Abstract
Ehrlichia chaffeensis, an obligatory intracellular gram-negative bacterium, must take up various nutrients and metabolic compounds because it lacks many genes involved in metabolism. Nutrient uptake by a gram-negative bacterium occurs primarily through pores or channels in the bacterial outer membrane. Here we demonstrate that isolated E. chaffeensis outer membranes have porin activities, as determined by a proteoliposome swelling assay. The activity was partially blocked by an antibody that recognizes the two most abundant outer membrane proteins, P28/OMP-19 and OMP-1F/OMP-18. Both proteins were predicted to have structural features characteristic of porins, including 12 transmembrane segments comprised of amphipathic and antiparallel β-strands. The sodium dodecyl sulfate stability of the two proteins was consistent with a β-barrel structure. Isolated native P28 and OMP-1F exhibited porin activities, with pore sizes similar to and larger than, respectively, that of OprF, which is the porin with the largest pore size known to date. E. chaffeensis experiences temperature changes during transmission by ticks. During the intracellular development of E. chaffeensis, both P28 and OMP-1F were expressed mostly in the mid-exponential growth phase at 37°C and the late-exponential growth phase at 28°C. The porin activity of proteoliposomes reconstituted with proteins from the outer membrane fractions derived from bacteria in the mid- and late-exponential growth phases at 28°C and 37°C correlated with the expression levels of P28 and OMP-1F. These results imply that P28 and OMP-1F function as porins with large pore sizes, suggesting that the differential expression of these two proteins might regulate nutrient uptake during intracellular E. chaffeensis development at both temperatures.
Ehrlichia chaffeensis, a pathogen that infects monocytes and macrophages, belongs to the order Rickettsiales, which is comprised of obligatory intracellular gram-negative bacteria. E. chaffeensis is transmitted to humans via a bite from its infected tick vector, Amblyomma americanum, and causes human monocytic ehrlichiosis (HME), an emerging and potentially fatal infectious disease that has been reported primarily in the United States and occasionally in other parts of the world (4, 17).
All members of Rickettsiales have limited biosynthetic capabilities due to the loss of genes required by free-living bacteria during reductive genome evolution (6). These bacteria, therefore, cannot survive extracellularly and are obliged to import most nutrients and metabolic products from their host cells. In order for small hydrophilic compounds, such as sugars, amino acids, or ions to pass through, the outer membrane of gram-negative bacteria have β-barrel proteins called porins that function as passive diffusion channels (12). To date, the only porin that has been identified in Rickettsiales is the major outer membrane protein of Anaplasma phagocytophilum, P44 (7). Identification of the analogous porin in E. chaffeensis can potentially be important in understanding the physiology of this pathogen.
The immunodominant P28/OMP-1 family of proteins are the most abundant outer membrane proteins in E. chaffeensis (16, 31). These proteins are encoded by a polymorphic 22-gene family (15). P28/OMP-1 paralogs are expressed in HME patients (15, 16, 26), dogs experimentally infected with E. chaffeensis (31), and the infected tick cell lines Ixodes scapularis ISE6 and A. americanum AAE2 (23). In fact, more diverse sets of E. chaffeensis P28/OMP-1 paralogs are expressed in infected dogs than in ticks at the transcriptional level (27) and more are expressed in the DH82 canine histiocyte cell line than in cultured tick cells at the protein level (23). Furthermore, immunization with recombinant P28 protects BALB/c mice from infection with E. chaffeensis (16). A monoclonal antibody against OMP-1g (P28/OMP-19) mediates the protection of SCID mice from fatal E. chaffeensis infection (11). However, the functions of P28/OMP-1 family proteins in the bacteria are unknown.
In the present study, we first examined an isolated outer membrane fraction of E. chaffeensis for porin activity by using an in vitro proteoliposome swelling assay. Second, as porins are generally major outer membrane proteins (12), we tested whether the two most abundant E. chaffeensis outer membrane proteins, P28/OMP-19 and OMP-1F/OMP-18, have the structural and physicochemical properties of porins and whether isolated native P28 and OMP-1F proteins have porin activities.
The developmental cycle of E. chaffeensis in DH82 cells cultured at 37°C (32) consists of two forms: small dense-cored cells (DCs), with cell binding activities and the ability to enter host cells, and larger reticulate cells (RCs) that are differentiated from DCs. RCs mature again into DCs, which are released upon host cell lysis. We further examined the temporal expressions of P28 and OMP-1F and the porin activities of outer membranes derived from different stages of E. chaffeensis intracellular development at 37°C by using synchronous cultures of E. chaffeensis in the human myelocytic cell line THP-1. The developmental stages we used were newly differentiated RCs and DCs. p30-10, an outer membrane protein gene of Ehrlichia canis, which was the only member of the p30 family detected in Rhipicephalus sanguineus ticks, was up-regulated when this bacterium was cultured at 25°C in DH82 cells (25). This result indicates that the expression of P28/OMP-1/P30 can be regulated by temperature, and as bacteria experience temperature changes during tick transmission, we investigated whether temperature influences P28 and OMP-1F temporal expression and porin activities in E. chaffeensis.
MATERIALS AND METHODS
Bacterial strains and culture.
Escherichia coli strains NovaBlue (Novagen, Madison, WI) and BL21(DE3) (Novagen) were cultured in Luria-Bertani broth (21) supplemented with 50 μg/ml kanamycin when required. E. chaffeensis Arkansas was propagated in human myelocytic THP-1 cells (ATCC, Manassas, VA) in RPMI-1640 medium supplemented with 10% fetal bovine serum and 2 mM l-glutamine at 37°C in 5% CO2 and 95% air. For nonsynchronous E. chaffensis culture, heavily infected (80 to 100% infected cells) THP-1 cells were mixed with uninfected THP-1 cells at a 1:10 to 1:5 ratio and incubated until 80 to 100% cells were infected. To synchronize the growth stages, host cell-free E. chaffeensis was isolated from highly infected THP-1 cells at 37°C (almost 100% of the cells were infected) by the sonication of harvested infected cells (twice for 5 s by using a W-380 sonicator at setting 2 [Heat Systems, Farmingdale, NY]) and centrifugation at 400 × g for 5 min without braking, followed by the filtration of the resulting supernatant through a 2.7-μm glass fiber membrane filter (Millipore, Billerica, MA). Bacteria were harvested from the filtrate by centrifugation at 10,000 × g for 10 min and added to fresh THP-1 cells. The cell culture was maintained at 37°C for 42 h until very small (<1-μm-diameter) morulae (microcolonies of bacteria) were detected in more than 80% of the THP-1 cells. Then half of the culture volume was transferred to 28°C, and the bacterial growth stage was monitored every 6 to 12 h by Diff-Quik staining (Baxter Scientific Products, Obetz, OH). The cells were harvested at the early exponential growth phase (∼1-μm-diameter morulae were detected in more than 80% of the THP-1 cells), the mid-exponential growth phase (1- to 3-μm-diameter morulae were detected), and the late exponential growth phase (>3-μm-diameter morulae were detected).
Cloning of E. chaffeensis p28 and omp-1F and expression of recombinant P28 (rP28) and recombinant OMP-1F (rOMP-1F) in E. coli.
Total DNA from THP-1 cells infected with E. chaffeensis was isolated with the QIAamp DNA blood mini kit (Qiagen, Valencia, CA). The DNA fragments encoding putative mature P28 and OMP-1F were amplified by PCR using the following primers: a combination of p28F (GCGGGATCCGGACCCAGCAGGTAGTGGTATT [which anneals nucleotides 76 to 96 of p28]) and p28R (CTTGCGGCCGCGAAAGCAAACCTTCCTCCAAG [which anneals nucleotides 823 to 843 of p28]) and a combination of omp-1FF (GCGGGATCCGGATGCAGTACAGAACGACAATGT [which anneals nucleotides 76 to 97 of omp-1F]) and omp-1FR (CTTGCGGCCGCAAAGTTAAACCTTCCTCCAAGTTC [which anneals nucleotides 817 to 840 of omp-1F]), respectively (BamHI/NotI sites are underlined). The cloned fragments encode the mature (without signal peptide) protein of P28, as determined by Edman degradation (16), and putative mature protein of OMP-1F, as predicted by the SignalP 3.0 program (www.cbs.dtu.dk/services/SignalP) (5). The PCR fragments were digested with BamHI/NotI and ligated into BamHI/NotI-digested pET33b (Novagen). Plasmids containing inserts of interest were selected by using E. coli strain NovaBlue, inserts were confirmed by DNA sequencing, and protein expression was induced in BL21(DE3), as described previously (3). The resulting plasmids were designated pET33b-p28 and pET33b-omp-1F.
Determination of bacterial numbers and Western blotting.
Total DNA was isolated from infected THP-1 cells at each stage of culture by using the QIAamp DNA blood mini kit, and the bacterial numbers in each culture were determined by quantifying the copy number of the 16S rRNA gene (found as a single copy in the E. chaffeensis genome) in each sample through quantitative PCR using the Brilliant Sybr green QPCR core reagent kit (Stratagene, Cedar Creek, TX) in a quantitative PCR instrument, Mx3000P (Stratagene), as described previously (3). Each DNA sample was assayed in triplicate. To compare protein amounts by Western blotting, protein levels in samples applied to sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis gels were normalized to bacterial numbers as determined by the quantitative PCR. After electrophoresis, proteins were transferred to a nitrocellulose membrane that was incubated with rabbit anti-rP28 antiserum at 1:500 dilutions at room temperature for 1 h (16) or with mouse monoclonal antibody Ec 56.5 (11) at 1:100 dilutions at room temperature for 1 h (kindly provided by G. M. Winslow at the Wadsworth Center, New York State Department of Health, Albany, NY), followed by horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G antibody (KPL, Gaithersburg, MD) or horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G antibody (KPL) at 1:1,000 dilutions at room temperature for 1 h. Bands were visualized by incubation of the membrane with ECL Western blotting detection reagents (Pierce, Rockford, IL). Images were captured with a LAS-3000 luminescent image analyzer (Fujifilm, Tokyo, Japan). Band intensity was quantified by using ImageGauge software (Fujifilm).
Preparation of the outer membrane fraction and solubilization of native outer membrane proteins.
Nonsynchronously or synchronously cultured E. chaffeensis was isolated from 3 × 107 to 6 × 107 infected THP-1 cells (80 to 100% infected cells), 0.1% Sarkosyl-insoluble outer membrane fraction was prepared, and outer membrane proteins were solubilized with 2% (wt/vol) n-octylglucoside (OGC), as described previously (7). Protein amounts were determined by the bicinchoninic acid protein assay (Pierce).
Isolation of native P28 and OMP-1F from the E. chaffeensis outer membrane fraction.
The outer membrane fraction prepared from 20 mg of isolated bacteria was suspended in 3 ml of 1% dithiothreitol in 10 mM Tris-HCl (pH 7.5) and incubated for 10 min at room temperature. Three milliliters of 3% iodoacetamide in 10 mM Tris-HCl (pH 7.5) was added to the suspension and incubated for an additional 10 min at room temperature. After centrifugation to pellet the outer membrane fraction at 18,000 × g for 10 min, the pellet was washed once with 10 mM Bis-Tris-HCl (pH 6.0) and then pelleted again. Proteins were solubilized in 1 ml of 2% OGC in 20 mM Bis-Tris-HCl (pH 6.0), 10 mM NaCl. The sample was centrifuged at 18,000 × g for 10 min, and the resulting supernatant containing solubilized outer membrane proteins was loaded onto a DEAE-Sepharose column (bed volume, 2 ml) equilibrated with 1% OGC in 20 mM Bis-Tris-HCl, pH 6.0, 10 mM NaCl. After the column was washed with the same buffer, proteins were eluted with a linear gradient from 10 mM to 0.5 M NaCl in 1% OGC, 20 mM Bis-Tris-HCl (pH 6.0). Each fraction (0.5 ml) was examined by Western blotting using anti-rP28 antiserum, and fractions containing P28 and OMP-1F were pooled. The protein sample was dialyzed against 1% OGC, 10 mM sodium phosphate buffer, pH 6.0, and loaded onto a hydroxyapatite column (bed volume, 2 ml) equilibrated with 1% OGC in 10 mM sodium phosphate, pH 6.0. After the column was washed with the same buffer, proteins were eluted with a linear gradient from 10 mM to 0.5 M sodium phosphate containing 1% OGC, followed by washing of the column with 1% OGC, 0.5 M sodium phosphate, pH 6.0. Each fraction (0.5 ml) was examined by SDS-polyacrylamide gel electrophoresis, followed by staining of the gel with GelCode Blue Stain Reagent (Pierce) or by Western blotting using anti-rP28 antiserum, and fractions containing P28 or OMP-1F were separately pooled. The purified proteins were desalted, and the buffer was changed with a PD-10 column (GE Healthcare, Piscataway, NJ) equilibrated with 1% OGC, 50 mM Tris-HCl, pH 8.0.
Proteoliposome swelling assay.
Two micrograms of OGC-solubilized outer membrane proteins, isolated native P28, or isolated native OMP-1F was reconstituted into proteoliposomes, and porin activity was determined by the proteoliposome swelling assay as described previously (7, 13, 14). Proteoliposome swelling, an indication of solute uptake, was monitored by the decrease in the optical density at 400 nm (OD400) after liposomes and solutes were mixed. The permeability of the proteoliposome to arabinose, glucose (monosaccharide), sucrose (disaccharide), stachyose (tetrasaccharide), and l-glutamine was examined. The inhibition of porin activity by anti-rP28 antiserum was examined as described previously (7). Briefly, half of the Sarkosyl-resistant outer membrane fraction was preincubated with 10 μl anti-rP28 antiserum, and the other half was preincubated with 10 μl normal rabbit serum for 1 h at room temperature. After the outer membrane fractions were washed to remove excess antibody, proteins were solubilized in 2% OGC, 2 μg solubilized outer membrane protein was reconstituted into proteoliposomes, and the proteoliposome swelling assay was performed.
Proteomic analysis.
Two micrograms each of isolated P28 and OMP-1F protein samples was digested with trypsin and analyzed by capillary liquid chromatography-nanospray tandem mass spectrometry, as described previously (5).
Secondary structure prediction.
The secondary structures of P28 and OMP-1F were predicted by measuring both hydrophobicity and the hydrophobic moment profile (9). Continuous peaks with more than six amino acid residues above the cutoff of 1.5 were considered transmembrane β-strands.
Statistical analysis.
The unpaired Student t test was applied to determine the differences between swelling rates. A P value of less than 0.05 was considered significant.
RESULTS
Porin activity of an isolated E. chaffeensis outer membrane fraction.
The porin activity was measured by the liposome swelling assay (13, 14) by using the outer membrane fraction derived from nonsynchronized E. chaffeensis cultured at 37°C. When the proteoliposome was mixed with 33 mM (isosmotic solute concentration) of the monosaccharide arabinose or glucose or with the disaccharide sucrose, swelling was observed, indicating that the solutes were transported efficiently (Fig. 1). While E. chaffeensis lacks genes for glycolysis (6), it has an incomplete tricarboxylic acid (TCA) cycle which can function with addition of l-glutamine. The E. chaffeensis outer membrane fraction was permeable to l-glutamine (Fig. 1), suggesting that the porin can feed the E. chaffeensis TCA cycle.
FIG. 1.
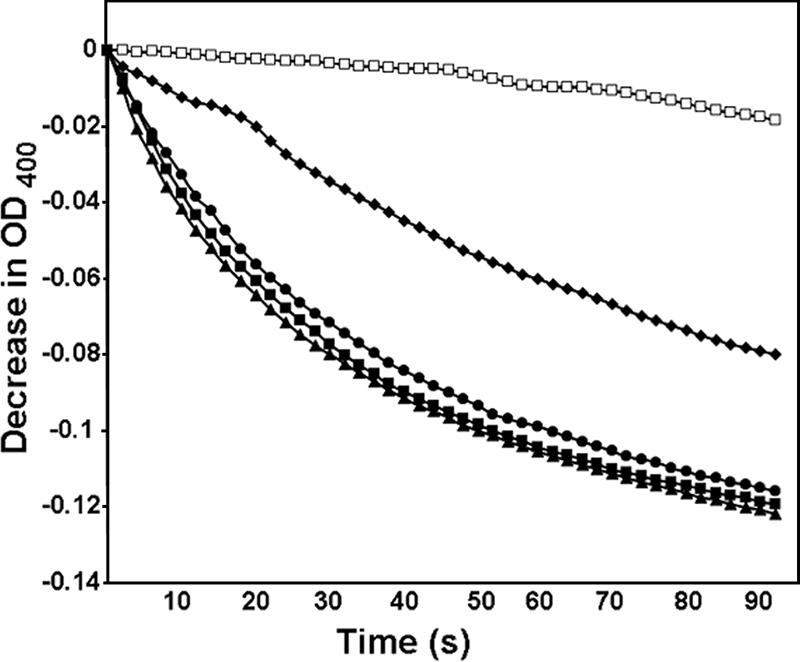
Porin activity of the E. chaffeensis outer membrane fraction. The diffusion rates of solutes into proteoliposomes reconstituted with E. chaffeensis outer membrane proteins were monitored as the decrease in OD400 when proteoliposomes were diluted in isosmotic solutions of 33 mM stachyose (Mr = 667) (□), 33 mM l-glutamine (Mr = 146) (▴), 33 mM arabinose (Mr = 150) (▪), 33 mM glucose (Mr = 180) (•), or 33 mM sucrose (Mr = 342) (⧫). The representative results of more than three independent experiments are shown.
Predicted secondary structure and physicochemical properties of P28 and OMP-1F as porins.
Porins are generally major proteins in gram-negative bacterial outer membranes (1). P28 was previously demonstrated to be a highly expressed surface-exposed protein in E. chaffeensis by using an antibody against rP28 (16). The antibody recognized two bands from E. chaffeensis lysates (Fig. 2A), a slightly less immunoreactive upper band of an apparent molecular mass 27 kDa, and a lower band of 26 kDa. The antibody also recognized rOMP-1F (Fig. 2A), consistent with the high amino acid sequence identity (77%) between P28 and OMP-1F. The rOMP-1F and rP28 were larger than native proteins due to 39 amino acids derived from the vector (Fig. 2A). The upper and lower bands in Fig. 2A correspond to native OMP-1F and P28, respectively, as preabsorption of the antibody with rOMP-1F led to a substantial decrease in the reactivity with rOMP-1F and the upper band derived from E. chaffeensis lysates, whereas it led to a lesser decrease in the reactivity with rP28 and the lower band derived from E. chaffeensis (compare Fig. 2A and B). These results suggest that the majority of anti-rP28 antiserum is rP28 specific.
FIG. 2.
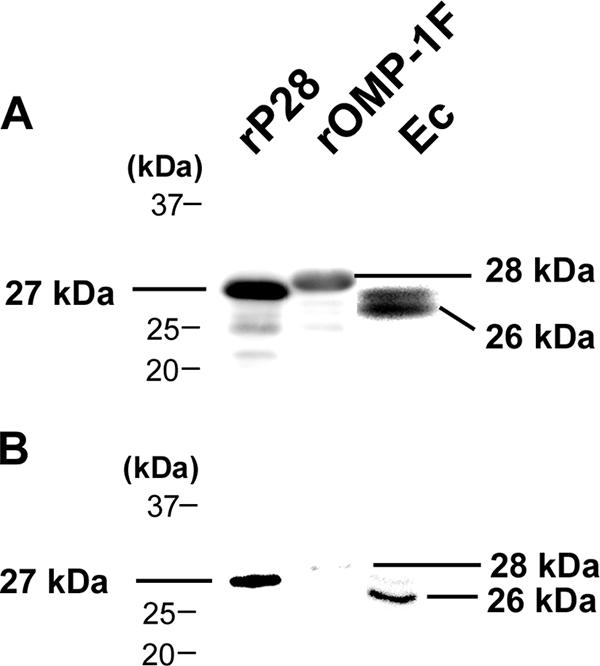
Identification of P28 and OMP-1F of E. chaffeensis. (A) Anti-rP28 antiserum recognized two bands from E. chaffeensis lysates (Ec) and rP28 (rP28) and rOMP-1F (rOMP-1F). Lysates of E. chaffeensis, BL21(DE3)/pET33b-p28, and pET33b-omp-1F were resolved by 12% SDS-polyacrylamide gel electrophoresis. Bands were detected by Western blotting using anti-rP28 antiserum. (B) Anti-rP28 antiserum preabsorbed with rOMP-1F recognized native P28 and rP28. Lysates of BL21(DE3)/pET33b-p28 (rP28), BL21(DE3)/pET33b-omp-1F (rOMP-1F), and isolated E. chaffeensis (Ec) were loaded onto a SDS-polyacrylamide stepwise reverse gradient gel consisting of 12% (lower) and 17% (upper) separation gel prepared as described previously (16). Western blotting was performed by using anti-rP28 antiserum preabsorbed with rOMP-1F.
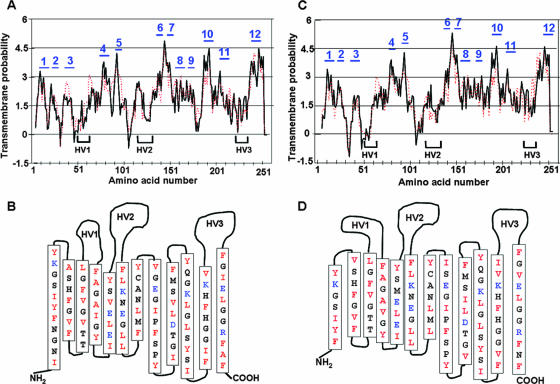
The secondary structures of P28 and OMP-1F have been predicted by measuring both hydrophobicity and the hydrophobic moment profile (9), which is thought to reasonably predict the structure of porins (12). Porins have transmembrane segments comprised of amphipathic and antiparallel β-strands (12). The following criteria were also used to predict a β-strand (9, 12, 22): (i) the length of the β-strand was between 7 and 16 amino acid residues, (ii) the strand number was an even value, with N and C termini of the proteins on the periplasmic side, (iii) the two end positions of each β-strand were occupied by lipophilic amino acids, (iv) each strand was amphipathic, (v) the β-strands were found in the conserved sequences among P28/OMP-1 family members (16), and (vi) β-strands were connected by short (<8 amino acids) “turns” on the periplasmic side and β-strands flanking the hypervariable regions were connected by long “loops” (>30 amino acids) on the external side. P28 and OMP-1F were both predicted to have 12 amphipathic transmembrane β-strands with girdles of lipophilic residues (Fig. 3A through D), which met well the criteria of a β-barrel structure. The C-terminal residue for both P28 and OMP-1F is phenylalanine, which is also true for most porins (12).
FIG. 3.
Predicted secondary structure of P28 and OMP-1F. (A and C) Membrane criterion profile of P28 (A) and OMP-1F (C). The solid black line shows normal β-strands, and the broken red line shows twisted β-strands. The x axis depicts the amino acid number starting from the N terminus of the mature proteins. Numbers in blue at the top of each peak are the numbers of predicted β-strands. Hypervariable regions (HV1, -2, and -3) of the proteins are shown. (B and D) Secondary structures of P28 (B) and OMP-1F (D) based on the results shown in panels A and C, respectively, and other criteria of porins. The residues shown are predicted to span the outer membrane; those in red are lipophilic, and those in blue are charged.
To determine whether P28 and OMP-1F form a β-barrel, the effect of detergent denaturation at room temperature (heat modifiability) of the proteins was examined. Due to the stability of the β-barrel structure, proteins containing a β-barrel are not fully denatured upon incubation with SDS-containing sample buffer at room temperature; therefore, such proteins migrate faster than do proteins of equivalent molecular weights that become denatured by heating at 95°C in the presence of SDS (24). The two proteins migrated more slowly after heat denaturation than after incubation at room temperature (Fig. 4), supporting the idea that P28 and OMP-1F each contain a β-barrel structure.
FIG. 4.
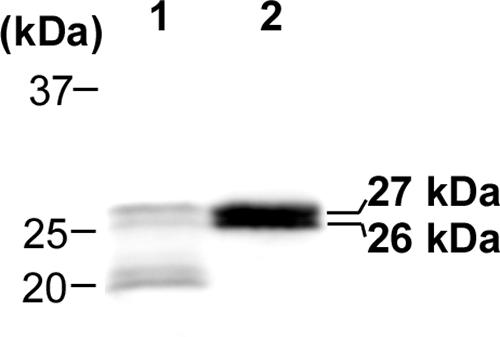
Heat modifiability of native P28 and OMP-1F. Lysates of isolated E. chaffeensis derived from a late-exponential-growth-phase culture grown at 28°C were mixed with SDS sample buffer and incubated for 5 min at room temperature (lane 1) or boiled for 5 min at 95°C (lane 2). Each sample was subjected to 12% SDS-polyacrylamide gel electrophoresis and Western blotting with rabbit anti-rP28 antiserum.
Isolation and porin activity of native P28 and OMP-1F.
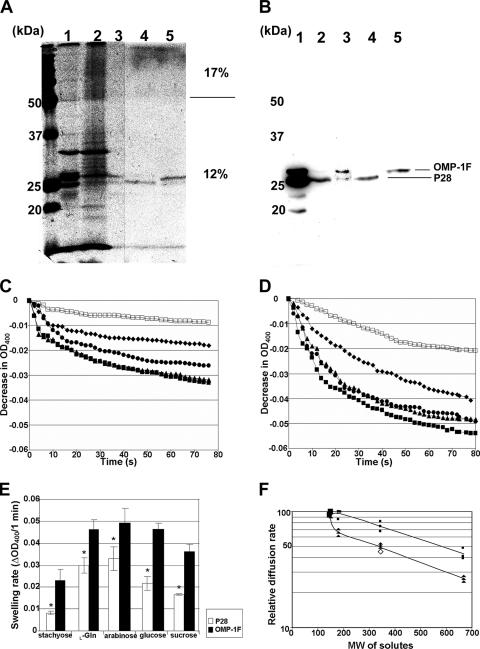
To experimentally prove that P28 and OMP-1F have porin activity, the two native proteins were isolated from the outer membrane fraction of E. chaffeensis harvested in the late exponential growth phase cultured at 28°C. Despite the difference in the calculated pIs of the two proteins (5.42 for P28 and 6.17 for OMP-1F [15]), the two proteins were not well separated with an anion exchange DEAE column equilibrated with pH 6.0 buffer (data not shown). However, the two proteins bound to the column, and most extracted outer membrane proteins were found in the flowthrough (Fig. 5A). After elution from the DEAE column, the two proteins could be separated by a hydroxyapatite column equilibrated with pH 6.0 buffer as two separate bands by GelCode Blue staining and Western blotting with anti-rP28 antiserum (Fig. 5A and B). The amounts of P28 and OMP-1F in the purified samples were estimated by using a monoclonal antibody (Ec 56.5) whose epitope resides in residues 65 to 70 (VFGLKQ) of P28/OMP-1g (11). Of all the E. chaffeensis proteins, this sequence is conserved only in P28 and OMP-1F. By comparing the band densities of 200 ng of purified samples and serially diluted rP28 or rOMP-1F (data not shown), we measured 142 ng of P28 in the isolated P28 sample and 166 ng of OMP-1F in the isolated OMP-1F sample. Thus, approximately 70% of the isolated P28 protein sample was P28, and 83% of the isolated OMP-1F protein sample was OMP-1F. Analysis by capillary liquid chromatography-nanospray tandem mass spectrometry confirmed that P28 and OMP-1F were major proteins in each of the isolated protein samples. The porin activities of the isolated proteins were determined by the liposome swelling assay with arabinose, l-glutamine, glucose, sucrose, and stachyose as solutes. Both proteins showed porin activity. OMP-1F displayed slightly higher porin activity than did P28, as the swelling rate of the proteoliposome containing OMP-1F was slightly faster than that of P28 in each solute (Fig. 5C, D, and E). The pore sizes of the two porins were estimated by plotting the logarithms of the diffusion rates for each solute against their molecular sizes. The pore size of P28 was estimated to be close to that of Pseudomonas aeruginosa OprF (13), whereas the pore size of OMP-1F was estimated as even larger (Fig. 5F).
FIG. 5.
Isolation of native P28 and OMP-1F from the E. chaffeensis outer membrane fraction and measurement of porin activity. (A and B) Outer membrane proteins extracted with 2% OGC (lane 1), DEAE column flowthrough (lane 2), DEAE column eluate (lane 3), hydroxyapatite column early eluate (lane 4), and hydroxyapatite column eluate with 0.5 M sodium phosphate buffer (lane 5) were loaded onto 12% (lower portion) and 17% (upper portion) stepwise gradient separation SDS-polyacrylamide gels. One gel was stained with GelCode Blue (A), and another gel was subjected to Western blotting (B) using rabbit anti-rP28 antiserum. (C and D) Two micrograms of isolated P28 (C) and OMP-1F (D) was reconstituted into proteoliposomes. The diffusion rates of solutes into the proteoliposomes were monitored by the decrease in the OD400 when proteoliposomes were diluted in 33 mM stachyose (Mr = 667) (□), 33 mM l-glutamine (Mr = 146) (▴), 33 mM arabinose (Mr = 150) (▪), 33 mM glucose (Mr = 180) (•), or 33 mM sucrose (Mr = 342) (⧫). The experiment was repeated twice independently in triplicates. Representative experiments are depicted in panels C and D. (E) Swelling rates (within 1 min) of proteoliposomes reconstituted with 2 μg each of purified P28 or OMP-1F in the presence of 33 mM solutes. The values are means ± standard deviations (error bars) (n = 3). Asterisks show significant differences (P < 0.05) between proteoliposomes reconstituted with native P28 and native OMP-1F. (F) Relative diffusion rates of various masses of solutes into proteoliposomes reconstituted with P28 (▴) or OMP-1F (▪). The average swelling rate in 1 min with arabinose as a solute was set at 100. The data for P. aeruginosa OprF (⋄) are from reference 13.
Differential expression of P28 and OMP-1F during E. chaffeensis intracellular development.
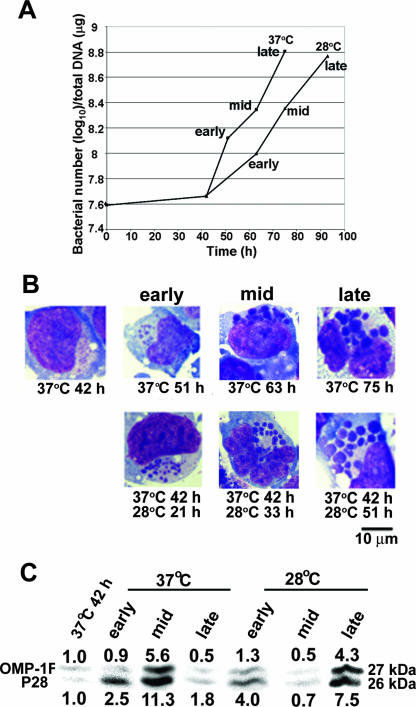
At 42 h postinfection at 37°C, small morulae (<1 μm in diameter) were detected in more than 80% of infected THP-1 cells (Fig. 6B). To compare P28 and OMP-1F expression at 37°C (corresponding to mammalian body temperature) and 28°C (the approximate temperature at which the organism develops in the tick), one-half of the infected cells was continuously cultured at 37°C and the remaining half of the culture was moved to 28°C. E. chaffeensis exponentially proliferated both at 37°C and 28°C after the 42-h incubation at 37°C, as determined by the quantity of the 16S rRNA gene (a single-copy gene in the E. chaffeensis chromosome) (Fig. 6A). The standard deviations of the three independent assays of quantitative PCR were less than 5% (data not shown). As E. chaffeensis growth was slower at 28°C than at 37°C, infected cells were harvested at three different time points: the early exponential phase (numbers of bacteria were two to three times the number at 42 h), mid-exponential phase (numbers of bacteria were about five times the number at 42 h), and late exponential phase (numbers of bacteria increased about 15-fold from the number at 42 h) of E. chaffeensis growth (Fig. 6A and B). While the development of bacterial microcolonies (morulae) at the early exponential growth phase was similar between two temperatures, at the mid-exponential growth phase at 28°C, colony sizes remained smaller than at 37°C, despite the similar numbers of bacteria (Fig. 6B). At the late exponential growth phase, the morula sizes at 28°C developed to the sizes seen at 37°C mid-exponential and late exponential growth phases (Fig. 6B).
FIG. 6.
Differential expression of P28 and OMP-1F by synchronized cultures of E. chaffeensis at 37°C and 28°C. (A) Host cell-free E. chaffeensis isolates were mixed with THP-1 cells and cultured at 37°C for 42 h until very small morulae were detected. One-half of the culture was maintained at 37°C, one-half was moved to 28°C, and incubation was continued at 37°C and 28°C, respectively. Infected cells were harvested at early exponential, mid-exponential, and late exponential growth phases. Bacterial cell numbers at each time point were determined by quantifying the 16S rRNA gene in each sample by using quantitative PCR. (B) A representative cell at each time point stained by Diff-Quik staining. Scale bar, 10 μm. (C) Expression of P28 and OMP-1F by E. chaffeensis at each 37°C and 28°C culture time point as detected by Western blotting using the mouse monoclonal antibody Ec 56.5 (11). The values below (P28) and above (OMP-1F) each band are the protein levels relative to the level measured at 42 h at 37°C, as determined by densitometric analysis.
The protein levels of P28 and OMP-1F increased in the mid-exponential growth phase and then decreased in the late exponential growth phase when cultured at 37°C, whereas the proteins were expressed at their highest levels in the late exponential growth phase when cultured at 28°C (Fig. 6C).
Porin activity differs between E. chaffeensis outer membrane fractions derived from the mid-exponential and late exponential growth phases at 37°C and 28°C.
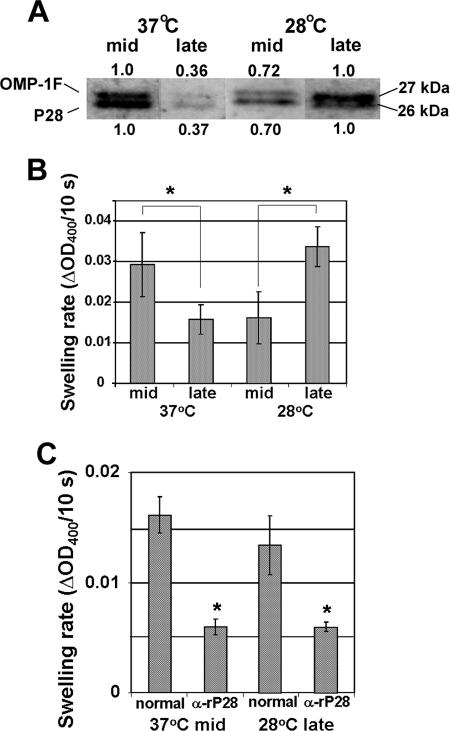
The P28 and OMP-1F levels were determined in the OGC-solubilized outer membrane fractions of E. chaffeensis derived from mid-exponential and late exponential growth phase cultures at 37°C and 28°C. In agreement with the result with the whole outer membrane fraction (Fig. 6C), P28 and OMP-1F levels were higher in the mid-exponential growth phase than in the late exponential growth phase at 37°C, whereas a higher level was observed in the late exponential growth phase at 28°C (Fig. 7A). Porin activities in the OGC-solubilized outer membrane fractions from different developmental stages of E. chaffeensis were determined by the liposome swelling assay with l-glutamine as a solute. For outer membrane fractions derived from the 37°C culture, the mid-exponential growth phase fraction showed higher porin activity than did the late exponential growth phase fraction. The converse was true for the outer membrane fractions derived from the 28°C culture: the late exponential growth phase fraction had higher porin activity than that of the mid-exponential growth phase fraction (Fig. 7B). The porin activities of the mid-exponential growth phase fraction at 28°C and the late exponential growth phase fraction at 37°C were similar, as were the activities of fractions from the mid-exponential growth phase at 37°C and the late exponential growth phase at 28°C (Fig. 7B). Thus, the porin activities corresponded approximately to the concentrations of P28 and OMP-1F.
FIG. 7.
Protein levels of P28 and OMP-1F and porin activity of E. chaffeensis OGC-extracted outer membrane fractions derived from different developmental stages. (A) The protein levels of P28 and OMP-1F in the OGC-extracted outer membrane fractions. E. chaffeensis cells were isolated from mid-exponential and late exponential growth stages at 37°C and 28°C, Sarkosyl-resistant outer membrane fractions from each E. chaffeensis sample were prepared, and outer membrane proteins were extracted with 2% OGC. One microgram of each sample was loaded onto an SDS-polyacrylamide gel, and the amounts of P28 and OMP-1F protein were assessed by Western blotting using rabbit anti-rP28 antiserum. The values below and above each band are protein levels of P28 and OMP-1F, respectively, relative to the level of protein in the mid-exponential growth phase at 37°C, as determined by densitometric analysis. (B) Porin activity detected in the OGC-extracted protein samples (2 μg), as determined by the proteoliposome swelling assay with l-glutamine as a solute. The decrease in OD400 within 10 s in the liposome swelling assay is shown. The values are means ± standard deviations (error bars) (n = 3). Asterisks show significant differences (P < 0.05) between swelling rates of proteoliposomes reconstituted with outer membrane proteins derived from mid-exponential and late exponential growth phases of E. chaffeensis. (C) Inhibition of porin activity by anti-rP28 antiserum. The Sarkosyl-resistant outer membranes derived from E. chaffeensis isolated from a mid-exponential growth phase culture at 37°C and a late exponential growth phase culture at 28°C were preincubated with 10 μl of anti-rP28 antiserum (α-rP28) or normal rabbit serum (normal). Two micrograms of protein from each sample was reconstituted into proteoliposomes. The decrease in OD400 within 10 s in the liposome swelling assay with l-glutamine as a solute is shown. The values are means ± standard deviations (error bars) (n = 3). Asterisks show significant differences (P < 0.05) between normal rabbit serum-treated and anti-rP28 antiserum-treated proteoliposomes.
Anti-rP28 antiserum partially inhibits porin activity.
To test the specificity of the porin activity in the proteoliposomes reconstituted with OGC-solubilized proteins, the outer membrane fractions derived from the 37°C mid-exponential growth phase and the 28°C late exponential growth phase, expressing the highest levels of P28 and OMP-1F at each temperature (Fig. 6C), were preincubated with anti-rP28 antiserum or with control rabbit serum. For both outer membrane fractions, the porin activities of the proteoliposomes reconstituted from anti-rP28 antiserum-treated outer membranes were reduced by approximately 50% compared to that of control proteoliposomes reconstituted from rabbit serum-treated outer membranes (Fig. 7C).
DISCUSSION
P28 and OMP-1F of E. chaffeensis exhibited many characteristic features of gram-negative bacterial porins (9, 12, 22). Membrane proteins with a β-barrel structure contain between 8 and 22 transmembrane β-strands (22). E. chaffeensis P28 and OMP-1F are each predicted to contain a β-barrel comprised of 12 transmembrane β-strands. In most cases, porin proteins have been demonstrated or predicted to contain 16 β-strands (12). An 18-β-strand porin has also been suggested (10, 33). While β barrel proteins with 8 β-strands, such as E. coli OmpX (29), Neisseria meningitidis NspA (28), and Comamonas acidovorans Omp21 (2), and a 10-β-strand protein, OpcA, of N. meningitidis (19), have been suggested to serve as outer membrane anchors rather than forming pores (2, 12), the proteoliposome swelling data presented here suggest that the 12 β-strands of P28 and OMP-1F form pores. Confirmation of the secondary structure of P28 and OMP-1F awaits crystal structure analysis.
P28/OMP-1 family proteins are encoded by a polymorphic multigene family of 22 genes (15), and 19 of the 22 proteins are surface exposed in E. chaffeensis (5). The paralogs contain conserved regions and three hypervariable regions (16, 20, 30). Most P28/OMP-1 paralogs are likely to fold in a manner similar to those of P28 and OMP-1F, with each paralog-specific region forming an extracellular loop and the conserved regions forming β-strands. The concentration of total P28/OMP-1 family proteins in the outer membrane may determine the overall transport capacity of this bacterium. About 70% of the protein in the purified P28 fraction and approximately 83% of the protein in the purified OMP-1F fraction were P28 and OMP-1F, respectively. At least 2 μg of protein is required to demonstrate liposome swelling activity (12), and the activity is proportional to the protein amount because porin is a membrane diffusion channel, not a catalytic enzyme. Anti-rP28 antiserum partially neutralized the porin activity. Thus, most of the porin activity of the purified P28 and OMP-1F samples is likely directly attributable to P28 and OMP-1F, respectively.
Despite the similarity of amino acid sequences and calculated molecular masses of the mature forms of P28 (27.7 kDa) and OMP-1F (27.9 kDa), the pore size of OMP-1F porin appeared to be larger than that of P28 porin. On the basis of the diffusion rates of the larger solutes, sucrose and stachyose, the pore sizes of both P28 and OMP-1F were estimated to be larger than that of E. coli OmpF (14). The pore size of P28 was similar to that of the free-living bacterium P. aeruginosa OprF (13), and the pore size of OMP-1F was even larger. To the best of our knowledge, a porin protein with a pore size larger than OprF has not been reported. The larger pore size of OMP-1F might allow E. chaffeensis to take up larger molecules, such as nucleoside triphosphates and polyamine required for intracellular growth.
Here we have shown that P28 and OMP-1F can allow efficient diffusion of l-glutamine. Like that of A. phagocytophilum (7), the TCA cycle of E. chaffeensis is incomplete because the gene for isocitrate dehydrogenase is missing from the genome (6). In order for the TCA cycle to function in both A. phagocytophilum and E. chaffeensis, these organisms must acquire exogenous 2-oxoglutarate or glutamate. E. chaffeensis has the enzyme GMP synthase (glutamine hydrolyzing) (GenBank no. YP_506951), which catalyzes the conversion of l-glutamine to l-glutamate. This observation suggests that like P44 in A. phagocytophilum, P28 and OMP-1F may feed the Ehrlichia TCA cycle and provide E. chaffeensis with carbon and energy production intermediates.
P28 and OMP-1F were differentially expressed during the intracellular developmental stages of E. chaffeensis. The differential expression pattern of P28 by E. chaffeensis cultured in THP-1 cells at 37°C was somewhat similar to that of the previously reported expression pattern in DH82 cells at 37°C, which showed that P28 was expressed in RCs and in the intermediate form between RCs and DCs, but not in DCs (32), despite the differences in the host cells and the methods of culture synchronization. Higher porin activity was observed at higher protein expression levels in the mid-exponential growth phase of bacteria cultured at 37°C, perhaps reflecting the massive uptake of nutrients required by the bacteria to allow proliferation. On the other hand, during the late exponential growth phase, bacteria cease to proliferate and differentiate into DCs, thus not requiring a substantial uptake of nutrients. Consistent with this view are the decreased levels of P28 and OMP-1F that we observed at the late exponential growth phase and the lower porin activity of the outer membrane fraction from bacteria cultured at 37°C.
We demonstrated that temperature can change P28 and OMP-1F expression and, as a result, overall outer membrane porin activity. Unexpectedly, the two proteins were expressed higher in the late exponential growth phase in organisms cultured at 28°C. When E. chaffeensis-infected monocytes in the blood meal are taken up by ticks on the mammalian skin surface, the tick midgut temperature is expected to be closer to 28°C than to 37°C. Thus, the higher porin activity at the late exponential growth stage at 28°C may be advantageous for E. chaffeensis to acquire as much nutrients as possible for survival and colonization in ticks.
As porins are essential for gram-negative bacteria, they are generally vaccine candidates for gram-negative bacteria, including facultative intracellular bacteria (8) as well as obligatory intracellular bacteria (18). Anti-rP28 antiserum was shown to facilitate E. chaffeensis clearance in immunocompetent mice (16), and a monoclonal antibody against OMP-1g (P28) has been shown to protect SCID mice from fatal E. chaffeensis infection (11), supporting the idea that E. chaffeensis P28 and/or OMP-1F may serve as vaccine candidates to prevent HME.
Acknowledgments
We thank G. M. Winslow for kindly providing the mouse monoclonal antibody Ec 56.5 and X. Wang for assistance with the proteoliposome swelling assay. We appreciate H. Nikaido for his valuable advice.
This work was supported by National Institutes of Health grants R01AI30010 and R01AI47407.
Footnotes
Published ahead of print on 21 March 2008.
REFERENCES
- 1.Achouak, W., T. Heulin, and J. M. Pagès. 2001. Multiple facets of bacterial porins. FEMS Microbiol. Lett. 1991-7. [DOI] [PubMed] [Google Scholar]
- 2.Baldermann, C., and H. Engelhardt. 2000. Expression, two-dimensional crystallization, and three-dimensional reconstruction of the β8 outer membrane protein Omp21 from Comamonas acidovorans. J. Struct. Biol. 13196-107. [DOI] [PubMed] [Google Scholar]
- 3.Cheng, Z., Y. Kumagai, M. Lin, C. Zhang, and Y. Rikihisa. 2006. Intra-leukocyte expression of two-component systems in Ehrlichia chaffeensis and Anaplasma phagocytophilum and effects of the histidine kinase inhibitor closantel. Cell. Microbiol. 81241-1252. [DOI] [PubMed] [Google Scholar]
- 4.Demma, L. J., R. C. Holman, J. H. McQuiston, J. W. Krebs, and D. L. Swerdlow. 2006. Human monocytic ehrlichiosis and human granulocytic anaplasmosis in the United States, 2001-2002. Ann. N. Y. Acad. Sci. 1078118-119. [DOI] [PubMed] [Google Scholar]
- 5.Ge, Y., and Y. Rikihisa. 2007. Surface-exposed proteins of Ehrlichia chaffeensis. Infect. Immun. 753833-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hotopp, J. C. D., M. Lin, R. Madupu, J. Crabtree, S. V. Angiuoli, J. Eisen, R. Seshadri, Q. Ren, M. Wu, T. R. Utterback, S. Smith, M. Lewis, H. Khouri, C. Zhang, H. Niu, Q. Lin, N. Ohashi, N. Zhi, W. Nelson, L. M. Brinkac, R. J. Dodson, M. J. Rosovitz, J. Sundaram, S. C. Daugherty, T. Davidsen, A. Durkin, M. Gwinn, D. H. Haft, J. D. Selengut, S. A. Sullivan, N. Zafar, L. Zhou, F. Benahmed, H. Forberger, R. Halpin, S. Mulligan, J. Robinson, O. White, Y. Rikihisa, and H. Tettelin. 2006. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang, H., X. Wang, T. Kikuchi, Y. Kumagai, and Y. Rikihisa. 2007. Porin activity of Anaplasma phagocytophilum outer membrane fraction and purified P44. J. Bacteriol. 1891998-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humphries, H. E., J. N. Williams, R. Blackstone, K. A. Jolley, H. M. Yuen, M. Christodoulides, and J. E. Heckels. 2006. Multivalent liposome-based vaccines containing different serosubtypes of PorA protein induce cross-protective bactericidal immune responses against Neisseria meningitidis. Vaccine 2436-44. [DOI] [PubMed] [Google Scholar]
- 9.Jeanteur, D., J. H. Lakey, and F. Pattus. 1991. The bacterial porin superfamily: sequence alignment and structure prediction. Mol. Microbiol. 52153-2164. [DOI] [PubMed] [Google Scholar]
- 10.Labesse, G., E. Garnotel, S. Bonnel, C. Dumas, J. M. Pages, and J. M. Bolla. 2001. MOMP, a divergent porin from Campylobacter: cloning and primary structural characterization. Biochem. Biophys. Res. Commun. 280380-387. [DOI] [PubMed] [Google Scholar]
- 11.Li, J. S., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, A. A. Reilly, F. K. Chu, and G. M. Winslow. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 1661855-1862. [DOI] [PubMed] [Google Scholar]
- 12.Nikaido, H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67593-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikaido, H., K. Nikaido, and S. Harayama. 1991. Identification and characterization of porins in Pseudomonas aeruginosa. J. Biol. Chem. 266770-779. [PubMed] [Google Scholar]
- 14.Nikaido, H., and E. Y. Rosenberg. 1983. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J. Bacteriol. 153241-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohashi, N., Y. Rikihisa, and A. Unver. 2001. Analysis of transcriptionally active gene clusters of major outer membrane protein multigene family in Ehrlichia canis and E. chaffeensis. Infect. Immun. 692083-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohashi, N., N. Zhi, Y. Zhang, and Y. Rikihisa. 1998. Immunodominant major outer membrane proteins of Ehrlichia chaffeensis are encoded by a polymorphic multigene family. Infect. Immun. 66132-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 1637-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pal, S., E. M. Peterson, and L. M. de la Maza. 2005. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 738153-8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prince, S. M., M. Achtman, and J. P. Derrick. 2002. Crystal structure of the OpcA integral membrane adhesin from Neisseria meningitidis. Proc. Natl. Acad. Sci. USA 993417-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reddy, G. R., C. R. Sulsona, A. F. Barbet, S. M. Mahan, M. J. Burridge, and A. R. Alleman. 1998. Molecular characterization of a 28 kDa surface antigen gene family of the tribe Ehrlichiae. Biochem. Biophys. Res. Commun. 247636-643. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Schulz, G. E. 2002. The structure of bacterial outer membrane proteins. Biochim. Biophys. Acta 1565308-317. [DOI] [PubMed] [Google Scholar]
- 23.Singu, V., L. Peddireddi, K. R. Sirigireddy, C. Cheng, U. Munderloh, and R. R. Ganta. 2006. Unique macrophage and tick cell-specific protein expression from the p28/p30-outer membrane protein multigene locus in Ehrlichia chaffeensis and Ehrlichia canis. Cell. Microbiol. 81475-1487. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara, E., M. Steiert, S. Rouhani, and H. Nikaido. 1996. Secondary structure of the outer membrane proteins OmpA of Escherichia coli and OprF of Pseudomonas aeruginosa. J. Bacteriol. 1786067-6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Unver, A., N. Ohashi, T. Tajima, R. W. Stich, D. Grover, and Y. Rikihisa. 2001. Transcriptional analysis of p30 major outer membrane multigene family of Ehrlichia canis in dogs, ticks, and cell culture at different temperatures. Infect. Immun. 696172-6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Unver, A., Y. Rikihisa, N. Ohashi, L. C. Cullman, R. Buller, and G. A. Storch. 1999. Western and dot blotting analyses of Ehrlichia chaffeensis indirect fluorescent-antibody assay-positive and -negative human sera by using native and recombinant E. chaffeensis and E. canis antigens. J. Clin. Microbiol. 373888-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Unver, A., Y. Rikihisa, R. W. Stich, N. Ohashi, and S. Felek. 2002. The omp-1 major outer membrane multigene family of Ehrlichia chaffeensis is differentially expressed in canine and tick hosts. Infect. Immun. 704701-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandeputte-Rutten, L., M. P. Bos, J. Tommassen, and P. Gros. 2003. Crystal structure of Neisserial surface protein A (NspA), a conserved outer membrane protein with vaccine potential. J. Biol. Chem. 27824825-24830. [DOI] [PubMed] [Google Scholar]
- 29.Vogt, J., and G. E. Schulz. 1999. The structure of the outer membrane protein OmpX from Escherichia coli reveals possible mechanisms of virulence. Structure 71301-1309. [DOI] [PubMed] [Google Scholar]
- 30.Yu, X. J., J. W. McBride, and D. H. Walker. 1999. Genetic diversity of the 28-kilodalton outer membrane protein gene in human isolates of Ehrlichia chaffeensis. J. Clin. Microbiol. 371137-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang, J. Z., H. Guo, G. M. Winslow, and X. J. Yu. 2004. Expression of members of the 28-kilodalton major outer membrane protein family of Ehrlichia chaffeensis during persistent infection. Infect. Immun. 724336-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, J. Z., V. L. Popov, S. Gao, D. H. Walker, and X. J. Yu. 2007. The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cell. Microbiol. 9610-618. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Q., J. C. Meitzler, S. Huang, and T. Morishita. 2000. Sequence polymorphism, predicted secondary structures, and surface-exposed conformational epitopes of Campylobacter major outer membrane protein. Infect. Immun. 685679-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]