Abstract
Upc2p, a transcription factor of the zinc cluster family, is an important regulator of sterol biosynthesis and azole drug resistance in Candida albicans. To better understand Upc2p function in C. albicans, we used genomewide location profiling to identify the transcriptional targets of Upc2p in vivo. A triple hemagglutinin epitope, introduced at the C terminus of Upc2p, conferred a gain-of-function effect on the fusion protein. Location profiling identified 202 bound promoters (P < 0.05). Overrepresented functional groups of genes whose promoters were bound by Upc2p included 12 genes involved in ergosterol biosynthesis (NCP1, ERG11, ERG2, and others), 18 genes encoding ribosomal subunits (RPS30, RPL32, RPL12, and others), 3 genes encoding drug transporters (CDR1, MDR1, and YOR1), 4 genes encoding transcription factors (INO2, ACE2, SUT1, and UPC2), and 6 genes involved in sulfur amino acid metabolism (MET6, SAM2, SAH1, and others). Bioinformatic analyses suggested that Upc2p binds to the DNA motif 5′-VNCGBDTR that includes the previously characterized Upc2p binding site 5′-TCGTATA. Northern blot analysis showed that increased binding correlates with increased expression for the analyzed Upc2p targets (ERG11, MDR1, CDR1, YOR1, SUT1, SMF12, and CBP1). The analysis of ERG11, MDR1, and CDR1 transcripts in wild-type and upc2Δ/upc2Δ strains grown under Upc2p-activating conditions (lovastatin treatment and hypoxia) showed that Upc2p regulates its targets in a complex manner, acting as an activator or as a repressor depending upon the target and the activating condition. Taken together, our results indicate that Upc2p is a key regulator of ergosterol metabolism. They also suggest that Upc2p may contribute to azole resistance by regulating the expression of drug efflux pump-encoding genes in addition to ergosterol biosynthesis genes.
Candida albicans is an important human fungal pathogen, in terms of both its clinical significance and its use as an experimental model for scientific investigation. This opportunistic pathogen is a natural component of the human flora, colonizing skin and the gastrointestinal and genitourinary tracts (4). Although many infections involve the colonization of surface mucosal membranes (oral thrush and vaginal candidiasis, for example), immunosuppressed patients can be subject to potentially lethal systemic infections (45).
Many antifungal drugs used to treat C. albicans infections function by targeting ergosterol, the analogue of cholesterol in mammalian cells and the major sterol of fungal cell membranes. Polyenes, such as amphotericin B, bind directly to ergosterol and perturb general membrane functions, resulting in low selectivity and high toxicity (2, 35). Azoles, including fluconazole and voriconazole, are more widely used and target the enzyme lanosterol demethylase (Erg11p) in the ergosterol biosynthesis pathway, with the consequence that ergosterol is depleted and replaced by unusual sterols, altering the fluidity of the membrane and the activities of membrane-bound proteins (e.g., enzymes involved in cell wall synthesis and transporters) (2, 35). However, the fungistatic rather than fungicidal activity of azole drugs leads to the frequent emergence of azole-resistant C. albicans cells, an important clinical problem, particularly in chronically immunosuppressed patients (those infected with human immunodeficiency virus or undergoing prolonged chemotherapy) (2, 39, 55). Molecular analyses of azole-resistant clinical isolates have shown that they carry mutations in the ERG11 gene and/or constitutively overexpress genes responsible for their drug-resistant phenotype: CDR1 and CDR2 (encoding homologous transporters of the ATP binding cassette family functioning as multidrug efflux pumps and phospholipid flippases), MDR1 (encoding a multidrug transporter of the major facilitator superfamily), PDR16 (encoding a phospholipid transfer protein), and ERG11 (2, 38, 39, 55). The stable overexpression of CDR1, CDR2, PDR16, and MDR1 in C. albicans clinical isolates results from the acquisition of activating mutations in transcription factors (17, 18, 57). It was shown previously that the constitutive overexpression of CDR1, CDR2, and PDR16 in azole-resistant isolates is due to gain-of-function mutations in the transcription factor Tac1p (11, 12, 59) and that MDR1 overexpression is the consequence of activating mutations in Mrr1p (31). ERG11 expression is controlled by the transcription factor Upc2p (uptake control 2) (28, 43); however, Upc2p-activating mutations in C. albicans clinical isolates have not been reported to date. A hallmark of the Tac1p, Mrr1p, and Upc2p proteins is that they belong to the zinc cluster family of transcription factors, which is found only in fungi (29). Thus, it appears that this family of regulators plays an important role in the adaptation of C. albicans cells to drug pressure, as well as to other general stresses.
Because sterol homeostasis involves the targets of many antifungal drugs, understanding the transcriptional control of this process in fungi is medically important. Sterol regulation in Saccharomyces cerevisiae has been studied previously (13, 48). In this yeast, sterol biosynthesis is an aerobic process (Erg11p needs oxygen during lanosterol demethylation). During aerobiosis, yeast does not import exogenous sterols (a characteristic referred to as aerobic sterol exclusion) (23). Under anaerobic conditions, sterol biosynthesis is compromised and cells become capable of taking up exogenous sterols. Key transcription factors regulating sterol biosynthesis and uptake in S. cerevisiae, including Upc2p, Ecm22p, and Sut1p, all of them members of the zinc cluster family, have been identified previously (15, 33). In response to sterol depletion, Upc2p and its paralogue Ecm22p bind to the promoters of the ERG2 and ERG3 genes via a sterol response element (SRE; TCGTATA) to activate their transcription (51). Upc2p also plays a role in the uptake of sterols under anaerobic conditions by regulating the expression of genes coding for cell wall mannoproteins (DAN/TIR) and ABC transporters (AUS1 and PDR11) (56). Interestingly, gain-of-function mutations in the C-terminal domain of Upc2p result in aerobic sterol uptake, most likely by preventing the interaction of Upc2p with a repressor, a situation that would mimic sterol deprivation (15). In line with this possibility, one recent report showed that hypoxia activates Upc2p as a result of sterol depletion, since ergosterol biosynthesis also requires oxygen (14).
In C. albicans, only one close homologue of S. cerevisiae Upc2p and Ecm22p has been identified (28, 43). We and others have shown previously that C. albicans cells lacking UPC2 are hypersensitive to azole drugs and fail to activate the expression of several ERG genes in response to azole treatment (28, 43). The upc2Δ/upc2Δ mutants show reduced cholesterol uptake relative to that of the wild-type strain, indicating that C. albicans Upc2 plays a role in sterol uptake as well (43), while displaying impaired growth under anaerobic conditions (28). The latter finding is important, since C. albicans is able to cause superficial skin infections as well as deep-seated infections, suggesting that its ability to switch between normoxia and hypoxia is a major determinant of its virulence (41). We showed previously that C. albicans Upc2p binds in vitro to an SRE located in the ERG2 promoter, TCGTATAA (28), while one recent study showed that azole-responsive enhancer elements (ARE), located in the ERG11 promoter (PERG11) and including a perfect SRE, are crucial for the induction of a PERG11-luciferase gene fusion (36). The activity of the ARE is UPC2 dependent, suggesting that Upc2p regulates ERG genes through direct binding to these ARE (36). In this study, we used genomewide location profiling, a technology that combines chromatin immunoprecipitation (ChIP) and DNA microarrays (chips), to identify genes whose promoters are bound in vivo by Upc2p in order to better understand Upc2p function in C. albicans.
MATERIALS AND METHODS
Strains and growth media.
The C. albicans strains used in this study are listed in Table 1. Strains SGY243 and its upc2Δ derivative were grown in yeast extract-peptone-dextrose (YPD) broth (Sigma-Aldrich, St. Louis, MO), supplemented or not with the drugs indicated below, at 30°C with vigorous shaking or under hypoxic conditions at 30°C in an anaerobic jar without shaking. The pCaEXP integrants (Table 1) were grown in synthetic complete (SC) medium lacking uracil (SC-ura) (42), in SC medium lacking uracil, methionine, and cysteine (SC-ura-met-cys) to induce the MET3 promoter (PMET3), or in SC-ura supplemented with methionine (2.5 mM) and cysteine (2.5 mM) (SC-ura+met+cys) under PMET3-repressing conditions. The Escherichia coli MC1061 bacterial strain was used for DNA cloning and the maintenance of the plasmid constructs.
TABLE 1.
Strains used in this study
| Strain | Parental strain | Genotype | Reference |
|---|---|---|---|
| SGY243 (parental) | ade2/ade2 Δura3::ADE2/Δura3::ADE2 | 22 | |
| SGY243 Δupc2 | SGY243 | upc2Δ4-178::hisG/upc2Δ5-177::hisG/upc2Δ4-528::hisG/upc2Δ5-701::hisG | 28 |
| SGY243-CaEXP-A | SGY243 | RP10::(pCaEXP) URA3 PMET3 | This study |
| SGY243-CaEXP-B | SGY243 | RP10::(pCaEXP) URA3 PMET3 | This study |
| SGY243-UPC2-A | SGY243 | RP10::(pCaEXP) URA3 PMET3-UPC2 | This study |
| SGY243-UPC2-B | SGY243 | RP10::(pCaEXP) URA3 PMET3-UPC2 | This study |
| SGY243-UPC2-HA-A | SGY243 | RP10::(pCaEXP) URA3 PMET3-UPC2-HA3 sequence | This study |
| SGY243-UPC2-HA-B | SGY243 | RP10::(pCaEXP) URA3 PMET3-UPC2-HA3 sequence | This study |
Generation of an HA-tagged Upc2p-expressing strain and derivatives.
A DNA fragment overlapping positions −63 to +2136 (relative to the ATG translation start site) of the C. albicans UPC2 gene was PCR amplified with Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA) from C. albicans strain SC5314 genomic DNA by using primers 5′-ATATGGATCCTTTCAACCACTATTACTACCT (which introduces a BamHI site, underlined) and 5′-TATACTGCAGctaGCGGCCGCCTTTCATATTCATAAACCCATT (which introduces sequentially a PstI site, underlined; a TAG stop codon, represented by lowercase letters; and a NotI site, underlined, respectively). The resulting fragment (2,225 bp) was digested with BamHI and PstI and cloned into the corresponding sites of plasmid pCaEXP (6), generating plasmid pCaEXP-UPC2. A triple hemagglutinin (HA3)-encoding sequence (111 bp) was released from plasmid pMPY-3×HA (40) by NotI enzymatic digestion and cloned into the NotI site of plasmid pCaEXP-UPC2, generating plasmid pCaEXP-UPC2-HA3. DNA sequencing was performed to confirm the in-frame cloning of the fragments and to ensure that no unintended mutations were introduced during the amplification process. The pCaEXP, pCaEXP-UPC2, and pCaEXP-UPC2-HA3 plasmids were digested with StuI, and the resulting fragments were individually used to transform strain SGY243 (Table 1).
C. albicans transformations.
C. albicans transformations were conducted as described by MacPherson et al. (28) by a modified standard lithium acetate procedure. The transformed cells were plated onto SC-ura plates and incubated for 3 days at 30°C.
Antifungal drugs and susceptibility testing.
Stock solutions of ketoconazole (Medisca) at a concentration of 0.25 mg/ml in dimethyl sulfoxide were prepared. Azole susceptibility testing was performed using spot assays. Cells were grown overnight on SC-ura-met-cys plates and resuspended in water to an optical density at 600 nm (OD600) of 0.1. Tenfold serial dilutions of each strain were spotted onto SC-ura-met-cys plates supplemented with 6 ng of ketoconazole/ml or with the solvent alone (dimethyl sulfoxide). The plates were incubated for 3 days at 30°C.
Total protein preparation and Western blotting.
Total protein was prepared as described for S. cerevisiae (37) from 2 OD units each of cultures of two independent strains carrying the vector alone (strains SGY243-CaEXP-A and SGY243-CaEXP-B) and two independent strains expressing the UPC2-HA3 sequence (SGY243-UPC2-HA-A and SGY243-UPC2-HA-B) (Table 1) grown overnight in SC-ura-met-cys (PMET3-inducing conditions) or in SC-ura+met+cys (PMET3-repressing conditions). Extracts were boiled for 1 min, and a 25-μl sample (out of 100 μl total) was separated by electrophoresis on a sodium dodecyl sulfate-10% polyacrylamide gel. Proteins were transferred onto a nitrocellulose membrane with a Trans Blot SD semidry transfer apparatus (Bio-Rad, Hercules, CA), and the membrane was incubated with a mouse anti-HA monoclonal antibody (12CA5; Roche) at a dilution of 1:1,000, followed by rabbit anti-mouse immunoglobulin G antibodies coupled to alkaline phosphatase (Bio-Rad). The membrane was then developed with 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt and nitroblue tetrazolium chloride substrates, as recommended by the manufacturer (Bio-Rad).
ChIP-on-chip (ChIP-chip) and data analysis.
Three independent cultures (50 ml each) of strains SGY243-CaEXP-A (untagged control strain) and SGY243-UPC2-HA-A (tagged strain) (Table 1) were grown overnight in SC-ura+met+cys, diluted to an OD600 of 0.005 in SC-ura-met-cys (to induce PMET3), and grown until the OD600 reached 1.0. The subsequent steps of DNA cross-linking, DNA shearing, ChIP, DNA labeling with the Cy dyes, hybridization to intergenic DNA microarrays, and data analysis were conducted exactly as described by Liu et al. (25). Briefly, cultures were treated with 1% formaldehyde (cross-linking) and snap-frozen in liquid nitrogen. Total cell extracts were prepared by bead beating, followed by sonication to shear the DNA. The extracts were then incubated at 4°C overnight with a mouse monoclonal anti-HA antibody (Santa Cruz Biotech) coupled to magnetic beads (pan-mouse immunoglobulin G Dynabeads; Dynal Biotech, Brown Deer, WI). Purified immunoprecipitated DNA (40 μl out of 50 μl in 10 mM Tris [pH 8.0]-1 mM EDTA) was used for amplification and labeling with Cy5/Cy3 dye (using Cy5/Cy3 monoreactive dye packs; Amersham Biosciences). Pools of labeled DNA from both the tagged strain (SGY243-UPC2-HA-A; Cy5 labeled) and the untagged control strain (SGY243-CaEXP-A; Cy3 labeled) were mixed and hybridized to a C. albicans intergenic DNA microarray described elsewhere (H. Hogues, H. Lavoie, A. Sellam, M. Mangos, T. Roemer, E. Purisima, A. Nantel, and M. Whiteway, submitted for publication). Scanning and data analyses (n = 3) were conducted as described previously (25).
Quantitative real-time PCR.
Quantitative PCR (Q-PCR) was performed with three independent ChIP samples each from strains SGY243-CaEXP-B and SGY243-UPC2-HA-B prepared as described above. Quantification of the recovered DNA was performed using the Quant-iT PicoGreen double-stranded DNA assay kit (Molecular Probes; Invitrogen) as previously described (25). The DNA concentrations ranged from 9.2 to 12.0 pg/μl for the tagged strain and 38.6 to 60.0 pg/μl for the untagged strain (in 25-μl total volumes). Q-PCR assays were conducted using the Universal ProbeLibrary (Roche Applied Science), the TaqMan (Integrated DNA Technologies [IDT]) (25), or the Sybr green (Applied Biosystems) methodology. The different primer and probe combinations used for Q-PCR are listed in Table 2.
TABLE 2.
Primers and probes used for quantitative real-time PCR binding assays
| Promoter | Primer or probea | Primer or probe sequence (5′-3′) | Location of fragment for amplificationb |
|---|---|---|---|
| ERG11 | F | TAAACGAGATAACGACATTATTAGGG | −458 to −372 |
| R | TGTCCATAGAGTGTAAGTTTGTTCAAT | ||
| Universal ProbeLibrary probe 5 (catalog no. 04685024001) | CAGCCACA | ||
| CDR1 | F | GGTGCACACACACACAAACACACA | −435 to −341 |
| R | TTGAGCTCCCACTATCCGATCCCTA | ||
| TaqMan probe | CCGCCCTCACTCTGTTCCATACAAAT | ||
| MDR1 | F | GGCGGATTTACTCCTGATACAACTC | −580 to −401 |
| R | GCGACGGGCTGTTGAGTAAACTAT | ||
| TaqMan probe | AGCTCGTTTAGTTGTTCCCATTCGCA | ||
| UPC2 | F | AACACGTGCCCATCTGTACCACAA | −985 to −786 |
| R | TGCAGAATGTGCGGCTCAGAAT | ||
| TaqMan probe | GGTTTACCTTGACCAACCAGAAATGGATCA | ||
| FUR1 | F | GGTGCTTTTGGGAGAATGAA | −987 to −913 |
| R | CTTCCTCAAAACAAAACTGCAA | ||
| Universal ProbeLibrary probe 27 (catalog no. 04687582001) | GCTGCCTG | ||
| SPS4c | F | TACAGTTGCCCCAGTCAACA | −636 to −574 |
| R | TGTCTTGGAACGGAAACTCA | ||
| Universal ProbeLibrary probe 15 (catalog no. 04685148001) | TCCTGCTC | ||
| SUT1d,e | F | GGCGAGGAAGAGTCATCCGAAATA | −1203 to −1057 |
| R | GGGCGTATGCTCTTCTTGCTTAGT | ||
| ACT1d | F | CTCACCAAGATTTATTGCCAAC | −865 to −619 |
| R | CACCCTACCCATTTGTCATATT |
F, forward primer; R, reverse primer. The TaqMan probes were from IDT, and the Universal ProbeLibrary probes were from Roche.
Position with respect to the ATG start codon.
orf19.7568.
Q-PCRs for the SUT1 and ACT1 promoters were performed using the Sybr green methodology.
orf19.4342.
Optimal specific primer sequences and probes for the promoters of ERG11 (target), FUR1 (control for statistical analyses), and SPS4 (orf19.7568; reference for normalization) were obtained using the Universal ProbeLibrary Web-based ProbeFinder software (version 2.34; Roche Applied Sciences) available on the Roche Applied Science website (http://www.universalprobelibrary.com) as previously described (25). TaqMan probes and specific forward and reverse primers for the CDR1, MDR1, and UPC2 target promoters were selected using the PrimerQuest tool from the IDT website (http://www.idtdna.com/Scitools/Applications/Primerquest/).
Q-PCR mixtures were prepared using the TaqMan universal PCR master mix according to the instructions of the manufacturer (Applied Biosystems, Inc.). For reactions using probes from the Universal ProbeLibrary, 6 pg of ChIP DNA, 250 nM (each) forward and reverse primers, 100 nM probe, 5 μl of the TaqMan universal PCR master mix, and water were combined in a final volume of 10 μl. Q-PCR mixtures using the TaqMan probe were prepared using the same conditions except that the probe and the primers (forward and reverse) were added to a final concentration of 100 and 200 nM, respectively. For Q-PCRs using the Sybr green methodology for the promoters of SUT1 (target) and ACT1 (reference for normalization), the conditions were as follows: 10 μl of 2× Sybr green master mix, 0.05 μl of 100 μM forward or reverse primer, 6 pg of DNA, and water were combined in a final volume of 20 μl.
Q-PCRs were performed in MicroAmp optical 384-well reaction plates (Applied Biosystems, Inc.) using an ABI 7900 HT real-time PCR instrument with 1 cycle at 95°C for 10 min and 40 cycles at 95°C for 15 s and 60°C for 1 min. Each biological replicate sample (from a total of three) was processed in duplicate. Data analysis was performed using the Sequence Detection System software (SDS 2.2.2; ABI). For each sample, threshold cycle (CT) values were determined using the SDS software. The levels of signal enrichment (n-fold) for the targets (ERG11, CDR1, MDR1, UPC2, and SUT1 promoters) were calculated using relative quantification according to the 2−ΔΔCt method, as follows: ΔCT = CT(target) − CT(reference), and ΔΔCT = ΔCT(test) − ΔCT(calibrator), where CT(reference) is the CT for the SPS4 or ACT1 promoter, ΔCT(test) is the ΔCT for the tagged ChIP sample, and ΔCT(calibrator) is the ΔCT for the untagged ChIP sample (26). The FUR1 promoter was used as a negative control to confirm the level of enrichment (n-fold) obtained using the 2−ΔΔCT method. Statistical analyses were performed using the R software (version 2.5.1; http://www.r-project.org), and the ΔΔCT values were compared using Welch's t test. The statistical significance threshold was set at an α value of 0.001.
Bioinformatic analyses.
For the analysis of overrepresented functional categories in the grouping of Upc2 targets (Table 3), 37 of the 202 Upc2-bound targets (P < 0.05) were removed from consideration, since their promoters were shared by two genes located on opposite strands, thus preventing us from identifying which of the two genes was regulated by Upc2. The sequences were then used as input data for functional grouping by the Candida Genome Database (CGD) Gene Ontology (GO) Term Finder tool (http://www.candidagenome.org/cgi-bin/GO/goTermFinder). The three ontology classifications “biological process,” “molecular function,” and “cellular component” were selected. GO Term Finder calculates a P value for the overrepresented GO categories (relative to data for the 4,096 annotated C. albicans genes) by using a binomial distribution (http://www.candidagenome.org/help/goTermFinder.html). If some GO categories corresponded to overlapping gene lists, the GO category corresponding to the largest number of genes was selected. The P value cutoff was <0.02. For motif discovery analyses (see Fig. 3), promoter sequences of up to 1.0 kb upstream of the ATG translation start sites of the 202 Upc2p-enriched promoters were retrieved from the Regulatory Sequence Analysis Tools database (http://rsat.ulb.ac.be/rsat/), such that overlap with neighboring genes was prevented, and these sequences were used as input for motif discovery in the Suite for Computational Identification of Promoter Elements (SCOPE) program (http://genie.dartmouth.edu/scope/) (7, 9). This program allows the accurate determination of potential transcription factor binding sites in a set of promoter sequences by using three different motif discovery algorithms (7, 9). To search for putative SRE sequences within the 202 target promoters (Table 4), the same sequences analyzed with the SCOPE program were used as input data for the Regulatory Sequence Analysis Tools pattern-matching tool (http://rsat.ulb.ac.be/rsat/) to identify matches to the DNA pattern TCGTATA.
TABLE 3.
Overrepresented functional categories in Upc2 ChIP-chip data (P < 0.02)
| GO categorya | Frequency of GO category for:
|
P valued | Genese | |
|---|---|---|---|---|
| Study sampleb | C. albicans genomec | |||
| Ergosterol metabolic process (GO:0008204, P)f | 7.8% (13) | 0.7% (29) | 3.0 × 10−10 | ERG10, ERG251, ERG2, ERG5, ERG11, ERG9, ERG25, NCP1, ERG6, ERG1, ERG24, ERG4, UPC2 |
| Cytosol (GO:0005829, C) | 13.3% (22) | 5.4% (221) | 9.6 × 10−5 | RPS30, orf19.3572.3, orf19.2309.2, RPL32, ERG10, GLK1, ATX1, RPL12, MET6, RPS1, orf19.4931.1, RPL15A, RPL17B, RPL19A, orf19.6882.1, RPL37B, RPL39, RPS13, orf19.2329.1, RPS23A, RPS6A, ACE2 |
| Lipid metabolic process (GO:0006629, P)f | 11.5% (19) | 4.9% (201) | 5.5 × 10−4 | ERG10, ERG251, ERG2, HSX11, ERG5, ERG11, orf19.1181, ERG9, ERG25, CDR1, orf19.4903, NCP1, ERG6, ERG1, ERG24, ERG4, UPC2, orf19.4982, orf19.2050 |
| Hyphal cell wall (GO:0030446, C) | 4.2% (7) | 0.8% (36) | 7.3 × 10−4 | AHP1, orf19.251, IPP1, MET6, SAM2, TSA1, TSA1B |
| Ribosome (GO:0005840, C) | 10.9% (18) | 4.7% (195) | 1.0 × 10−3 | RPS30, orf19.3572.3, orf19.2309.2, RPL32, RPL12, RPS1, orf19.4931.1, RPL15A, RPL17B, RPL19A, orf19.6882.1, RPL37B, RPL39, RPS13, orf19.2329.1, RPS23A, RPS6A, EFT2 |
| Structural molecule activity (GO:0005198, F) | 12.7% (21) | 6.3% (257) | 1.8 × 10−3 | RPS30, orf19.3572.3, orf19.2309.2, RPL32, RPL12, orf19.6665, orf19.3583, RPS1, orf19.4931.1, RPL15A, RPL17B, RPL19A, orf19.6882.1, RPL37B, RPL39, RPS13, orf19.2329.1, RPS23A, RPS6A, CDC12, orf19.121 |
| Thioredoxin peroxidase activity (GO:0008379, F) | 1.8% (3) | 0.1% (7) | 3.0 × 10−3 | AHP1, TSA1, TSA1B |
| Drug binding (GO:0008144, F) | 1.8% (3) | 0.1% (8) | 4.4 × 10−3 | ERG11, CDR1, EFT2 |
| Endoplasmic reticulum (GO:0005783, C) | 9.6% (16) | 4.9% (200) | 7.6 × 10−3 | ERG251, ERG2, ERG5, ERG11, orf19.1181, KAR2, ERG9, ERG25, orf19.4903, ERG6, CPY1, ERG1, ERG24, ERG4, orf19.1376, orf19.5830 |
| Pyrimidine nucleoside metabolic process (GO:0006213, P) | 1.2% (2) | 0.0% (4) | 1.2 × 10−2 | URA3, orf19.1137 |
| Drug transporter activity (GO:0015238, F) | 1.8% (3) | 0.2% (12) | 1.3 × 10−2 | MDR1, CDR1, YOR1 |
| Soluble fraction (GO:0005625, C) | 5.4% (9) | 2.2% (92) | 1.3 × 10−2 | ERG10, AHP1, GRP2, IPP1, IMH3, MET6, HSP60, EFT2, ASC1 |
| Positive regulation of transcription from RNA polymerase II promoter (GO:0045944, P) | 2.4% (4) | 0.7% (23) | 1.5 × 10−2 | INO2, SHA3, ACE2, UPC2 |
| Sulfur metabolic process (GO:0006790, P) | 3.6% (6) | 1.2% (51) | 1.8 × 10−2 | BIO2, CYS3, GCS1, MET6, SAM2, SAH1 |
| Lipid particle (GO:0005811, C) | 2.4% (4) | 0.6% (25) | 1.9 × 10−2 | ERG6, ERG1, orf19.4982, orf19.2050 |
Grouping of the Upc2p targets identified in ChIP-chip data according to GO terminology by using the online CGD GO Term Finder tool (http://www.candidagenome.org/cgi-bin/GO/goTermFinder). The GO accession number, followed by the corresponding ontology classification for each GO category, is shown in parentheses. P, biological process; C, cellular component; F, molecular function.
The number of genes in each category is shown in parentheses. Percentages were calculated based on the number of genes in each GO category divided by the total number of genes in the study sample (165 genes).
The number of genes in the annotated genome associated with each category is shown in parentheses. Percentages were calculated based on the number of genes in each category divided by the total number of annotated genes of the C. albicans genome, according to the CGD (4,096 genes).
P values for the overrepresented categories were calculated using a binomial distribution, as described on the GO Term Finder tool website (http://www.candidagenome.org/help/goTermFinder.shtml). The P value cutoff used was <0.02.
Gene names or orf19 nomenclature according to the CGD. Some genes were associated with more than one GO category.
The selection criteria for GO term groups with overlapping gene lists (see Materials and Methods for details) were not applied to these two groups, in order to show that “ergosterol metabolic process” was the most significantly overrepresented functional category.
FIG. 3.
Motif logo of a conserved consensus sequence in the Upc2p target promoters. DNA sequences of the 202 Upc2p-enriched promoters were retrieved from the Regulatory Sequence Analysis Tools website (http://rsat.ulb.ac.be/rsat/; see Materials and Methods for details) and used as input in the SCOPE program (http://genie.dartmouth.edu/scope/) for motif discovery (7, 9).
TABLE 4.
Upc2p target promoters containing a perfect putative SRE (TCGTATA)
| Functional category and ORFa | Gene nameb | Binding ratioc | P valued | Starting positione | Ending positione | Strandf |
|---|---|---|---|---|---|---|
| Lipid metabolic process | ||||||
| orf19.5178 | ERG5 | 4.0 | 0.0000 | −577 | −571 | − |
| orf19.3732 | ERG25 | 7.8 | 0.0000 | −429 | −423 | − |
| orf19.922 | ERG11 | 8.2 | 0.0000 | −232 | −226 | + |
| orf19.922 | ERG11 | 8.2 | 0.0000 | −486 | −480 | − |
| orf19.1631 | ERG6 | 3.0 | 0.0001 | −555 | −549 | + |
| orf19.391 | UPC2 | 1.9 | 0.0052 | −437 | −431 | − |
| orf19.1598 | ERG24 | 1.6 | 0.0265 | −139 | −133 | − |
| orf19.2050 | TGL1 | 1.6 | 0.0306 | −9 | −3 | − |
| orf19.4631 | ERG252 | 2.6 | 0.0318 | −499 | −493 | − |
| orf19.4631 | ERG252 | 2.6 | 0.0318 | −119 | −113 | − |
| orf19.406 | ERG1 | 1.5 | 0.0354 | −373 | −367 | + |
| orf19.1591 | ERG10 | 1.5 | 0.0377 | −264 | −258 | + |
| Organelle organization and biogenesis | ||||||
| orf19.7221 | SET3 | 7.1 | 0.0008 | −682 | −676 | + |
| orf19.7221 | SET3 | 7.1 | 0.0008 | −541 | −535 | + |
| orf19.7221 | SET3 | 7.1 | 0.0008 | −718 | −712 | − |
| orf19.3363 | VTC4 | 3.0 | 0.0135 | −601 | −595 | − |
| orf19.121 | ARC18 | 1.7 | 0.0226 | −459 | −453 | + |
| orf19.6942 | ORC3 | 1.5 | 0.0394 | −156 | −150 | + |
| Vesicle-mediated transport | ||||||
| orf19.2911 | SEC3 | 1.5 | 0.0447 | −745 | −739 | + |
| orf19.1376 | SSO2 | 1.5 | 0.0477 | −811 | −805 | − |
| Translation | ||||||
| orf19.3415.1 | RPL32 | 1.7 | 0.0239 | −473 | −467 | + |
| orf19.493 | RPL15A | 1.6 | 0.0397 | −241 | −235 | + |
| Amino acid and derivative metabolic process | ||||||
| orf19.3911 | SAH1 | 2.5 | 0.0005 | −383 | −377 | − |
| orf19.657 | SAM2 | 2.3 | 0.0064 | −521 | −515 | + |
| Unknown or other | ||||||
| orf19.1691 | 3.7 | 0.0000 | −713 | −707 | − | |
| orf19.6852 | 2.5 | 0.0006 | −295 | −289 | − | |
| orf19.1350 | 18.5 | 0.0027 | −742 | −736 | + | |
| orf19.6348 | 2.0 | 0.0037 | −826 | −820 | + | |
| orf19.1563 | ECM3 | 2.0 | 0.0039 | −309 | −303 | − |
| orf19.664 | 4.5 | 0.0108 | −472 | −466 | + | |
| orf19.1958 | 1.8 | 0.0140 | −171 | −165 | − | |
| orf19.4659 | PRP21 | 1.9 | 0.0158 | −600 | −594 | − |
| orf19.2286 | 1.7 | 0.0176 | −100 | −94 | + | |
| orf19.5491.1g | ATP14 | 1.7 | 0.0192 | −282 | −276 | − |
| orf19.3781 | 1.6 | 0.0236 | −337 | −331 | + | |
| orf19.4357g | 1.9 | 0.0240 | −219 | −213 | + | |
| orf19.4214 | 1.8 | 0.0342 | −584 | −578 | + | |
| orf19.3705 | 1.5 | 0.0377 | −415 | −409 | − | |
| orf19.3659g | 1.5 | 0.0406 | −366 | −360 | + | |
| orf19.496 | MSH1 | 1.5 | 0.0493 | −346 | −340 | + |
| orf19.496 | MSH1 | 1.5 | 0.0493 | −209 | −203 | − |
orf19 nomenclature according to the CGD assembly 19 version. Grouping of the SRE-containing promoter sequences into functional categories was carried out using the CGD GO Slim Mapper tool (http://www.candidagenome.org/cgi-bin/GO/goTermMapper).
Gene name according to the CGD (http://www.candidagenome.org/) or the National Research Council of Canada C. albicans database (http://candida.bri.nrc.ca/candida/index.cfm?page=CaGeneSearch).
Binding ratio from the ChIP-chip data. Probes were spotted in duplicate onto the ChIP-chip arrays (Hogues et al., submitted). The binding ratio with the most significant P value is shown.
P value for the corresponding binding ratio.
Limits (starting and ending positions) of the putative SRE sequence relative to the ATG translation start site.
+, sense strand; −, antisense strand.
The intergenic oligonucleotide probe corresponds to a common promoter of two adjacent genes.
Northern blotting.
C. albicans strains SGY243-CaEXP-B, SGY243-UPC2-B, and SGY243-UPC2-HA-B were grown overnight at 30°C in 10 ml of SC-ura-met-cys medium, diluted to an OD600 of 0.005 in 100 ml of the same medium, and grown to an OD600 of 1.0. The cells were subsequently harvested and frozen at −80°C prior to RNA extraction. For lovastatin treatment, strain SGY243 (parental wild type) and the upc2Δ/upc2Δ derivative (Table 1) were grown overnight at 30°C in 10 ml of YPD medium, diluted to an OD600 of 0.16 in 50 ml of the same medium, and exposed or not to 25 μg of hydrolyzed lovastatin/ml for 24 h. Lovastatin (Sigma) was hydrolyzed in ethanolic NaOH (15% [vol/vol] ethanol, 0.25% [wt/vol] NaOH) as previously described (27) except that the incubation time at 60°C was 2.5 h. Aliquots of stock solutions (20 mg/ml) were stored at −20°C. For growth under hypoxic conditions, the strains were pregrown under the same conditions and diluted to an OD600 of 0.16 in 250-ml flasks containing 50 ml of YPD medium. The flasks were then incubated in a BBL GasPak anaerobic jar under conditions described by the manufacturer (BD Biosciences) at 30°C for 24 h without shaking. Cell samples of 40 to 60 OD600 units (from the lovastatin-treated, hypoxic, and control cells) were then collected and stored at −80°C prior to RNA extraction. Total RNA was extracted using the hot-phenol method (58). Northern blotting was performed as previously described (38). The UPC2, ERG11, CDR1, MDR1, and ACT1 probes used for the Northern blot experiments have been described elsewhere (28, 38). The YOR1 probe consisted of a 32P-radiolabeled, 4,486-bp PCR-amplified fragment from C. albicans CAI4 genomic DNA overlapping positions −16 to +4470 relative to the ATG translation start site of the YOR1 open reading frame (ORF). The CBP1, SMF12, and SUT1 probes consisted of 32P-radiolabeled, 525-, 254-, and 279-bp PCR-amplified fragments from SC5314 genomic DNA overlapping positions +945 to +1469, +1 to +254, and +715 to +993 (relative to the ATG translation start site) of each ORF, respectively. The membranes were exposed to a Fujifilm imaging-plate screen for 24 h. The signal was quantified using the Multi Gauge program, version 2.3 (Fujifilm). The membranes were subsequently exposed to Kodak XAR films at −80°C.
RESULTS
Epitope tagging of Upc2p.
We used the URA3-based pCaEXP vector (6) to construct a C. albicans strain carrying an HA3 epitope at the C terminus of Upc2p (Fig. 1). The pCaEXP expression system allows regulated gene expression in C. albicans by cloning genes under the control of the MET3 promoter, which is repressed in the presence of methionine and activated in its absence (6). This system also permits high-efficiency integration at the RP10 locus (6). The UPC2 gene was PCR amplified using a primer that introduces a NotI site upstream of the stop codon and was cloned into pCaEXP. The NotI site enabled the subsequent in-frame cloning of a 111-bp NotI DNA fragment coding for HA3 (see Materials and Methods for details) (Fig. 1A). Western blotting showed that the expression of the tagged proteins, from two independent clones, was induced in the absence of methionine and repressed in its presence (Fig. 1B, lanes 3 to 6), while the negative control strain did not show any signal (Fig. 1B, lanes 1 and 2). The strains were also phenotypically characterized on PMET3-inducing medium supplemented or not with ketoconazole (Fig. 1C). Interestingly, while the expression of ectopic UPC2 did not significantly alter the azole resistance of the cells compared to that of the control strain (Fig. 1C, middle panel), the strain expressing the UPC2-HA3 allele showed marked resistance to ketoconazole (Fig. 1C, lower panel). This result indicates that the HA3 tag introduced at the C terminus of Upc2p acts as a gain-of-function mutation, probably preventing Upc2p from interacting with a repressor, a situation mimicking sterol deprivation, as previously suggested for S. cerevisiae Upc2p (15) (see Discussion).
FIG. 1.
Strategy for Upc2p epitope tagging and characterization of the Upc2p-HA3-expressing strains by Western blotting and spot assays. (A) Schematic representation of the UPC2-HA3 sequence integration cassette. Upc2p was tagged at its C terminus with a triple HA epitope (dark gray box) in C. albicans strain SGY243 by using the pCaEXP expression system (6), which allows the integration of the cassette carrying the UPC2-HA3 allele (UPC2-HA; light gray box) within the RP10 locus (black box). The expression of the UPC2-HA3 allele was under the control of PMET3 (open arrow), which is induced in the absence of methionine. (B) Western blot analyses of total protein extracts from the control (vector) or the UPC2-HA3 sequence-expressing clone SGY243-UPC2-HA-A (clone A) or SGY243-UPC2-HA-B (clone B) grown in the absence (−) or in the presence (+) of 2.5 mM methionine. The proteins were analyzed with a mouse monoclonal anti-HA antibody. The molecular mass markers are indicated on the left. (C) The drug resistance profiles of C. albicans integrants carrying the empty vector (vector), untagged UPC2 (UPC2), and the UPC2-HA3 sequence (UPC2-HA3) were analyzed by a spot assay on SC-ura-met-cys plates in the absence (no drug) or in the presence of ketoconazole (0.06 μg/ml).
Identification of Upc2p binding sites in vivo.
We performed location profiling (n = 3) of the UPC2-HA3 sequence-expressing strain (SGY243-UPC2-HA-B) relative to the empty vector control strain (SGY243-CaEXP-B) (Table 1) (see Materials and Methods for details). Under these conditions, we expected to identify the transcriptional targets of a constitutively active form of Upc2p. Using a P value cutoff of <0.05, we found 202 bound targets with binding ratios of 1.5 and above, the most significantly enriched target being the ergosterol biosynthesis gene NCP1 (exhibiting 5.7-fold enrichment) (see Table S1 in the supplemental material for the complete data set). Thirty-seven of 202 target promoters were common to two genes located on opposite strands (i.e., they shared the same probe on the chip), preventing us from identifying which one of the two genes was a target of Upc2p.
We used the GO Term Finder tool from the CGD (http://www.candidagenome.org/cgi-bin/GO/goTermFinder) to identify functional groupings among the 165 bound targets with unshared promoters and to determine which functional categories were significantly overrepresented relative to the annotations of the C. albicans genome (see Materials and Methods for details). As expected, Upc2p binding was significantly enriched at promoters of ergosterol biosynthesis genes, including ERG5 (4.0-fold), ERG25 (7.8-fold), ERG11 (8.2-fold), ERG6 (3.0-fold), NCP1 (5.7-fold), ERG9 (1.9-fold), ERG24 (1.6-fold), ERG4 (1.6-fold), ERG2 (1.8-fold), ERG251 (2.6-fold), ERG1 (1.5-fold), and ERG10 (1.5-fold) (see Table S1 in the supplemental material), grouped into the functional category “ergosterol metabolic process,” which itself was the most overrepresented category among the GO categories identified (P = 3 × 10−10) (Table 3). These results highlight a major role for Upc2p in controlling the ergosterol biosynthesis pathway. The Upc2p gene promoter was also enriched with bound Upc2p (1.9-fold), suggesting an autoregulatory loop controlling UPC2 expression (Table 3). Genes grouped into the GO category “ergosterol metabolic process” were also found in the parent overrepresented category “lipid metabolic process” (P = 5.5 × 10−4), in addition to six genes involved in or predicted to be involved in glycosylphosphatidylinositol anchor biosynthesis (orf19.4903), glucosylceramide synthesis (HSX11), lipid mobilization (orf19.4982 and orf19.2050, encoding putative triglyceride lipases highly homologous to the S. cerevisiae TGL1 product), phospholipid metabolism (orf19.1181, encoding a putative diacylglycerol pyrophosphate phosphatase), and lipid trafficking (CDR1). Upc2p also appeared to be implicated in the integrity of the hyphal cell wall, as a significant proportion of the enriched targets were localized or predicted to be localized at the hyphal cell wall. Strikingly, enrichment with bound Upc2p was observed for the promoters of 17 genes implicated in the assembly of ribosomal subunits, including 11 genes encoding proteins implicated in the assembly of the large subunit and 6 genes involved in the assembly of the small subunit (Table 3). These genes were grouped into the overrepresented GO category “ribosome,” in addition to EFT2, encoding a putative translation elongation factor (for a total of 18 genes; P = 1.0 × 10−3). Another overrepresented category was “positive regulation of transcription from RNA polymerase II promoter” (P = 1.5 × 10−2). The finding that Upc2p binds to promoters of transcription factor-encoding genes suggests that Upc2p may indirectly regulate other metabolic pathways, at least those implicating INO2 (putative regulator of ribosomal protein-encoding genes) and ACE2 (putative regulator of morphogenesis). SHA3, although encoding a serine/threonine kinase, was associated with the GO category “positive regulation of transcription from RNA polymerase II promoter,” probably because it was predicted previously to modulate the activity of a transcription factor regulating hexose transporter-encoding genes in S. cerevisiae (50). Another transcription factor-encoding gene whose promoter was significantly bound by Upc2p is SUT1 (orf19.4342), a zinc cluster regulator which was shown previously to control sterol uptake in S. cerevisiae (33), although this gene was not associated with the GO category “positive regulation of transcription from RNA polymerase II promoter.” Interestingly, Upc2p bound also to the promoters of the drug efflux pump-encoding genes CDR1 (1.6-fold), MDR1 (4.2-fold), and YOR1 (1.6-fold). This result constitutes a new finding with respect to the transcriptional regulation of CDR1 and MDR1 and azole resistance. Finally, a significant proportion of bound promoters were grouped into different categories, including thioredoxin peroxidase activity, the pyrimidine nucleoside metabolic process, and the sulfur metabolic process (Table 3).
To confirm our ChIP-chip results, the UPC2-HA3 sequence-expressing and control strains used for the ChIP-chip experiment were subjected to ChIP (three biological replicates), and the recovered DNA samples were analyzed by quantitative real-time PCR analysis (see Materials and Methods for details) (Fig. 2). We confirmed the enrichment of the promoters of ERG11 (25.1-fold), CDR1 (4.4-fold), MDR1 (9.8-fold), UPC2 (2.1-fold), and orf19.4342 (SUT1; 2.2-fold) with bound Upc2p (Fig. 2).
FIG. 2.
Quantification of the in vivo enrichment of Upc2p binding at selected promoter targets using Q-PCR. The SGY243-CaEXP-B and SGY243-UPC2-HA-B strains were subjected to ChIP (three biological replicates), and the recovered DNA samples were analyzed by Q-PCR using Universal ProbeLibrary probes (Roche Diagnostics) for the ERG11, FUR1, and SPS4 (reference) promoters, TaqMan probes for the CDR1, MDR1, and UPC2 promoters, and the Sybr green dye for the SUT1 and ACT1 (reference) promoters. Levels of enrichment (n-fold) presented on a log scale were as follows: 25.1 for ERG11 (95% confidence interval of 17.8 to 35.2), 4.4 for CDR1 (95% confidence interval of 3.9 to 5.1), 9.8 for MDR1 (95% confidence interval of 8.3 to 11.5), 2.1 for UPC2 (95% confidence interval of 2.0 to 2.2), 2.2 for SUT1 (95% confidence interval of 2.0 to 2.4), and 1.5 for FUR1 (95% confidence interval of 1.5 to 1.5), which was used as a negative control. Error bars denote standard deviations. All five tested promoters were significantly enriched compared to the FUR1 control, as determined by Welch's two-sample t test (α = 0.001).
Identification of potential Upc2p binding motifs.
We have previously shown that C. albicans Upc2p binds in vitro to the SRE 5′-TCGTATA, found in the promoter of the ERG2 gene (28). Using an in vivo reporter transactivation assay, one recent study showed that an ARE including one perfect SRE is involved in the UPC2-dependent induction of a PERG11-Renilla reniformis luciferase gene fusion (36). We thus searched for the SRE motif (TCGTATA) in the promoters of the 202 genes identified by ChIP-chip, using up to 1,000 bp of the promoter sequence upstream of the ATG translation start site and preventing overlap with neighboring genes (i.e., by clipping upstream sequences when a predicted upstream ORF was located within positions −1000 to −1 relative to the ATG codon). This analysis identified 40 matches, for a total of 36 SRE-containing promoters, including the promoters of eight ERG genes in addition to UPC2 (Table 4). As a control, we searched for the TCGTATA motif in equivalent promoter regions from the 6,045 ORFs of the C. albicans genome and found an average of 12.3 of 202 promoters containing this motif, yielding a 2.9-fold enrichment for the presence of putative SREs in the Upc2p targets (data not shown). The fact that only 36 of the 202 enriched promoter sequences contained putative SREs suggested that Upc2p may recognize different motifs in the promoters of its targets. We thus conducted a motif search analysis using the SCOPE program (http://genie.dartmouth.edu/scope/), which allows the accurate detection of conserved putative transcription factor binding sites among a given set of promoter sequences by using three independent motif discovery algorithms (7, 9) (see Materials and Methods for details). We found the following three highest-scoring motifs: AYAGCCCKH (significance value of 47.2; 12.9% coverage), VNCGBDTR (significance value of 47.1; 82.2% coverage), and GAAAAA (significance value of 35.0; 95.5% coverage). Interestingly, the VNCGBDTR motif (Fig. 3), which was found in 166 of 202 promoters, includes the previously characterized SRE motif TCGTATA (where underlining highlights identical residues) present in several ERG genes (Table 4), suggesting that the SRE is a variant of a more general recognition sequence for Upc2p.
Increased in vivo Upc2p binding correlates with increased expression of Upc2p targets.
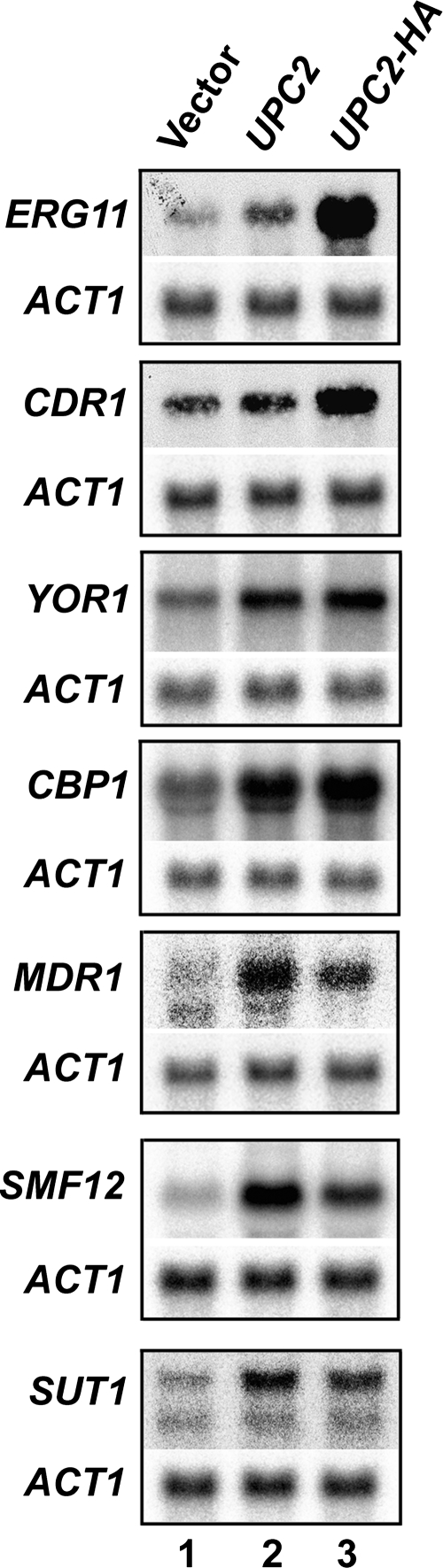
To test whether the expression of the Upc2p target genes identified by ChIP-chip was also modulated, we prepared total RNA from strains SGY243-CaEXP-B (vector control), SGY243-UPC2-B (expressing the UPC2 gene), and SGY243-UPC2-HA-B (expressing the UPC2-HA3 allele) (Table 1) grown under the same conditions as those for the ChIP-chip experiments. We then performed Northern blot analyses using probes for different Upc2p targets (Fig. 4). All the tested genes displayed basal levels of expression in the control strain (Fig. 4, lane 1). As expected, ERG11 expression was strongly upregulated as a consequence of the activating effect of the HA3 tag, consistent with the role of Upc2p as a transcriptional regulator of the ERG11 gene (28, 43). Interestingly, we found that the Upc2p target genes responded differentially to UPC2 and UPC2-HA3 sequence overexpression (Fig. 4, lanes 2 and 3). ERG11 and CDR1 were upregulated mostly as a consequence of the activating effect of the HA3 tag in Upc2p, and MDR1, SMF12, and SUT1 were upregulated mostly as a consequence of Upc2p overexpression, whereas YOR1 and CBP1 were upregulated at similar levels under both conditions. The ACT1 control gene showed no change in expression (Fig. 4). Taken together, these results showed that increased binding of Upc2p-HA3 to its targets correlates with their increased expression.
FIG. 4.
Northern blot analysis of the SGY243-derived integrants. Total RNA was prepared from strains SGY243-UPC2-HA-B (UPC2-HA) and SGY243-UPC2-B (UPC2) and the control SGY243-CaEXP-B strain (vector). RNA samples (20 μg) were electrophoresed, transferred onto nylon membranes, and probed with the indicated probes. The membranes were deprobed and subsequently rehybridized with the ACT1 control probe. The membranes were exposed to a phosphoscreen for 24 h.
Transcriptional response of ERG11, MDR1, and CDR1 to Upc2p-activating signals.
Upc2 in S. cerevisiae was shown previously to activate the expression of its target genes under hypoxic conditions or in cells treated with specific inhibitors of the ergosterol biosynthetic pathway, such as lovastatin (a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor) and azoles (14). Some experimental evidence supports the idea that Upc2p in C. albicans is similarly activated. First, ERG11 is upregulated following azole or lovastatin treatment (46) or during hypoxia (41). Second, C. albicans upc2Δ/upc2Δ mutants display altered growth on a medium containing azole drugs or lacking oxygen (28). Our finding that Upc2p bound in vivo to the promoters of genes involved in clinical azole resistance, namely, ERG11, MDR1, and CDR1, prompted us to determine whether the expression of these genes was regulated in response to Upc2p-activating signals. The SGY243 wild-type strain and its upc2Δ/upc2Δ mutant derivative were grown for 24 h in the presence of lovastatin, under hypoxic conditions, or without treatment (control). Total RNA from treated and untreated cells was prepared and subjected to Northern blotting using UPC2-, ERG11-, MDR1-, and CDR1-specific probes (Fig. 5). UPC2 displayed a basal level of expression in untreated wild-type cells (Fig. 5, lane 1), and UPC2 expression in the upc2Δ/upc2Δ mutant was undetectable (Fig. 5, lane 2). UPC2 expression was strongly induced upon treatment with lovastatin or during hypoxia (Fig. 5, lanes 3 and 5), potentially as a consequence of an autoregulatory mechanism and/or the activation of another transcription factor acting upstream of Upc2p. As expected, the ERG11 gene was upregulated upon lovastatin treatment, as well as under hypoxic conditions (Fig. 5, lanes 3 and 5). This induction was totally abolished in the upc2Δ/upc2Δ mutant, demonstrating that Upc2p is essential for the drug- or hypoxia-induced upregulation of ERG11 (Fig. 5, lanes 4 and 6). In the presence of lovastatin, MDR1 expression in the wild-type strain was slightly induced (Fig. 5, lane 3) yet that in the upc2Δ/upc2Δ mutant was strongly upregulated (Fig. 5, lane 4). Conversely, while MDR1 expression in wild-type cells grown under hypoxic conditions was slightly induced (Fig. 5, lane 5), this induction was abolished in the upc2Δ/upc2Δ mutant (Fig. 5, lane 6). These observations demonstrate that Upc2p is a transcriptional regulator of the MDR1 gene, acting as a transcriptional repressor upon lovastatin treatment and as a transcriptional activator during hypoxia. Interestingly, we found that Upc2p participates in maintaining the basal expression levels of CDR1 (in untreated cells), as a slight but reproducible decrease in CDR1 basal expression in the upc2Δ/upc2Δ mutant compared to that in the wild-type strain was observed when the signal was normalized with the ACT1 control signal (Fig. 5, compare lanes 1 and 2). Furthermore, CDR1 expression in the wild-type strain was downregulated by lovastatin (Fig. 5, lanes 1 and 3), and this downregulation was abolished in the upc2Δ/upc2Δ mutant (Fig. 5, compare lanes 3 and 4), suggesting that Upc2p acts as a repressor of CDR1 in the presence of lovastatin. Finally, CDR1 expression in the wild-type strain and that in the upc2Δ/upc2Δ mutant were induced at similar levels by hypoxia (Fig. 5, lanes 5 and 6), indicating that hypoxia-induced CDR1 expression is mediated by another regulator. Since SGY243 was found to carry four copies of the UPC2 gene (29), suggesting that partial or complete chromosome 1 tetrasomy may have occurred in that strain, we repeated these experiments using strain BWP17 and its upc2Δ/upc2Δ mutant derivative (43). Similar results were obtained (data not shown), indicating that aneuploidy did not affect gene expression in an atypical way in the SGY243 background. Taken together, our results show that Upc2p is a transcriptional regulator of the azole resistance genes ERG11, CDR1, and MDR1. They also reveal that Upc2p regulates the expression of its targets in a complex manner, acting as a transcriptional activator or a repressor, depending upon the target and the activating signal.
FIG. 5.
Northern blot analysis of the SGY243 and upc2Δ/upc2Δ strains. The wild-type SGY243 strain (WT) and the upc2Δ homozygous mutant (upc2Δ/Δ) were treated or not treated (control) with 25 μg of hydrolyzed lovastatin/ml or grown under hypoxic conditions for 24 h in YPD medium. Total RNA was prepared, and RNA samples (25 μg) were electrophoresed, transferred onto nylon membranes, and probed with UPC2-, ERG11-, MDR1-, and CDR1-specific probes (as indicated on the left). C. albicans 18S rRNA ethidium bromide staining (18S) was used as a loading control. The membranes were deprobed and rehybridized with the ACT1 probe as a transfer control. The membranes were exposed to a phosphoscreen for 24 h.
DISCUSSION
In the present study, we used the ChIP-chip technology to identify genes whose promoters are bound by C. albicans Upc2p in vivo with the goal of better understanding the biological function of Upc2p. In the course of these experiments, we found that tagging Upc2p at its C terminus conferred a hyperactive state on the fusion protein. This was not surprising, as several transcription factors of the zinc cluster family become hyperactive when mutated. Some examples include S. cerevisiae Pdr1p and Pdr3p, implicated in pleiotropic drug resistance (8, 34, 44), War1p, involved in response to weak acids (19), and Leu3p, involved in branched-chain-amino-acid biosynthesis and ammonia assimilation (52, 53). Furthermore, such activating mutations are naturally selected in azole-resistant C. albicans isolates, resulting in the hyperactivity of the zinc cluster transcription factor Tac1p (11, 12, 59) or Mrr1p (31). It has been shown previously that replacing amino acids at the C terminus of S. cerevisiae Upc2p with residues containing bulky lateral chains results in transcriptional hyperactivity (15). Thus, the presence of the bulky HA3 tag may act similarly and prevent C. albicans Upc2p from interacting with a repressor, a situation which would mimic sterol deprivation, as proposed for S. cerevisiae Upc2p (15). Whether this repressor molecule is a distinct protein or an intrinsic negative regulatory domain in Upc2p remains to be determined.
Our Upc2p-HA3 expression system has some advantages and limitations. For instance, the pCaEXP system permits high levels of expression of the fusion gene at any time during the growth of the cells, simply by removing methionine from the medium. This property is an advantage for nuclear proteins that are expressed at very low levels and are difficult to immunoprecipitate. Also, the constitutive transcriptional activity of Upc2p conferred by the HA tag allowed us to identify the targets of Upc2p in the absence of inducers, a situation that would mimic Upc2p gain-of-function mutations in azole-resistant clinical strains. Such mutations have not been reported yet, although some azole-resistant strains overexpress ERG11 (11, 54), potentially as a consequence of mutations in Upc2p. One recent study with S. cerevisiae used a similar expression system to identify the transcriptional targets of the zinc cluster regulator Leu3p and showed that overexpressing as well as constitutively activating a Leu3p-maltose binding protein fusion increases both the sensitivity and the relevance of the ChIP-chip results (49). Furthermore, it has been shown previously that the overexpression of transcription factors mimics their physiological activation and is a reliable, straightforward strategy to identify their transcriptional targets (10). However, this approach often leads to cell growth inhibition, probably due to signals that sense aberrant pathway activation and inhibit the cell cycle (10, 47). In fact, a growth inhibition effect on cells overexpressing UPC2 or the UPC2-HA3 gene was observed, as their colony sizes were smaller than that of the control cells (Fig. 1C, left panels). Since Upc2p was not expressed at its normal endogenous levels and was not physiologically activated, our binding data may not exactly reflect the activity of Upc2p upon treatment with its inducers (such as lovastatin, azoles, and growth under hypoxic conditions) or may be somewhat different from the natural binding sites of Upc2p. It is also possible that some Upc2p targets were missed by this strategy. Nevertheless, our results showing that endogenous Upc2p controls the expression of ERG11, MDR1, and CDR1 (Fig. 5) suggest that the genes that were identified by ChIP-chip using our system are bona fide Upc2p transcriptional targets.
Our study demonstrates that C. albicans Upc2p binds in vivo to the promoters of several ergosterol biosynthesis genes and other genes involved or predicted to be involved in lipid metabolism, grouped into the most significantly overrepresented functional category “ergosterol metabolic process,” as well as into the parental GO category “lipid metabolic process” (Table 3). This result highlights a major role for Upc2p in sterol homeostasis. We found that Upc2p binds in vivo to the promoter of the SUT1 gene (orf19.4342) (Table 3; Fig. 2), encoding an atypical zinc cluster regulator highly homologous to S. cerevisiae Sut1p. It has been shown previously that S. cerevisiae Sut1p controls sterol uptake (5, 33), suggesting that, in C. albicans, Upc2p and Sut1p may interact in a sterol regulatory network. In S. cerevisiae, sterol uptake occurs through the upregulation of the ATP binding cassette transporters Aus1p and Pdr11p or the Dan/Tir mannoproteins under anaerobic conditions (1, 3, 56). A search for candidate proteins which would function similarly in C. albicans, using BLASTP analyses, did not detect clear Dan/Tir homologues but identified five proteins with high levels of homology to Aus1p and Pdr11p, namely, Snq2p, Cdr1p, Cdr2p, Cdr11p, and Cdr4p. Our ChIP-chip data showed enrichment with bound Upc2p at the promoters of CDR1 (1.6-fold enrichment; P = 0.03) and CDR4 (1.4-fold enrichment; P = 0.06) (see Table S1 in the supplemental material). Thus, one could hypothesize that the two corresponding ABC transporters are candidates for sterol transport in C. albicans. Interestingly, among the hypoxic genes whose promoters were bound in vivo by Upc2p was CBP1, which was shown previously to encode a corticosteroid binding protein in C. albicans (30). This finding is important as C. albicans appears to take up steroids from the host under hypoxic conditions, possibly as metabolic precursors of sterols. Northern blotting showed that CBP1 induction under hypoxic conditions was strictly UPC2 dependent (data not shown). This result suggests that Upc2p plays a role in corticosteroid uptake from mammals and reflects a potential role for Upc2p in the adaptation of C. albicans to hypoxic conditions in the host.
An important finding was that Upc2p acts as a transcriptional activator or as a repressor of MDR1, depending on the activating signal (Fig. 5). Studies of S. cerevisiae showed that the zinc cluster regulator Hap1p, which controls the expression of ERG genes (ERG2, ERG5, and ERG11) in response to hypoxia, can act as a transcriptional activator or as a repressor, depending on heme levels (20). The conversion of Hap1p into a transcriptional repressor occurs through the recruitment of the Tup1p-Ssn6p corepressor complex (20). Thus, it is possible that Upc2p uses a similar mechanism to repress the expression of some of its target genes. Also, it will be interesting to find out how Upc2p interacts with Mrr1p to control MDR1 expression (30).
Among the overrepresented functional categories of genes whose promoters were bound by Upc2p were “sulfur metabolic process,” “ribosome,” and “positive regulation of transcription from RNA polymerase II promoter” (Table 3). Two of the six genes involved in the sulfur metabolic process, SAM2 and MET6, encode enzymes catalyzing reactions involved in the biosynthesis of precursors of the Erg6p substrate S-adenosylmethionine (16). This finding may explain why Upc2p binds to the promoters of genes involved in the sulfur metabolic process. Upc2p also binds to the promoters of genes encoding components of the ribosomal subunits, suggesting a role for Upc2p in translation. As ergosterol is an important component of the membranes in yeast, altering the ergosterol biosynthetic process would have major impacts on the general metabolism within the cell. Thus, it would not be surprising that Upc2p directly controls processes that would be affected as a result of sterol deprivation in the cell, such as translation. This possibility is also reflected by our finding that Upc2p binds to the promoters of transcription factor-encoding genes, such as the Ace2p gene, which is implicated in the regulation of several metabolic pathways, including glycolysis, respiration, and filamentation (32), and the Ino2p gene, which is predicted to be involved in regulating the expression of ribosomal genes in C. albicans (21).
As Upc2p is activated upon treatment with azoles or during growth under hypoxic conditions, we expected an overlap between our ChIP-chip data and data from genomewide expression profiling of the C. albicans response to azole treatment or hypoxia (16, 24, 41). Previous genomewide transcriptional profiling of the C. albicans response to hypoxia showed that six genes involved in ergosterol metabolism, namely, ERG11, ERG251, ERG3, ERG5, UPC2, and ERG10, are significantly upregulated relative to the corresponding genes in untreated cells (41). Interestingly, we showed here that the upregulation of ERG11 during hypoxia was strictly UPC2 dependent (Fig. 5). Furthermore, five of these six genes had their promoters bound in vivo by Upc2p in our present study (Table 3), suggesting that their induction by hypoxia was mediated by Upc2p as well. We also found other genes which both were upregulated under hypoxia and had their promoters bound by Upc2p in our study, including CBP1 (orf19.7323), GRP2 (orf19.4309), CPY1 (orf19.1339), orf19.1964, orf19.251, and AHP1 (orf19.2762). Several genes whose promoters were bound in vivo by Upc2p in the present study were also shown to be upregulated in response to azoles, including the ergosterol biosynthetic genes ERG2, ERG10, ERG11, ERG25, ERG251, and NCP1 (16, 24). Interestingly, a large number of ribosomal genes were found to be differentially upregulated 2.5- to 5.2-fold upon exposure to itraconazole (16), and among these genes, RPL15A, RPL14B, RPS17B, RPL32, and RPL17B also had their promoters bound by Upc2p (Table 3). Genes involved in the sulfur metabolic process, one of the overrepresented functional groups of genes whose promoters are bound by Upc2p, including SAM2, MET6, SAH1, and BIO2, were also found to be modulated upon itraconazole treatment (16). Thus, our study highlights how the ChIP-chip technology can help provide mechanistic explanations of expression array data.
Supplementary Material
Acknowledgments
We are indebted to Raphaëlle Lambert and Pierre Chagnon from the IRIC's genomic platform for their support with the Q-PCR experiments. We also thank Theodore C. White for the BWP17 strain and upc2 mutant derivative and Jean Barbeau for the GasPak anaerobic jar.
This work was supported by research grants from the Canadian Institutes of Health Research (CIHR) to M.R. (MT-15679 and HOP-67260) and to F.R. (MOP-82891). Work by S.L. was supported by research grants from the Natural Sciences and Engineering Research Council of Canada (NSERC; 312023-2005) and from CIHR (CTP-79843). S.Z. and S.S. are supported by doctoral studentships from the Fonds de la Recherche en Santé du Québec (FRSQ). S.D. is supported by a doctoral studentship from the Institut de Recherches Cliniques de Montréal/CIHR Cancer Research Program. F.R. holds a CIHR new-investigator award.
Footnotes
Published ahead of print on 4 April 2008.
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1.Abramova, N. E., B. D. Cohen, O. Sertil, R. Kapoor, K. J. Davies, and C. V. Lowry. 2001. Regulatory mechanisms controlling expression of the DAN/TIR mannoprotein genes during anaerobic remodeling of the cell wall in Saccharomyces cerevisiae. Genetics 1571169-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins, R. A. 2005. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med. Mycol. 43285-318. [DOI] [PubMed] [Google Scholar]
- 3.Alimardani, P., M. Regnacq, C. Moreau-Vauzelle, T. Ferreira, T. Rossignol, B. Blondin, and T. Berges. 2004. SUT1-promoted sterol uptake involves the ABC transporter Aus1 and the mannoprotein Dan1 whose synergistic action is sufficient for this process. Biochem. J. 381195-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berman, J., and P. E. Sudbery. 2002. Candida albicans: a molecular revolution built on lessons from budding yeast. Nat. Rev. Genet. 3918-930. [DOI] [PubMed] [Google Scholar]
- 5.Bourot, S., and F. Karst. 1995. Isolation and characterization of the Saccharomyces cerevisiae SUT1 gene involved in sterol uptake. Gene 16597-102. [DOI] [PubMed] [Google Scholar]
- 6.Care, R. S., J. Trevethick, K. M. Binley, and P. E. Sudbery. 1999. The MET3 promoter: a new tool for Candida albicans molecular genetics. Mol. Microbiol. 34792-798. [DOI] [PubMed] [Google Scholar]
- 7.Carlson, J. M., A. Chakravarty, C. E. DeZiel, and R. H. Gross. 2007. SCOPE: a web server for practical de novo motif discovery. Nucleic Acids Res. 35W259-W264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvajal, E., H. B. van den Hazel, A. Cybularz-Kolaczkowska, E. Balzi, and A. Goffeau. 1997. Molecular and phenotypic characterization of yeast PDR1 mutants that show hyperactive transcription of various ABC multidrug transporter genes. Mol. Gen. Genet. 256406-415. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarty, A., J. M. Carlson, R. S. Khetani, and R. H. Gross. 2007. A novel ensemble learning method for de novo computational identification of DNA binding sites. BMC Bioinformatics. 8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua, G., Q. D. Morris, R. Sopko, M. D. Robinson, O. Ryan, E. T. Chan, B. J. Frey, B. J. Andrews, C. Boone, and T. R. Hughes. 2006. Identifying transcription factor functions and targets by phenotypic activation. Proc. Natl. Acad. Sci. USA 10312045-12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coste, A., A. Selmecki, A. Forche, D. Diogo, M. E. Bougnoux, C. d'Enfert, J. Berman, and D. Sanglard. 2007. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot. Cell. [DOI] [PMC free article] [PubMed]
- 12.Coste, A., V. Turner, F. Ischer, J. Morschhauser, A. Forche, A. Selmecki, J. Berman, J. Bille, and D. Sanglard. 2006. A mutation in Tac1p, a transcription factor regulating CDR1 and CDR2, is coupled with loss of heterozygosity at chromosome 5 to mediate antifungal resistance in Candida albicans. Genetics 1722139-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daum, G., N. D. Lees, M. Bard, and R. Dickson. 1998. Biochemistry, cell biology and molecular biology of lipids of Saccharomyces cerevisiae. Yeast 141471-1510. [DOI] [PubMed] [Google Scholar]
- 14.Davies, B. S., and J. Rine. 2006. A role for sterol levels in oxygen sensing in Saccharomyces cerevisiae. Genetics 174191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies, B. S., H. S. Wang, and J. Rine. 2005. Dual activators of the sterol biosynthetic pathway of Saccharomyces cerevisiae: similar activation/regulatory domains but different response mechanisms. Mol. Cell. Biol. 257375-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Backer, M. D., T. Ilyina, X. J. Ma, S. Vandoninck, W. H. Luyten, and H. Vanden Bossche. 2001. Genomic profiling of the response of Candida albicans to itraconazole treatment using a DNA microarray. Antimicrob. Agents Chemother. 451660-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Deken, X., and M. Raymond. 2004. Constitutive activation of the PDR16 promoter in a Candida albicans azole-resistant clinical isolate overexpressing CDR1 and CDR2. Antimicrob. Agents Chemother. 482700-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Micheli, M., J. Bille, C. Schueller, and D. Sanglard. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 431197-1214. [DOI] [PubMed] [Google Scholar]
- 19.Gregori, C., B. Bauer, C. Schwartz, A. Kren, C. Schuller, and K. Kuchler. 2007. A genetic screen identifies mutations in the yeast WAR1 gene, linking transcription factor phosphorylation to weak-acid stress adaptation. FEBS J. 2743094-3107. [DOI] [PubMed] [Google Scholar]
- 20.Hickman, M. J., and F. Winston. 2007. Heme levels switch the function of Hap1 of Saccharomyces cerevisiae between transcriptional activator and transcriptional repressor. Mol. Cell. Biol. 277414-7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoppen, J., M. Dietz, G. Warsow, R. Rohde, and H. J. Schuller. 2007. Ribosomal protein genes in the yeast Candida albicans may be activated by a heterodimeric transcription factor related to Ino2 and Ino4 from S. cerevisiae. Mol. Genet. Genomics 278317-330. [DOI] [PubMed] [Google Scholar]
- 22.Kelly, R., S. M. Miller, M. B. Kurtz, and D. R. Kirsch. 1987. Directed mutagenesis in Candida albicans: one-step gene disruption to isolate ura3 mutants. Mol. Cell. Biol. 7199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis, T. A., F. R. Taylor, and L. W. Parks. 1985. Involvement of heme biosynthesis in control of sterol uptake by Saccharomyces cerevisiae. J. Bacteriol. 163199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, T. T., R. E. Lee, K. S. Barker, R. E. Lee, L. Wei, R. Homayouni, and P. D. Rogers. 2005. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother. 492226-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, T. T., S. Znaidi, K. S. Barker, L. Xu, R. Homayouni, S. Saidane, J. Morschhauser, A. Nantel, M. Raymond, and P. D. Rogers. 2007. Genome-wide expression and location analyses of the Candida albicans Tac1p regulon. Eukaryot. Cell 62122-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz, R. T., and L. W. Parks. 1990. Effects of lovastatin (mevinolin) on sterol levels and on activity of azoles in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 341660-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacPherson, S., B. Akache, S. Weber, X. De Deken, M. Raymond, and B. Turcotte. 2005. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob. Agents Chemother. 491745-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacPherson, S., M. Larochelle, and B. Turcotte. 2006. A fungal family of transcriptional regulators: the zinc cluster proteins. Microbiol. Mol. Biol. Rev. 70583-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malloy, P. J., X. Zhao, N. D. Madani, and D. Feldman. 1993. Cloning and expression of the gene from Candida albicans that encodes a high-affinity corticosteroid-binding protein. Proc. Natl. Acad. Sci. USA 901902-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morschhauser, J., K. S. Barker, T. T. Liu, J. Blass-Warmuth, R. Homayouni, and P. D. Rogers. 2007. The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog. 3e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulhern, S. M., M. E. Logue, and G. Butler. 2006. Candida albicans transcription factor Ace2 regulates metabolism and is required for filamentation in hypoxic conditions. Eukaryot. Cell 52001-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ness, F., S. Bourot, M. Regnacq, R. Spagnoli, T. Berges, and F. Karst. 2001. SUT1 is a putative Zn[II]2Cys6-transcription factor whose upregulation enhances both sterol uptake and synthesis in aerobically growing Saccharomyces cerevisiae cells. Eur. J. Biochem. 2681585-1595. [PubMed] [Google Scholar]
- 34.Nourani, A., D. Papajova, A. Delahodde, C. Jacq, and J. Subik. 1997. Clustered amino acid substitutions in the yeast transcription regulator Pdr3p increase pleiotropic drug resistance and identify a new central regulatory domain. Mol. Gen. Genet. 256397-405. [DOI] [PubMed] [Google Scholar]
- 35.Odds, F. C., A. J. Brown, and N. A. Gow. 2003. Antifungal agents: mechanisms of action. Trends Microbiol. 11272-279. [DOI] [PubMed] [Google Scholar]
- 36.Oliver, B. G., J. L. Song, J. H. Choiniere, and T. C. White. 2007. cis-acting elements within the Candida albicans ERG11 promoter mediate the azole response through transcription factor Upc2p. Eukaryot. Cell 62231-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose, M. D., F. Winston, and P. Hieter. 1990. Methods in yeast genetics: a laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, NY.
- 38.Saidane, S., S. Weber, X. De Deken, G. St. Germain, and M. Raymond. 2006. PDR16-mediated azole resistance in Candida albicans. Mol. Microbiol. 601546-1562. [DOI] [PubMed] [Google Scholar]
- 39.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 273-85. [DOI] [PubMed] [Google Scholar]
- 40.Schneider, B. L., W. Seufert, B. Steiner, Q. H. Yang, and A. B. Futcher. 1995. Use of polymerase chain reaction epitope tagging for protein tagging in Saccharomyces cerevisiae. Yeast 111265-1274. [DOI] [PubMed] [Google Scholar]
- 41.Setiadi, E. R., T. Doedt, F. Cottier, C. Noffz, and J. F. Ernst. 2006. Transcriptional response of Candida albicans to hypoxia: linkage of oxygen sensing and Efg1p-regulatory networks. J. Mol. Biol. 361399-411. [DOI] [PubMed] [Google Scholar]
- 42.Sherman, F. 1991. Getting started with yeast. Methods Enzymol. 1943-21. [DOI] [PubMed] [Google Scholar]
- 43.Silver, P. M., B. G. Oliver, and T. C. White. 2004. Role of Candida albicans transcription factor Upc2p in drug resistance and sterol metabolism. Eukaryot. Cell 31391-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonics, T., Z. Kozovska, D. Michalkova-Papajova, A. Delahodde, C. Jacq, and J. Subik. 2000. Isolation and molecular characterization of the carboxy-terminal pdr3 mutants in Saccharomyces cerevisiae. Curr. Genet. 38248-255. [DOI] [PubMed] [Google Scholar]
- 45.Sims, C. R., L. Ostrosky-Zeichner, and J. H. Rex. 2005. Invasive candidiasis in immunocompromised hospitalized patients. Arch. Med. Res. 36660-671. [DOI] [PubMed] [Google Scholar]
- 46.Song, J. L., C. N. Lyons, S. Holleman, B. G. Oliver, and T. C. White. 2003. Antifungal activity of fluconazole in combination with lovastatin and their effects on gene expression in the ergosterol and prenylation pathways in Candida albicans. Med. Mycol. 41417-425. [DOI] [PubMed] [Google Scholar]
- 47.Sopko, R., D. Huang, N. Preston, G. Chua, B. Papp, K. Kafadar, M. Snyder, S. G. Oliver, M. Cyert, T. R. Hughes, C. Boone, and B. Andrews. 2006. Mapping pathways and phenotypes by systematic gene overexpression. Mol. Cell 21319-330. [DOI] [PubMed] [Google Scholar]
- 48.Sturley, S. L. 2000. Conservation of eukaryotic sterol homeostasis: new insights from studies in budding yeast. Biochim. Biophys. Acta 1529155-163. [DOI] [PubMed] [Google Scholar]
- 49.Tang, L., X. Liu, and N. D. Clarke. 2006. Inferring direct regulatory targets from expression and genome location analyses: a comparison of transcription factor deletion and overexpression. BMC Genomics 7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vagnoli, P., and L. F. Bisson. 1998. The SKS1 gene of Saccharomyces cerevisiae is required for long-term adaptation of snf3 null strains to low glucose. Yeast 14359-369. [DOI] [PubMed] [Google Scholar]
- 51.Vik, A., and J. Rine. 2001. Upc2p and Ecm22p, dual regulators of sterol biosynthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 216395-6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, D., Y. Hu, F. Zheng, K. Zhou, and G. B. Kohlhaw. 1997. Evidence that intramolecular interactions are involved in masking the activation domain of transcriptional activator Leu3p. J. Biol. Chem. 27219383-19392. [DOI] [PubMed] [Google Scholar]
- 53.Wang, D., F. Zheng, S. Holmberg, and G. B. Kohlhaw. 1999. Yeast transcriptional regulator Leu3p. Self-masking, specificity of masking, and evidence for regulation by the intracellular level of Leu3p. J. Biol. Chem. 27419017-19024. [DOI] [PubMed] [Google Scholar]
- 54.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 411482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilcox, L. J., D. A. Balderes, B. Wharton, A. H. Tinkelenberg, G. Rao, and S. L. Sturley. 2002. Transcriptional profiling identifies two members of the ATP-binding cassette transporter superfamily required for sterol uptake in yeast. J. Biol. Chem. 27732466-32472. [DOI] [PubMed] [Google Scholar]
- 57.Wirsching, S., S. Michel, G. Kohler, and J. Morschhauser. 2000. Activation of the multiple drug resistance gene MDR1 in fluconazole-resistant, clinical Candida albicans strains is caused by mutations in a trans-regulatory factor. J. Bacteriol. 182400-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wodicka, L., H. Dong, M. Mittmann, M. H. Ho, and D. J. Lockhart. 1997. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat. Biotechnol. 151359-1367. [DOI] [PubMed] [Google Scholar]
- 59.Znaidi, S., X. De Deken, S. Weber, T. Rigby, A. Nantel, and M. Raymond. 2007. The zinc cluster transcription factor Tac1p regulates PDR16 expression in Candida albicans. Mol. Microbiol. 66440-452. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.