrosome maturation and actin-dependent asymmetric protein localization during mitosis. Curr. Biol. 12640-647. [DOI] [PubMed] [Google Scholar]
- 4.Biggins, S., and A. W. Murray. 2001. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 153118-3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bischoff, J. R., L. Anderson, Y. Zhu, K. Mossie, L. Ng, B. Souza, B. Schryver, P. Flanagan, F. Clairvoyant, C. Ginther, C. S. Chan, M. Novotny, D. J. Slamon, and G. D. Plowman. 1998. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 173052-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishop, J. D., and J. M. Schumacher. 2002. Phosphorylation of the carboxyl terminus of inner centromere protein (INCENP) by the Aurora B kinase stimulates Aurora B kinase activity. J. Biol. Chem. 27727577-27580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bretschneider, T., J. Jonkman, J. Kohler, O. Medalia, K. Barisic, I. Weber, E. H. Stelzer, W. Baumeister, and G. Gerisch. 2002. Dynamic organization of the actin system in the motile cells of Dictyostelium. J. Muscle Res. Cell Motil. 23639-649. [DOI] [PubMed] [Google Scholar]
- 8.Brown, J. R., K. K. Koretke, M. L. Birkeland, P. Sanseau, and D. R. Patrick. 2004. Evolutionary relationships of Aurora kinases: implications for model organism studies and the development of anti-cancer drugs. BMC Evol. Biol. 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buvelot, S., S. Y. Tatsutani, D. Vermaak, and S. Biggins. 2003. The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. J. Cell Biol. 160329-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmena, M., and W. C. Earnshaw. 2003. The cellular geography of aurora kinases. Nat. Rev. Mol. Cell. Biol. 4842-854. [DOI] [PubMed] [Google Scholar]
- 11.Castro, A., Y. Arlot-Bonnemains, S. Vigneron, J. C. Labbe, C. Prigent, and T. Lorca. 2002. APC/Fizzy-Related targets Aurora-A kinase for proteolysis. EMBO Rep. 3457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan, C. S., and D. Botstein. 1993. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics 135677-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheetham, G. M., R. M. Knegtel, J. T. Coll, S. B. Renwick, L. Swenson, P. Weber, J. A. Lippke, and D. A. Austen. 2002. Crystal structure of aurora-2, an oncogenic serine/threonine kinase. J. Biol. Chem. 27742419-42422. [DOI] [PubMed] [Google Scholar]
- 14.Chen, Q., G. S. Lakshmikanth, J. A. Spudich, and A. De Lozanne. 2007. The localization of INCENP at the cleavage furrow is dependent on Kif12 and involves interactions of the N-terminus of INCENP with the actin cytoskeleton. Mol. Biol. Cell 183366-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen, Q., H. Li, and A. De Lozanne. 2006. Contractile ring-independent localization of DdINCENP, a protein important for spindle stability and cytokinesis. Mol. Biol. Cell 17779-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Lozanne, A., and J. A. Spudich. 1987. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 2361086-1091. [DOI] [PubMed] [Google Scholar]
- 17.DeLuca, J. G., W. E. Gall, C. Ciferri, D. Cimini, A. Musacchio, and E. D. Salmon. 2006. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 127969-982. [DOI] [PubMed] [Google Scholar]
- 18.Demidov, D., D. Van Damme, D. Geelen, F. R. Blattner, and A. Houben. 2005. Identification and dynamics of two classes of aurora-like kinases in Arabidopsis and other plants. Plant Cell 17836-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eichinger, L., J. A. Pachebat, G. Glockner, M. A. Rajandream, R. Sucgang, M. Berriman, J. Song, R. Olsen, K. Szafranski, Q. Xu, B. Tunggal, S. Kummerfeld, M. Madera, B. A. Konfortov, F. Rivero, A. T. Bankier, R. Lehmann, N. Hamlin, R. Davies, P. Gaudet, P. Fey, K. Pilcher, G. Chen, D. Saunders, E. Sodergren, P. Davis, A. Kerhornou, X. Nie, N. Hall, C. Anjard, L. Hemphill, N. Bason, P. Farbrother, B. Desany, E. Just, T. Morio, R. Rost, C. Churcher, J. Cooper, S. Haydock, N. van Driessche, A. Cronin, I. Goodhead, D. Muzny, T. Mourier, A. Pain, M. Lu, D. Harper, R. Lindsay, H. Hauser, K. James, M. Quiles, M. Madan Babu, T. Saito, C. Buchrieser, A. Wardroper, M. Felder, M. Thangavelu, D. Johnson, A. Knights, H. Loulseged, K. Mungall, K. Oliver, C. Price, M. A. Quail, H. Urushihara, J. Hernandez, E. Rabbinowitsch, D. Steffen, M. Sanders, J. Ma, Y. Kohara, S. Sharp, M. Simmonds, S. Spiegler, A. Tivey, S. Sugano, B. White, D. Walker, J. Woodward, T. Winckler, Y. Tanaka, G. Shaulsky, M. Schleicher, G. Weinstock, A. Rosenthal, E. C. Cox, R. L. Chisholm, R. Gibbs, W. F. Loomis, M. Platzer, R. R. Kay, J. Williams, P. H. Dear, A. A. Noegel, B. Barrell, and A. Kuspa. 2005. The genome of the social amoeba Dictyostelium discoideum. Nature 43543-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faix, J., I. Weber, U. Mintert, J. Kohler, F. Lottspeich, and G. Marriott. 2001. Recruitment of cortexillin into the cleavage furrow is controlled by Rac1 and IQGAP-related proteins. EMBO J. 203705-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fischle, W., B. S. Tseng, H. L. Dormann, B. M. Ueberheide, B. A. Garcia, J. Shabanowitz, D. F. Hunt, H. Funabiki, and C. D. Allis. 2005. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 4381116-1122. [DOI] [PubMed] [Google Scholar]
- 22.Francisco, L., and C. S. Chan. 1994. Regulation of yeast chromosome segregation by Ipl1 protein kinase and type 1 protein phosphatase. Cell. Mol. Biol. Res. 40207-213. [PubMed] [Google Scholar]
- 23.Goto, H., T. Kiyono, Y. Tomono, A. Kawajiri, T. Urano, K. Furukawa, E. A. Nigg, and M. Inagaki. 2006. Complex formation of Plk1 and INCENP required for metaphase-anaphase transition. Nat. Cell Biol. 8180-187. [DOI] [PubMed] [Google Scholar]
- 24.Graf, R., C. Daunderer, and M. Schliwa. 2000. Dictyostelium DdCP224 is a microtubule-associated protein and a permanent centrosomal resident involved in centrosome duplication. J. Cell Sci. 1131747-1758. [DOI] [PubMed] [Google Scholar]
- 25.Graf, R., C. Daunderer, and I. Schulz. 2004. Molecular and functional analysis of the dictyostelium centrosome. Int. Rev. Cytol. 241155-202. [DOI] [PubMed] [Google Scholar]
- 26.Graf, R., U. Euteneuer, T. H. Ho, and M. Rehberg. 2003. Regulated expression of the centrosomal protein DdCP224 affects microtubule dynamics and reveals mechanisms for the control of supernumerary centrosome number. Mol. Biol. Cell 144067-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gruneberg, U., R. Neef, R. Honda, E. A. Nigg, and F. A. Barr. 2004. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 166167-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hannak, E., M. Kirkham, A. A. Hyman, and K. Oegema. 2001. Aurora-A kinase is required for centrosome maturation in Caenorhabditis elegans. J. Cell Biol. 1551109-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirota, T., J. J. Lipp, B. H. Toh, and J. M. Peters. 2005. Histone H3 serine 10 phosphorylation by Aurora B causes HP1 dissociation from heterochromatin. Nature 4381176-1180. [DOI] [PubMed] [Google Scholar]
- 30.Honda, R., R. Korner, and E. A. Nigg. 2003. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell 143325-3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaitna, S., M. Mendoza, V. Jantsch-Plunger, and M. Glotzer. 2000. Incenp and an aurora-like kinase form a complex essential for chromosome segregation and efficient completion of cytokinesis. Curr. Biol. 101172-1181. [DOI] [PubMed] [Google Scholar]
- 32.Kaller, M., U. Euteneuer, and W. Nellen. 2006. Differential effects of heterochromatin protein 1 isoforms on mitotic chromosome distribution and growth in Dictyostelium discoideum. Eukaryot. Cell 5530-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaller, M., B. Foldesi, and W. Nellen. 2007. Localization and organization of protein factors involved in chromosome inheritance in Dictyostelium discoideum. Biol. Chem. 388355-365. [DOI] [PubMed] [Google Scholar]
- 34.Kim, J. H., J. S. Kang, and C. S. Chan. 1999. Sli15 associates with the ipl1 protein kinase to promote proper chromosome segregation in Saccharomyces cerevisiae. J. Cell Biol. 1451381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kimura, M., Y. Matsuda, T. Yoshioka, and Y. Okano. 1999. Cell cycle-dependent expression and centrosome localization of a third human aurora/Ipl1-related protein kinase, AIK3. J. Biol. Chem. 2747334-7340. [DOI] [PubMed] [Google Scholar]
- 36.Kwak, E., N. Gerald, D. A. Larochelle, K. K. Vithalani, M. L. Niswonger, M. Maready, and A. De Lozanne. 1999. LvsA, a protein related to the mouse beige protein, is required for cytokinesis in Dictyostelium. Mol. Biol. Cell 104429-4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lakshmikanth, G. S., H. M. Warrick, and J. A. Spudich. 2004. A mitotic kinesin-like protein required for normal karyokinesis, myosin localization to the furrow, and cytokinesis in Dictyostelium. Proc. Natl. Acad. Sci. USA 10116519-16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larochelle, D. A., K. K. Vithalani, and A. De Lozanne. 1996. A novel member of the rho family of small GTP-binding proteins is specifically required for cytokinesis. J. Cell Biol. 1331321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leverson, J. D., H. K. Huang, S. L. Forsburg, and T. Hunter. 2002. The Schizosaccharomyces pombe aurora-related kinase Ark1 interacts with the inner centromere protein Pic1 and mediates chromosome segregation and cytokinesis. Mol. Biol. Cell 131132-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li, X., G. Sakashita, H. Matsuzaki, K. Sugimoto, K. Kimura, F. Hanaoka, H. Taniguchi, K. Furukawa, and T. Urano. 2004. Direct association with inner centromere protein (INCENP) activates the novel chromosomal passenger protein, Aurora-C. J. Biol. Chem. 27947201-47211. [DOI] [PubMed] [Google Scholar]
- 41.Littlepage, L. E., and J. V. Ruderman. 2002. Identification of a new APC/C recognition domain, the A box, which is required for the Cdh1-dependent destruction of the kinase Aurora-A during mitotic exit. Genes Dev. 162274-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackay, A. M., A. M. Ainsztein, D. M. Eckley, and W. C. Earnshaw. 1998. A dominant mutant of inner centromere protein (INCENP), a chromosomal protein, disrupts prometaphase congression and cytokinesis. J. Cell Biol. 140991-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntosh, J. R., U. P. Roos, B. Neighbors, and K. L. McDonald. 1985. Architecture of the microtubule component of mitotic spindles from Dictyostelium discoideum. J. Cell Sci. 7593-129. [DOI] [PubMed] [Google Scholar]
- 44.Moens, P. B. 1976. Spindle and kinetochore morphology of Dictyostelium discoideum. J. Cell Biol. 68113-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norden, C., M. Mendoza, J. Dobbelaere, C. V. Kotwaliwale, S. Biggins, and Y. Barral. 2006. The NoCut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell 12585-98. [DOI] [PubMed] [Google Scholar]
- 46.O'Halloran, T. J. 2000. Membrane traffic and cytokinesis. Traffic 1921-926. [PubMed] [Google Scholar]
- 47.Petersen, J., and I. M. Hagan. 2003. S. pombe aurora kinase/survivin is required for chromosome condensation and the spindle checkpoint attachment response. Curr. Biol. 13590-597. [DOI] [PubMed] [Google Scholar]
- 48.Petersen, J., J. Paris, M. Willer, M. Philippe, and I. M. Hagan. 2001. The S. pombe aurora-related kinase Ark1 associates with mitotic structures in a stage dependent manner and is required for chromosome segregation. J. Cell Sci. 1144371-4384. [DOI] [PubMed] [Google Scholar]
- 49.Reinders, Y., I. Schulz, R. Graf, and A. Sickmann. 2006. Identification of novel centrosomal proteins in Dictyostelium discoideum by comparative proteomic approaches. J. Proteome Res. 5589-598. [DOI] [PubMed] [Google Scholar]
- 50.Roos, U. P., M. De Brabander, and J. De Mey. 1984. Indirect immunofluorescence of microtubules in Dictyostelium discoideum. A study with polyclonal and monoclonal antibodies to tubulins. Exp. Cell Res. 151183-193. [DOI] [PubMed] [Google Scholar]
- 51.Sandall, S., F. Severin, I. X. McLeod, J. R. Yates III, K. Oegema, A. Hyman, and A. Desai. 2006. A Bir1-Sli15 complex connects centromeres to microtubules and is required to sense kinetochore tension. Cell 1271179-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schumacher, J. M., N. Ashcroft, P. J. Donovan, and A. Golden. 1998. A highly conserved centrosomal kinase, AIR-1, is required for accurate cell cycle progression and segregation of developmental factors in Caenorhabditis elegans embryos. Development 1254391-4402. [DOI] [PubMed] [Google Scholar]
- 53.Schumacher, J. M., A. Golden, and P. J. Donovan. 1998. AIR-2: an Aurora/Ipl1-related protein kinase associated with chromosomes and midbody microtubules is required for polar body extrusion and cytokinesis in Caenorhabditis elegans embryos. J. Cell Biol. 1431635-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart, S., and G. Fang. 2005. Destruction box-dependent degradation of aurora B is mediated by the anaphase-promoting complex/cyclosome and Cdh1. Cancer Res. 658730-8735. [DOI] [PubMed] [Google Scholar]
- 55.Sugimoto, K., T. Urano, H. Zushi, K. Inoue, H. Tasaka, M. Tachibana, and M. Dotsu. 2002. Molecular dynamics of Aurora-A kinase in living mitotic cells simultaneously visualized with histone H3 and nuclear membrane protein importinα. Cell Struct. Funct. 27457-467. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka, T. U., N. Rachidi, C. Janke, G. Pereira, M. Galova, E. Schiebel, M. J. Stark, and K. Nasmyth. 2002. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108317-329. [DOI] [PubMed] [Google Scholar]
- 57.Terada, Y., M. Tatsuka, F. Suzuki, Y. Yasuda, S. Fujita, and M. Otsu. 1998. AIM-1: a mammalian midbody-associated protein required for cytokinesis. EMBO J. 17667-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tu, X., P. Kumar, Z. Li, and C. C. Wang. 2006. An aurora kinase homologue is involved in regulating both mitosis and cytokinesis in Trypanosoma brucei. J. Biol. Chem. 2819677-9687. [DOI] [PubMed] [Google Scholar]
- 59.Ueda, M., M. Schliwa, and U. Euteneuer. 1999. Unusual centrosome cycle in Dictyostelium: correlation of dynamic behavior and structural changes. Mol. Biol. Cell 10151-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vader, G., R. H. Medema, and S. M. Lens. 2006. The chromosomal passenger complex: guiding Aurora-B through mitosis. J. Cell Biol. 173833-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van de Weerdt, B. C., and R. H. Medema. 2006. Polo-like kinases: a team in control of the division. Cell Cycle 5853-864. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.