Abstract
Horizontal gene transfer (HGT) is thought to occur frequently in bacteria in nature and to play an important role in bacterial evolution, contributing to the formation of new species. To gain insight into the frequency of HGT in Vibrionaceae and its possible impact on speciation, we assessed the incidence of interspecies transfer of the lux genes (luxCDABEG), which encode proteins involved in luminescence, a distinctive phenotype. Three hundred three luminous strains, most of which were recently isolated from nature and which represent 11 Aliivibrio, Photobacterium, and Vibrio species, were screened for incongruence of phylogenies based on a representative housekeeping gene (gyrB or pyrH) and a representative lux gene (luxA). Strains exhibiting incongruence were then subjected to detailed phylogenetic analysis of horizontal transfer by using multiple housekeeping genes (gyrB, recA, and pyrH) and multiple lux genes (luxCDABEG). In nearly all cases, housekeeping gene and lux gene phylogenies were congruent, and there was no instance in which the lux genes of one luminous species had replaced the lux genes of another luminous species. Therefore, the lux genes are predominantly vertically inherited in Vibrionaceae. The few exceptions to this pattern of congruence were as follows: (i) the lux genes of the only known luminous strain of Vibrio vulnificus, VVL1 (ATCC 43382), were evolutionarily closely related to the lux genes of Vibrio harveyi; (ii) the lux genes of two luminous strains of Vibrio chagasii, 21N-12 and SB-52, were closely related to those of V. harveyi and Vibrio splendidus, respectively; (iii) the lux genes of a luminous strain of Photobacterium damselae, BT-6, were closely related to the lux genes of the lux-rib2 operon of Photobacterium leiognathi; and (iv) a strain of the luminous bacterium Photobacterium mandapamensis was found to be merodiploid for the lux genes, and the second set of lux genes was closely related to the lux genes of the lux-rib2 operon of P. leiognathi. In none of these cases of apparent HGT, however, did acquisition of the lux genes correlate with phylogenetic divergence of the recipient strain from other members of its species. The results indicate that horizontal transfer of the lux genes in nature is rare and that horizontal acquisition of the lux genes apparently has not contributed to speciation in recipient taxa.
Horizontal gene transfer (HGT), the acquisition of genes by a member of one bacterial lineage from another lineage, is widely believed to play a major role in bacterial evolutionary divergence and speciation (29, 39, 40, 46). As a possibly common process, HGT also is thought to result in reticulate evolution, precluding or at least seriously complicating reconstruction of bacterial phylogenies (6, 17). The actual frequency of HGT in nature and its influence on bacterial evolution, however, are controversial; many believe that HGT is uncommon, at best has only minor or infrequent effects on the process of bacterial speciation, and does not prevent reconstruction of bacterial phylogenies (23, 24, 36, 38). An intermediate view is that HGT occurs frequently between bacterial lineages, contributing various strain-specific genes and thereby increasing the pan-genome of a bacterial species, but that the bacterium's core genome is inherited vertically and rarely perturbed by HGT (15, 41, 50, 51, 58).
Bacterial luminescence, a distinctive, easily observable phenotype of members of Vibrionaceae and certain other bacteria, provides a readily tractable subject for evaluating the frequency of HGT events in nature and the possible impact of those events on bacterial evolution and speciation. The genes for bacterial light production, luxCDABEG, are present in bacteria as a conserved, contiguous, and coordinately expressed set of genes, the lux operon. The luxA and luxB genes code for the α and β subunits of the light-emitting enzyme luciferase; luxC, luxD, and luxE specify the enzymatic components of a fatty acid reductase complex necessary for synthesis and recycling of an aldehyde that, along with O2 and FMNH2, serves as a substrate for luciferase; and luxG, present in most luminous bacteria, codes for a flavin reductase (20). Associated with the lux operons of certain species of the “fischeri group,” recently reclassified as members of a new genus, Aliivibrio (61), are regulatory genes, luxR and luxI, specifying a quorum-sensing transcriptional activator and an acyl-homoserine lactone synthase. An additional gene, luxF, which codes for a nonfluorescent flavoprotein, is present in the lux operons of some luminous bacteria. Also contiguous with the lux genes in some bacteria are genes for synthesis of riboflavin, forming a lux-rib operon, luxCDAB(F)EG-ribEBHA, which is located adjacent to the chromosomal putA gene (coding for proline dehydrogenase) (3). Recently, many strains of Photobacterium leiognathi were found to carry two complete, physically separate, and apparently functional lux-rib operons, one adjacent to putA and the other located elsewhere on the chromosome and flanked by putative transposase genes (3). The conserved gene content and gene order of the luminescence system in bacteria and the high levels of amino acid sequence identities of the Lux proteins among luminous bacteria suggest a common evolutionary origin for the lux operon genes.
The presence of the luxCDABEG genes is scattered taxonomically in extant bacteria. Most luminous bacteria are members of Vibrionaceae (Gammaproteobacteria), with luminous species in the genera Aliivibrio, Photobacterium, and Vibrio. Members of two other Gammaproteobacteria families, Shewanella hanedai and Shewanella woodyi (Shewanellaceae) and Photorhabdus species (Enterobacteriaceae), also are luminous. This pattern of incidence suggests that the lux genes arose either in the ancestor of the Vibrionaceae, followed by horizontal transfer to members of the Shewanellaceae and Enterobacteriaceae, or earlier, in the ancestor of all three families. Most of the species in these three families, however, lack the lux genes, and for many of the luminous species, some or most strains are nonluminous (20). An evolutionary scenario that might account for the scattered incidence of lux genes is vertical inheritance with loss of these genes from several lineages leading to extant species of Vibrionaceae, Shewanellaceae, and Enterobacteriaceae. Alternatively, the lux genes, regardless of their origin, might have been acquired by many strains and species via horizontal transfer. A combination of loss from many taxa and acquisition by horizontal transfer by other taxa also could account for the scattered incidence of lux genes in bacteria. However, neither scenario has been tested phylogenetically. The only available evidence on these questions, which suggests that Photorhabdus and luminous Shewanella species acquired their lux genes by horizontal transfer from a member of Vibrionaceae (20, 27, 34), is based on analysis of nucleotide and amino acid sequence similarities, criteria that lack a rigorous evolutionary basis and therefore do not provide definitive evidence for or against HGT.
To address these issues and gain insight into the frequency of horizontal transfer of the lux genes and its impact on speciation, we used an evolutionary approach to test for and characterize instances of lux gene horizontal transfer in Vibrionaceae. Over 300 luminous strains, most of which were newly isolated from nature, were examined for incongruence of phylogenies based on multiple housekeeping genes and multiple lux genes. The results, which indicate that horizontal transfer of the lux genes in nature apparently is rare and has not contributed to speciation in recipient taxa, support the scenario of vertical inheritance with multiple loss.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains examined in detail in this study are listed in Table 1. For routine culture, strains were grown at room temperature (20 to 22°C) with aeration in LSW-70 (21), which contained 10 g tryptone, 5 g yeast extract, 350 ml double-strength artificial seawater (44), 650 ml deionized water, and, for solid medium, 15 g·liter−1 agar.
TABLE 1.
Bacterial strains used in this studya
| Strain | Origin | Reference(s) or source |
|---|---|---|
| Aliivibrio fischeri ATCC 7744Tb | Seawater | 32 |
| Aliivibrio logei ATCC 29985Tb | Gut of arctic mussel | 10 |
| Aliivibrio salmonicida ATCC 43839Tb | Farmed Atlantic salmon, Norway | 22 |
| Aliivibrio wodanis ATCC BAA-104T | Atlantic salmon with winter ulcer disease, Norway | 42 |
| Photobacterium damselae BT-6b | Aquarium seawater, Sesoko Island, Okinawa, Japan | This study |
| Photobacterium leiognathi lnuch.13.1b | Light organ of leiognathid fish, Wakasa Bay, Honshu, Japan | 3 |
| Photobacterium mandapamensis | ||
| ajapo.4.20b | Light organ of acropomatid fish, Saga, Shikoku, Japan | 33 |
| ajapo.4.22b,c | Light organ of acropomatid fish, Saga, Shikoku, Japan | 33 |
| ajapo.5.37b,c | Light organ of acropomatid fish, Saga, Shikoku, Japan | 33 |
| Vibrio chagasii | ||
| LMG 21353T | Intestine of larval fish, Norway | 60 |
| LMG 13219 | Rotifer, Greece | 60 |
| LMG 13237 | Seawater, Greece | 60 |
| 21N-12b | Seawater, 250-m depth at 21°N, Pacific Ocean | This study |
| SB-52b | Coastal seawater, Suruga Bay, Honshu, Japan | This study |
| Vibrio cholerae | ||
| ATCC 14547b | Fish, River Elbe, Germany | 54 |
| manz.1.1b | Coastal seawater, Manzanita, OR | This study |
| manz.1.2b | Coastal seawater, Manzanita, OR | This study |
| Vibrio harveyi ATCC 14126Tb | Amphipod (Talorchestia sp.), Woods Hole, MA | 10 |
| Vibrio orientalis ATCC 33934Tb | Seawater, Yellow Sea, China | 62 |
| Vibrio parahaemolyticus ATCC 17802T | Clinical (food poisoning), Japan | 9 |
| Vibrio rotiferianus LMG 21460T | Rotifer (Brachionus plicatilis), Belgium | 31 |
| Vibrio splendidus ATCC 33125Tb | Marine fish, The Netherlands | 54 |
| Vibrio vulnificus | ||
| ATCC 27562T (B9629) | Clinical (blood), Florida; E typed | 8 |
| ATCC 43382 (VVL1)b | Clinical (fatal), Florida; E type | 48 |
| CMCP6 | Clinical, Korea; C typed | 35, 55 |
| YJ016 | Clinical, Taiwan; C type | 14, 55 |
| 300 1A1 | Seawater; E type | 55 |
| C7184 | Clinical (blood); C type | 52, 55 |
| 395R | Clinical; E type | 55 |
| 300 1C1 | Seawater; C type | 55 |
| SS109B-3B2 | Oyster; E type | 55 |
| SS108C-5C1 | Oyster; E type | 55 |
| SPRC10143 | Clinical (fatal), California; C type | 55 |
Strains for which DNA sequences were obtained in this study. See also Table 2 for strains from which previously obtained sequences were analyzed in this study.
Bioluminescent; all other strains listed here are nonluminous and apparently lack lux genes.
Previously identified incorrectly as P. leiognathi based on sequence analysis of lux-rib2 genes (33).
Isolation and screening of luminous bacteria.
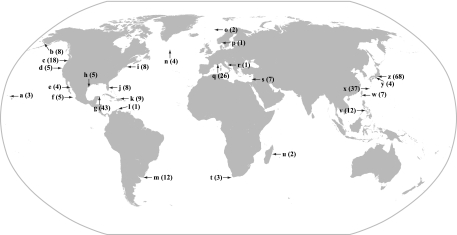
Bacterial strains newly isolated in this study were taken primarily from coastal and open-ocean seawater at various locations worldwide (Fig. 1). Samples of aseptically collected seawater were spread on LSW-70 agar plates made with 40 g·liter−1 agar (7), incubated overnight at 18 to 25°C, and examined for luminous colonies, which were then picked and purified on the same medium. Purified strains were stored in cryoprotective medium at −75°C (20).
FIG. 1.
Collection locations of luminous strains screened for horizontal transfer of lux genes. Collection locations are as follows: (a) Hawaii; (b) Alaska; (c) Manzanita, OR; (d) Pacific Ocean off the coast of northern California; (e) Guaymas Basin, Gulf of California; (f) western Pacific Ocean at 21°N; (g) Cancun, Mexico; (h) Galveston, TX; (i) Woods Hole, MA; (j) Fort Lauderdale and Atlantic Ocean off the coast of Florida; (k) Dominican Republic; (l) Caracasbaai, Curacao; (m) José Ignacio, Uruguay; (n) North Atlantic; (o) North Sea; (p) Oslo Harbor, Norway; (q) Il de Bendor, Provence, France; (r) Adriatic Sea; (s) Habonim Beach, Israel; (t) Cape Town, South Africa; (u) Madagascar; (v) Tigbauan, Panay, and Sangat Island, Palawan, Philippines; (w) Tungkang, Taiwan; (x) Nakagusuku Bay and Sesoko Island, Okinawa, Japan; (y) Ago Bay, Honshu, Japan; and (z) Suruga Bay, Honshu, Japan. Species, number of strains, and (in parentheses) letter designations of the locations from which each species was collected are as follows: A. fischeri, nine (h, x, z); Aliivibrio logei, eight (i); P. damselae, one (x); Photobacterium kishitanii, 14 (a, e, j, q, t, z); P. leiognathi, 13 (j, v, x, y, z); P. mandapamensis, 51 (g, j, w, x, z); Photobacterium phosphoreum, 20 (b, d, n, o, r); V. chagasii, two (f, z); V. cholerae, 17 (c); V. harveyi, 166 (c, e, f, g, h, k, l, m, p, q, s, u, v, w, x, y, z); Vibrio orientalis, two (h). Also, see Table 1 for collection information for V. vulnificus (11 strains) and for strains of light-organ symbiotic P. mandapamensis (three strains).
For photometric detection of luminescence, cells were taken from early-stationary-phase broth cultures or overnight cultures on plates of LSW-70 agar (15 g·liter−1 agar), and their luminescence was monitored with a Turner BioSystems (Sunnyvale, CA) 20/20 luminometer operated at high sensitivity.
Genomic DNA extraction, PCR, and sequencing.
Extraction of genomic DNA from overnight broth cultures and amplification of housekeeping genes gyrB, recA, and pyrH (specifying gyrase subunit B, recombinase A, and uridylate kinase, respectively) and of the luxCDABEG genes followed previously described protocols (2, 59). Primer sequences and amplification conditions for each gene and strain are provided in the supplemental material. PCR products were visualized using electrophoresis on 1% agarose gels stained with ethidium bromide and were purified using a QIAquick PCR purification kit (Qiagen, Valencia, CA). PCR products were sequenced using the respective PCR primers, and sequencing was carried out by the staff of the University of Michigan Sequencing Core using dye terminator cycle sequencing on a Perkin-Elmer (Waltham, MA) ABI 3730 or 3700 DNA analyzer.
Phylogenetic analysis.
Provisional species identifications were based on sequence analysis of gyrB or pyrH, and instances of lux gene horizontal transfer were identified provisionally by incongruence between phylogenies based on gyrB or pyrH and luxA. Nucleotide and amino acid identities were calculated using PAUP* (57). For detailed phylogenetic analyses based on multiple housekeeping genes and multiple lux genes, concatenated sequences of gyrB, recA, and pyrH and of luxCDABEG genes were analyzed (Table 2). DNA sequences were aligned by reference to the inferred amino acid sequences. Characters from genes unavailable for analysis were treated as missing data, and spacer regions between lux genes were omitted from the analysis. The luxF sequence was treated as missing data in taxa lacking this gene. Sequence data were analyzed simultaneously with TNT (30). Outgroup sequences were from Photorhabdus luminescens subsp. laumondii TTO1 (18). Jackknife resampling values (25) were calculated in TNT with 10,000 resampling replicates.
TABLE 2.
GenBank accession numbers for sequences used in this study
| Strain | GenBank accession no. or reference for sequence of:
|
|||
|---|---|---|---|---|
| recA | gyrB | pyrH | lux operon genesc | |
| Aliivibrio fischeri ATCC 7744T | AJ842417 | AY455874 | EF415528 | AY341062 (luxCDABE) |
| Aliivibrio fischeri ES114a | VF0535 | VF0012 | VF1960 | VFA0923 to VFA0918 (luxCDABEG) |
| Aliivibrio logei ATCC 29985T | AJ842457 | EF380255 | EF380234 | EF576941 (luxAB) |
| Aliivibrio salmonicida ATCC 43839T | EF380243 | EF380256 | EU118245 | AF452135 (luxCDABE) |
| Aliivibrio wodanis ATCC BAA-104T | EF380244 | EF380257 | EU118246 | NA |
| Photobacterium angustum ATCC 25915T | AJ842354 | AY455890 | EF380235 | NA |
| Photobacterium damselae subsp. damselae ATCC 33539T | AJ842357 | AY455889 | EF380236 | NA |
| Photobacterium damselae BT-6 | EU118224 | EU118205 | EU118247 | EU122290 (luxDABE) |
| Photobacterium iliopiscarium ATCC 51760T | AJ842362 | AY455878 | EF380237 | NA |
| Photobacterium kishitanii ATCC BAA-1194T | EF415552 | AY455877 | EF415536 | DQ988874 (luxCDABFEG) |
| Photobacterium leiognathi ATCC 25521T | AJ842364 | AY455879 | EF380238 | M63594 (luxCDABEG) |
| Photobacterium leiognathi lnuch.13.1 | EU118227 | EU118211 | EU118253 | EF536338 (luxCDABEG1) |
| EF536332 (luxCDABEG2) | ||||
| Photobacterium mandapamensis ATCC 27561T | EF415548 | AY455883 | EF415532 | DQ988878 (luxCDABFEG) |
| Photobacterium mandapamensis | EU118221 | EU118202 | EU118237 | EU122285 (luxCDABFEG1) |
| ajapo.4.20 | EU122286 (luxCDABFEG2) | |||
| Photobacterium phosphoreum ATCC 11040T | EF415550 | AY455875 | EF380239 | DQ988873 (luxCDABFEG) |
| Photorhabdus luminescens subsp. laumondii TTO1a | plu1249 | plu0004 | plu0674 | plu2079 to plu2083 (luxCDABE) |
| Shewanella hanedai ATCC 33224T | NA | AF005693 | NA | AB058949 (luxCDABEG) |
| Shewanella woodyi ATCC 51908Ta | SWooDRAFT_4188 | SWooDRAFT_0158 | SWooDRAFT_4084 | SWooDRAFT_2999 to SWooDRAFT_3004 (luxCDABEG) |
| Vibrio aestuarianus ATCC 35048T | AJ842369 | NA | 59b | NA |
| Vibrio alginolyticus ATCC 17749T | AJ842373 | AF007288 | 59 | NA |
| Vibrio anguillarum ATCC 19264T | AJ842375 | NA | 59 | NA |
| Vibrio brasiliensis LMG 20546T | AJ842376 | NA | 59 | NA |
| Vibrio campbellii ATCC 25920T | AJ842377 | AB014950 | EF596641 | NA |
| Vibrio chagasii LMG 21353T | AJ842385 | AJ577820 | EU118252 | NA |
| Vibrio chagasii 21N-12 | EU118217 | EU118198 | EU118233 | EU122293 (luxDABE) |
| Vibrio chagasii SB52 | EU118230 | EU118214 | EU118256 | EU122294 (luxDABE) |
| Vibrio chagasii LMG 13219 | AJ842383 | EU118208 | EU118250 | NA |
| Vibrio chagasii LMG 13237 | AJ842384 | EU118209 | EU118251 | NA |
| Vibrio cholerae ATCC 14035T | AJ842386 | NA | 59 | NA |
| Vibrio cholerae ATCC 14547 | EU118222 | EU118203 | EU118239 | AB115761 (luxCDABEG) |
| Vibrio cholerae manz.1.1 | EU118228 | EU118212 | EU118254 | EU122291 (luxDABE) |
| Vibrio cholerae manz.1.2 | EU118229 | EU118213 | EU118255 | EU122292 (luxDABE) |
| Vibrio cholerae N16961a | VC_0543 | VC_0015 | VC_2258 | NA |
| Vibrio coralliilyticus ATCC BAA-450T | AJ842402 | NA | 59 | NA |
| Vibrio cyclitrophicus ATCC 700982T | AJ842405 | AM162562 | NA | NA |
| Vibrio diabolicus LMG 19805T | AJ842407 | NA | 59 | NA |
| Vibrio diazotrophicus ATCC 33466T | AJ842411 | AB014951 | 59 | NA |
| Vibrio fluvialis ATCC 33809T | AJ842419 | NA | 59 | NA |
| Vibrio fortis LMG 21557T | AJ842422 | NA | 59 | NA |
| Vibrio furnissii ATCC 35016T | AJ842427 | NA | 59 | NA |
| Vibrio harveyi ATCC 14126T | DQ648369 | DQ648280 | EU118238 | EU122288 (luxCDABEG) |
| Vibrio hispanicus LMG 13240T | AJ842445 | NA | 59 | NA |
| Vibrio ichthyoenteri ATCC 700023T | AJ842446 | NA | 59 | NA |
| Vibrio kanaloae LMG 20539T | AJ842450 | AJ577821 | 59 | NA |
| Vibrio lentus ATCC BAA-539T | AJ842452 | AM162564 | 59 | NA |
| Vibrio mediterranei ATCC 43341T | AJ842459 | EF380258 | 59 | NA |
| Vibrio mimicus ATCC 33653T | AJ842468 | EF380259 | EU118242 | NA |
| Vibrio mytili ATCC 51288T | AJ842472 | NA | 59 | NA |
| Vibrio natriegens ATCC 14048T | AJ842473 | NA | 59 | NA |
| Vibrio navarrensis ATCC 51183T | AJ842474 | NA | 59 | NA |
| Vibrio neptunius LMG 20536T | AJ842478 | NA | 59 | NA |
| Vibrio nereis ATCC 25917T | AJ842479 | NA | 59 | NA |
| Vibrio ordalii ATCC 33509T | AJ842482 | NA | 59 | NA |
| Vibrio orientalis ATCC 33934T | AJ842485 | EF380260 | EU118243 | EU122287, AB058948 (luxCDABE) |
| Vibrio pacinii LMG 19999T | AJ842486 | NA | 59 | NA |
| Vibrio parahaemolyticus ATCC 17802T | AJ842490 | AF007287 | EU118240 | NA |
| Vibrio pelagius ATCC 25916T | AJ842495 | AB014954 | 59 | NA |
| Vibrio pomeroyi LMG 20537T | AJ842497 | AJ577822 | 59 | NA |
| Vibrio proteolyticus ATCC 15338T | AJ842499 | NA | 59 | NA |
| Vibrio rotiferianus LMG 21460T | AJ842501 | EU118210 | EF596722 | NA |
| Vibrio shilonii ATCC BAA-91T | AJ842507 | NA | 59 | NA |
| Vibrio splendidus ATCC 33125T | AJ842511 | EF380261 | EU118241 | EF536342 (luxAB) |
| Vibrio tasmaniensis LMG 20012T | AJ842515 | AJ577823 | 59 | NA |
| Vibrio tubiashii ATCC 19109T | AJ842518 | NA | 59 | NA |
| Vibrio vulnificus ATCC 27562T | AJ842523 | AY705491 | 59 | NA |
| Vibrio vulnificus VVL1 (ATCC 43382) | EU118223 | EU118204 | EU118244 | EU122289 (luxCDABEG) |
| Vibrio vulnificus 300 1C1 | EU118218 | EU118200 | EU118235 | NA |
| Vibrio vulnificus SS108C 5C1 | EU118232 | EU118216 | EU118258 | NA |
| Vibrio vulnificus 395R | EU118220 | EU118199 | EU118234 | NA |
| Vibrio vulnificus C71840 | EU118225 | EU118206 | EU118248 | NA |
| Vibrio vulnificus 301 1A1 | EU118219 | EU118201 | EU118236 | NA |
| Vibrio vulnificus ENV1 | EU118226 | EU118207 | EU118249 | NA |
| Vibrio vulnificus SPRC 10143 | EU118231 | EU118215 | EU118257 | NA |
| Vibrio vulnificus CMCP6a | VV1_1591 | VV1_0996 | VV1_1861 | NA |
| Vibrio vulnificus YJ016a | VV2805 | VV0014 | VV2555 | NA |
| Vibrio xuii LMG 21346T | AJ842529 | NA | 59 | NA |
| Vibrio sp. strain BB120a | VIBHAR_03513 | VIBHAR_00445 | VIBHAR_03235 | VIBHAR_06244-06339 |
| Uncultured symbiont of Kryptophanaron alfredi | NA | NA | NA | M36597 (luxAB) |
For A. fischeri ES114, P. luminescens subsp. laumondii TTO1, S. woodyi ATCC 51908T, V. cholerae N16961, V. vulnificus CMCP6, V. vulnificus YJ016, and Vibrio sp. strain BB120, locus tags from genome sequencing projects are given.
Some pyrH sequences were reported previously (59) and can be obtained from the multilocus sequence analysis identification page for Vibrionaceae at http://www.taxvibrio.lncc.br.
The specific lux genes analyzed are listed in parentheses. NA, not available.
Nucleotide sequence accession numbers.
GenBank accession numbers for sequences obtained in this study are EU118198 to EU118258 and EU122285 to EU122294. A complete list of accession numbers for sequences used in this study is given in Table 2.
RESULTS
Predominantly vertical inheritance of the lux genes in Vibrionaceae.
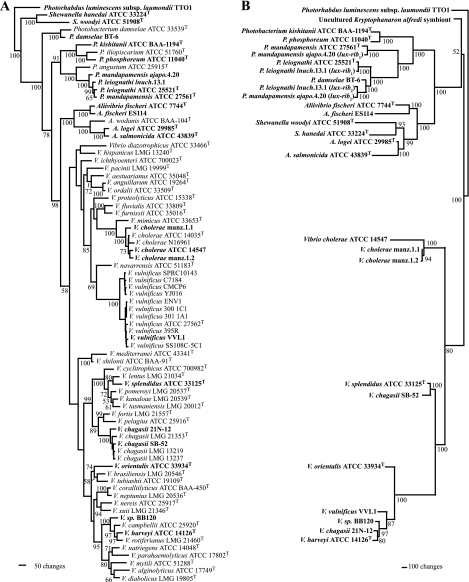
To distinguish between multiple losses and frequent horizontal acquisition as evolutionary scenarios accounting for the scattered incidence of lux genes, we screened luminous bacterial strains for congruence between phylogenies based on gyrB or pyrH, representative housekeeping genes, and luxA, a representative lux gene. Strains exhibiting incongruence were then subjected to detailed phylogenetic analysis based on multiple housekeeping genes and multiple lux genes to more rigorously test and characterize putative horizontal transfer events. Three hundred three luminous strains, representing 11 species of Aliivibrio, Photobacterium, and Vibrio, were examined; these strains were isolated from coastal and open-ocean seawater and skin and intestines of fish from many locations worldwide (Fig. 1). Based on sequence analysis of the gyrB or pyrH gene, most of the strains were identified as members of species previously identified as luminous or as having luminous representatives. For nearly all of the strains, phylogenies based on gyrB or pyrH and luxA were found to be congruent (Fig. 2). Furthermore, no instance in which the lux genes of a luminous species had replaced the lux genes of another luminous species was identified, i.e., lux gene sequences of strains identified using housekeeping genes as belonging to known luminous species were typical of that species. These results indicate that the lux genes are predominantly vertically inherited and are stable in most extant luminous taxa. Therefore, multiple losses instead of frequent acquisition by horizontal transfer most likely account for the scattered incidence of lux genes in Vibrionaceae. Notable in this regard is Vibrio cholerae, a species in which most strains lack the lux genes (16, 20, 49, 53). For luminous strains of V. cholerae, phylogenies based on gyrB and luxA were congruent, and phylogenetic analysis of multiple housekeeping gene sequences did not separate luminous and nonluminous strains (Fig. 2). Therefore, the lux genes apparently have been lost from many strains of this species. Exceptions to the general pattern of phylogenetic congruence were found, however, as described below.
FIG. 2.
Phylogenetic trees of the three housekeeping genes gyrB, recA, and pyrH (A) and the lux operon genes (B); luminescent taxa are shown in bold. The housekeeping gene analysis resulted in 36 equally parsimonious trees. Tree length, 8327; consistency index (CI), 0.247; retention index (RI), 0.675. The jackknife values are shown at the nodes; some values for V. vulnificus, V. cholerae, and V. chagasii clades were omitted for clarity. Parsimony analysis of the lux operon genes sequences resulted in a single tree: tree length, 14311; CI, 0.546; RI, 0.732. Each of the housekeeping genes separately yielded the same relationship of VVL1 to the other V. vulnificus strains (data not shown).
Horizontal acquisition of lux genes by Vibrio vulnificus VVL1.
Vibrio vulnificus VVL1 (ATCC 43382), a human pathogen (48, 55) (Table 1), differs from other strains of V. vulnificus in being bioluminescent, and it is the only luminous member of Vibrionaceae documented to have caused a fatal human infection. To determine if other strains of V. vulnificus might be luminous but cryptically so, 10 strains representing environmental and clinical genotypes of this species (Table 1) (55) were tested for the presence of the lux genes. When grown under conditions in which VVL1 produces a high level of luminescence, none of the additional strains was visibly luminous, and none produced light that could be detected with a sensitive photometer. Furthermore, none yielded a luxA or luxB amplicon when tested with PCR primers effective for amplifying these genes from VVL1 and other luminous bacteria. The genomes of two of these strains, YJ016 and CMCP6, have been fully sequenced (14), and neither contains lux genes. These results establish that VVL1, of the 11 strains of V. vulnificus examined, is unique in producing luminescence and carrying lux genes. Preliminary sequence analysis revealed VVL1 lux genes to be similar in nucleotide and inferred amino acid sequences to Vibrio harveyi lux genes (Table 3). This similarity, the uniqueness of luminescence in VVL1 among examined V. vulnificus strains, and the lack of gyrB-luxA congruence suggested that VVL1 acquired its lux genes by horizontal transfer.
TABLE 3.
Nucleotide and inferred amino acid sequence identities of lux genes
| Genes and recipient strain | Comparison straina | % Identity
|
|
|---|---|---|---|
| Nucleotide | Amino acid | ||
| luxDAB | |||
| Vibrio vulnificus VVL1 | Vibrio harveyi ATCC 14126T | 94.9 | 97.1 |
| Vibrio chagasii 21N-12 | Vibrio harveyi ATCC 14126T | 96.6 | 97.9 |
| Vibrio chagasii SB-52 | Vibrio splendidus ATCC 33125T | 91.5 | 97.5 |
| Photobacterium damselae BT-6 | Photobacterium leiognathi lnuch.13.1 (lux-rib2) | 95.4 | 95.9 |
| Photobacterium mandapamensis ajapo.4.20 | Photobacterium leiognathi lnuch.13.1 (lux-rib2) | 99.3 | 99.4 |
| Shewanella hanedai ATCC 33224T | Aliivibrio fischeri ATCC 7744T | 73.6 | 77.2 |
| Aliivibrio fischeri ES114 | 72.9 | 74.0 | |
| Aliivibrio logei ATCC 29985T | 77.5 | 81.6 | |
| Aliivibrio salmonicida ATCC 43839T | 79.1 | 84.9 | |
| Shewanella woodyi ATCC 51908T | Aliivibrio fischeri ATCC 7744T | 73.3 | 79.7 |
| Aliivibrio fischeri ES114 | 71.1 | 75.7 | |
| Aliivibrio logei ATCC 29985T | 75.9 | 83.0 | |
| Aliivibrio salmonicida ATCC 43839T | 76.9 | 82.9 | |
| luxCDABEb | |||
| Photorhabdus luminescens TTO1 | Aliivibrio fischeri ATCC 7744T | 63.4 | 62.3 (luxDABE) |
| Aliivibrio fischeri ES114 | 63.9 | 61.1 | |
| Aliivibrio logei ATCC 29985T | 64.5 | 62.0 (luxAB) | |
| Aliivibrio salmonicida ATCC 43839T | 65.2 | 64.0 | |
| Photobacterium kishitanii ATCC BAA-1194T | 63.8 | 59.7 | |
| Photobacterium leiognathi ATCC 25521T | 61.4 | 58.8 | |
| Photobacterium mandapamensis ATCC 27561T | 63.4 | 61.3 | |
| Photobacterium phosphoreum ATCC 11040T | 63.9 | 59.3 | |
| Shewanella hanedai ATCC 33224T | 64.6 | 63.2 | |
| Shewanella woodyi ATCC 51908T | 62.5 | 61.6 | |
| Vibrio cholerae ATCC 14547T | 65.2 | 66.8 | |
| Vibrio harveyi ATCC 14126T | 65.0 | 67.4 | |
| Vibrio orientalis ATCC 33934T | 65.4 | 67.5 | |
| Vibrio splendidus ATCC 33125T | 70.4 | 75.4 (luxAB) | |
The listed comparison strains are representative of a species.
Unless noted otherwise in parentheses in final column.
To test this possibility phylogenetically, we reconstructed the evolutionary relationships of VVL1 with other luminous and nonluminous members of Vibrionaceae. A phylogeny based on the housekeeping genes gyrB, recA, and pyrH, analysis of which robustly resolves members of Vibrionaceae (2, 59), was constructed and compared with a phylogeny based on the luxCDABEG genes for luminous species (Fig. 2; Table 2). The analysis of housekeeping genes placed VVL1 within the clade formed by the other V. vulnificus strains, confirming its species identification by other criteria (48). Phylogenetic placement of VVL1 based on the lux genes, however, did not match that based on the housekeeping genes (Fig. 2). The lux genes of VVL1 instead were evolutionarily very closely related to those of V. harveyi, a species that, based on the divergence in sequences of gyrB, recA, and pyrH, is phylogenetically distinct from V. vulnificus. The discordance between housekeeping and lux gene phylogenies supports the hypothesis that VVL1 acquired its lux genes by horizontal transfer, and the data indicate that V. harveyi was the likely donor of those genes. These results are the first demonstration based on phylogenetic criteria of an apparent interspecies horizontal transfer of the lux genes from a luminous to a nonluminous bacterium.
Horizontal acquisition of lux genes in Vibrio chagasii.
Vibrio chagasii is a species for which luminous strains had not previously been reported (60). Luminous strains 21N-12 and SB-52, isolated from seawater off the coast of Mexico and from Suruga Bay, Japan, respectively (Fig. 1; Table 1), were provisionally identified by gyrB sequence analysis as members of V. chagasii. Previously characterized strains of V. chagasii, LMG 21353T, LMG 13219, and LMG 13237, were tested and found not to produce luminescence and to not carry luxA or luxB genes. Preliminary sequence analysis revealed similarity of the lux genes of 21N-12 and SB-52 to lux genes of V. harveyi and Vibrio splendidus, respectively (Table 3). This similarity, the apparently atypical luminescence of 21N-12 and SB-52, and the lack of gyrB-luxA congruence suggested that these strains acquired their lux genes by horizontal transfer. Phylogenetic analysis based on multiple housekeeping genes (gyrB, recA, and pyrH) confirmed the identification of 21N-12 and SB-52 as V. chagasii (Fig. 2), but phylogenetic placement of 21N-12 and SB-52 based on the housekeeping genes did not match that based on the lux genes. Instead, the lux genes of 21N-12 were closely related to those of V. harveyi, and the lux genes of SB-52 were closely related to those of V. splendidus (Fig. 2). These results support the hypothesis that 21N-12 and SB-52 acquired their lux genes horizontally, with V. harveyi and V. splendidus, respectively, as the likely donors.
Horizontal acquisition of lux genes in Photobacterium damselae.
Photobacterium damselae, like V. chagasii, is a species for which luminous strains had not previously been reported (28). A luminous strain, BT-6, isolated from seawater in Okinawa (Fig. 1; Table 1), was provisionally identified by gyrB sequence analysis as P. damselae. Preliminary analysis revealed nucleotide and amino acid sequence similarities of the BT-6 lux genes to lux genes of the lux-rib2 operon of P. leiognathi (3) (Table 3). Phylogenetic analysis based on multiple housekeeping genes, including the 16S rRNA gene, and lux genes confirmed the identification of BT-6 as P. damselae and indicated that the lux-rib2 operon of P. leiognathi was the likely source of the BT-6 lux genes (Fig. 2). This is the first reported instance of a likely transfer of the lux genes of the lux-rib2 operon of P. leiognathi to another species.
Merodiploidy and horizontal acquisition of lux genes in Photobacterium mandapamensis.
Photobacterium mandapamensis is a luminous species that is closely related to P. leiognathi; these two species can be distinguished by molecular phylogenetic criteria, by some phenotypic traits, and by their different host species ranges (1, 33). Strain ajapo.4.20, isolated from the light organ of an acropomatid fish (Table 1), the primary symbiont of which is P. mandapamensis (33), was found here to carry two lux-rib operons. One operon, lux-rib1, was located adjacent to putA, the common and putatively ancestral chromosomal location for lux-rib genes in Photobacterium (3). Sequence analysis of genes of this operon confirmed the identification of this strain as P. mandapamensis. The lux genes of the second lux-rib operon, located elsewhere in the genome, were found however to be closely related to those of the P. leiognathi lux-rib2 operon (Fig. 2). These results provide a third example of natural merodiploidy of the lux-rib operon in luminous bacteria and the second instance of apparent horizontal transfer of the lux genes of the lux-rib2 operon of P. leiognathi to another species. Two other strains of P. mandapamensis, ajapo.4.22 and ajapo.5.37, also were found here to be merodiploid for the lux-rib operon; as with ajapo.4.20, the second lux-rib operons of these strains were closely related to lux-rib2 of P. leiognathi. In a previous report (33), these two strains had been identified incorrectly as P. leiognathi based on phylogenetic analysis of the lux-rib2 operon.
Interfamily horizontal transfer of lux genes.
Similarities in the inferred amino acid sequences of lux genes in members of other families of Gammaproteobacteria, i.e., Shewanella hanedai and Shewanella woodyi (Shewanellaceae) and Photorhabdus species (Enterobacteriaceae) (Table 3), have been interpreted as evidence of horizontal acquisition of lux genes to these bacteria from species of Vibrionaceae (20, 27, 34). To test these proposed instances of interfamily HGT using evolutionary criteria, we compared phylogenies based on housekeeping and lux genes for Shewanella, Photorhabdus, and luminous members of Vibrionaceae. The lux genes of S. hanedai and S. woodyi were found to be closely related to those of members of Aliivibrio (Fig. 2). These results are consistent with horizontal transfer of the lux genes from a member of the Aliivibrio lineage to S. hanedai and S. woodyi. Analysis of the sequences of the luxR and luxI genes of S. hanedai, Allivibrio fischeri, and Allivibrio salmonicida (45), which revealed a close relationship among these genes (data not shown), further supports this conclusion. In contrast, phylogenies based on housekeeping and lux genes for Photorhabdus were congruent (Fig. 2). Whether Photorhabdus acquired lux genes by horizontal transfer therefore remains unresolved. Consistent with this result, nucleotide and amino acid identities of the luxCDABE genes of P. luminescens TTO1 with those of other species of luminous bacteria are relatively low (Table 3).
DISCUSSION
The results of this study indicate that acquisition of the bacterial lux genes by HGT in nature apparently is rare. HGT is thought to occur very commonly in bacteria (29, 39, 40, 46), and luminescence, a distinctive, easily observed phenotype, provides direct access to bacteria from nature that bear functional lux genes. However, despite an extensive sampling of luminous members of Vibrionaceae isolated from a variety of habitats and locations worldwide, we found only four instances consistent with HGT, two in Vibrio, with transfer of the lux genes from luminous to nonluminous species, and two in Photobacterium, with transfer of genes of the lux-rib2 operon of P. leiognathi to other Photobacterium species. No instance in which the lux genes of one luminous species had replaced those of another luminous species was found, so homologous gene replacement may be even more rare than horizontal gene acquisition (11, 47). Phylogenetic analysis also supported a hypothesized interfamily transfer of the lux genes from members of a third Vibrionaceae genus, Aliivibrio, to species of Shewanella (Shewanellaceae) but did not confirm a postulated horizontal acquisition of lux genes by Photorhabdus (Enterobacteriaceae). Strains that had acquired lux genes horizontally in all cases were otherwise phylogenetically indistinguishable from nonluminous strains of that species; thus, none of the identified instances of apparent horizontal acquisition of lux genes has led to evolutionary divergence of the recipient lineage. These results indicate that the bacterial lux genes are transferred primarily by vertical inheritance and that the rare instances of apparent horizontal acquisition, although contributing to the pan-genome of recipient species, have not played an obvious role in their speciation. If the same is true for other genes, HGT may be uncommon in and have no major impact on the evolution of Vibrionaceae.
Previously proposed instances of lux gene HGT (20, 27, 34) were based primarily on inferred amino acid sequence similarity comparisons. Such comparisons, which identify the best similarity matches for genes from different organisms, provide a rapid screen for possible HGT events (Table 3), but they are not reliable for evolutionary inference (5, 23). We therefore used phylogenetic reconstruction, i.e., character state methods, to more rigorously test and characterize putative horizontal transfer events. Incongruent housekeeping gene and lux gene phylogenetic trees provide strong evidence that the bacterial lux genes have been acquired horizontally in the few cases described here. Although events other than HGT can give rise to incongruent trees (5), they are unlikely to account for the instances of apparent lux HGT described here.
A possible criticism of our results is that analysis of lux genes underestimates the occurrence of HGT, because the lux genes may be subject to special selection. A strong counterpoint to this view, however, is that the bacterial lux genes do not appear to have evolved differently from housekeeping genes. There is no evidence for a separate evolutionary history for the lux genes, other than their apparent loss from many species and strains of Vibrionaceae. Sequence divergence of the lux genes, individually and collectively, resolves the same evolutionary relationships as housekeeping genes, although sometimes with a better delimitation of closely related species and clades (1, 2, 4, 33; also this study). Therefore, the bacterial lux genes do not appear to be subject to special selection or to have undergone genetic drift different from that experienced by core housekeeping genes. We nonetheless cannot exclude the possibility that horizontal transfer of other genes in Vibrionaceae is more common than that seen for the lux genes.
In this regard, horizontal acquisition of lux genes may be more common than the four instances identified here. Detection of bacteria bearing the lux genes was based on visual screening for luminescence, a phenotype that requires the presence and a high level of expression of the luxCDABEG genes. Several classes of lux HGT events that would not be detected by this screening are possible. Bacteria that acquired only some of the lux genes, e.g., luxCDA or luxAB, or those in which the luxCDABEG genes had mutations would be dark or would produce too low a level of luminescence to be detected visually, as would those strains unable to transcribe the lux genes at a high level in culture, such as those lacking necessary regulatory genes or regulatory factors, and those in which the activities of the Lux proteins do not lead to high levels of luminescence in laboratory culture (12, 26, 49, 53). PCR and hybridization screening methods therefore may reveal additional instances of lux horizontal transfer, as presumably will whole-genome sequence analysis of members of Vibrionaceae and other bacterial families. Regardless, the work reported here establishes with evolutionary criteria that horizontal transfer of the lux genes apparently has occurred but that the transfer of functional lux genes is rare.
Many factors likely influence and possibly limit the horizontal acquisition of lux genes and their stable, functional retention by nonluminous bacteria. The examples reported here suggest that HGT may be more common among closely related bacteria. Other factors include the availability of lux DNA in the recipient's environment, the susceptibility of acquired lux DNA to degradation by restriction endonucleases of the recipient cell, constraints on integration of lux DNA into the recipient genome, and the extent of physiological compatibility of the luminescence proteins in the recipient cell (11, 37, 56). Once acquired, stable retention of the lux genes in a functional state might be curtailed in the absence of strong positive selection for the activities of the luxCDABEG gene products by the accumulation of mutations, by excision, and by reduced fitness of progeny of the recipient cell (11, 37). Even if levels of lux DNA in the recipient's environment are high, this multistep winnowing process could sharply limit the incidence of lux HGT. Furthermore, given the costs associated with replication and expression of the bacterial lux genes (19) and given that the metabolic and physiological benefits of the luminescence system for bacteria are not well understood (20), it is difficult to envision the selective pressures that would lead to the fixation of lux genes in progeny of recipient cells and the subsequent spread of these genes from isolated individuals and patchy populations to the global population of a recipient species (11). We therefore speculate that the examples of apparent lux HGT shown here are the rare stable instances of an otherwise highly transient process of infrequent lux gene acquisition that typically is followed by rapid loss.
A possible exception to this “infrequent acquisition-rapid loss” scenario is the situation with the lux-rib2 operon of P. leiognathi. In at least one P. leiognathi strain, the lux-rib2 operon is flanked by putative transposase genes, suggesting mobility of lux-rib2 via transposon-mediated transfer (3). Here, we identified a strain of P. damselae, a nonluminous bacterium, and strains of P. mandapamensis, a luminous species, that bear the P. leiognathi lux-rib2 genes. The implication of these instances of apparent lux HGT is that the lux-rib2 genes might be highly mobile and therefore might be transferred frequently to nonluminous and other luminous bacteria. Furthermore, at least in P. leiognathi, the merodiploid state appears to be stable over ecological time (3).
In contrast to the situation for other bacteria examined here, the origin of lux genes in Photorhabdus remains obscure. The conserved gene order, luxCDABE, and inferred amino acid sequence similarities of the luxA and luxB genes with those of V. harveyi and other bacteria suggest horizontal acquisition of the lux genes from a member of Vibrionaceae (27). Because the chromosomal locations of lux genes in ecologically distinct strains of P. luminescens apparently differ (43), horizontal transfer might have occurred more than once (27), although genomic rearrangements also could account for the apparently different chromosomal locations. A recent report, based on similarity analysis of inferred amino acid sequences, presents a more complicated scenario, in which the luxCDABE genes were transferred from a gram-positive bacterium to V. cholerae and then later were transferred from V. cholerae to P. luminescens (34). The lack of evolutionary analysis, the invoking of an unknown gram-positive bacterium as the source of the P. luminescens lux genes, and the multiple steps involved weaken the possible validity of this scenario. Furthermore, we demonstrate here that lux and housekeeping gene phylogenies for P. luminescens are congruent (Fig. 2). A more likely scenario, therefore, is that the lux genes were acquired by Photorhabdus from a member of Vibrionaceae in the distant past, followed by substantial sequence divergence of the Photorhabdus lux genes. This scenario is consistent with the generally low lux nucleotide and amino acid sequence similarities of Photorhabdus and Aliivibrio, Photobacterium, and Vibrio species (Table 3) and with the observed congruence of lux and housekeeping gene phylogenies.
Supplementary Material
Acknowledgments
We thank Claudine Vereecke (BCCM/LMG Bacteria Collection) for providing the LMG strains of V. chagasii, Thomas Rosche for assistance with strains of V. vulnificus, and Elizabeth Warner for typing strain VVL1. The following individuals facilitated seawater sampling or provided seawater samples: Holger Jannasch (deceased) and captain and crew of the R/V Atlantis II, Celia Lavilla-Torres, Seishi Kimura, Tetsuo Yoshino, Atsushi Fukui, Prosanta Chakrabarty, John Sparks, Julian Adams, Heather Adams, and Karen Beeri. DNA sequencing was carried out by the staff of the University of Michigan Sequencing Core.
This work was supported by grant DEB 0413441 from the National Science Foundation.
Footnotes
Published ahead of print on 21 March 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ast, J. C., and P. V. Dunlap. 2004. Phylogenetic analysis of the lux operon distinguishes two evolutionarily distinct clades of Photobacterium leiognathi. Arch. Microbiol. 181352-361. [DOI] [PubMed] [Google Scholar]
- 2.Ast, J. C., and P. V. Dunlap. 2005. Phylogenetic resolution and habitat specificity of members of the Photobacterium phosphoreum species group. Environ. Microbiol. 71641-1654. [DOI] [PubMed] [Google Scholar]
- 3.Ast, J. C., H. Urbanczyk, and P. V. Dunlap. 2007. Natural merodiploidy of the lux-rib operon of Photobacterium leiognathi from coastal waters of Honshu, Japan. J. Bacteriol. 1896148-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ast, J. C., I. Cleenwerck, K. Engelbeen, H. Urbanczyk, F. L. Thompson, P. De Vos, and P. V. Dunlap. 2007. Photobacterium kishitanii sp. nov., a luminous marine bacterium symbiotic with deep-sea fishes. Int. J. Syst. Evol. Microbiol. 572073-2078. [DOI] [PubMed] [Google Scholar]
- 5.Avise, J. C. 2004. Molecular markers, natural history, and evolution, 2nd ed. Sinauer Associates, Sunderland, MA.
- 6.Bapteste, E., Y. Boucher, J. Leigh, and W. F. Doolittle. 2004. Phylogenetic reconstruction and lateral gene transfer. Trends Microbiol. 12406-411. [DOI] [PubMed] [Google Scholar]
- 7.Baumann, P., A. L. Furniss, and J. V. Lee. 1984. Genus Vibrio Pacini 1854, p. 518-538. In N. R. Kreig and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 8.Baumann, P., L. Baumann, and J. L. Reichelt. 1973. Taxonomy of marine bacteria: Beneckea parahaemolytica and Beneckea alginolytica. J. Bacteriol. 1131144-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baumann, P., L. Baumann, and M. Mandell. 1971. Taxonomy of marine bacteria: the genus Beneckea. J. Bacteriol. 107268-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumann, P., L. Baumann, S. S. Bang, and M. J. Woolkalis. 1980. Reevaluation of the taxonomy of Vibrio, Beneckea, and Photobacterium: abolition of the genus Beneckea. Curr. Microbiol. 4127-132. [Google Scholar]
- 11.Berg, O. G., and C. G. Kurland. 2002. Evolution of microbial genomes: sequence acquisition and loss. Mol. Biol. Evol. 192265-2272. [DOI] [PubMed] [Google Scholar]
- 12.Boettcher, K. J., and E. G. Ruby. 1990. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 173701-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatzidaki-Livanis, M., M. A. Hubbard, K. Gordon, V. J. Harwood, and A. C. Wright. 2006. Genetic distinctions among clinical and environmental strains of Vibrio vulnificus. Appl. Environ. Microbiol. 726136-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, C. Y., K. M. Wu, Y. C. Chang, C. H. Chang, H. C. Tsai, T. L. Liao, Y. M. Liu, H. J. Chen, A. B. Shen, J. C. Li, T. L. Su, C. P. Shao, C. T. Lee, L. I. Hor, and S. F. Tsai. 2003. Comparative genome analysis of Vibrio vulnificus, a marine pathogen. Genome Res. 132577-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daubin, V., N. A. Moran, and H. Ochman. 2003. Phylogenetics and the cohesion of bacterial genomes. Science 301829-832. [DOI] [PubMed] [Google Scholar]
- 16.Desmarchieler, P. M., and J. L. Reichelt. 1981. Phenotypic characterization of clinical and environmental isolates of Vibrio cholerae from Australia. Curr. Microbiol. 5123-127. [Google Scholar]
- 17.Doolittle, W. F. 1999. Phylogenetic classification and the universal tree. Science 2842124-2128. [DOI] [PubMed] [Google Scholar]
- 18.Duchaud, E., C. Rusniok, L. Frangeul, C. Buchrieser, A. Givaudan, S. Taourit, S. Bocs, C. Boursaux-Eude, M. Chandler, J. F. Charles, E. Dassa, R. Derose, S. Derzelle, G. Freyssinet, S. Gaudriault, C. Medigue, A. Lanois, K. Powell, P. Siguier, R. Vincent, V. Wingate, M. Zouine, P. Glaser, N. Boemare, A. Danchin, and F. Kunst. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 211307-1313. [DOI] [PubMed] [Google Scholar]
- 19.Dunlap, P. V., and E. P. Greenberg. 1991. Role of intercellular chemical communication in the Vibrio fischeri-monocentrid fish symbiosis, p. 219-253. In M. Dworkin (ed.), Microbial cell-cell interactions. American Society for Microbiology, Washington, DC.
- 20.Dunlap, P. V., and K. Kita-Tsukamoto. 2006. Luminous bacteria, p. 863-892. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, a handbook on the biology of bacteria, 3rd ed., vol. 2. Ecophysiology and biochemistry. Springer, New York, NY. [Google Scholar]
- 21.Dunlap, P. V., A. Jiemjit, J. C. Ast, M. M. Pearce, R. R. Marques, and C. R. Lavilla-Pitogo. 2004. Genomic polymorphism in symbiotic populations of Photobacterium leiognathi. Environ. Microbiol. 6145-158. [DOI] [PubMed] [Google Scholar]
- 22.Egidius, E., R. Wiik, K. Andersen, K. A. Hoff, and B. Hjeltnes. 1986. Vibrio salmonicida sp. nov., a new fish pathogen. Int. J. Syst. Bacteriol. 36518-520. [Google Scholar]
- 23.Eisen, J. A. 2000. Horizontal gene transfer among microbial genomes: new insights from complete genome analysis. Curr. Opin. Genet. Dev. 10606-611. [DOI] [PubMed] [Google Scholar]
- 24.Eisen, J. A., and C. M. Fraser. 2003. Phylogenomics: intersection of evolution and genomics. Science 3001706-1707. [DOI] [PubMed] [Google Scholar]
- 25.Farris, J. S., V. A. Albert, M. Källersjö, D. Lipscomb, and A. G. Kluge. 1996. Parsimony jackknifing outperforms neighbor-joining. Cladistics 1299-124. [DOI] [PubMed] [Google Scholar]
- 26.Fidopiastis, P. M., H. Sorum, and E. G. Ruby. 1999. Cryptic luminescence in the cold-water fish pathogen Vibrio salmonicida. Arch. Microbiol. 171205-209. [DOI] [PubMed] [Google Scholar]
- 27.Forst, S., B. Dowds, N. Boemare, and E. Stackebrandt. 1997. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 5147-72. [DOI] [PubMed] [Google Scholar]
- 28.Gauthier, G., B. Lafay, R. Ruimy, V. Breittmayer, J. L. Nicolas, M. Gauthier, and R. Christen. 1995. Small-subunit rRNA sequences and whole DNA relatedness concur for the reassignment of Pasteurella piscicida (Snieszko et al.) Janssen and Surgalla to the genus Photobacterium as Photobacterium damsela subsp. piscicida. Int. J. Syst. Bacteriol. 45139-144. [DOI] [PubMed] [Google Scholar]
- 29.Gogarten, J. P., W. F. Doolittle, and J. G. Lawrence. 2002. Prokaryotic evolution in light of gene transfer. Mol. Biol. Evol. 192226-2238. [DOI] [PubMed] [Google Scholar]
- 30.Goloboff, P. A., J. S. Farris, and K. C. Nixon. 10 August 2006, revision date. TNT: tree analysis using new technology, version 1.0. http://www.zmuc.dk/public/phylogeny/TNT/.
- 31.Gomez-Gil, B., F. L. Thompson, C. C. Thompson, and J. Swings. 2003. Vibrio rotiferianus sp. nov., isolated from cultures of the rotifer Brachionus plicatilis. Int. J. Syst. Microbiol. 53239-243. [DOI] [PubMed] [Google Scholar]
- 32.Hendrie, M. S., W. Hodgkiss, and J. M. Shewan. 1970. The identification, taxonomy and classification of luminous bacteria. J. Gen. Microbiol. 64151-169. [Google Scholar]
- 33.Kaeding, A. J., J. C. Ast, M. M. Pearce, H. Urbanczyk, S. Kimura, H. Endo, M. Nakamura, and P. V. Dunlap. 2007. Phylogenetic diversity and cosymbiosis in the bioluminescent symbioses of “Photobacterium mandapamensis”. Appl. Environ. Microbiol. 733173-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasai, S., K. Okada, A. Hoshino, T. Iida, and T. Honda. 2007. Horizontal transfer of the lux gene cluster. J. Biochem. 141231-237. [DOI] [PubMed] [Google Scholar]
- 35.Kim, Y. R., S. E. Lee, C. M. Kim, S. Y. Kim, E. K. Shin, D. H. Shin, S. S. Chung, H. E. Choy, A. Progulske-Fox, J. D. Hillman, M. Handfield, and J. H. Rhee. 2003. Characterization and pathogenic significance of Vibrio vulnificus antigens preferentially expressed in septicemic patients. Infect. Immun. 715461-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurland, C. G. 2000. Something for everyone: horizontal gene transfer in evolution. EMBO Rep. 192-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kurland, C. G. 2005. What tangled web: barriers to rampant horizontal gene transfer. Bioessays 27741-747. [DOI] [PubMed] [Google Scholar]
- 38.Kurland, C. G., B. Canback, and O. G. Berg. 2003. Horizontal gene transfer: a critical view. Proc. Natl. Acad. Sci. USA 1009658-9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawrence, J. G. 2001. Catalyzing bacterial speciation: correlating lateral transfer with genetic headroom. Syst. Biol. 50479-496. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence, J. G., and H. Hendrickson. 2003. Lateral gene transfer: when will adolescence end? Mol. Microbiol. 50739-749. [DOI] [PubMed] [Google Scholar]
- 41.Lerat, E., V. Daubin, H. Ochman, and N. A. Moran. 2005. Evolutionary origins of genomic repertoires in bacteria. PLoS Biol. 3e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lunder, T., H. Sørum, G. Holstad, A. G. Steigerwalt, P. Mowinckel, and D. J. Brenner. 2000. Phenotypic and genotypic characterization of Vibrio viscosus sp. nov. and Vibrio wodanis sp. nov. isolated from Atlantic salmon (Salmo salar) with ‘winter ulcer’. Int. J. Syst. Evol. Microbiol. 50427-450. [DOI] [PubMed] [Google Scholar]
- 43.Meighen, E. A., and R. B. Szittner. 1992. Multiple repetitive elements and organization of the lux operons of luminescent terrestrial bacteria. J. Bacteriol. 1745371-5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nealson, K. H. 1978. Isolation, identification and manipulation of luminous bacteria. Methods Enzymol. 57153-166. [Google Scholar]
- 45.Nelson, E. J., H. S. Tunsjø, P. M. Fidopiastis, H. Sørum, and E. G. Ruby. 2007. A novel lux operon in the cryptically bioluminescent fish pathogen Vibrio salmonicida is associated with virulence. Appl. Environ. Microbiol. 731825-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ochman, H., J. G. Lawrence, and E. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405299-304. [DOI] [PubMed] [Google Scholar]
- 47.Ochman, H., E. Lerat, and V. Daubin. 2005. Examining bacterial species under the specter of gene transfer and exchange. Proc. Natl. Acad. Sci. USA 1026595-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oliver, J. D., D. M. Roberts, V. K. White, M. A. Dry, and L. M. Simpson. 1986. Bioluminescence in a strain of the human pathogenic bacterium Vibrio vulnificus. Appl. Environ. Microbiol. 521209-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palmer, L. M., and R. R. Colwell. 1991. Detection of luciferase gene sequence in nonluminescent Vibrio cholerae by colony hybridization and polymerase chain reaction. Appl. Environ. Microbiol. 571286-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paulsen, I. T., L. Banerjei, G. S. Myers, K. E. Nelson, R. Seshadri, T. D. Read, D. E. Fouts, J. A. Eisen, S. R. Gill, J. F. Heidelberg, H. Tettelin, R. J. Dodson, L. Umayam, L. Brinkac, M. Beanan, S. Daugherty, R. T. DeBoy, S. Durkin, J. Kolonay, R. Madupu, W. Nelson, J. Vamathevan, B. Tran, J. Upton, T. Hansen, J. Shetty, H. Khouri, T. Utterback, D. Radune, K. A. Ketchum, B. A. Dougherty, and C. M. Fraser. 2003. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 2992071-2074. [DOI] [PubMed] [Google Scholar]
- 51.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409529-533. [DOI] [PubMed] [Google Scholar]
- 52.Poole, M. D., and J. D. Oliver. 1978. Experimental pathogenicity and mortality in ligated ileal loop studies of the newly reported halophilic lactose-positive Vibrio species. Infect. Immun. 20126-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramaiah, N., J. Chun, J. Ravel, W. L. Straube, R. T. Hill, and R. R. Colwell. 2000. Detection of luciferase gene sequence in nonluminescent bacteria from Chesapeake Bay. FEMS Microbiol. Ecol. 3327-34. [DOI] [PubMed] [Google Scholar]
- 54.Reichelt, J. L., P. Baumann, and L. Baumann. 1976. Study of genetic relationships among marine species of the genera Beneckea and Photobacterium by means of in vitro DNA/DNA hybridization. Arch. Microbiol. 110101-120. [DOI] [PubMed] [Google Scholar]
- 55.Rosche, T. M., Y. Yano, and J. D. Oliver. 2005. A rapid and simple PCR analysis indicates there are two subgroups of Vibrio vulnificus which correlate with clinical or environmental isolation. Microbiol. Immunol. 49381-389. [DOI] [PubMed] [Google Scholar]
- 56.Sorek, R., Y. Zhu, C. J. Creevey, M. P. Francino, P. Bork, and E. M. Rubin. 2007. Genome-wide experimental determination of barriers to horizontal gene transfer. Science 3181449-1452. [DOI] [PubMed] [Google Scholar]
- 57.Swofford, D. L. 2003. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0B10. Sinauer Associates, Sunderland, MA.
- 58.Tettelin, H., V. Masignani, M. J. Cieslewicz, C. Donati, D. Medini, N. L. Ward, S. V. Angiuoli, J. Crabtree, A. L. Jones, A. S. Durkin, R. T. Deboy, T. M. Davidsen, M. Mora, M. Scarselli, I. Margarit y Ros, J. D. Peterson, C. R. Hauser, J. P. Sundaram, W. C. Nelson, R. Madupu, L. M. Brinkac, R. J. Dodson, M. J. Rosovitz, S. A. Sullivan, S. Daugherty, D. H. Haft, J. Selengut, M. L. Gwinn, L. Zhou, N. Zafar, H. Khouri, D. Radune, G. Dimitrov, K. Watkins, K. J. O'Connor, S. Smith, T. R. Utterback, O. White, C. E. Rubens, G. Grandi, L. C. Madoff, D. L. Kasper, J. L. Telford, M. R. Wessels, R. Rappuoli, and C. M. Fraser. 2005. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome”. Proc. Natl. Acad. Sci. USA 10213950-13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thompson, F. L., D. Gevers, C. C. Thompson, P. Dawyndt, S. Naser, B. Hoste, C. B. Munn, and J. Swings. 2005. Phylogeny and molecular identification of vibrios on the basis of multilocus sequence analysis. Appl. Environ. Microbiol. 715107-5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson, F. L., C. C. Thompson, Y. Li, B. Gomez-Gil, J. Vandenberghe, B. Hoste, and J. Swings. 2003. Vibrio kanaloae sp. nov., Vibrio pomeroyi sp. nov. and Vibrio chagasii sp. nov., from sea water and marine animals. Int. J. Syst. Evol. Microbiol. 53753-759. [DOI] [PubMed] [Google Scholar]
- 61.Urbanczyk, H., J. C. Ast, M. J. Higgins, J. Carson, and P. V. Dunlap. 2007. Reclassification of Vibrio fischeri, Vibrio logei, Vibrio salmonicida and Vibrio wodanis as Aliivibrio fischeri gen. nov., comb. nov., Aliivibrio logei comb. nov., Aliivibrio salmonicida comb. nov., and Aliivibrio wodanis comb. nov. Int. J. Syst. Evol. Microbiol. 572823-2829. [DOI] [PubMed] [Google Scholar]
- 62.Yang, Y., L. P. Yeh, Y. Cao, L. Baumann, P. Baumann, J. S. Tang, and B. Beaman. 1983. Characterization of marine luminous bacteria isolated off the coast of China and description of Vibrio orientalis sp. nov. Curr. Microbiol. 895-100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.